Effects of Replacing Soybean Meal with Cottonseed Meal, Peanut Meal, Rapeseed Meal, or Distillers’ Dried Grains with Solubles on the Growth Performance, Nutrient Digestibility, Serum Parameters, and Rumen Fermentation in Growing Lambs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Diets
2.2. Digestibility Trial
2.3. Blood and Rumen Sample Collection
2.4. Laboratory Analysis
2.5. DNA Extraction and Sequencing
2.6. Bioinformatics Analysis
2.7. Statistical Analysis
3. Results
3.1. Growth Performance and Apparent Nutrient Digestibility
3.2. Serum Parameters
3.3. Rumen Fermentation Parameters
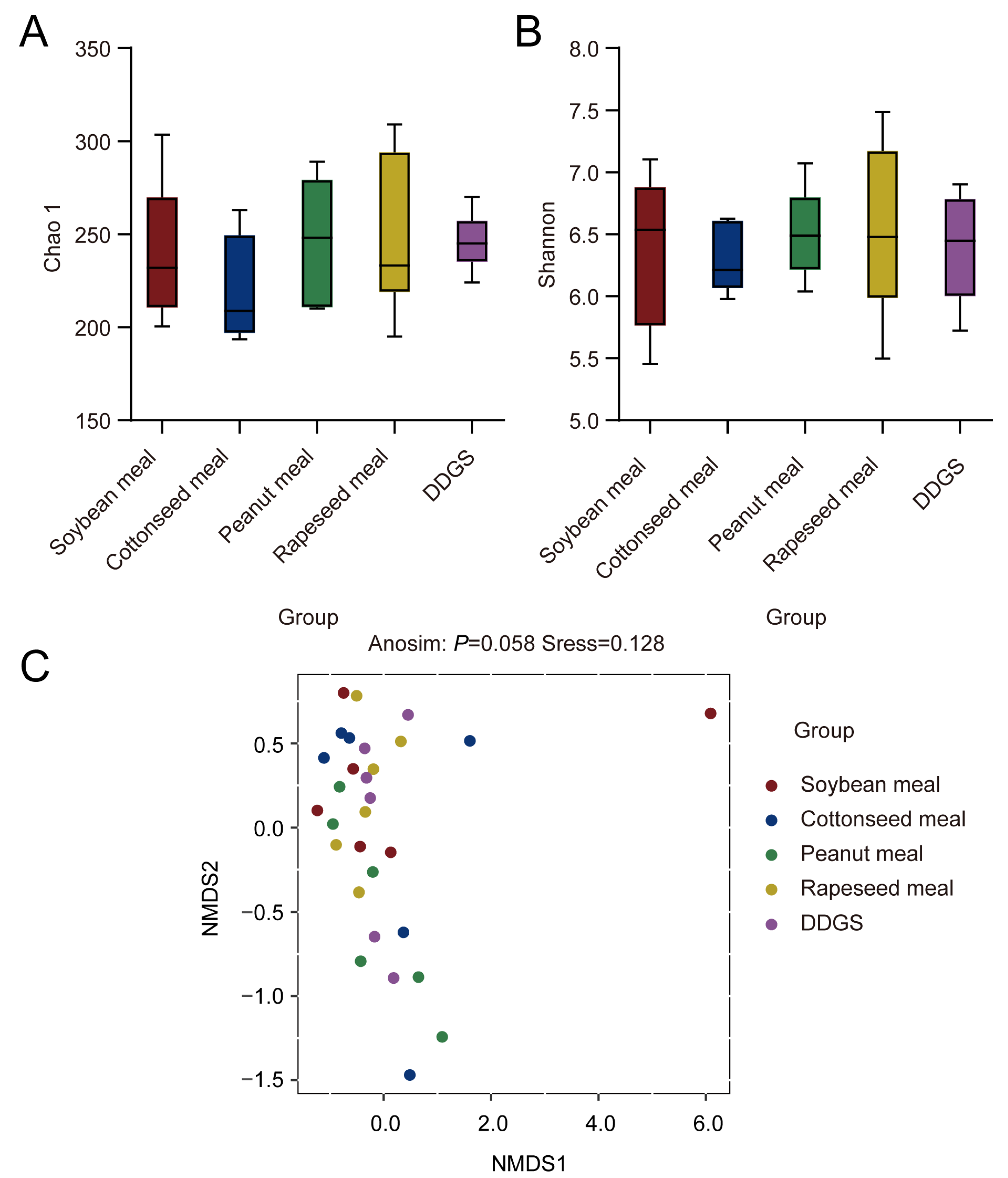
3.4. Changes in Rumen Microbiota and Taxonomic Composition
4. Discussion
4.1. Growth Performance and Nutrient Digestibility
4.2. Serum Parameters
4.3. Rumen Fermentation and Bacterial Community
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Danes, M.A.; Chagas, L.J.; Pedroso, A.M.; Santos, F.A. Effect of protein supplementation on milk production and metabolism of dairy cows grazing tropical grass. J. Dairy Sci. 2013, 96, 407–419. [Google Scholar] [CrossRef]
- Schogor, A.L.B.; Palin, M.F.; Santos, G.T.; Benchaar, C.; Petit, H.V. β-glucuronidase activity and enterolactone concentration in ruminal fluid, plasma, urine, and milk of Holstein cows fed increased levels of flax (Linum usitatissimum) meal. Anim. Feed Sci. Tech. 2017, 223, 23–29. [Google Scholar] [CrossRef]
- Hao, X.Y.; Yu, S.C.; Mu, C.T.; Wu, X.D.; Zhang, C.X.; Zhao, J.X.; Zhang, J.X. Replacing soybean meal with flax seed meal: Effects on nutrient digestibility, rumen microbial protein synthesis and growth performance in sheep. Animal 2020, 14, 1841–1848. [Google Scholar] [CrossRef]
- Broderick, G.A.; Kerkman, T.M.; Sullivan, H.M.; Dowd, M.K.; Funk, P.A. Effect of replacing soybean meal protein with protein from upland cottonseed, Pima cottonseed, or extruded Pima cottonseed on production of lactating dairy cows. J. Dairy Sci. 2013, 96, 2374–2386. [Google Scholar] [CrossRef]
- Santos, F.S.D.; Signoretti, R.D.; Oliveira, J.S.; Silva, G.T.D.; Rufino, M.O.A.; Souza, C.G.; Pinheiro, J.K.; Gonzaga Neto, S. Effect of replacing soybean meal with peanut meal on milk production and fat composition in lactating dairy cows. Trop. Anim. Health Prod. 2022, 54, 80. [Google Scholar] [CrossRef]
- Dingyuan, F.; Jianjun, Z. Nutritional and anti-nutritional composition of rapeseed meal and its utilization as a feed ingredient for animal. In Proceedings of the International Rapeseed Congress, Wuhan, China, 26–30 March 2007. [Google Scholar]
