Evaluation of Safe Insertion Angles for Spinal Needles and Safe Intensity of the Holmium:YAG Laser during Percutaneous Laser Disc Ablations in Feline Cadavers
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
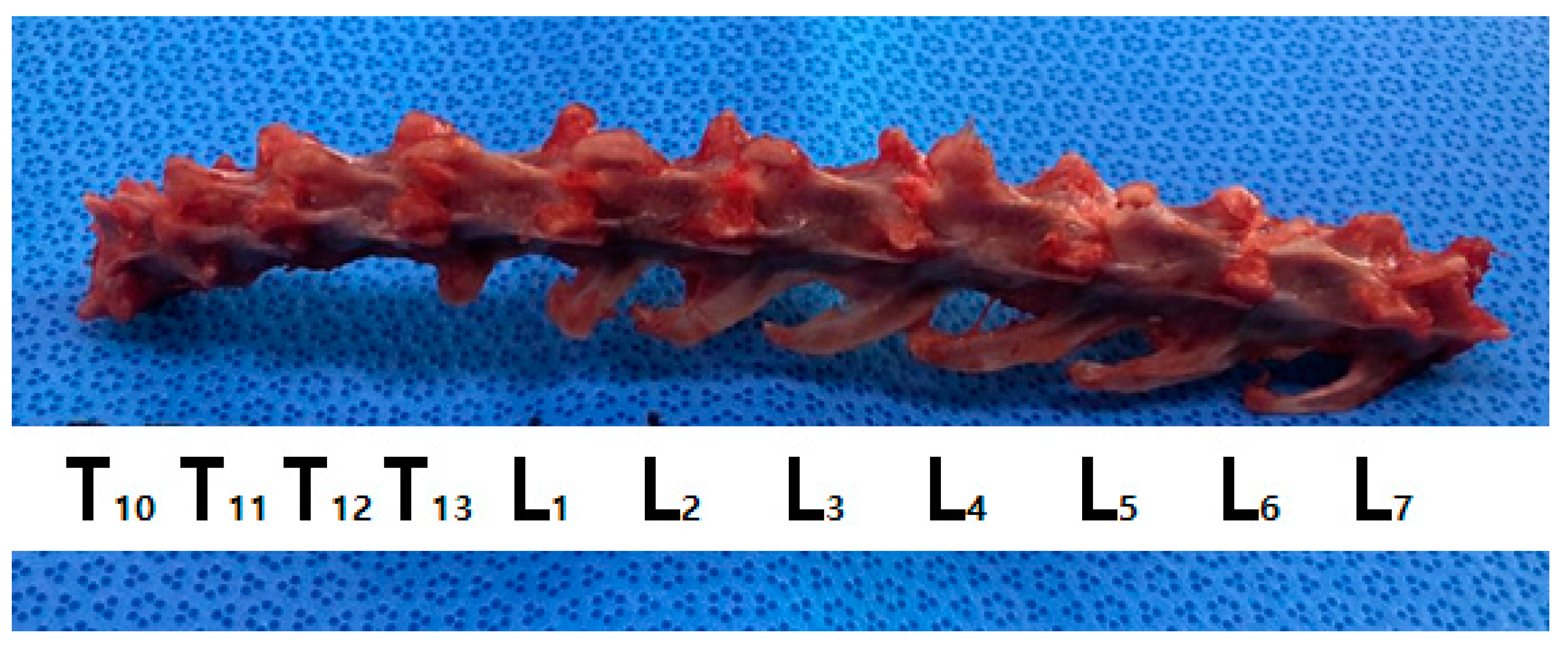
2.1. Preparation of the Vertebral Bodies in Cadavers
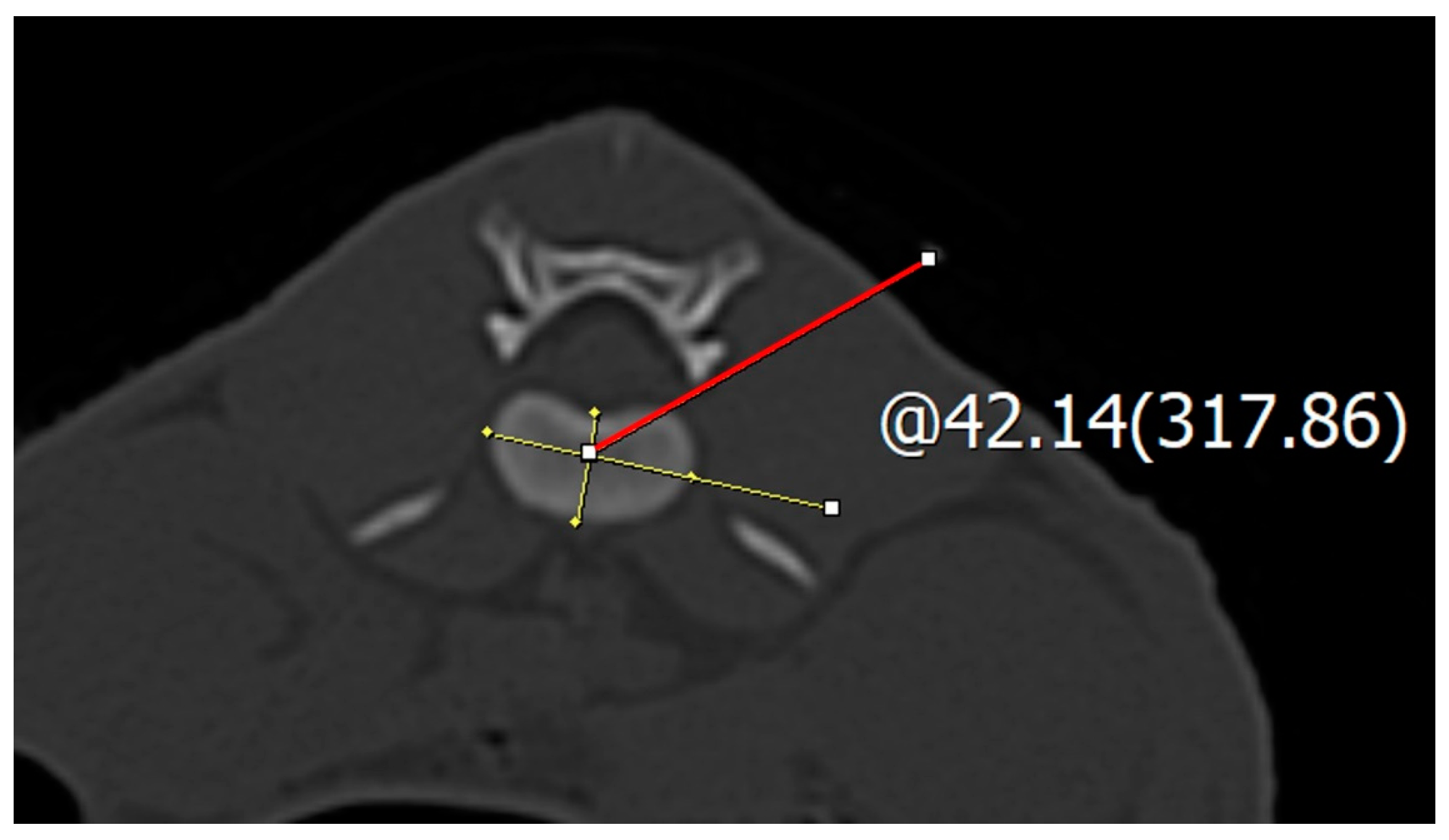
2.2. Spinal Needle Safe Corridor Evaluation
2.3. Safe Corridor Evaluation at the Thoracic Region
2.4. Safe Corridor Evaluation at the Lumbar Region
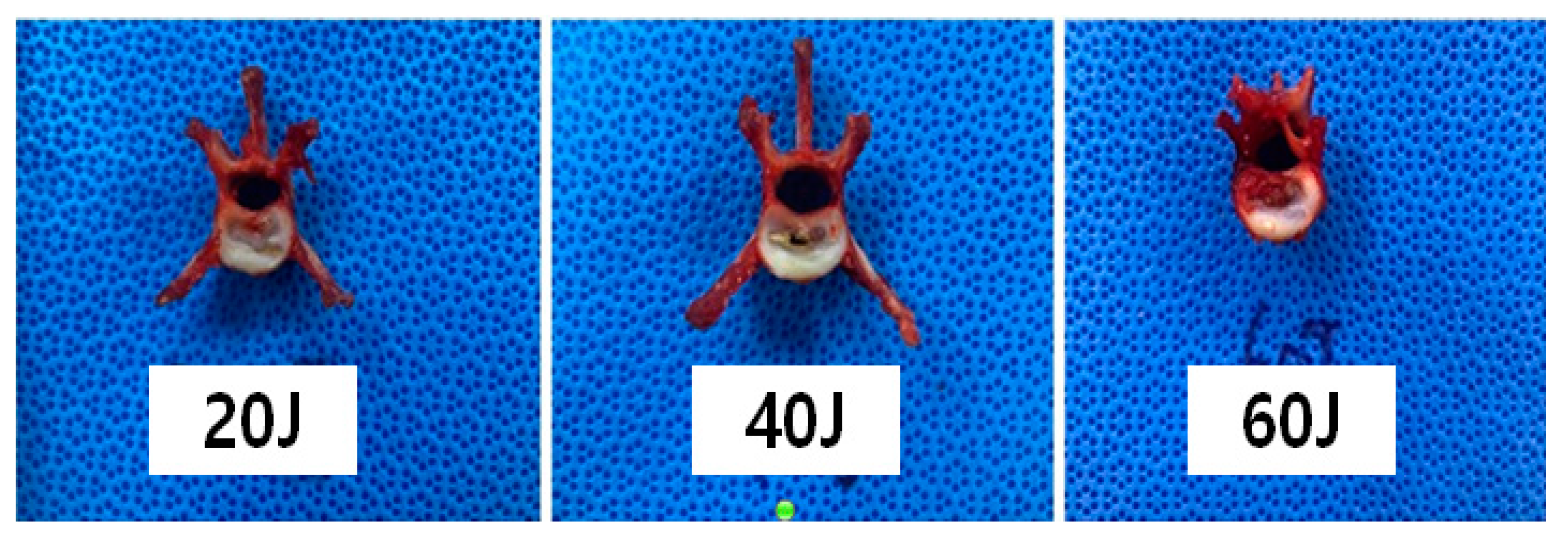
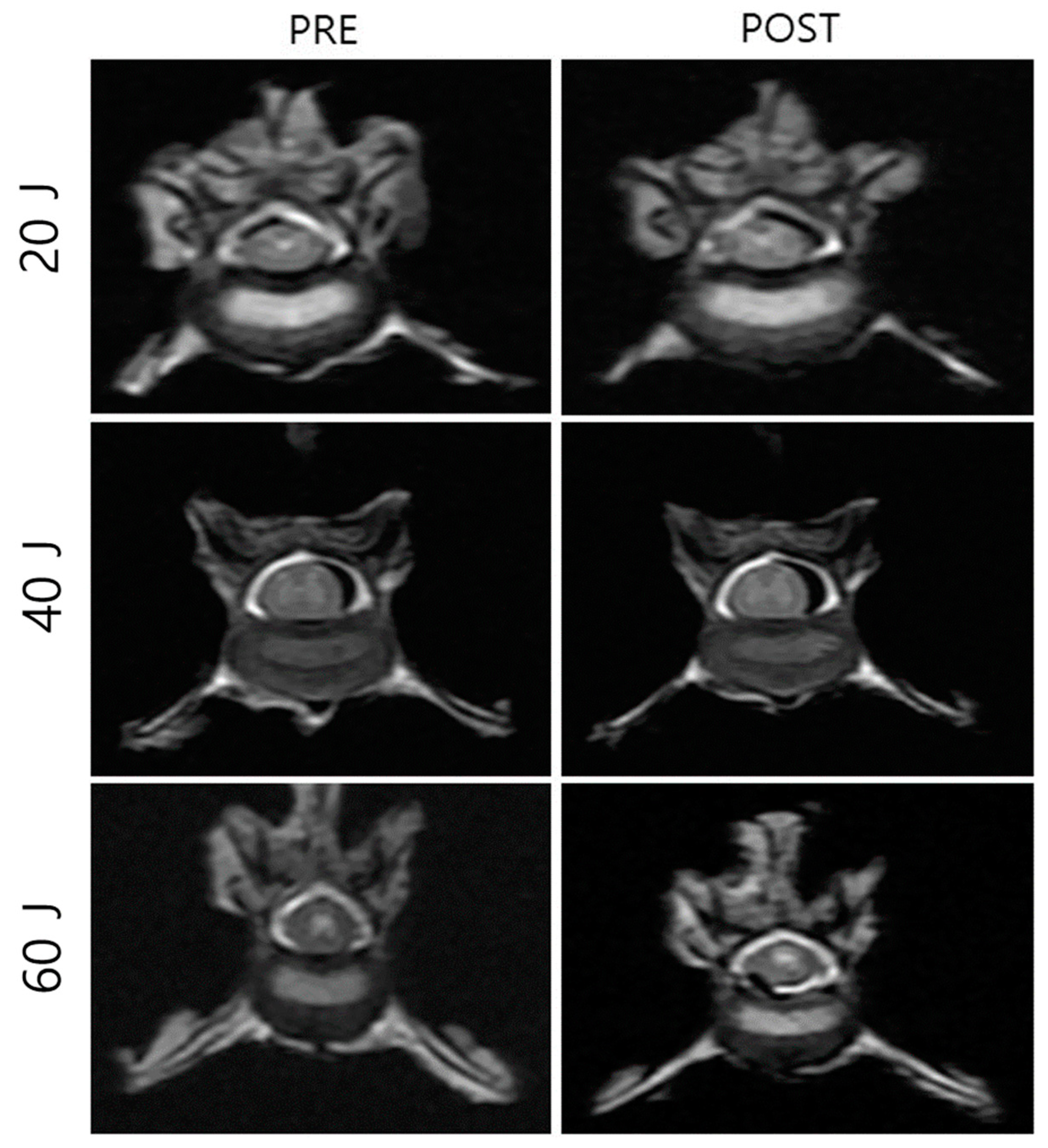
2.5. Macroscopic Evaluation According to Laser Intensity
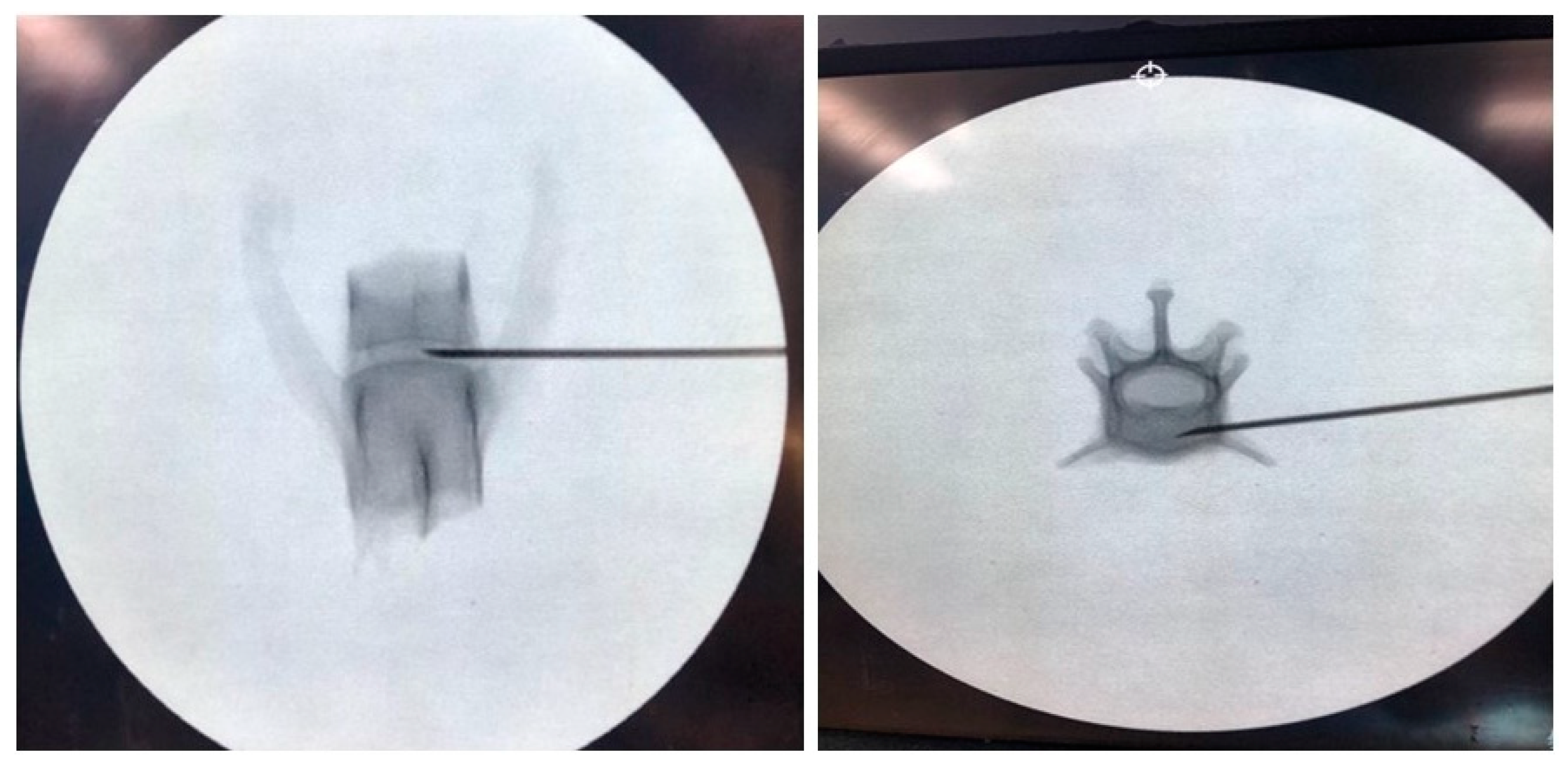
2.6. Cadaver Preparation and Computed Tomography
2.7. Vaporized NP Weight According to the Total Energy
2.8. Histopathological Analysis
2.9. Statistical Analysis
3. Results
3.1. Safe Corridor Angle at the Thoracolumbar Region
3.2. NP Changes after Laser Treatment
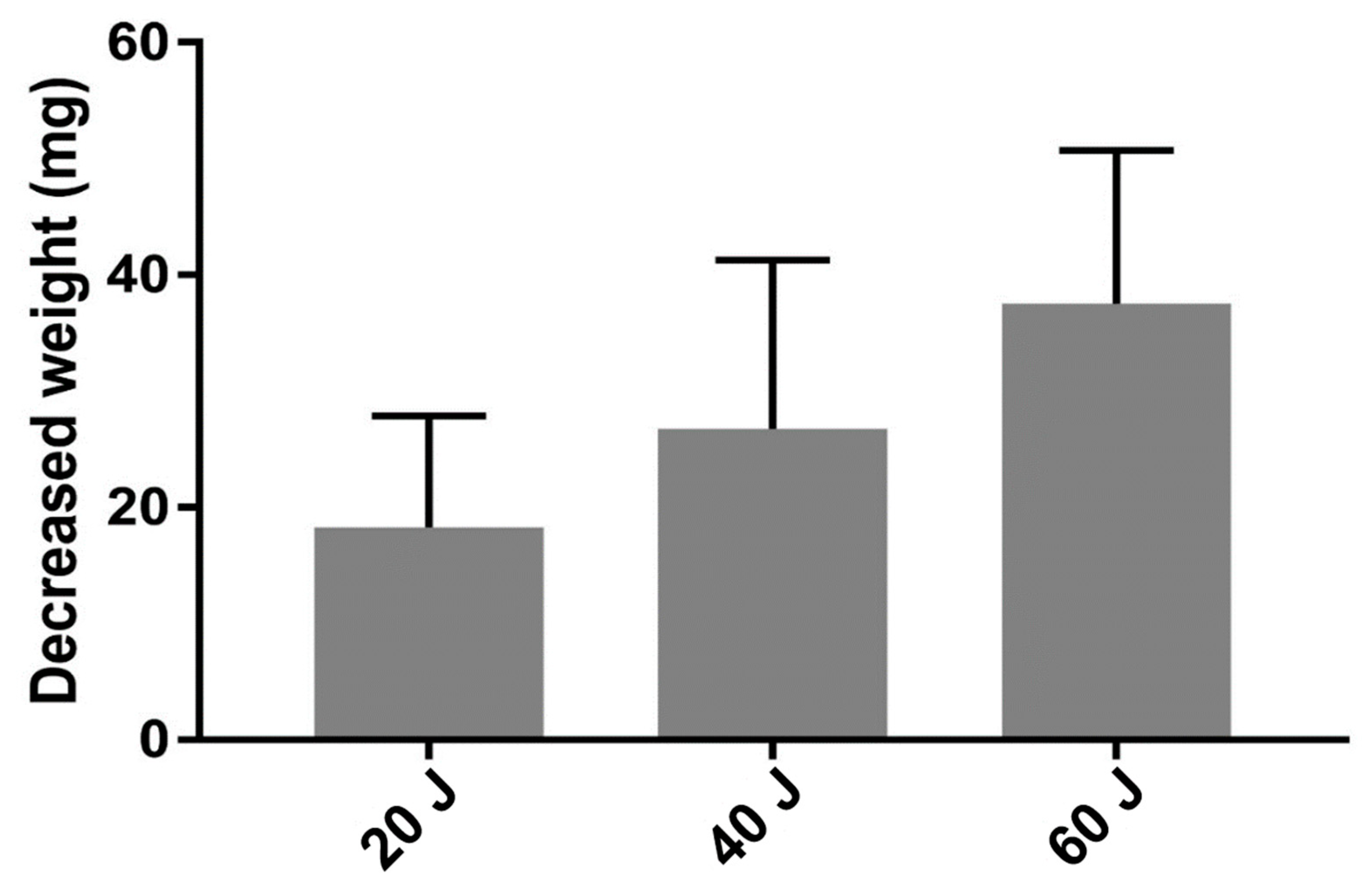
3.3. Vaporized NP Weight According to Total Energy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jeong, I.-S.; Rahman, M.M.; Choi, G.-C.; Seo, B.-S.; Lee, G.-J.; Kim, S.; Kim, N.S. A retrospective study of canine cervical disk herniation and the beneficial effects of rehabilitation therapy after ventral slot decompression. Veterinární Medicína 2019, 64, 251–259. [Google Scholar] [CrossRef]
- Hamilton-Bennett, S.E.; Behr, S. Clinical presentation, magnetic resonance imaging features, and outcome in 6 cats with lumbar degenerative intervertebral disc extrusion treated with hemilaminectomy. Vet. Surg. 2019, 48, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Crowe, Y.C.; Child, G.; Lam, R.; McGregor, R. Congenital block vertebrae and intervertebral disc protrusion in a young cat. JFMS Open Rep. 2019, 5, 2055116919868037. [Google Scholar] [CrossRef] [PubMed]
