Microbial Matryoshka: Addressing the Relationship between Pathogenic Flagellated Protozoans and Their RNA Viral Endosymbionts (Family Totiviridae)
Abstract
Simple Summary
Abstract
1. Introduction
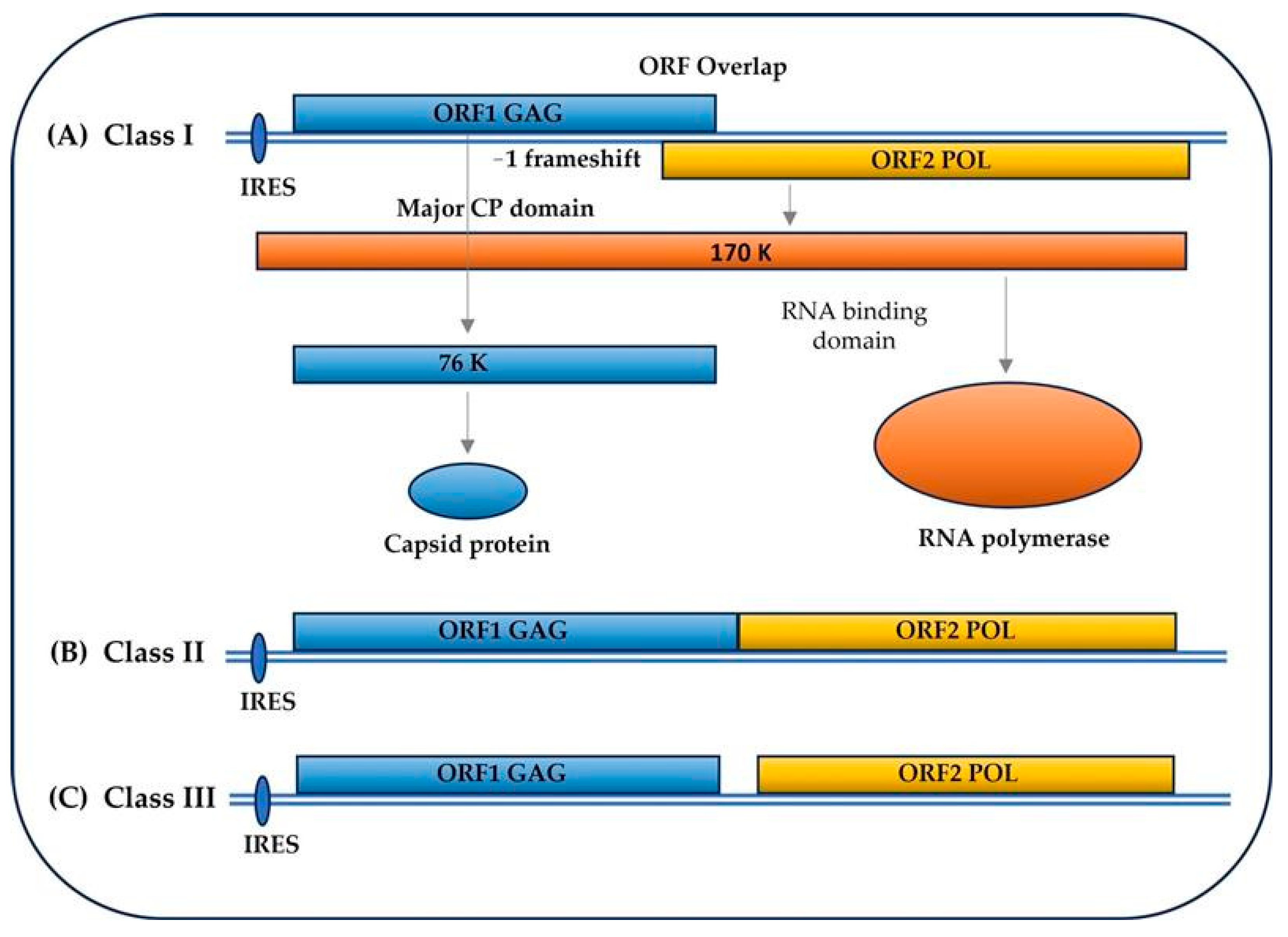
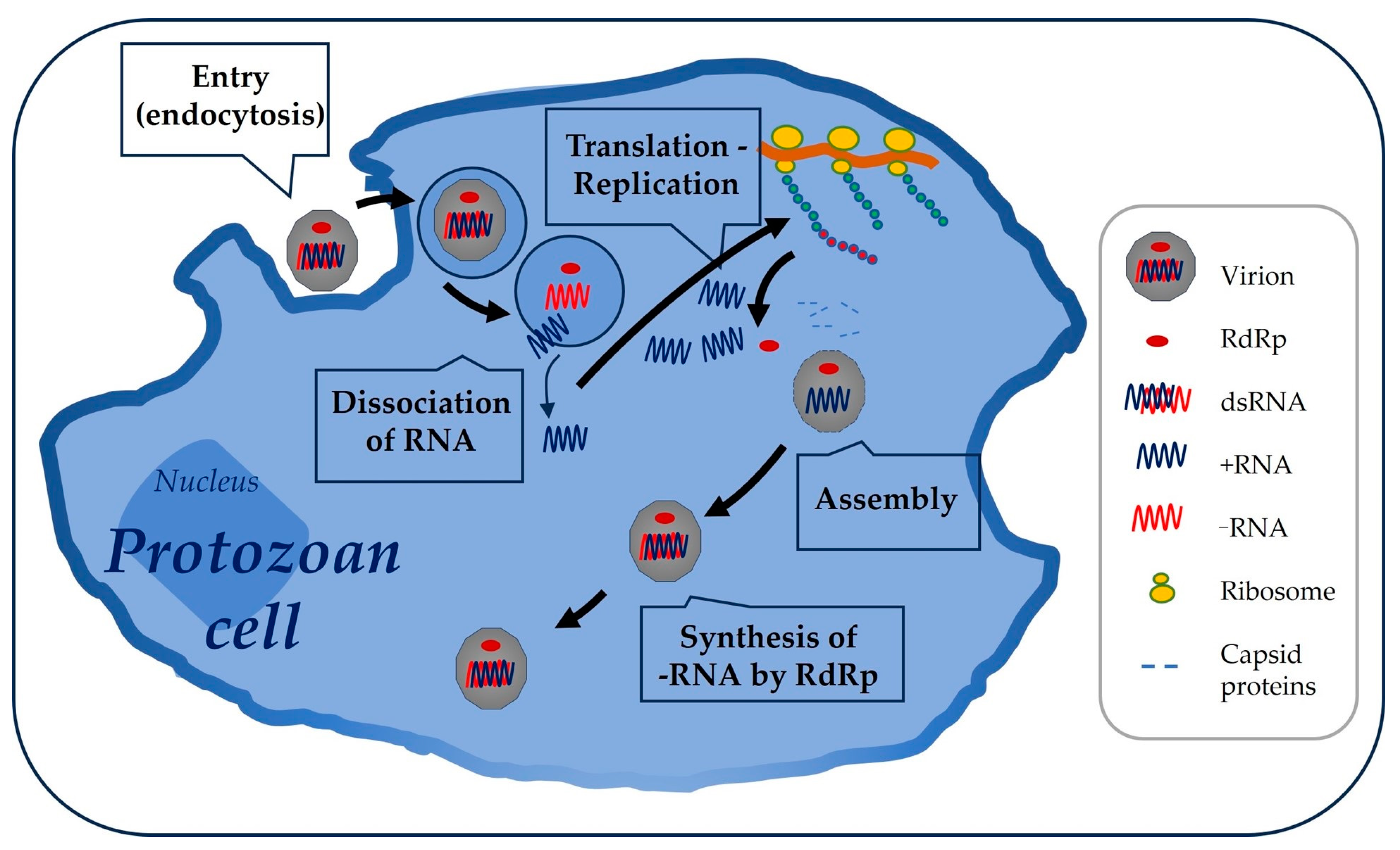
2. Totiviridae Family: Viral and Genomic Structure, Cycle, and Other Details
3. Flagellated Protozoans of Health Relevance
3.1. Giardia-Giardiavirus
3.1.1. Endosymbiotic Relationship
3.1.2. Endosymbiotic Modulation of Virulence and Immune Response
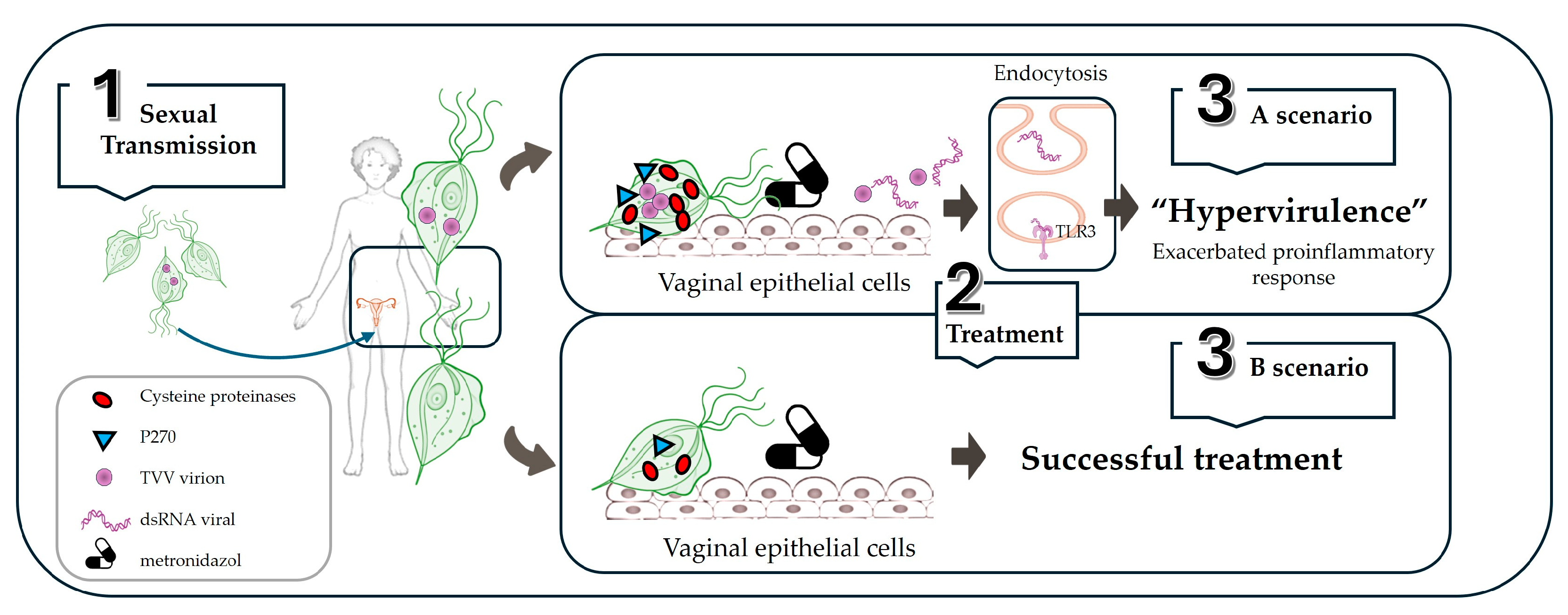
3.2. Trichomonas-Trichomonasvirus
3.2.1. Endosymbiotic Relationship
3.2.2. Endosymbiont Modulation of Virulence and Immune Response
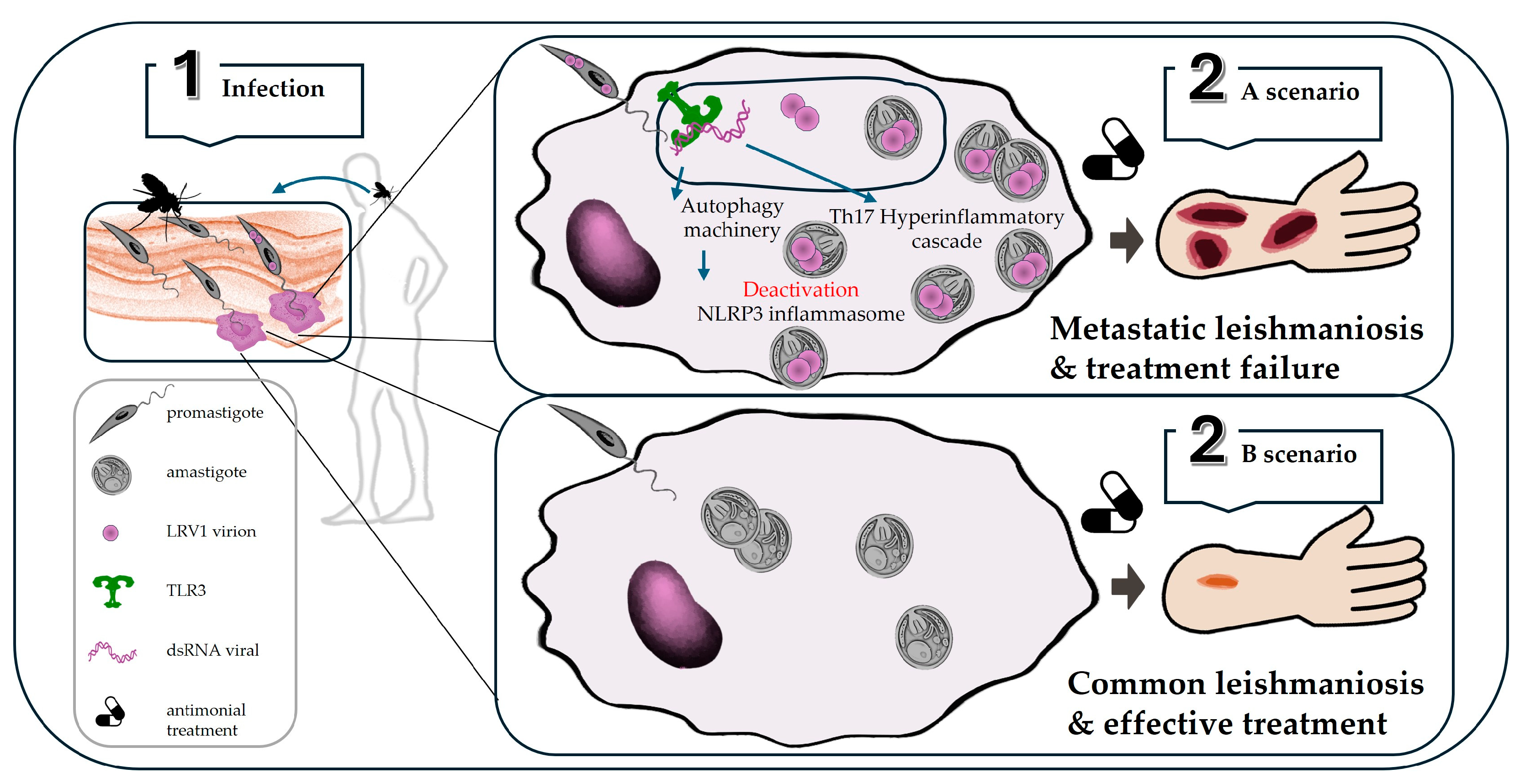
3.3. Leishmania-Leishmaniavirus
3.3.1. Endosymbiotic Relationship
3.3.2. Endosymbiont Modulation of Virulence and Immune Response
3.4. Trypanosoma and Other Suspected VLPs
4. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LPG | Lipophosphoglycan |
| iNOS | Inducible nitric oxide synthase |
| NO | Nitric oxide |
| ROS | Reactive oxygen species |
| TLR | Toll-like receptor |
References
- Capela, R.; Moreira, R.; Lopes, F. An Overview of Drug Resistance in Protozoal Diseases. Int. J. Mol. Sci. 2019, 20, 5748. [Google Scholar] [CrossRef] [PubMed]
- Janssen, M.E.; Takagi, Y.; Parent, K.N.; Cardone, G.; Nibert, M.L.; Baker, T.S. Three-dimensional structure of a protozoal double-stranded RNA virus that infects the enteric pathogen Giardia lamblia. J. Virol. 2015, 89, 1182–1194. [Google Scholar] [CrossRef] [PubMed]
- de Lima, J.G.S.; Lima, J.P.M.S.; Lanza, D.C.F. Giardiavirus (Totiviridae). In Encyclopedia of Virology; Elsevier Science: Amsterdam, The Netherlands, 2021; pp. 582–588. [Google Scholar]
- Hillman, B.I.; Cohen, A.B. Totiviruses (Totiviridae). In Encyclopedia of Virology; Elsevier Science: Amsterdam, The Netherlands, 2021; pp. 648–657. [Google Scholar]
- Billker, O.; Charon, J.; Grigg, M.J.; Eden, J.-S.; Piera, K.A.; Rana, H.; William, T.; Rose, K.; Davenport, M.P.; Anstey, N.M.; et al. Novel RNA viruses associated with Plasmodium vivax in human malaria and Leucocytozoon parasites in avian disease. PLoS Pathog. 2019, 15, e1008216. [Google Scholar] [CrossRef]
- Wang, A.L.; Wang, C.C. Discovery of a specific double-stranded RNA virus in Giardia lamblia. Mol. Biochem. Parasitol. 1986, 21, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Li, X.; Zhang, N.; Li, J.; Zhao, N.; Gao, M.; Zhang, X.; Wang, X.; Zhao, P.; Li, L.; et al. Multiple Regulations of Parasitic Protozoan Viruses: A Double-Edged Sword for Protozoa. MBio 2023, 14, e0264222. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Arreaza, A.; Haenni, A.L.; Dunia, I.; Avilan, L. Viruses of parasites as actors in the parasite-host relationship: A “menage a trois”. Acta Trop. 2017, 166, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Ives, A.; Ronet, C.; Prevel, F.; Ruzzante, G.; Fuertes-Marraco, S.; Schutz, F.; Zangger, H.; Revaz-Breton, M.; Lye, L.-F.; Hickerson, S.M.; et al. Leishmania RNA Virus Controls the Severity of Mucocutaneous Leishmaniasis. Science 2011, 331, 775–778. [Google Scholar] [CrossRef] [PubMed]
- Klebanoff, M.A.; Carey, J.C.; Hauth, J.C.; Hillier, S.L.; Nugent, R.P.; Thom, E.A.; Ernest, J.M.; Heine, R.P.; Wapner, R.J.; Trout, W.; et al. Failure of Metronidazole to Prevent Preterm Delivery among Pregnant Women with Asymptomatic Trichomonas vaginalis Infection. N. Engl. J. Med. 2001, 345, 487–493. [Google Scholar] [CrossRef]
- Zangger, H.; Ronet, C.; Desponds, C.; Kuhlmann, F.M.; Robinson, J.; Hartley, M.A.; Prevel, F.; Castiglioni, P.; Pratlong, F.; Bastien, P.; et al. Detection of Leishmania RNA virus in Leishmania parasites. PLoS Neglected Trop. Dis. 2013, 7, e2006. [Google Scholar] [CrossRef]
- Fasel, N.; Fichorova, R.N.; Lee, Y.; Yamamoto, H.S.; Takagi, Y.; Hayes, G.R.; Goodman, R.P.; Chepa-Lotrea, X.; Buck, O.R.; Murray, R.; et al. Endobiont Viruses Sensed by the Human Host—Beyond Conventional Antiparasitic Therapy. PLoS ONE 2012, 7, e48418. [Google Scholar] [CrossRef]
- Stevens, A.; Muratore, K.; Cui, Y.; Johnson, P.J.; Zhou, Z.H.; Hendrickson, W.A. Atomic Structure of the Trichomonas vaginalis Double-Stranded RNA Virus 2. MBio 2021, 12, e02924-20. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, K.; Ferreira, R.J.; Larsson, D.S.D.; Maia, F.R.N.C.; Isawa, H.; Sawabe, K.; Murata, K.; Hajdu, J.; Iwasaki, K.; Kasson, P.M.; et al. Acquired Functional Capsid Structures in Metazoan Totivirus-like dsRNA Virus. Structure 2020, 28, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Parent, K.N.; Takagi, Y.; Cardone, G.; Olson, N.H.; Ericsson, M.; Yang, M.; Lee, Y.; Asara, J.M.; Fichorova, R.N.; Baker, T.S.; et al. Structure of a Protozoan Virus from the Human Genitourinary Parasite Trichomonas vaginalis. MBio 2013, 4, e00056-13. [Google Scholar] [CrossRef] [PubMed]
- Graves, K.J.; Ghosh, A.P.; Kissinger, P.J.; Muzny, C.A. Trichomonas vaginalis virus: A review of the literature. Int. J. STD AIDS 2019, 30, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.W.; Chu, Y.D.; Tai, J.H. Characterization of Trichomonas vaginalis virus proteins in the pathogenic protozoan T. vaginalis. Arch. Virol. 2014, 143, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Furfine, E.S.; Wang, C.C. Transfection of the Giardia lamblia Double-Stranded RNA Virus into Giardia lamblia by Electroporation of a Single-Stranded RNA Copy of the Viral Genome. Mol. Cell. Biol. 2023, 10, 3659–3663. [Google Scholar] [CrossRef]
- Olendraite, I.; Brown, K.; Firth, A.E. Identification of RNA Virus-Derived RdRp Sequences in Publicly Available Transcriptomic Data Sets. Mol. Biol. Evol. 2023, 40, msad060. [Google Scholar] [CrossRef] [PubMed]
- Lagunas-Rangel, F.A.; Kameyama-Kawabe, L.Y.; Bermudez-Cruz, R.M. Giardiavirus: An update. Parasitol. Res. 2021, 120, 1943–1948. [Google Scholar] [CrossRef] [PubMed]
- Dinman, J.D.; Icho, T.; Wickner, R.B. A-1 ribosomal frameshift in a double-stranded RNA virus of yeast forms a gag-pol fusion protein. Proc. Natl. Acad. Sci. USA 1991, 88, 174–178. [Google Scholar] [CrossRef]