- Chelkapally, S.C.; Terrill, T.H.; Estrada-Reyes, Z.M.; Ogunade, I.M.; Pech-Cervantes, A.A. Effects of dietary inclusion of dry distillers grains with solubles on performance, carcass characteristics, and nitrogen metabolism in meat sheep: A meta-analysis. Front. Vet. Sci. 2023, 10, 1141068. [Google Scholar] [CrossRef]
- Anderson, J.L.; Schingoethe, D.J.; Kalscheur, K.F.; Hippen, A.R. Evaluation of dried and wet distillers grains included at two concentrations in the diets of lactating dairy cows. J. Dairy Sci. 2006, 89, 3133–3142. [Google Scholar] [CrossRef]
- Shuai, C.; Chen, D.; Yu, B.; Luo, Y.; Zheng, P.; Huang, Z.; Yu, J.; Mao, X.; Yan, H.; He, J. Effect of fermented rapeseed meal on growth performance, nutrient digestibility, and intestinal health in growing pigs. Anim. Nutr. 2023, 15, 420–429. [Google Scholar] [CrossRef]
- Woźniak, B.; Witek, S.; Matraszek-Żuchowska, I.; Sell, B.; Posyniak, A. The effect of diet enriched with rapeseed meal on endogenous thiouracil contents in urine of calves. Res. Vet. Sci. 2021, 136, 192–197. [Google Scholar] [CrossRef]
- Wang, C.; Liu, C.; Zhang, G.W.; Du, H.S.; Wu, Z.Z.; Liu, Q.; Guo, G.; Huo, W.J.; Zhang, J.; Pei, C.X.; et al. Effects of rumen-protected folic acid and betaine supplementation on growth performance, nutrient digestion, rumen fermentation and blood metabolites in Angus bulls. Br. J. Nutr. 2020, 123, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Ji, S.; Duan, C.; Ju, S.; Zhang, Y.; Yan, H.; Liu, Y. Rumen fluid transplantation affects growth performance of weaned lambs by altering gastrointestinal microbiota, immune function and feed digestibility. Animal 2021, 15, 100076. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis, 17th ed.; AOAC: Arlington, VA, USA, 2000. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Weatherburn, M.W. Phenol-hypochlorite reaction for determination of ammonia. Anal. Chem. 1967, 39, 971–974. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ekiz, B.; Ergul Ekiz, E.; Yalçıntan, H.; Koçak, O.; Yılmaz, A.; Güneş, H. The effects of transport stress on certain welfare parameters and behaviours in Red Karaman, Imroz, Sakız and Karakul rams. J. Fac. Vet. Med. Istanb. Univ. 2012, 38, 15–28. [Google Scholar]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glockner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Grice, E.A.; Kong, H.H.; Conlan, S.; Deming, C.B.; Davis, J.; Young, A.C.; Program, N.C.S.; Bouffard, G.G.; Blakesley, R.W.; Murray, P.R.; et al. Topographical and temporal diversity of the human skin microbiome. Science 2009, 324, 1190–1192. [Google Scholar] [CrossRef]