- Taylor-Brown, F.E.; De Decker, S. Presumptive acute non-compressive nucleus pulposus extrusion in 11 cats: Clinical features, diagnostic imaging findings, treatment and outcome. J. Feline Med. Surg. 2017, 19, 21–26. [Google Scholar] [CrossRef] [PubMed]
- De Decker, S.; Warner, A.S.; Volk, H.A. Prevalence and breed predisposition for thoracolumbar intervertebral disc disease in cats. J. Feline Med. Surg. 2017, 19, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Van Meervenne, S.; Haers, H.; Vissers, B.; Bosmans, T.; van Ham, L. Favorable outcome of conservative treatment in a cat with T9T10 intervertebral disk disease. Vlaams Diergeneeskd. Tijdschr. 2010, 79, 143–146. [Google Scholar] [CrossRef]
- Knipe, M.F.; Vernau, K.M.; Hornof, W.J.; LeCouteur, R.A. Intervertebral disc extrusion in six cats. J. Feline Med. Surg. 2001, 3, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Kathmann, I.; Cizinauskas, S.; Rytz, U.; Lang, J.; Jaggy, A. Spontaneous lumbar intervertebral disc protrusion in cats: Literature review and case presentations. J. Feline Med. Surg. 2000, 2, 207–212. [Google Scholar] [CrossRef]
- Choi, K.H.; Hill, S.A. Acupuncture treatment for feline multifocal intervertebral disc disease. J. Feline Med. Surg. 2009, 11, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Lamb, C.R.; Wesselingh, K.; Targett, M.P. Acute intervertebral disc extrusion in a cat: Clinical and MRI findings. J. Feline Med. Surg. 2002, 4, 65–68. [Google Scholar] [CrossRef]