- Zhao, M.; Xu, L.; Bowers, H.; Schott, E.J. Characterization of Two Novel Toti-Like Viruses Co-infecting the Atlantic Blue Crab, Callinectes sapidus, in Its Northern Range of the United States. Front. Microbiol. 2022, 13, 855750. [Google Scholar] [CrossRef]
- Bekaert, M.; Rousset, J.-P. An Extended Signal Involved in Eukaryotic −1 Frameshifting Operates through Modification of the E Site tRNA. Mol. Cell 2005, 17, 61–68. [Google Scholar] [CrossRef]
- Khalifa, M.E.; MacDiarmid, R.M. A Novel Totivirus Naturally Occurring in Two Different Fungal Genera. Front. Microbiol. 2019, 10, 2318. [Google Scholar] [CrossRef]
- Carrion, R.; Ro, Y.T.; Patterson, J.L. Leishmaniaviruses. In Encyclopedia of Virology; Elsevier Science: Amsterdam, The Netherlands, 2008; pp. 220–224. [Google Scholar]
- Goodman, R.P.; Ghabrial, S.A.; Fichorova, R.N.; Nibert, M.L. Trichomonasvirus: A new genus of protozoan viruses in the family Totiviridae. Arch. Virol. 2010, 156, 171–179. [Google Scholar] [CrossRef]
- Wang, A.L.; Wang, C.C. Viruses of parasitic protozoa. Parasitol. Today 1991, 7, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Graves, K.J.; Ghosh, A.P.; Schmidt, N.; Augostini, P.; Secor, W.E.; Schwebke, J.R.; Martin, D.H.; Kissinger, P.J.; Muzny, C.A. Trichomonas vaginalis Virus Among Women With Trichomoniasis and Associations With Demographics, Clinical Outcomes, and Metronidazole Resistance. Clin. Infect. Dis. 2019, 69, 2170–2176. [Google Scholar] [CrossRef] [PubMed]
- Hartley, M.-A.; Ronet, C.; Zangger, H.; Beverley, S.M.; Fasel, N. Leishmania RNA virus: When the host pays the toll. Front. Cell. Infect. Microbiol. 2012, 2, 99. [Google Scholar] [CrossRef]
- Yu, D.; Wang, C.C.; Wang, A.L. Maturation of giardiavirus capsid protein involves posttranslational proteolytic processing by a cysteine protease. J. Virol. 1995, 69, 2825–2830. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.-C.; Cheng, W.-H.; Ku, F.-M.; Tsai, C.-Y.; Huang, P.-J.; Lee, C.-C.; Yeh, Y.-M.; Rada, P.; Hrdý, I.; Narayanasamy, R.K.; et al. Identification of Endosymbiotic Virus in Small Extracellular Vesicles Derived from Trichomonas vaginalis. Genes 2022, 13, 531. [Google Scholar] [CrossRef]
- Rada, P.; Hrdý, I.; Zdrha, A.; Narayanasamy, R.K.; Smutná, T.; Horáčková, J.; Harant, K.; Beneš, V.; Ong, S.-C.; Tsai, C.-Y.; et al. Double-Stranded RNA Viruses Are Released From Trichomonas vaginalis Inside Small Extracellular Vesicles and Modulate the Exosomal Cargo. Front. Microbiol. 2022, 13, 893692. [Google Scholar] [CrossRef]
- Atayde, V.D.; Hassani, K.; da Silva Lira Filho, A.; Borges, A.R.; Adhikari, A.; Martel, C.; Olivier, M. Leishmania exosomes and other virulence factors: Impact on innate immune response and macrophage functions. Cell. Immunol. 2016, 309, 7–18. [Google Scholar] [CrossRef]
- Olivier, M.; Zamboni, D.S. Leishmania Viannia guyanensis, LRV1 virus and extracellular vesicles: A dangerous trio influencing the faith of immune response during muco-cutaneous leishmaniasis. Curr. Opin. Immunol. 2020, 66, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Kerviel, A.; Zhang, M.; Altan-Bonnet, N. A New Infectious Unit: Extracellular Vesicles Carrying Virus Populations. Annu. Rev. Cell Dev. Biol. 2021, 37, 171–197. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Lozano-Amado, D.; Chowdhury, D.; Singh, U. Extracellular Vesicles and Their Impact on the Biology of Protozoan Parasites. Trop. Med. Infect. Dis. 2023, 8, 448. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Xiao, L. Zoonotic potential and molecular epidemiology of Giardia species and giardiasis. Clin. Microbiol. Rev. 2011, 24, 110–140. [Google Scholar] [CrossRef] [PubMed]
- Fink, M.Y.; Shapiro, D.; Singer, S.M. Giardia lamblia: Laboratory Maintenance, Lifecycle Induction, and Infection of Murine Models. Curr. Protoc. Microbiol. 2020, 57, e102. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.K.C.; Leung, A.A.M.; Wong, A.H.C.; Sergi, C.M.; Kam, J.K.M. Giardiasis: An Overview. Recent Pat Inflamm. Allergy Drug Discov. 2019, 13, 134–143. [Google Scholar] [CrossRef]
- Nosala, C.; Dawson, S.C. The Critical Role of the Cytoskeleton in the Pathogenesis of Giardia. Curr. Clin. Microbiol. Rep. 2015, 2, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Muhsen, K.; Levine, M.M. A systematic review and meta-analysis of the association between Giardia lamblia and endemic pediatric diarrhea in developing countries. Clin. Infect. Dis. 2012, 55 (Suppl. 4), S271–S293. [Google Scholar] [CrossRef] [PubMed]
- Hamnes, I.S.; Gjerde, B.K.; Robertson, L.J. A longitudinal study on the occurrence of Cryptosporidium and Giardia in dogs during their first year of life. Acta Vet. Scand. 2007, 49, 22. [Google Scholar] [CrossRef]
- Ryan, U.M.; Feng, Y.; Fayer, R.; Xiao, L. Taxonomy and molecular epidemiology of Cryptosporidium and Giardia—A 50 year perspective (1971–2021). Int. J. Parasitol. 2021, 51, 1099–1119. [Google Scholar] [CrossRef]
- Currie, S.L.; Stephenson, N.; Palmer, A.S.; Jones, B.L.; Hawkins, G.; Alexander, C.L. Under-reporting giardiasis: Time to consider the public health implications. Epidemiol. Infect. 2017, 145, 3007–3011. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zahedi, A.; Field, D.; Ryan, U. Molecular typing of Giardia duodenalis in humans in Queensland—First report of Assemblage E. Parasitology 2017, 144, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Kraft, M.R.; Klotz, C.; Bücker, R.; Schulzke, J.-D.; Aebischer, T. Giardia’s Epithelial Cell Interaction In Vitro: Mimicking Asymptomatic Infection? Front. Cell. Infect. Microbiol. 2017, 7, 421. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.L.; Yang, H.M.; Shen, K.A.; Wang, C.C. Giardiavirus double-stranded RNA genome encodes a capsid polypeptide and a gag-pol-like fusion protein by a translation frameshift. Proc. Natl. Acad. Sci. USA 1993, 90, 8595–8599. [Google Scholar] [CrossRef] [PubMed]
- Sepp, T.; Wang, A.L.; Wang, C.C. Giardiavirus-resistant Giardia lamblia lacks a virus receptor on the cell membrane surface. J. Virol. 1994, 68, 1426–1431. [Google Scholar] [CrossRef] [PubMed]