- Looft, T.; Johnson, T.A.; Allen, H.K.; Bayles, D.O.; Alt, D.P.; Stedtfeld, R.D.; Sul, W.J.; Stedtfeld, T.M.; Chai, B.; Cole, J.R.; et al. In-feed antibiotic effects on the swine intestinal microbiome. Proc. Natl. Acad. Sci. USA 2012, 109, 1691–1696. [Google Scholar] [CrossRef]
- Sami, A.S.; Schuster, M.; Schwarz, F.J. Performance, carcass characteristics and chemical composition of beef affected by lupine seed, rapeseed meal and soybean meal. J. Anim. Physiol. Anim. Nutr. 2010, 94, 465–473. [Google Scholar] [CrossRef]
- Yang, C.; Tian, X.; Li, J.; Yan, H.; Duan, C.; Zhang, Y.; Ji, S.; Liu, Y. Effect of different protein sources in vitro rumen fermentation parameters and amino acid content in sheep. Feed Res. 2023, 46, 5–11. (In Chinese) [Google Scholar] [CrossRef]
- Correia, B.R.; de Carvalho, G.G.; Oliveira, R.L.; Pires, A.J.; Ribeiro, O.L.; Silva, R.R.; Leão, A.G.; Oliveira, P.A. Intake, digestibility, performance, and nitrogen metabolism of feedlot-finished young bulls (Bos indicus) fed diets containing peanut cake. J. Anim. Sci. 2016, 94, 4720–4727. [Google Scholar] [CrossRef]
- Webb, N.W.; Bernard, J.K.; Tao, S. Production responses to diets supplemented with soybean meal, expeller soybean meal, or dry-extruded cottonseed cake by lactating dairy cows. Appl. Anim. Sci. 2019, 35, 543–549. [Google Scholar] [CrossRef]
- Alhadas, H.M.; Filhos, S.C.V.; Tedeschi, L.O.; Vilela, R.S.R.; Souza, G.A.P. In situ evaluation of dried distillers grains (DDG) and of diets containing different levels of DDG inclusion replacing soybean meal, urea and corn, and development of alternative methods to estimate in vivo digestibility of diets. Livest. Sci. 2021, 253, 104706. [Google Scholar] [CrossRef]
- Allen, M.S.; Bradford, B.J.; Oba, M. Board Invited Review: The hepatic oxidation theory of the control of feed intake and its application to ruminants. J. Anim. Sci. 2009, 87, 3317–3334. [Google Scholar] [CrossRef]
- Carvalho, S.; Dias, F.D.; Pires, C.C.; Brutti, D.; Lopes, J.; Santos, D.; Barcelos, R.D.; Macari, S.; Wommer, T.P.; Griebler, L. Ingestive behavior of lambs texel and ideal fed soybean hulls. Arch. De Zootec. 2014, 63, 55–64. [Google Scholar] [CrossRef][Green Version]