- Malik, Y.; Konar, M.; Wernick, M.; Howard, J.; Forterre, F. Chronic intervertebral disk herniation associated with fused vertebrae treated by vertebral lateral corpectomy in a cat. Vet. Comp. Orthop. Traumatol. 2009, 22, 170–173. [Google Scholar] [CrossRef] [PubMed]
- Maritato, K.C.; Colon, J.A.; Mauterer, J.V. Acute non-ambulatory tetraparesis attributable to cranial cervical intervertebral disc disease in a cat. J. Feline Med. Surg. 2007, 9, 494–498. [Google Scholar] [CrossRef] [PubMed]
- McConnell, J.F.; Garosi, L.S. Intramedullary intervertebral disk extrusion in a cat. Vet. Radiol. Ultrasound 2004, 45, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Chow, K.; Beatty, J.A.; Voss, K.; Barrs, V.R. Probable lumbar acute non-compressive nucleus pulposus extrusion in a cat with acute onset paraparesis. J. Feline Med. Surg. 2012, 14, 764–767. [Google Scholar] [CrossRef] [PubMed]
- Vallefuoco, R.; Manassero, M.; Leperlier, D.; Scotti, S.; Viateau, V.; Moissonnier, P. Surgical repair of thoraco-lumbar vertebral fracture-luxations in eight cats using screws and polymethylmethacrylate fixation. Vet. Comp. Orthop. Traumatol. 2014, 27, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Wu, P.H.; Jang, I.T. Lumbar Endoscopic Unilateral Laminotomy for Bilateral Decompression Outside-In Approach: A Proctorship Guideline with 12 Steps of Effectiveness and Safety. Neurospine 2020, 17, S99–S109. [Google Scholar] [CrossRef] [PubMed]
- Blamoutier, A. Surgical discectomy for lumbar disc herniation: Surgical techniques. Orthop. Traumatol. Surg. Res. 2013, 99, S187–S196. [Google Scholar] [CrossRef] [PubMed]
- Guevar, J.; Olby, N. Minimally invasive microsurgical decompression of an intervertebral disc protrusion in a dog. Vet. Surg. 2020, 49 (Suppl. 1), O86–O92. [Google Scholar] [CrossRef] [PubMed]