- de Lima, J.G.S.; Teixeira, D.G.; Freitas, T.T.; Lima, J.; Lanza, D.C.F. Evolutionary origin of 2A-like sequences in Totiviridae genomes. Virus. Res. 2019, 259, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Gong, P.; Li, J.; Zhang, X.; Zou, X.; Tuo, W.; Liu, Q.; Wang, Q.; Zhang, G.; Chen, L.; et al. Giardia canis: Ultrastructural analysis of G. canis trophozoites transfected with full length G. canis virus cDNA transcripts. Exp. Parasitol. 2009, 123, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Mata, C.P.; Rodriguez, J.M.; Suzuki, N.; Caston, J.R. Structure and assembly of double-stranded RNA mycoviruses. Adv. Virus. Res. 2020, 108, 213–247. [Google Scholar] [CrossRef] [PubMed]
- Marucci, G.; Zullino, I.; Bertuccini, L.; Camerini, S.; Cecchetti, S.; Pietrantoni, A.; Casella, M.; Vatta, P.; Greenwood, A.D.; Fiorillo, A.; et al. Re-Discovery of Giardiavirus: Genomic and Functional Analysis of Viruses from Giardia duodenalis Isolates. Biomedicines 2021, 9, 654. [Google Scholar] [CrossRef]
- Kinsella, C.M.; Bart, A.; Deijs, M.; Broekhuizen, P.; Kaczorowska, J.; Jebbink, M.F.; van Gool, T.; Cotten, M.; van der Hoek, L. Entamoeba and Giardia parasites implicated as hosts of CRESS viruses. Nat. Commun. 2020, 11, 4620. [Google Scholar] [CrossRef]
- Krupovic, M.; Varsani, A. Naryaviridae, Nenyaviridae, and Vilyaviridae: Three new families of single-stranded DNA viruses in the phylum Cressdnaviricota. Arch. Virol. 2022, 167, 2907–2921. [Google Scholar] [CrossRef] [PubMed]
- Schoch, C.L.; Ciufo, S.; Domrachev, M.; Hotton, C.L.; Kannan, S.; Khovanskaya, R.; Leipe, D.; McVeigh, R.; O’Neill, K.; Robbertse, B.; et al. NCBI Taxonomy: A comprehensive update on curation, resources and tools. Database 2020, 2020, baaa062. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.; Li, X.; Wu, W.; Cao, L.; Zhao, P.; Li, X.; Ren, B.; Li, J.; Zhang, X. A Novel MicroRNA From the Translated Region of the Giardiavirus rdrp Gene Governs Virus Copy Number in Giardia duodenalis. Front. Microbiol. 2020, 11, 569412. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.L.; Miller, R.L.; Wang, C.C. Antibodies to the Giardia lamblia double-stranded RNA virus major protein can block the viral infection. Mol. Biochem. Parasitol. 1988, 30, 225–232. [Google Scholar] [CrossRef] [PubMed]
- De Jonckheere, J.F.; Gordts, B. Occurrence and transfection of a Giardia virus. Mol. Biochem. Parasitol. 1987, 23, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Tai, J.H.; Ong, S.J.; Chang, S.C.; Su, H.M. Giardiavirus enters Giardia lamblia WB trophozoite via endocytosis. Exp. Parasitol. 1993, 76, 165–174. [Google Scholar] [CrossRef]
- Abodeely, M.; DuBois, K.N.; Hehl, A.; Stefanic, S.; Sajid, M.; de Souza, W.; Attias, M.; Engel, J.C.; Hsieh, I.; Fetter, R.D.; et al. A Contiguous Compartment Functions as Endoplasmic Reticulum and Endosome/Lysosome in Giardia lamblia. Eukaryot. Cell 2009, 8, 1665–1676. [Google Scholar] [CrossRef]
- Natali, L.; Luna Pizarro, G.; Moyano, S.; de la Cruz-Thea, B.; Musso, J.; Rópolo, A.S.; Eichner, N.; Meister, G.; Musri, M.M.; Feliziani, C.; et al. The Exosome-like Vesicles of Giardia Assemblages A, B, and E Are Involved in the Delivering of Distinct Small RNA from Parasite to Parasite. Int. J. Mol. Sci. 2023, 24, 9559. [Google Scholar] [CrossRef]
- Miska, K.B.; Jenkins, M.C.; Trout, J.M.; Santin, M.; Fayer, R. Detection and comparison of Giardia virus (GLV) from different assemblages of Giardia duodenalis. J. Parasitol. 2009, 95, 1197–1200. [Google Scholar] [CrossRef]
- Banik, G.; Stark, D.; Rashid, H.; Ellis, J. Recent Advances in Molecular Biology of Parasitic Viruses. Infect. Disord. -Drug Targets 2015, 14, 155–167. [Google Scholar] [CrossRef]
- Barrow, P.; Dujardin, J.C.; Fasel, N.; Greenwood, A.D.; Osterrieder, K.; Lomonossoff, G.; Fiori, P.L.; Atterbury, R.; Rossi, M.; Lalle, M. Viruses of protozoan parasites and viral therapy: Is the time now right? Virol. J. 2020, 17, 142. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.L.; Wang, A.L.; Wang, C.C. Purification and characterization of the Giardia lamblia double-stranded RNA virus. Mol. Biochem. Parasitol. 1988, 28, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, C.; Zhang, X.; Li, J.; Gong, P.; Wang, X.; Li, X.; Wang, X.; Zhang, X.; Cheng, S.; et al. A potential role for Giardia chaperone protein GdDnaJ in regulating Giardia proliferation and Giardiavirus replication. Parasites Vectors 2023, 16, 168. [Google Scholar] [CrossRef] [PubMed]
- Serradell, M.C.; Gargantini, P.R.; Saura, A.; Oms, S.R.; Rupil, L.L.; Berod, L.; Sparwasser, T.; Luján, H.D.; Adams, J.H. Cytokines, Antibodies, and Histopathological Profiles during Giardia Infection and Variant-Specific Surface Protein-Based Vaccination. Infect. Immun. 2018, 86, e00773-17. [Google Scholar] [CrossRef] [PubMed]
- Serradell, M.C.; Rupil, L.L.; Martino, R.A.; Prucca, C.G.; Carranza, P.G.; Saura, A.; Fernández, E.A.; Gargantini, P.R.; Tenaglia, A.H.; Petiti, J.P.; et al. Efficient oral vaccination by bioengineering virus-like particles with protozoan surface proteins. Nat. Commun. 2019, 10, 361. [Google Scholar] [CrossRef] [PubMed]
- Pu, X.; Li, X.; Cao, L.; Yue, K.; Zhao, P.; Wang, X.; Li, J.; Zhang, X.; Zhang, N.; Zhao, Z.; et al. Giardia duodenalis Induces Proinflammatory Cytokine Production in Mouse Macrophages via TLR9-Mediated p38 and ERK Signaling Pathways. Front. Cell Dev. Biol. 2021, 9, 694675. [Google Scholar] [CrossRef] [PubMed]
- Rowley, J.; Vander Hoorn, S.; Korenromp, E.; Low, N.; Unemo, M.; Abu-Raddad, L.J.; Chico, R.M.; Smolak, A.; Newman, L.; Gottlieb, S.; et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: Global prevalence and incidence estimates, 2016. Bull. World Health Organ. 2019, 97, 548–562P. [Google Scholar] [CrossRef] [PubMed]
- Benchimol, M.; da Silva Fontes, R.; Burla Dias, Â.J. Tritrichomonas foetus damages bovine oocytes in vitro. Vet. Res. 2007, 38, 399–408. [Google Scholar] [CrossRef]
- Yao, C.; Köster, L.S. Tritrichomonas foetus infection, a cause of chronic diarrhea in the domestic cat. Vet. Res. 2015, 46, 35. [Google Scholar] [CrossRef]