- Bastos, M.P.; de Carvalho, G.G.; Pires, A.J.; Silva, R.R.; Filho, A.E.; Dos Santos Ede, J.; Chagas, D.M.; Barroso, D.S.; Filho, G.A. Ingestive behavior and nitrogen balance of confined santa ines lambs fed diets containing soybean hulls. Asian-Australas. J. Anim. Sci. 2014, 27, 24–29. [Google Scholar] [CrossRef][Green Version]
- Chen, T.; Chen, D.; Tian, G.; Zheng, P.; Yu, B. Effects of soluble and insoluble dietary fiber supplementation on growth performance, nutrient digestibility, intestinal microbe and barrier function in weaning piglet. Ani. Feed Sci. Tech. 2019, 260, 114335. [Google Scholar] [CrossRef]
- Chishti, G.A.; Mitchell, L.K.; Dennis, T.S.; Hill, T.M.; Suarez-Mena, F.X.; Heinrichs, A.J. Starch-protein interaction in the rumen of weaned dairy calves. J. Dairy Sci. 2021, 104, 5445–5456. [Google Scholar] [CrossRef]
- Rehemujiang, H.; Rehemujiang, K.; Tuoxunjiang, H.; Lin, Z.; Yimamu, A.; Jie, G.L. Winter grazing on cotton stubble affects grazing behavior, feed intake, production, and health of small ruminants. Small Rumin. Res. 2022, 209, 106635. [Google Scholar] [CrossRef]
- Morgan, S.E. Gossypol as a toxicant in livestock. Vet. Clin. N. Am. Food Anim. Pract. 1989, 5, 251–262. [Google Scholar] [CrossRef]
- Barraza, M.L.; Coppock, C.E.; Brooks, K.N.; Wilks, D.L.; Saunders, R.G.; Latimer, G.W., Jr. Iron sulfate and feed pelleting to detoxify free gossypol in cottonseed diets for dairy cattle. J. Dairy Sci. 1991, 74, 3457–3467. [Google Scholar] [CrossRef]
- Junxia, W.; Haitao, Z.; Qihui, Y.; Beiping, T.; Xiaohui, D.; Shuyan, C.; Hongyu, L.; Shuang, Z. Effects of replacing soybean meal with cottonseed meal on growth, feed utilization and non-specific immune enzyme activities for juvenile white shrimp. Aquac. Rep. 2020, 16, 100255. [Google Scholar] [CrossRef]
- Liu, C.; Qu, Y.H.; Guo, P.T.; Xu, C.C.; Ma, Y.; Luo, H.L. Effects of dietary supplementation with alfalfa (Medicago sativa L.) saponins on lamb growth performance, nutrient digestibility, and plasma parameters. Anim. Feed Sci. Tech. 2018, 236, 98–106. [Google Scholar] [CrossRef]
- Kim, J.S.; Ingale, S.L.; Hosseindoust, A.R.; Lee, S.H.; Lee, J.H.; Chae, B.J. Effects of mannan level and β-mannanase supplementation on growth performance, apparent total tract digestibility and blood metabolites of growing pigs. Animal 2017, 11, 202–208. [Google Scholar] [CrossRef]
- Wanapat, M.; Boonnop, K.; Promkot, C.; Cherdthong, A. Effects of alternative protein sources on rumen microbes and productivity of dairy cows. Maejo Int. J. Sci. Technol. 2011, 5, 13–23. [Google Scholar] [CrossRef]