- Lockwood, A.A.; Griffon, D.J.; Gordon-Evans, W.; Matheson, J.A.; Barthelemy, N.; Schaeffer, D.J. Comparison of two minimally invasive approaches to the thoracolumbar spinal canal in dogs. Vet. Surg. 2014, 43, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Jeong, I.S.; Piao, Z.; Rahman, M.M.; Kim, S.; Kim, N.S. Canine thoracolumbar intervertebral disk herniation and rehabilitation therapy after surgical decompression: A retrospective study. J. Adv. Vet. Anim. Res. 2019, 6, 394–402. [Google Scholar] [CrossRef]
- Leblond, G.; Gaitero, L.; Moens, N.M.; Zur Linden, A.; James, F.M.K.; Monteith, G.; Runciman, J. Canine atlantoaxial optimal safe implantation corridors—description and validation of a novel 3D presurgical planning method using OsiriX™. BMC Vet. Res. 2016, 12, 188. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Lin, L.; Kong, X.; Zhao, W.; Tang, L.; Li, J.; Ouyang, J. Construction and accuracy assessment of patient-specific biocompatible drill template for cervical anterior transpedicular screw (ATPS) insertion: An in vitro study. PLoS ONE 2013, 8, e53580. [Google Scholar] [CrossRef] [PubMed]
- Kamimura, M.; Ebara, S.; Itoh, H.; Tateiwa, Y.; Kinoshita, T.; Takaoka, K. Cervical pedicle screw insertion: Assessment of safety and accuracy with computer-assisted image guidance. J. Spinal Disord. 2000, 13, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, S.C.; Kramer, D.L.; Balderston, R.A.; Vaccaro, A.R.; Foley, K.F.; Albert, T.J. Placement of pedicle screws in the human cadaveric cervical spine: Comparative accuracy of three techniques. Spine (Phila. Pa 1976) 2000, 25, 1655–1667. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.Y.; Gu, T.; Zhang, C.; Wang, D.L.; He, Q.; Ruan, D.K. Computed tomography-guided nucleus pulposus biopsy for canine intervertebral disc degeneration preparation: A radiology and histology study. Spine J. 2016, 16, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Sumner-Smith, G. The spine. In Feline Orthopedic Surgery and Musculoskeletal Disease; Montavon, P.M., Voss, K., Langley-Hobbs, S.J., Eds.; Elsevier: Edinburgh, UK, 2009; pp. 407–422. [Google Scholar]
- Vallefuoco, R.; Bedu, A.S.; Manassero, M.; Viateau, V.; Niebauer, G.; Moissonnier, P. Computed tomographic study of the optimal safe implantation corridors in feline thoraco-lumbar vertebrae. Vet. Comp. Orthop. Traumatol. 2013, 26, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Bartels, K. Use of lasers in veterinary surgery and percutaneous laser disc ablation. In Advances in Intervertebral Disc Disease in Dogs and Cats; Fingeroth, J.M., Thomas, W.B., Eds.; John Wiley & Sons: Ames, Iowa, 2014; pp. 269–278. [Google Scholar] [CrossRef]
- Dillingham, M.F.; Price, J.M.; Fanton, G.S. Holmium laser surgery. Orthopedics 1993, 16, 563–566. [Google Scholar] [CrossRef] [PubMed]
- Quigley, M.R.; Maroon, J.C. Laser discectomy: A review. Spine J. 1994, 19, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Mayer, H.M.; Brock, M.; Berlien, H.P.; Weber, B. Percutaneous endoscopic laser discectomy (PELD). A new surgical technique for non-sequestrated lumbar discs. Acta Neurochir. Suppl. 1992, 54, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Jacques, S.L. Laser-tissue interactions. Photochemical, photothermal, and photomechanical. Surg. Clin. N. Am. 1992, 72, 531–558. [Google Scholar] [CrossRef]