- Kissinger, P. Trichomonas vaginalis: A review of epidemiologic, clinical and treatment issues. BMC Infect. Dis. 2015, 15, 307. [Google Scholar] [CrossRef]
- Sutton, M.; Sternberg, M.; Koumans, E.H.; McQuillan, G.; Berman, S.; Markowitz, L. The Prevalence of Trichomonas vaginalis Infection among Reproductive-Age Women in the United States, 2001–2004. Clin. Infect. Dis. 2007, 45, 1319–1326. [Google Scholar] [CrossRef]
- Bolumburu, C.; Zamora, V.; Muñoz-Algarra, M.; de la Cruz Conty, M.L.; Escario, J.A.; Ibáñez-Escribano, A. Impact of COVID-19 Pandemic on the Trends of Trichomonas vaginalis Infection in a Tertiary Hospital of Madrid, Spain. Microorganisms 2024, 12, 620. [Google Scholar] [CrossRef] [PubMed]
- Mercer, F.; Johnson, P.J. Trichomonas vaginalis: Pathogenesis, Symbiont Interactions, and Host Cell Immune Responses. Trends Parasitol. 2018, 34, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhao, W.; Wang, H.; Wang, Y.; Li, J.; Wu, X. Trichomonas vaginalis infection-associated risk of cervical cancer: A meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 228, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-Y.; Su, R.-Y.; Chung, C.-H.; Huang, K.-Y.; Lin, H.-A.; Wang, J.-Y.; Chen, C.-C.; Chien, W.-C.; Lin, H.-C. Association between trichomoniasis and prostate and bladder diseases: A population-based case–control study. Sci. Rep. 2022, 12, 15358. [Google Scholar] [CrossRef] [PubMed]
- Tolbert, M.K.; Gookin, J.L. Mechanisms of Tritrichomonas foetus Pathogenicity in Cats with Insights from Venereal Trichomonosis. J. Vet. Intern. Med. 2016, 30, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Fichorova, R.; Fraga, J.; Rappelli, P.; Fiori, P.L. Trichomonas vaginalis infection in symbiosis with Trichomonasvirus and Mycoplasma. Res. Microbiol. 2017, 168, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Benchimol, M.; Monteiro, S.P.; Chang, T.H.; Alderete, J.F. Virus in Trichomonas—An ultrastructural study. Parasitol. Int. 2002, 51, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Gomes Vancini, R.; Benchimol, M. Appearance of virus-like particles in Tritrichomonas foetus after drug treatment. Tissue Cell 2005, 37, 317–323. [Google Scholar] [CrossRef]
- Gerhold, R.W.; Allison, A.B.; Sellers, H.; Linnemann, E.; Chang, T.H.; Alderete, J.F. Examination for double-stranded RNA viruses in Trichomonas gallinae and identification of a novel sequence of a Trichomonas vaginalis virus. Parasitol. Res. 2009, 105, 775–779. [Google Scholar] [CrossRef]
- Bahadory, S.; Aminizadeh, S.; Taghipour, A.; Bokharaei-Salim, F.; Khanaliha, K.; Razizadeh, M.H.; Soleimani, A.; beikzadeh, L.; Khatami, A. A systematic review and meta-analysis on the global status of Trichomonas vaginalis virus in Trichomonas vaginalis. Microb. Pathog. 2021, 158, 105058. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Chung, P.-R.; Hwang, M.-K.; Choi, E.Y. Double-stranded RNA virus in Korean Isolate IH-2 of Trichomonas vaginalis. Korean J. Parasitol. 2007, 45, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Malla, N.; Kaul, P.; Sehgal, R.; Gupta, I. The presence of dsRNA virus in Trichomonas vaginalis isolates from symptomatic and asymptomatic Indian women and its correlation with in vitro metronidazole sensitivity. Indian J. Med. Microbiol. 2011, 29, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.L.; Wang, C.C. A linear double-stranded RNA in Trichomonas vaginalis. J. Biol. Chem. 1985, 260, 3697–3702. [Google Scholar] [CrossRef] [PubMed]
- Bessarab, I.N.; Liu, H.-W.; Ip, C.-F.; Tai, J.-H. The Complete cDNA Sequence of a Type II Trichomonas vaginalis Virus. Virology 2000, 267, 350–359. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bessarab, I.N.; Nakajima, R.; Liu, H.-W.; Tai, J.-H. Identification and characterization of a type III Trichomonas vaginalis virus in the protozoan pathogen Trichomonas vaginalis. Arch. Virol. 2010, 156, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Goodman, R.P.; Freret, T.S.; Kula, T.; Geller, A.M.; Talkington, M.W.T.; Tang-Fernandez, V.; Suciu, O.; Demidenko, A.A.; Ghabrial, S.A.; Beach, D.H.; et al. Clinical Isolates of Trichomonas vaginalis Concurrently Infected by Strains of Up to Four Trichomonasvirus Species (Family Totiviridae). J. Virol. 2011, 85, 4258–4270. [Google Scholar] [CrossRef] [PubMed]
- Manny, A.R.; Hetzel, C.A.; Mizani, A.; Nibert, M.L. Discovery of a Novel Species of Trichomonasvirus in the Human Parasite Trichomonas vaginalis Using Transcriptome Mining. Viruses 2022, 14, 548. [Google Scholar] [CrossRef]
- Jehee, I.; van der Veer, C.; Himschoot, M.; Hermans, M.; Bruisten, S. Direct detection of Trichomonas vaginalis virus in Trichomonas vaginalis positive clinical samples from the Netherlands. J. Virol. Methods 2017, 250, 1–5. [Google Scholar] [CrossRef]
- Vaňáčová, Ŝ.; Tachezy, J.A.N.; Kulda, J.; Flegr, J. Characterization of Trichomonad Species and Strains by PCR Fingerprinting. J. Eukaryot. Microbiol. 2007, 44, 545–552. [Google Scholar] [CrossRef]
- Ghedin, E.; Conrad, M.D.; Gorman, A.W.; Schillinger, J.A.; Fiori, P.L.; Arroyo, R.; Malla, N.; Dubey, M.L.; Gonzalez, J.; Blank, S.; et al. Extensive Genetic Diversity, Unique Population Structure and Evidence of Genetic Exchange in the Sexually Transmitted Parasite Trichomonas vaginalis. PLoS Neglected Trop. Dis. 2012, 6, e1573. [Google Scholar] [CrossRef]
- Hampl, V.; Vaňáčová, Š.; Kulda, J.; Flegr, J. Concordance between genetic relatedness and phenotypic similarities of Trichomonas vaginalis strains. BMC Evol. Biol. 2001, 1, 11. [Google Scholar] [CrossRef] [PubMed]
- da Luz Becker, D.; dos Santos, O.; Frasson, A.P.; de Vargas Rigo, G.; Macedo, A.J.; Tasca, T. High rates of double-stranded RNA viruses and Mycoplasma hominis in Trichomonas vaginalis clinical isolates in South Brazil. Infect. Genet. Evol. 2015, 34, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Fraga, J.; Rojas, L.; Sariego, I.; Fernández-Calienes, A. Double-stranded RNA viral infection in Cuban Trichomonas vaginalis isolates. Braz. J. Infect. Dis. 2005, 9, 521–524. [Google Scholar] [CrossRef] [PubMed]