- Rebelo Lucas, R.; Luna Irene, C.; Messana Juliana, D.; Araujo Rafael, C.; Simioni Tiago, A.; Granja-Salcedo Yury, T.; Vito Elias, S.; Lee, C.; Teixeira Izabelle, A.M.A.; Rooke John, A.; et al. Effect of replacing soybean meal with urea or encapsulated nitrate with or without elemental sulfur on nitrogen digestion and methane emissions in feedlot cattle. Anim. Feed Sci. Technol. 2019, 257, 114293. [Google Scholar] [CrossRef]
- Abdallah, A.; Zhang, P.; Abubakari, A.H.; Elemba, E.; Zhong, Q.; Sun, Z. Reclamation of Astragalus By-Product through Dietary Inclusion in Ruminant Diets: Effects on Growth Performance, Nutrient Digestibility, Rumen Fermentation, Blood Biochemical Parameters, and Humoral Immune Response in Sheep. Evid.-Based Complement. Altern. Med. 2019, 2019, 8530961. [Google Scholar] [CrossRef]
- Reynolds, C.K.; Kristensen, N.B. Nitrogen recycling through the gut and the nitrogen economy of ruminants: An asynchronous symbiosis. J. Anim. Sci. 2008, 86, E293–E305. [Google Scholar] [CrossRef]
- Panah, F.M.; Lashkari, S.; Frydendahl Hellwing, A.L.; Larsen, M.; Weisbjerg, M.R. Effects of toasting and decortication of oat on nutrient digestibility in the rumen and small intestine and on amino acid supply in dairy cows. J. Dairy Sci. 2020, 103, 1484–1499. [Google Scholar] [CrossRef]
- Dos Santos, F.S.; Signoretti, R.D.; de Oliveira, J.S.; da Silva, G.T.; de Oliveira Alves Rufino, M.; de Souza, C.G.; Pinheiro, J.K.; Gonzaga Neto, S. Nitrogen balance and microbial protein synthesis in dairy cows fed with peanut meal to replace soybean meal. Trop. Anim. Health Prod. 2022, 54, 335. [Google Scholar] [CrossRef]
- Silva, R.V.M.M.; Carvalho, G.G.P.d.; Pires, A.J.V.; Pereira, M.L.A.; Pereira, L.; Campos, F.S.; Perazzo, A.F.; Bezerra, L.S.; Moreira, J.V.; Rufino, L.M.D.A. Nitrogen balance, microbial protein synthesis and ingestive behavior of lambs fed diets containing cottonseed cake in substitution of soybean meal. Semin. Ciências Agrárias 2016, 37, 2155–2166. [Google Scholar] [CrossRef][Green Version]
- Ahmad, A.A.; Yang, C.; Zhang, J.; Kalwar, Q.; Liang, Z.; Li, C.; Du, M.; Yan, P.; Long, R.; Han, J.; et al. Effects of Dietary Energy Levels on Rumen Fermentation, Microbial Diversity, and Feed Efficiency of Yaks (Bos grunniens). Front. Microbiol. 2020, 11, 625. [Google Scholar] [CrossRef]
- Van Hecke, T.; De Vrieze, J.; Boon, N.; De Vos, W.H.; Vossen, E.; De Smet, S. Combined Consumption of Beef-Based Cooked Mince and Sucrose Stimulates Oxidative Stress, Cardiac Hypertrophy, and Colonic Outgrowth of Desulfovibrionaceae in Rats. Mol. Nutr. Food Res. 2019, 63, e1800962. [Google Scholar] [CrossRef]