| No. | Breeds | Sex | Body Weight (kg) | Experiment | Total Energy |
|---|---|---|---|---|---|
| 1 | KSH * | Castrated male | 3.5 | Laser intensity and nucleus pulposus weight | 20 J |
| 2 | KSH | Spayed female | 4 | Laser intensity and nucleus pulposus weight | 20 J |
| 3 | KSH | Spayed female | 4.2 | Total energy and nucleus pulposus weight | 40 J |
| 4 | KSH | Castrated male | 3.8 | Total energy and nucleus pulposus weight | 40 J |
| 5 | KSH | Castrated male | 4.1 | Nucleus pulposus weight | 40 J |
| 6 | KSH | Castrated male | 4.3 | Nucleus pulposus weight | 60 J |
| 7 | KSH | Spayed female | 3.9 | Nucleus pulposus weight | 60 J |
| No. | T10–11 IVDs | T11–12 IVDs | T12–13 IVDs | T13–L1 IVDs | L1–2 IVDs | L2–3 IVDs | L3–4 IVDs | L4–5 IVDs | L5–6 IVDs | L6–7 IVDs |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Rib~Acc | Rib~Acc | Rib~Acc | Rib~Acc | DP~Acc | DP~Acc | DP~Acc | DP~Acc | DP~Cau | - |
| 2 | Rib~Acc | Rib~Acc | Rib~Acc | Rib~Acc | DP~Acc | DP~Acc | DP~Acc | DP~Acc | DP~Cau | - |
| 3 | Rib~Acc | Rib~Cau | Rib~Acc | Rib~Acc | DP~Acc | DP~Acc | DP~Acc | DP~Acc | DP~Acc | - |
| 4 | Rib~Cau | Rib~Acc | Rib~Acc | Rib~Acc | DP~Acc | DP~Acc | DP~Acc | DP~Acc | DP~Cau | - |
| 5 | Rib~Acc | Rib~Acc | Rib~Acc | Rib~Acc | DP~Acc | DP~Acc | DP~Acc | DP~Acc | DP~Acc | - |
| 6 | Rib~Acc | Rib~Acc | Rib~Acc | Rib~Acc | DP~Acc | DP~Acc | DP~Acc | DP~Acc | DP~Cau | - |
| 7 | Rib~Acc | Rib~Cau | Rib~Acc | Rib~Acc | DP~Cau | DP~Acc | DP~Cau | DP~Acc | DP~Cau | - |
| 8 | Rib~Cau | Rib~Acc | Rib~Acc | Rib~Acc | DP~Acc | DP~Acc | DP~Acc | DP~Acc | DP~Acc | - |
| 9 | Rib~Acc | Rib~Acc | Rib~Acc | Rib~Acc | DP~Acc | DP~Acc | DP~Acc | DP~Acc | DP~Acc | - |