- Fraga, J.N.; Rojas, L.; Sariego, I.; Fernández-Calienes, A.; Núñez, F.A. Double-Stranded RNA Viral Infection of Trichomonas vaginalis and Association with Clinical Presentation. Acta Protozool. 2007, 46, 93–98. [Google Scholar]
- Fraga, J.; Rojas, L.; Sariego, I.; Fernández-Calienes, A.; Nuñez, F.A. Species typing of Cuban Trichomonas vaginalis virus by RT-PCR, and association of TVV-2 with high parasite adhesion levels and high pathogenicity in patients. Arch. Virol. 2012, 157, 1789–1795. [Google Scholar] [CrossRef]
- Fraga, J.; Rojas, L.; Sariego, I.; Fernández-Calienes, A. Genetic characterization of three Cuban Trichomonas vaginalis virus. Phylogeny of Totiviridae family. Infect. Genet. Evol. 2012, 12, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Hussien, E.M.; El-Sayed, H.Z.; El-Moamly, A.A.; Helmy, M.M.; Shaban, M.M. Molecular characterization of Egyptian Trichomonas vaginalis clinical isolates by HSP70 restriction fragment length polymorphism. J. Egypt. Soc. Parasitol. 2005, 35, 699–710. [Google Scholar] [PubMed]
- El-Gayar, E.K.; Mokhtar, A.B.; Hassan, W.A. Molecular characterization of double-stranded RNA virus in Trichomonas vaginalis Egyptian isolates and its association with pathogenicity. Parasitol. Res. 2016, 115, 4027–4036. [Google Scholar] [CrossRef]
- Margarita, V.; Marongiu, A.; Diaz, N.; Dessì, D.; Fiori, P.L.; Rappelli, P. Prevalence of double-stranded RNA virus in Trichomonas vaginalis isolated in Italy and association with the symbiont Mycoplasma hominis. Parasitol. Res. 2019, 118, 3565–3570. [Google Scholar] [CrossRef]
- Heidary, S.; Bandehpour, M.; Valadkhani, Z.; Seyyed-Tabaee, S.; Haghighi, A.; Abadi, A.; Kazemi, B. Double-Stranded RNA Viral Infection in Tehran Trichomonas vaginalis Isolates. Iran. J. Parasitol. 2013, 8, 60–64. [Google Scholar] [PubMed]
- Khanaliha, K.; Masoumi-Asl, H.; Bokharaei-Salim, F.; Tabatabaei, A.; Naghdalipoor, M. Double-stranded RNA viral infection of Trichomonas vaginalis (TVV1) in Iranian isolates. Microb. Pathog. 2017, 109, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Bokharaei-Salim, F.; Esteghamati, A.; Khanaliha, K.; Esghaei, M.; Donyavi, T.; Salemi, B. The First Detection of Co-Infection of Double-Stranded RNA Virus 1, 2 and 3 in Iranian Isolates of Trichomonas vaginalis. Iran. J. Parasitol. 2020, 15, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Masha, S.C.; Cools, P.; Crucitti, T.; Sanders, E.J.; Vaneechoutte, M. Molecular typing of Trichomonas vaginalis isolates by actin gene sequence analysis and carriage of T. vaginalis viruses. Parasites Vectors 2017, 10, 537. [Google Scholar] [CrossRef] [PubMed]
- Rivera, W.L.; Justo, C.A.C.; Relucio-San Diego, M.A.C.V.; Loyola, L.M. Detection and molecular characterization of double-stranded RNA viruses in Philippine Trichomonas vaginalis isolates. J. Microbiol. Immunol. Infect. 2017, 50, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Justo, C.A.C.; Relucio-San Diego, M.A.C.V.; Rivera, W.L. Metronidazole Susceptibility and TVV-infection of Trichomonas vaginalis from Metro Manila and Angeles City, Philippines. Acta Medica Philipp. 2018, 52, 516–520. [Google Scholar] [CrossRef]
- Ertabaklar, H.; Malatyali, E.; Özün Özbay, E.P.; Yildiz, İ.; Sİnecen, M.; Ertuğ, S.; Bozdoğan, B.; Güçlü, Ö. Microsatellite-Based Genotyping, Analysis of Population Structure, Presence of Trichomonas vaginalis Virus (TVV) and Mycoplasma hominis in T. vaginalis Isolates from Southwest of Turkey. Iran. J. Parasitol. 2021, 16, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Weber, B. Double stranded RNA virus in South African Trichomonas vaginalis isolates. J. Clin. Pathol. 2003, 56, 542–543. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, A.; Wang, C.C.; Alderete, J.F. Trichomonas vaginalis phenotypic variation occurs only among trichomonads infected with the double-stranded RNA virus. J. Exp. Med. 1987, 166, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Snipes, L.J.; Gamard, P.M.; Narcisi, E.M.; Beard, C.B.; Lehmann, T.; Secor, W.E. Molecular Epidemiology of Metronidazole Resistance in a Population of Trichomonas vaginalis Clinical Isolates. J. Clin. Microbiol. 2000, 38, 3004–3009. [Google Scholar] [CrossRef]
- Wendel, K.A.; Rompalo, A.M.; Erbelding, E.J.; Chang, T.H.; Alderete, J.F. Double-Stranded RNA Viral Infection of Trichomonas vaginalis Infecting Patients Attending a Sexually Transmitted Diseases Clinic. J. Infect. Dis. 2002, 186, 558–561. [Google Scholar] [CrossRef]
- Khoshnan, A.; Alderete, J.F. Trichomonas vaginalis with a double-stranded RNA virus has upregulated levels of phenotypically variable immunogen mRNA. J. Virol. 1994, 68, 4035–4038. [Google Scholar] [CrossRef]
- Provenzano, D.; Khoshnan, A.; Alderete, J.F. Involvement of dsRNA virus in the protein compositionand growth kinetics of host Trichomonas vaginalis. Arch. Virol. 2014, 142, 939–952. [Google Scholar] [CrossRef]
- Petrin, D.; Delgaty, K.; Bhatt, R.; Garber, G. Clinical and Microbiological Aspects of Trichomonas vaginalis. Clin. Microbiol. Rev. 1998, 11, 300–317. [Google Scholar] [CrossRef]
- Hernández, H.M.; Marcet, R.; Sarracent, J. Biological roles of cysteine proteinases in the pathogenesis of Trichomonas vaginalis. Parasite 2014, 21, 54. [Google Scholar] [CrossRef]
- He, D.; Pengtao, G.; Li Jianhua, Y.J.; Zhang Guocai, L.H.; Xichen, Z. Differential Protein Expressions in Virus-Infected and Uninfected Trichomonas vaginalis. Korean J. Parasitol. 2017, 55, 121–128. [Google Scholar] [CrossRef]
- Schwebke, J.R.; Barrientes, F.J. Prevalence of Trichomonas vaginalis isolates with resistance to metronidazole and tinidazole. Antimicrob. Agents Chemother. 2006, 50, 4209–4210. [Google Scholar] [CrossRef]
- Hong, A.; Zampieri, R.A.; Shaw, J.J.; Floeter-Winter, L.M.; Laranjeira-Silva, M.F. One Health Approach to Leishmaniases: Understanding the Disease Dynamics through Diagnostic Tools. Pathogens 2020, 9, 809. [Google Scholar] [CrossRef]