| Ingredients, % DM | Dietary Treatment | ||||
|---|---|---|---|---|---|
| Soybean Meal | Cottonseed Meal | Peanut Meal | Rapeseed Meal | DDGS 1 | |
| Peanut vine | 22.50 | 22.50 | 23.00 | 17.50 | 17.80 |
| Corn | 55.80 | 55.50 | 56.00 | 54.50 | 39.00 |
| Soybean meal | 16.70 | ||||
| Cottonseed cake | 17.00 | ||||
| Peanut cake | 16.00 | ||||
| Rapeseed cake | 23.00 | ||||
| DDGSs | 37.70 | ||||
| NaHCO3 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Premix 2 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Diet Composition | |||||
| Dry matter | 89.57 | 89.20 | 89.93 | 89.02 | 90.09 |
| Metabolizable energy 3, MJ kg−1 DM | 12.10 | 12.07 | 12.00 | 12.02 | 12.19 |
| Starch content | 35.01 | 34.50 | 35.83 | 34.50 | 25.65 |
| Crude protein | 15.14 | 15.32 | 15.16 | 15.16 | 15.43 |
| Neutral detergent fiber | 40.16 | 40.35 | 39.61 | 37.63 | 40.50 |
| Acid detergent fiber | 17.65 | 19.06 | 21.49 | 18.57 | 20.29 |
| Ether extract | 3.07 | 2.98 | 3.33 | 3.26 | 2.93 |
| Ash | 8.59 | 8.95 | 8.84 | 9.01 | 8.89 |
| Calcium | 0.85 | 0.83 | 0.84 | 0.91 | 0.91 |
| Phosphorus | 0.31 | 0.31 | 0.30 | 0.33 | 0.38 |
| Items 1 | Groups | ||||||
|---|---|---|---|---|---|---|---|
| Soybean Meal | Cottonseed Meal | Peanut Meal | Rapeseed Meal | DDGS | SEM | p-Value | |
| Growth performance | |||||||
| IW, kg | 38.6 | 37.3 | 39.8 | 39.3 | 38.62 | 0.403 | 0.325 |
| FW, kg | 51.4 | 50.4 | 52.3 | 53.1 | 51.54 | 0.548 | 0.599 |
| ADG, g/d | 246.4 | 252.1 | 248.9 | 266.9 | 251.40 | 5.829 | 0.687 |
| ADFI, kg/d | 1.46 b | 1.64 a | 1.63 a | 1.69 a | 1.64 a | 0.012 | <0.001 |
| Feed efficiency 2 | 5.94 a | 6.46 b | 6.80 c | 6.32 b | 6.51 b | 0.045 | <0.001 |
| Apparent nutrient digestibility, % | |||||||
| DM | 83.4 a | 82.6 a | 68.5 c | 83.4 a | 77.9 b | 0.513 | <0.001 |
| GE | 75.4 a | 71.2c | 60.7 d | 72.6 b | 71.1 c | 0.697 | <0.001 |
| CP | 84.6 a | 80.3c | 74.2 d | 83.0 b | 79.4 c | 0.440 | <0.001 |
| EE | 82.8 | 80.6 | 85.0 | 81.6 | 80.2 | 1.307 | 0.203 |
| NDF | 58.9 | 62.4 | 59.1 | 63.4 | 60.2 | 1.243 | 0.284 |
| ADF | 23.1 | 29.6 | 28.2 | 33.3 | 24.0 | 1.856 | 0.263 |
| Items | Groups | ||||||
|---|---|---|---|---|---|---|---|
| Soybean Meal | Cottonseed Meal | Peanut Meal | Rapeseed Meal | DDGS | SEM | p-Value | |
| Immunoglobulin, mg/mL | |||||||
| Immunoglobulin A | 1.52 | 1.81 | 1.66 | 1.72 | 1.51 | 0.045 | 0.176 |