| 10 | Rib~Acc | Rib~Acc | Rib~Acc | Rib~Acc | DP~Acc | DP~Acc | DP~Cau | DP~Acc | DP~Cau | - |
| No. | T10–11 IVDs | T11–12 IVDs | T12–13 IVDs | T13–L1 IVDs | ||||
|---|---|---|---|---|---|---|---|---|
| S † (°) | F b (°) | S (°) | F (°) | S (°) | F (°) | S (°) | F (°) | |
| 1 | 8.35 | 37.43 | 7.45 | 34.47 | 9.36 | 33.25 | 8.46 | 39.42 |
| 2 | 7.83 | 34.52 | 9.72 | 35.12 | 7.48 | 33.55 | 9.62 | 41.53 |
| 3 | 9.23 | 36.68 | 10.53 | 39.78 | 8.34 | 33.53 | * | 37.35 |
| 4 | 5.71 | 37.74 | 14.34 | 41.39 | 14.45 | 32.48 | 10.63 | 36.53 |
| 5 | 5.41 | 33.87 | 5.35 | 40.27 | 15.58 | 38.76 | 11.47 | 41.34 |
| 6 | 7.85 | 30.58 | 10.15 | 40.36 | 8.51 | 38.86 | 4.48 | 40.00 |
| 7 | 5.95 | 37.58 | 8.19 | 35.62 | 8.75 | 40.47 | 3.19 | 38.56 |
| 8 | 6.96 | 32.75 | 8.41 | 34.23 | 8.43 | 43.63 | 9.89 | 39.53 |
| 9 | 8.79 | 30.35 | 10.43 | 36.54 | 10.43 | 33.89 | 10.35 | 35.61 |
| 10 | 4.79 | 32.84 | 12.63 | 34.73 | 14.62 | 42.95 | * | 41.35 |
| Mean | 7.09 | 34.43 | 9.72 | 37.25 | 10.60 | 37.14 | 8.51 | 39.12 |
| SD | 1.55 | 2.83 | 2.57 | 2.85 | 3.07 | 4.29 | 3.03 | 2.09 |
| 20 J | 40 J | 60 J | |
|---|---|---|---|
| Pre weight | 3470.40 ± 228.57 | 4025.73 ± 181.09 | 3950.75 ± 212.18 |
| Post weight | 3452.15 ± 229.09 | 3998.97 ± 180.90 | 3912.25 ± 211.51 |
| Difference weight | 18.25 ± 2.15 | 26.77 ± 2.65 | 38.50 ± 2.49 |
| No. | L1–2 | L2–3 | L3–4 | L4–5 | L5–6 | L6–7 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S a (°) | F b (°) | S (°) | F (°) | S (°) | F (°) | S (°) | F (°) | S (°) | F (°) | S (°) | F (°) | |
| 1 | * | 41.45 | * | 43.23 | * | 44.53 | * | 42.52 | * | 44.23 | * | * |
| 2 | * | 40.36 | * | 41.53 | * | 42.72 | * | 42.73 | * | 41.32 | * | * |