- Laing, G.; Vigilato, M.A.N.; Cleaveland, S.; Thumbi, S.M.; Blumberg, L.; Salahuddin, N.; Abdela-Ridder, B.; Harrison, W. One Health for neglected tropical diseases. Trans. R. Soc. Trop. Med. Hyg. 2021, 115, 182–184. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.J.; Liao, C.X.; Li, L.M. Introduction for One Health Joint Plan of Action (2022–2026). Zhonghua Liu Xing Bing Xue Za Zhi 2023, 44, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Picado, A.; Pires, M.; Wright, B.; Kaye, P.M.; da Conceição, V.; Churchill, R.C. The impact of leishmaniasis on mental health and psychosocial well-being: A systematic review. PLoS ONE 2019, 14, e0223313. [Google Scholar] [CrossRef]
- Melo, G.D.; Silva, J.E.S.; Grano, F.G.; Souza, M.S.; Machado, G.F. Leishmania infection and neuroinflammation: Specific chemokine profile and absence of parasites in the brain of naturally-infected dogs. J. Neuroimmunol. 2015, 289, 21–29. [Google Scholar] [CrossRef]
- Melo, G.D.; Goyard, S.; Fiette, L.; Boissonnas, A.; Combadiere, C.; Machado, G.F.; Minoprio, P.; Lang, T. Unveiling Cerebral Leishmaniasis: Parasites and brain inflammation in Leishmania donovani infected mice. Sci. Rep. 2017, 7, 8454. [Google Scholar] [CrossRef]
- Kantzanou, M.; Karalexi, M.A.; Theodoridou, K.; Kostares, E.; Kostare, G.; Loka, T.; Vrioni, G.; Tsakris, A. Prevalence of visceral leishmaniasis among people with HIV: A systematic review and meta-analysis. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 42, 1–12. [Google Scholar] [CrossRef]
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef]
- Gupta, A.K.; Das, S.; Kamran, M.; Ejazi, S.A.; Ali, N. The pathogenicity and virulence of Leishmani-interplay of virulence factors with host defenses. Virulence 2022, 13, 903–935. [Google Scholar] [CrossRef]
- Atayde, V.D.; Aslan, H.; Townsend, S.; Hassani, K.; Kamhawi, S.; Olivier, M. Exosome Secretion by the Parasitic Protozoan Leishmania within the Sand Fly Midgut. Cell Rep. 2015, 13, 957–967. [Google Scholar] [CrossRef]
- Croft, S.L.; Molyneux, D.H. Studies on the ultrastructure, virus-like particles and infectivity of Leishmania hertigi. Ann. Trop. Med. Parasitol. 1979, 73, 213–226. [Google Scholar] [CrossRef]
- Kostygov, A.Y.; Yurchenko, V. Revised classification of the subfamily Leishmaniinae (Trypanosomatidae). Folia Parasitol. 2017, 64. [Google Scholar] [CrossRef]
- Grybchuk, D.; Kostygov, A.Y.; Macedo, D.H.; d’Avila-Levy, C.M.; Yurchenko, V. RNA viruses in trypanosomatid parasites: A historical overview. Mem. Do Inst. Oswaldo Cruz 2018, 113, e170487. [Google Scholar] [CrossRef]
- Zeltins, A. Construction and Characterization of Virus-like Particles: A Review. Mol. Biotechnol. 2012, 53, 92–107. [Google Scholar] [CrossRef]
- Croft, S.L.; Chance, M.L.; Gardener, P.J. Ultrastructural and biochemical characterization of stocks of Endotrypanum. Ann. Trop. Med. Parasitol. 2016, 74, 585–589. [Google Scholar] [CrossRef]
- Tarr, P.I.; Aline, R.F.; Smiley, B.L.; Scholler, J.; Keithly, J.; Stuart, K. LR1: A candidate RNA virus of Leishmania. Proc. Natl. Acad. Sci. USA 1988, 85, 9572–9575. [Google Scholar] [CrossRef]
- Stuart, K.D.; Weeks, R.; Guilbride, L.; Myler, P.J. Molecular organization of Leishmania RNA virus 1. Proc. Natl. Acad. Sci. USA 1992, 89, 8596–8600. [Google Scholar] [CrossRef]
- Weeks, R.S.; Patterson, J.L.; Stuart, K.; Widmer, G. Transcribing and replicating particles in a double-stranded RNA virus from Leishmania. Mol. Biochem. Parasitol. 1992, 52, 207–213. [Google Scholar] [CrossRef]
- Clos, J.; Cantanhêde, L.M.; Fernandes, F.G.; Ferreira, G.E.M.; Porrozzi, R.; Ferreira, R.G.M.; Cupolillo, E. New insights into the genetic diversity of Leishmania RNA Virus 1 and its species-specific relationship with Leishmania parasites. PLoS ONE 2018, 13, e0198727. [Google Scholar] [CrossRef]
- Scheffter, S.M.; Ro, Y.T.; Chung, I.K.; Patterson, J.L. The Complete Sequence of Leishmania RNA Virus LRV2-1, a Virus of an Old World Parasite Strain. Virology 1995, 212, 84–90. [Google Scholar] [CrossRef]
- Bates, P.A.; Zangger, H.; Hailu, A.; Desponds, C.; Lye, L.-F.; Akopyants, N.S.; Dobson, D.E.; Ronet, C.; Ghalib, H.; Beverley, S.M.; et al. Leishmania aethiopica Field Isolates Bearing an Endosymbiontic dsRNA Virus Induce Pro-inflammatory Cytokine Response. PLoS Neglected Trop. Dis. 2014, 8, e2836. [Google Scholar] [CrossRef][Green Version]
- Nalçacı, M.; Karakuş, M.; Yılmaz, B.; Demir, S.; Özbilgin, A.; Özbel, Y.; Töz, S. Detection of Leishmania RNA virus 2 in Leishmania species from Turkey. Trans. R. Soc. Trop. Med. Hyg. 2019, 113, 410–417. [Google Scholar] [CrossRef]
- Moin-Vaziri, V.; Zare, F.; Seyyed Tabaei, S.J.; Saberi, R.; Hajjaran, H. Successful Isolation of Leishmania RNA Virus (LRV) from Leishmania major in a Cutaneous Leishmaniasis Focus in Central Iran: An Update on Cases. Acta Parasitol. 2022, 67, 1290–1298. [Google Scholar] [CrossRef]
- Hajjaran, H.; Mahdi, M.; Mohebali, M.; Samimi-Rad, K.; Ataei-Pirkooh, A.; Kazemi-Rad, E.; Naddaf, S.R.; Raoofian, R. Detection and molecular identification of leishmania RNA virus (LRV) in Iranian Leishmania species. Arch. Virol. 2016, 161, 3385–3390. [Google Scholar] [CrossRef]
- Rêgo, F.D.; da Silva, E.S.; Lopes, V.V.; Teixeira-Neto, R.G.; Belo, V.S.; Fonseca Júnior, A.A.; Pereira, D.A.; Pena, H.P.; Laurenti, M.D.; Araújo, G.V.; et al. First report of putative Leishmania RNA virus 2 (LRV2) in Leishmania infantum strains from canine and human visceral leishmaniasis cases in the southeast of Brazil. Mem. Do Inst. Oswaldo Cruz 2023, 118, e230071. [Google Scholar] [CrossRef]
- Ferreira, T.R.; Sacks, D.L. Experimental Hybridization in Leishmania: Tools for the Study of Genetic Exchange. Pathogens 2022, 11, 580. [Google Scholar] [CrossRef]
- Heeren, S.; Maes, I.; Sanders, M.; Lye, L.-F.; Adaui, V.; Arevalo, J.; Llanos-Cuentas, A.; Garcia, L.; Lemey, P.; Beverley, S.M.; et al. Diversity and dissemination of viruses in pathogenic protozoa. Nat. Commun. 2023, 14, 8343. [Google Scholar] [CrossRef]
- Klocek, D.; Grybchuk, D.; Tichá, L.; Votýpka, J.; Volf, P.; Kostygov, A.Y.; Yurchenko, V. Evolution of RNA viruses in trypanosomatids: New insights from the analysis of Sauroleishmania. Parasitol. Res. 2023, 122, 2279–2286. [Google Scholar] [CrossRef]