| Immunoglobulin G | 13.43 a, b | 13.37 a, b | 14.20 a | 10.28 c | 12.22 b | 0.391 | <0.001 |
| Immunoglobulin M | 1.76 a | 1.70 b | 1.73 a, b | 1.72 a, b | 1.75 a | 0.007 | 0.016 |
| Metabolism | |||||||
| Glucose, mmol/L | 5.02 | 5.38 | 4.71 | 4.99 | 4.94 | 0.135 | 0.670 |
| Blood urea nitrogen, mmol/L | 8.74 a | 7.41 a | 6.05 b | 5.37 b | 8.21 a | 0.300 | <0.001 |
| Total protein, g/L | 83.00 a | 80.42 a | 80.73 a | 69.02 b | 78.98 a | 1.122 | <0.001 |
| Albumin, g/L | 27.74 a | 28.69 a | 28.00 a | 24.37 b | 26.80 a, b | 0.500 | 0.033 |
| Globulin, g/L | 55.31 a | 48.35 a, b | 52.41 a, b | 44.79 b | 49.10 a, b | 1.208 | 0.050 |
| Total cholesterol, mmol/L | 1.45 | 1.55 | 1.48 | 1.55 | 1.76 | 0.058 | 0.532 |
| Aspartate aminotransferase, U/L | 6.95 a, b | 8.38 a | 6.20 b | 7.04 a, b | 7.57 a, b | 0.300 | 0.188 |
| Alanine aminotransferase, U/L | 1.87 b | 3.40 a | 1.95 b | 1.91 b | 1.73 b | 1.576 | <0.001 |
| Items 1, % | Groups | ||||||
|---|---|---|---|---|---|---|---|
| Soybean Meal | Cottonseed Meal | Peanut Meal | Rapeseed Meal | DDGS | SEM | p-Value | |
| pH | 6.18 | 6.27 | 6.35 | 6.20 | 6.13 | 0.685 | 0.303 |
| NH3-N, mg/dL | 20.68 a | 12.55 b | 11.52 b | 11.18 b | 11.01 b | 0.712 | <0.001 |
| MCP, mg/dL | 9.09 a | 8.10 a, b | 8.79 a, b | 8.42 a, b | 7.75 b | 0.161 | 0.049 |
| Acetate, mmol/L | 31.33 | 34.50 | 27.26 | 32.57 | 28.76 | 1.957 | 0.803 |
| Propionate, mmol/L | 32.95 | 27.92 | 21.92 | 34.38 | 28.58 | 1.852 | 0.234 |
| Butyrate, mmol/L | 10.31 | 10.29 | 8.96 | 10.38 | 8.03 | 0.858 | 0.8993 |
| Valerate, mmol/L | 1.61 | 2.17 | 2.05 | 1.89 | 2.03 | 1.579 | 0.850 |
| Isobutyrate | 0.69 | 0.13 | 0.27 | 0.09 | 0.03 | 0.103 | 0.267 |
| Isovalerate | 0.85 | 0.54 | 0.88 | 0.40 | 0.25 | 0.107 | 0.254 |
| Total volatile fatty acids, mmol/L | 77.74 | 75.55 | 61.34 | 79.70 | 67.68 | 3.471 | 0.438 |
| Acetate/Propionate | 1.38 | 1.32 | 1.31 | 0.95 | 1.03 | 0.124 | 0.778 |
| Items 1 | Groups | ||||||
|---|---|---|---|---|---|---|---|
| Soybean Meal | Cottonseed Meal | Peanut Meal | Rapeseed Meal | DDGS | SEM | p-Value | |
| Firmicutes | 45.14 | 54.04 | 56.21 | 43.24 | 47.32 | 1.939 | 0.138 |
| Bacteroidota | 38.35 | 30.74 | 30.38 | 43.18 | 32.21 | 1.692 | 0.319 |
| Proteobacteria | 10.55 | 10.09 | 10.17 | 12.34 | 12.89 | 1.024 | 0.764 |
| Actinobacteriota | 4.34 | 6.45 | 5.66 | 4.76 | 5.44 | 0.787 | 0.285 |
| Firmicutes/Bacteroidota | 1.22 | 2.27 | 1.98 | 1.17 | 1.56 | 0.167 | 0.151 |
| Items 1 | Groups | ||||||
|---|---|---|---|---|---|---|---|