| 3 | * | 42.64 | * | 46.12 | * | 42.63 | * | 40.85 | * | 43.26 | * | * |
| 4 | * | 41.84 | * | 42.62 | * | 43.63 | * | 41.75 | * | 43.62 | * | * |
| 5 | * | 43.25 | * | 40.32 | * | 41.53 | * | 45.23 | * | 42.34 | * | * |
| 6 | * | 40.62 | * | 42.32 | * | 40.23 | * | 41.54 | * | 43.53 | * | * |
| 7 | * | 45.12 | * | 43.62 | * | 42.21 | * | 43.18 | * | 44.23 | * | * |
| 8 | * | 41.63 | * | 45.23 | * | 43.21 | * | 41.34 | * | 43.62 | * | * |
| 9 | * | 42.21 | * | 39.87 | * | 42.74 | * | 43.23 | * | 41.23 | * | * |
| 10 | * | 40.00 | * | 39.24 | * | 41.64 | * | 40.03 | * | 42.61 | * | * |
| Mean | 41.91 | 42.41 | 42.51 | 42.24 | 43.00 | |||||||
| SD | 1.52 | 2.25 | 1.20 | 1.47 | 1.09 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piao, Z.; Kim, Y.-u.; Ko, J.; Lee, J.; Choi, D.; Kim, N. Evaluation of Safe Insertion Angles for Spinal Needles and Safe Intensity of the Holmium:YAG Laser during Percutaneous Laser Disc Ablations in Feline Cadavers. Vet. Sci. 2024, 11, 325. https://doi.org/10.3390/vetsci11070325
Piao Z, Kim Y-u, Ko J, Lee J, Choi D, Kim N. Evaluation of Safe Insertion Angles for Spinal Needles and Safe Intensity of the Holmium:YAG Laser during Percutaneous Laser Disc Ablations in Feline Cadavers. Veterinary Sciences. 2024; 11(7):325. https://doi.org/10.3390/vetsci11070325
Chicago/Turabian StylePiao, Zhenglin, Young-ung Kim, Jongchan Ko, Jumjae Lee, Daeyoung Choi, and Namsoo Kim. 2024. "Evaluation of Safe Insertion Angles for Spinal Needles and Safe Intensity of the Holmium:YAG Laser during Percutaneous Laser Disc Ablations in Feline Cadavers" Veterinary Sciences 11, no. 7: 325. https://doi.org/10.3390/vetsci11070325