- Saberi, R.; Fakhar, M.; Hajjaran, H.; Ataei-Pirkooh, A.; Mohebali, M.; Taghipour, N.; Ziaei Hezarjaribi, H.; Moghadam, Y.; Bagheri, A. Presence and diversity of Leishmania RNA virus in an old zoonotic cutaneous leishmaniasis focus, northeastern Iran: Haplotype and phylogenetic based approach. Int. J. Infect. Dis. 2020, 101, 6–13. [Google Scholar] [CrossRef]
- Kumari, D.; Mahajan, S.; Kour, P.; Singh, K. Virulence factors of Leishmania parasite: Their paramount importance in unraveling novel vaccine candidates and therapeutic targets. Life Sci. 2022, 306, 120829. [Google Scholar] [CrossRef]
- Faria, M.S.; Reis, F.C.; Lima, A.P. Toll-like receptors in leishmania infections: Guardians or promoters? J. Parasitol. Res. 2012, 2012, 930257. [Google Scholar] [CrossRef]
- Jafarzadeh, A.; Nemati, M.; Sharifi, I.; Nair, A.; Shukla, D.; Chauhan, P.; Khorramdelazad, H.; Sarkar, A.; Saha, B. Leishmania species-dependent functional duality of Toll-like receptor 2. IUBMB Life 2019, 71, 1685–1700. [Google Scholar] [CrossRef]
- Murray, H.W.; Zhang, Y.; Zhang, Y.; Raman, V.S.; Reed, S.G.; Ma, X. Regulatory actions of Toll-like receptor 2 (TLR2) and TLR4 in Leishmania donovani infection in the liver. Infect. Immun. 2013, 81, 2318–2326. [Google Scholar] [CrossRef]
- He, J.; Huang, F.; Liao, X.; Zhang, J.; Wei, S.; Xiao, Y.; Zheng, X.; Zhu, Z.; Chen, D.; Chen, J. TLR9 agonist CpG ODN 2395 promotes the immune response against Leishmania donovani in obesity and undernutrition mice. Acta Trop. 2023, 242, 106921. [Google Scholar] [CrossRef]
- Matsumoto, M.; Oshiumi, H.; Seya, T. Antiviral responses induced by the TLR3 pathway. Rev. Med. Virol. 2011, 21, 67–77. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, J.; Zhao, Y.; Ma, X.; Yi, H. Toll-like receptor 3 (TLR3) regulation mechanisms and roles in antiviral innate immune responses. J. Zhejiang Univ.-Sci. B 2021, 22, 609–632. [Google Scholar] [CrossRef]
- Kariyawasam, R.; Grewal, J.; Lau, R.; Purssell, A.; Valencia, B.M.; Llanos-Cuentas, A.; Boggild, A.K. Influence of Leishmania RNA Virus 1 on Proinflammatory Biomarker Expression in a Human Macrophage Model of American Tegumentary Leishmaniasis. J. Infect. Dis. 2017, 216, 877–886. [Google Scholar] [CrossRef]
- Kopelyanskiy, D.; Desponds, C.; Prevel, F.; Rossi, M.; Migliorini, R.; Snaka, T.; Eren, R.O.; Claudinot, S.; Lye, L.F.; Pasparakis, M.; et al. Leishmania guyanensis suppressed inducible nitric oxide synthase provoked by its viral endosymbiont. Front. Cell. Infect. Microbiol. 2022, 12, 944819. [Google Scholar] [CrossRef]
- Grybchuk, D.; Akopyants, N.S.; Kostygov, A.Y.; Konovalovas, A.; Lye, L.-F.; Dobson, D.E.; Zangger, H.; Fasel, N.; Butenko, A.; Frolov, A.O.; et al. Viral discovery and diversity in trypanosomatid protozoa with a focus on relatives of the human parasite Leishmania. Proc. Natl. Acad. Sci. USA 2017, 115, E506–E515. [Google Scholar] [CrossRef]
- Rossi, M.; Fasel, N. How to master the host immune system? Leishmania parasites have the solutions! Int. Immunol. 2018, 30, 103–111. [Google Scholar] [CrossRef]
- de Carvalho, R.V.H.; Lima-Júnior, D.S.; de Oliveira, C.V.; Zamboni, D.S. Endosymbiotic RNA virus inhibits Leishmania-induced caspase-11 activation. iScience 2021, 24, 102004. [Google Scholar] [CrossRef]
- de Carvalho, R.V.H.; Lima-Junior, D.S.; da Silva, M.V.G.; Dilucca, M.; Rodrigues, T.S.; Horta, C.V.; Silva, A.L.N.; da Silva, P.F.; Frantz, F.G.; Lorenzon, L.B.; et al. Leishmania RNA virus exacerbates Leishmaniasis by subverting innate immunity via TLR3-mediated NLRP3 inflammasome inhibition. Nat. Commun. 2019, 10, 5273. [Google Scholar] [CrossRef] [PubMed]
- Hartley, M.A.; Eren, R.O.; Rossi, M.; Prevel, F.; Castiglioni, P.; Isorce, N.; Desponds, C.; Lye, L.F.; Beverley, S.M.; Drexler, S.K.; et al. Leishmania guyanensis parasites block the activation of the inflammasome by inhibiting maturation of IL-1beta. Microb. Cell 2018, 5, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Fasel, N. The criminal association of Leishmania parasites and viruses. Curr. Opin. Microbiol. 2018, 46, 65–72. [Google Scholar] [CrossRef]
- Ginouvès, M.; Couppié, P.; Simon, S.; Bourreau, E.; Rogier, S.; Brousse, P.; Travers, P.; Pommier de Santi, V.; Demar, M.; Briolant, S.; et al. Leishmaniavirus genetic diversity is not related to leishmaniasis treatment failure. Clin. Microbiol. Infect. 2021, 27, e281–e286. [Google Scholar] [CrossRef]
- Dutra, W.O.; Valencia, B.M.; Lau, R.; Kariyawasam, R.; Jara, M.; Ramos, A.P.; Chantry, M.; Lana, J.T.; Boggild, A.K.; Llanos-Cuentas, A. Leishmania RNA virus-1 is similarly detected among metastatic and non-metastatic phenotypes in a prospective cohort of American Tegumentary Leishmaniasis. PLoS Neglected Trop. Dis. 2022, 16, e0010162. [Google Scholar] [CrossRef]
- Bourreau, E.; Ginouves, M.; Prévot, G.; Hartley, M.-A.; Gangneux, J.-P.; Robert-Gangneux, F.; Dufour, J.; Sainte-Marie, D.; Bertolotti, A.; Pratlong, F.; et al. Presence of Leishmania RNA Virus 1 in Leishmania guyanensis Increases the Risk of First-Line Treatment Failure and Symptomatic Relapse. J. Infect. Dis. 2016, 213, 105–111. [Google Scholar] [CrossRef]
- Adaui, V.; Lye, L.-F.; Akopyants, N.S.; Zimic, M.; Llanos-Cuentas, A.; Garcia, L.; Maes, I.; De Doncker, S.; Dobson, D.E.; Arevalo, J.; et al. Association of the Endobiont Double-Stranded RNA Virus LRV1 With Treatment Failure for Human Leishmaniasis Caused by Leishmania braziliensisin Peru and Bolivia. J. Infect. Dis. 2016, 213, 112–121. [Google Scholar] [CrossRef]
- Jha, B.; Reverte, M.; Ronet, C.; Prevel, F.; Morgenthaler, F.D.; Desponds, C.; Lye, L.F.; Owens, K.L.; Scarpellino, L.; Dubey, L.K.; et al. In and out: Leishmania metastasis by hijacking lymphatic system and migrating immune cells. Front. Cell. Infect. Microbiol. 2022, 12, 941860. [Google Scholar] [CrossRef]
- Silverman, N.; Lafleur, A.; Olivier, M. Viral endosymbiotic infection of protozoan parasites: How it influences the development of cutaneous leishmaniasis. PLoS Pathog. 2022, 18, e1010910. [Google Scholar] [CrossRef]