| Soybean Meal | Cottonseed Meal | Peanut Meal | Rapeseed Meal | DDGS | SEM | p-Value | |
| Prevotella 7 | 26.98 | 28.06 | 20.25 | 21.93 | 20.66 | 2.020 | 0.713 |
| Dialister | 8.31 | 7.12 | 5.97 | 8.33 | 5.80 | 0.627 | 0.569 |
| Lachnospiracea NK3A20 group | 5.76 | 11.56 | 7.81 | 6.09 | 9.05 | 0.889 | 0.242 |
| Unclassified Selenomonadaceae | 5.24 | 4.63 | 2.78 | 6.45 | 6.87 | 0.679 | 0.410 |
| Prevotella | 4.00 | 6.98 | 5.48 | 3.48 | 6.96 | 1.098 | 0.829 |
| Succinivibrionaceae UCG 001 | 2.22 | 5.68 | 2.10 | 3.11 | 5.13 | 0.700 | 0.391 |
| Rikenellaceae RC9 gut group | 3.97 | 2.33 | 2.99 | 3.92 | 2.20 | 0.392 | 0.472 |
| Olsenella | 1.33 | 3.53 | 3.71 | 3.27 | 3.46 | 0.372 | 0.177 |
| Unclassified Lachnospiraceae | 1.72 | 3.20 | 1.66 | 1.44 | 1.35 | 0.282 | 0.259 |
| Succinivibrio | 1.19 | 0.51 | 2.03 | 0.13 | 0.49 | 0.318 | 0.388 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, X.; Chen, M.; Yang, C.; Duan, C.; Ji, S.; Yan, H.; Liu, Y.; Zhang, Y. Effects of Replacing Soybean Meal with Cottonseed Meal, Peanut Meal, Rapeseed Meal, or Distillers’ Dried Grains with Solubles on the Growth Performance, Nutrient Digestibility, Serum Parameters, and Rumen Fermentation in Growing Lambs. Vet. Sci. 2024, 11, 322. https://doi.org/10.3390/vetsci11070322
Yin X, Chen M, Yang C, Duan C, Ji S, Yan H, Liu Y, Zhang Y. Effects of Replacing Soybean Meal with Cottonseed Meal, Peanut Meal, Rapeseed Meal, or Distillers’ Dried Grains with Solubles on the Growth Performance, Nutrient Digestibility, Serum Parameters, and Rumen Fermentation in Growing Lambs. Veterinary Sciences. 2024; 11(7):322. https://doi.org/10.3390/vetsci11070322
Chicago/Turabian StyleYin, Xuejiao, Meijing Chen, Caihong Yang, Chunhui Duan, Shoukun Ji, Hui Yan, Yueqin Liu, and Yingjie Zhang. 2024. "Effects of Replacing Soybean Meal with Cottonseed Meal, Peanut Meal, Rapeseed Meal, or Distillers’ Dried Grains with Solubles on the Growth Performance, Nutrient Digestibility, Serum Parameters, and Rumen Fermentation in Growing Lambs" Veterinary Sciences 11, no. 7: 322. https://doi.org/10.3390/vetsci11070322
APA StyleYin, X., Chen, M., Yang, C., Duan, C., Ji, S., Yan, H., Liu, Y., & Zhang, Y. (2024). Effects of Replacing Soybean Meal with Cottonseed Meal, Peanut Meal, Rapeseed Meal, or Distillers’ Dried Grains with Solubles on the Growth Performance, Nutrient Digestibility, Serum Parameters, and Rumen Fermentation in Growing Lambs. Veterinary Sciences, 11(7), 322. https://doi.org/10.3390/vetsci11070322