- Vieira, C.B.; Praca, Y.R.; Bentes, K.; Santiago, P.B.; Silva, S.M.M.; Silva, G.D.S.; Motta, F.N.; Bastos, I.M.D.; de Santana, J.M.; de Araujo, C.N. Triatomines: Trypanosomatids, Bacteria, and Viruses Potential Vectors? Front. Cell. Infect. Microbiol. 2018, 8, 405. [Google Scholar] [CrossRef]
- Stoco, P.H.; Wagner, G.; Talavera-Lopez, C.; Gerber, A.; Zaha, A.; Thompson, C.E.; Bartholomeu, D.C.; Luckemeyer, D.D.; Bahia, D.; Loreto, E.; et al. Genome of the avirulent human-infective trypanosome—Trypanosoma rangeli. PLoS Negl. Trop. Dis. 2014, 8, e3176. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Presas, A.M.; Padilla-Noriega, L.; Becker, I.; Robert, L.; Jimenez, J.A.; Solano, S.; Delgado, J.; Tato, P.; Molinari, J.L. Enveloped and non-enveloped viral-like particles in Trypanosoma cruzi epimastigotes. Rev. Do Inst. Med. Trop. Sao Paulo 2017, 59, e46. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, M.A.; Rodrigues, J.R.; Roy, S.W.; Sehgal, R.N.M. Novel RNA viruses associated with avian haemosporidian parasites. PLoS ONE 2022, 17, e0269881. [Google Scholar] [CrossRef]
- Queiroz, V.F.; Tatara, J.M.; Botelho, B.B.; Rodrigues, R.A.L.; Almeida, G.M.F.; Abrahao, J.S. The consequences of viral infection on protists. Commun. Biol. 2024, 7, 306. [Google Scholar] [CrossRef] [PubMed]
- McInally, S.G.; Hagen, K.D.; Nosala, C.; Williams, J.; Nguyen, K.; Booker, J.; Jones, K.; Dawson, S.C.; Wickens, M.P. Robust and stable transcriptional repression in Giardiausing CRISPRi. Mol. Biol. Cell 2019, 30, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Kushnir, N.; Streatfield, S.J.; Yusibov, V. Virus-like particles as a highly efficient vaccine platform: Diversity of targets and production systems and advances in clinical development. Vaccine 2012, 31, 58–83. [Google Scholar] [CrossRef]
- Fujiwara, R.T.; Castiglioni, P.; Hartley, M.-A.; Rossi, M.; Prevel, F.; Desponds, C.; Utzschneider, D.T.; Eren, R.-O.; Zangger, H.; Brunner, L.; et al. Exacerbated Leishmaniasis Caused by a Viral Endosymbiont can be Prevented by Immunization with Its Viral Capsid. PLoS Negl. Trop. Dis. 2017, 11, e0005240. [Google Scholar] [CrossRef]


| Country | Type of Sample | TVV Presence | TVV Type Isolates | Co-Infections % (n) * | Ref. |
|---|---|---|---|---|---|
| Austria, Brazil, China, Czech Republic, Estonia, Slovakia, Sweden, and USA | NR | 44.4% (8/18) | NR | NR | [93] |
| Australia, Chile, India, Italy, Mexico, Papua New Guinea, Southern Africa, and USA | Vaginal swabs | 30.3% (67/221) | NR | NR | [94] |
| Czech Rep., Slovakia, USA, China, Brazil, Estonia, Sweden, and Austria | Vaginal samples + 3 ATCC isolates | 40% (8/20) | NR | NR | [95] |
| Brazil | Urine samples + 2 ATCC isolates | 90.9% (30/33) | TVV1 = 24 TVV2 = 9 TVV3 = 11 TVV4 = 3 | 36.7% (11/30) | [96] |
| Cuba | Vaginal samples | 55% (22/40) | NR | NR | [97] |
| Cuba | Vaginal exudates | 55% (22/40) | NR | NR | [98] |
| Cuba | NR | 56.7% (21/37) | TVV1 = 19 TVV2 = 15 | 14.3% (3/21) | [99] |
| Cuba | Vaginal samples | 100% (3/3) | TVV1 = 1 TVV2 = 2 | NR | [100] |
| Egypt | Vaginal swabs | 35% (7/20) | NR | NR | [101] |
| Egypt | Vaginal swabs | 20% (8/40) | TVV2 = 5 TVV4 = 3 | 0% (0/8) | [102] |
| India | Vaginal swabs and urine samples | 100% (30/30) | NR | NR | [86] |
| Italy | NR | 50% (24/48) | TVV1 = 17 TVV2 = 19 TVV3 = 13 TVV4 = 2 | 75% (18/24) | [103] |
| Iran | Vaginal discharge and urine samples | 17.4% (8/46) | TVV1 = 8 | NR | [104] |
| Iran | Vaginal swabs | 50% (4/8) | TVV1 = 4 | 0% (0/4) | [105] |
| Iran | Vaginal samples | 44.4% (4/9) | TVV1 = 4 TVV2 = 1 TVV3 = 1 | 25% (1/4) | [106] |
| Kenya | Vaginal swabs | 43.5% (10/22) | TVV1 = 9 TVV2 = 6 TVV3 = 4 TVV4 = 3 | 90% (9/10) | [107] |
| Korea | NR | 14% (4/22) | NR | NR | [85] |
| Netherlands | Cervicovaginal + urine samples | 50.4% (60/119) | TVV1 = 42 TVV2 = 26 TVV3 = 34 | 51.7% (31/60) | [92] |
| Philippines | Vaginal swabs | 18.7% (18/96) | TVV1 = 12 TVV2 = 12 TVV3 = 5 TVV4 = 6 | 33.3% (6/18) | [108] |
| Philippines | Vaginal swabs | 30.9% (13/42) | NR | NR | [109] |
| Turkey | Vaginal swabs | 16.7% (5/30) | NR | NR | [110] |
| South Africa | Clinical samples | 81.9% (59/72) | NR | NR | [111] |
| USA | NR | 50% (14/28) | NR | NR | [112] |
| USA | Vaginal swabs and clinical samples | 50% (55/109) | NR | NR | [113] |
| USA | Vaginal swabs and urine samples | 75% (21/28) | NR | NR | [114] |
| USA | Vaginal swabs | 40% (142/355) | NR | NR | [28] |
| USA | Vaginal swabs | 100% (5/5) | TVV1 = 5 TVV2 = 3 TVV3 = 3 TVV4 = 4 | 100% (5/5) | [90] |
| USA | Vaginal swabs | 81.2% (13/16) | NR | 84.6% (11/13) | [12] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibañez-Escribano, A.; Gomez-Muñoz, M.T.; Mateo, M.; Fonseca-Berzal, C.; Gomez-Lucia, E.; Perez, R.G.; Alunda, J.M.; Carrion, J. Microbial Matryoshka: Addressing the Relationship between Pathogenic Flagellated Protozoans and Their RNA Viral Endosymbionts (Family Totiviridae). Vet. Sci. 2024, 11, 321. https://doi.org/10.3390/vetsci11070321
Ibañez-Escribano A, Gomez-Muñoz MT, Mateo M, Fonseca-Berzal C, Gomez-Lucia E, Perez RG, Alunda JM, Carrion J. Microbial Matryoshka: Addressing the Relationship between Pathogenic Flagellated Protozoans and Their RNA Viral Endosymbionts (Family Totiviridae). Veterinary Sciences. 2024; 11(7):321. https://doi.org/10.3390/vetsci11070321
Chicago/Turabian StyleIbañez-Escribano, Alexandra, Maria Teresa Gomez-Muñoz, Marta Mateo, Cristina Fonseca-Berzal, Esperanza Gomez-Lucia, Raquel Garcia Perez, Jose M. Alunda, and Javier Carrion. 2024. "Microbial Matryoshka: Addressing the Relationship between Pathogenic Flagellated Protozoans and Their RNA Viral Endosymbionts (Family Totiviridae)" Veterinary Sciences 11, no. 7: 321. https://doi.org/10.3390/vetsci11070321
APA StyleIbañez-Escribano, A., Gomez-Muñoz, M. T., Mateo, M., Fonseca-Berzal, C., Gomez-Lucia, E., Perez, R. G., Alunda, J. M., & Carrion, J. (2024). Microbial Matryoshka: Addressing the Relationship between Pathogenic Flagellated Protozoans and Their RNA Viral Endosymbionts (Family Totiviridae). Veterinary Sciences, 11(7), 321. https://doi.org/10.3390/vetsci11070321










