Lactobacillus Genus Complex Probiotic-Induced Changes on the Equine Clitoral Microbiome
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mare Selection
2.2. Sample Collection
2.3. LGC Probiotic Gel
2.4. Microbiome Sampling and Probiotic Treatment
2.5. Post-Treatment Breeding Trial
2.6. DNA Extraction and Quality Control
2.7. DNA Sequencing and Data Analysis
3. Results
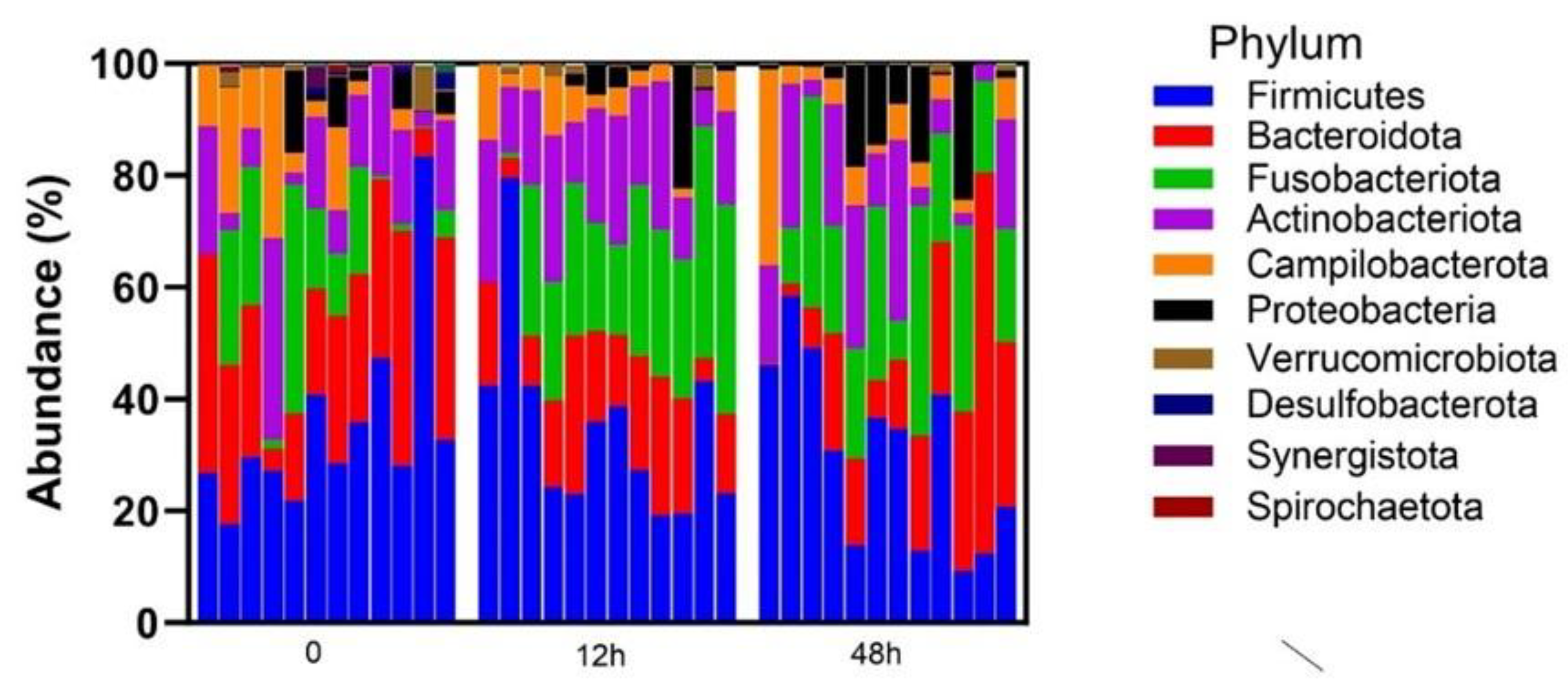
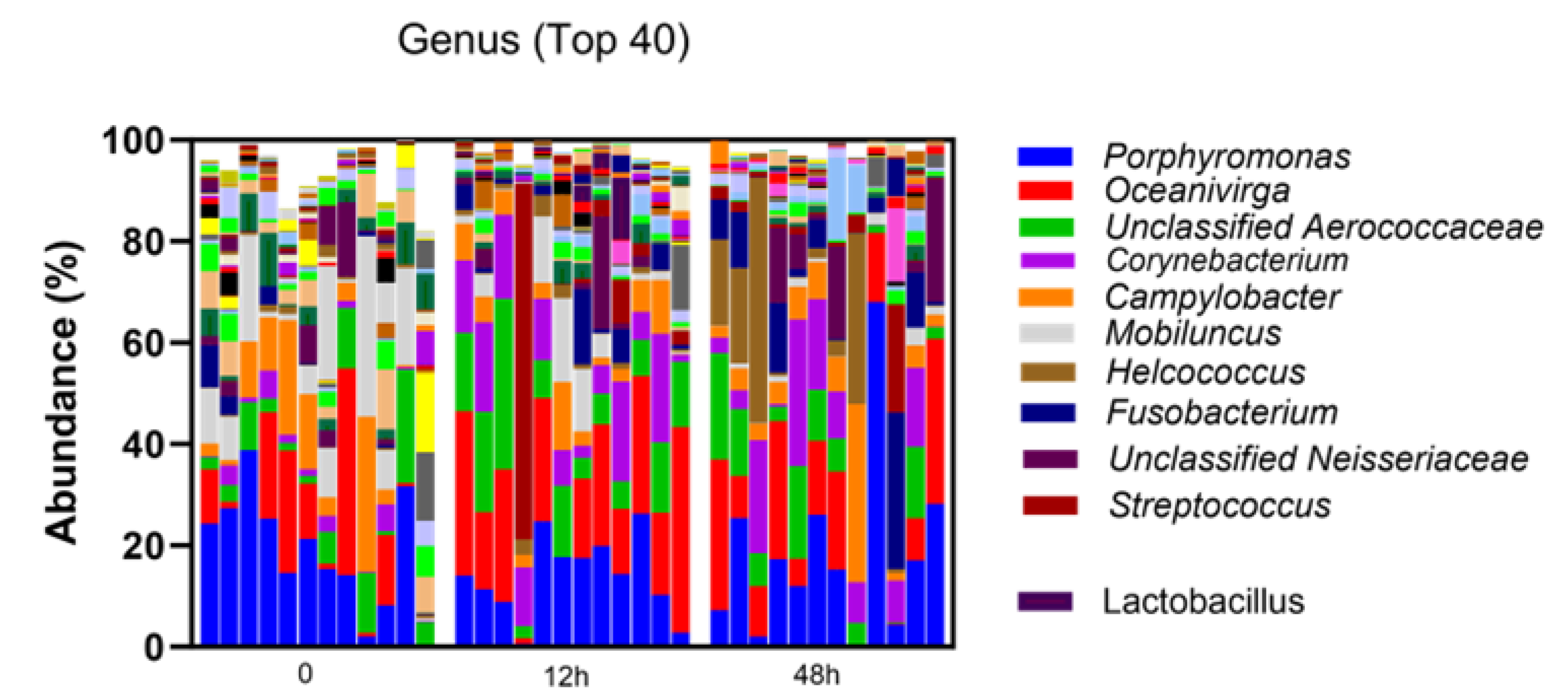
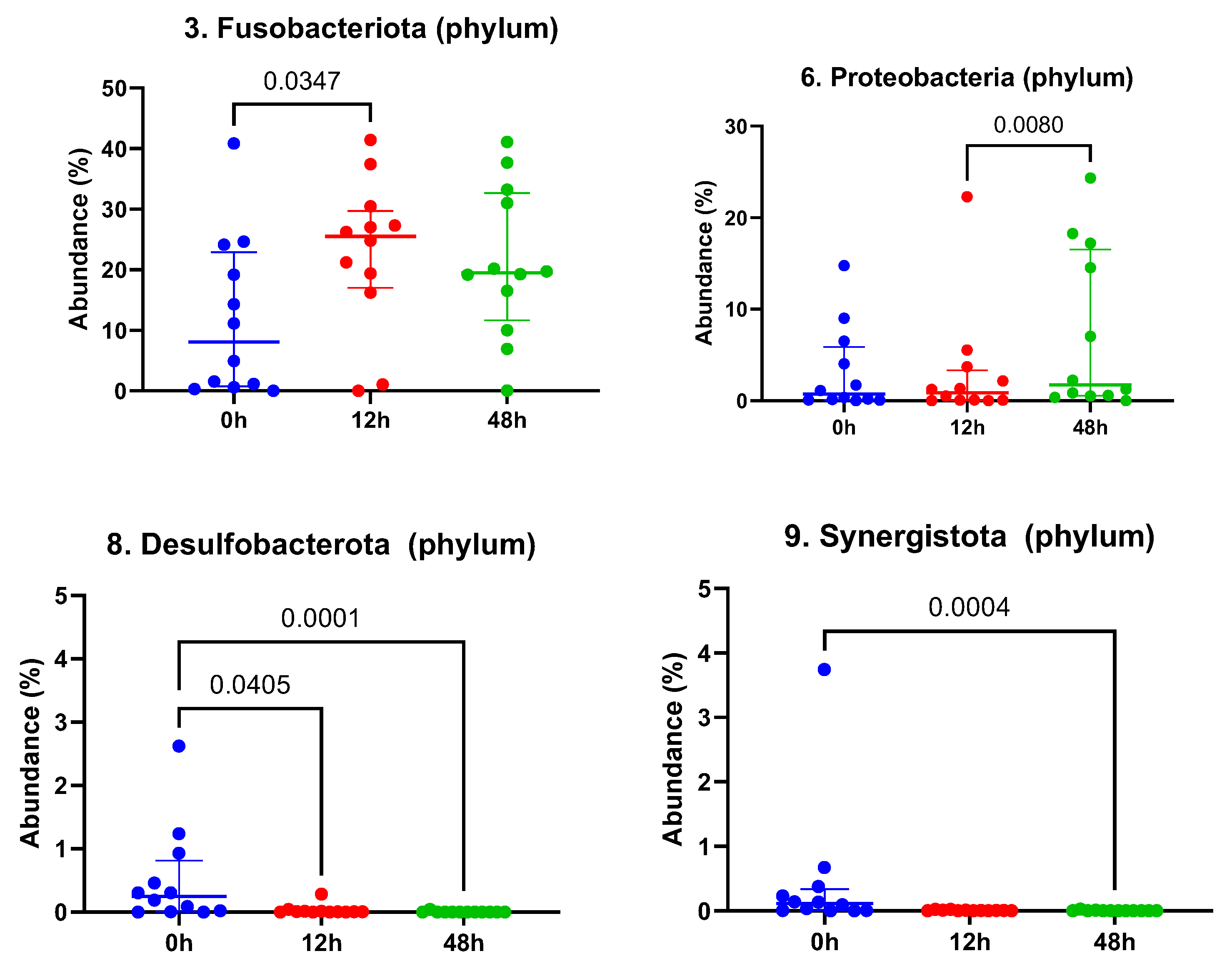
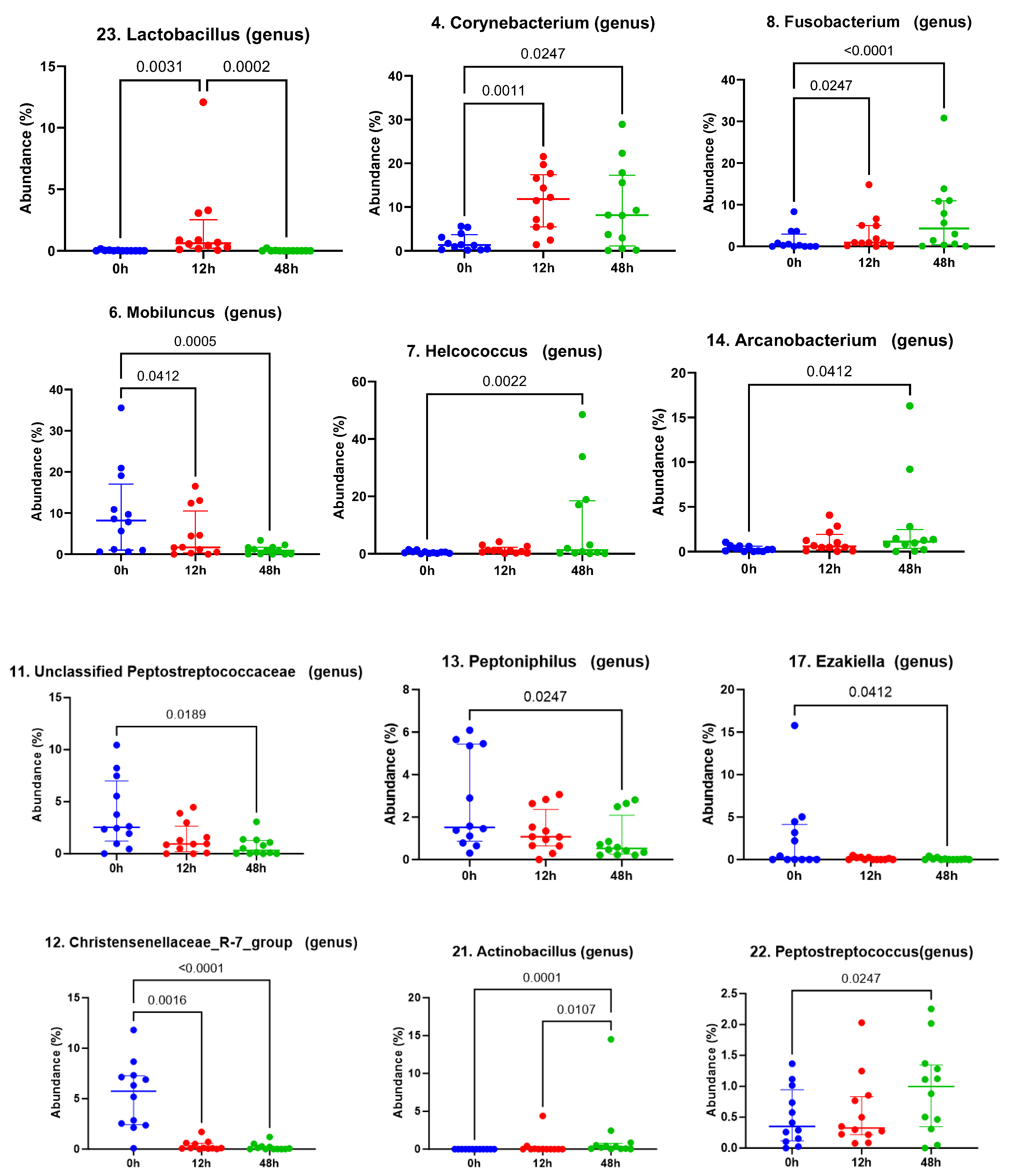
3.1. Taxonomic Abundance
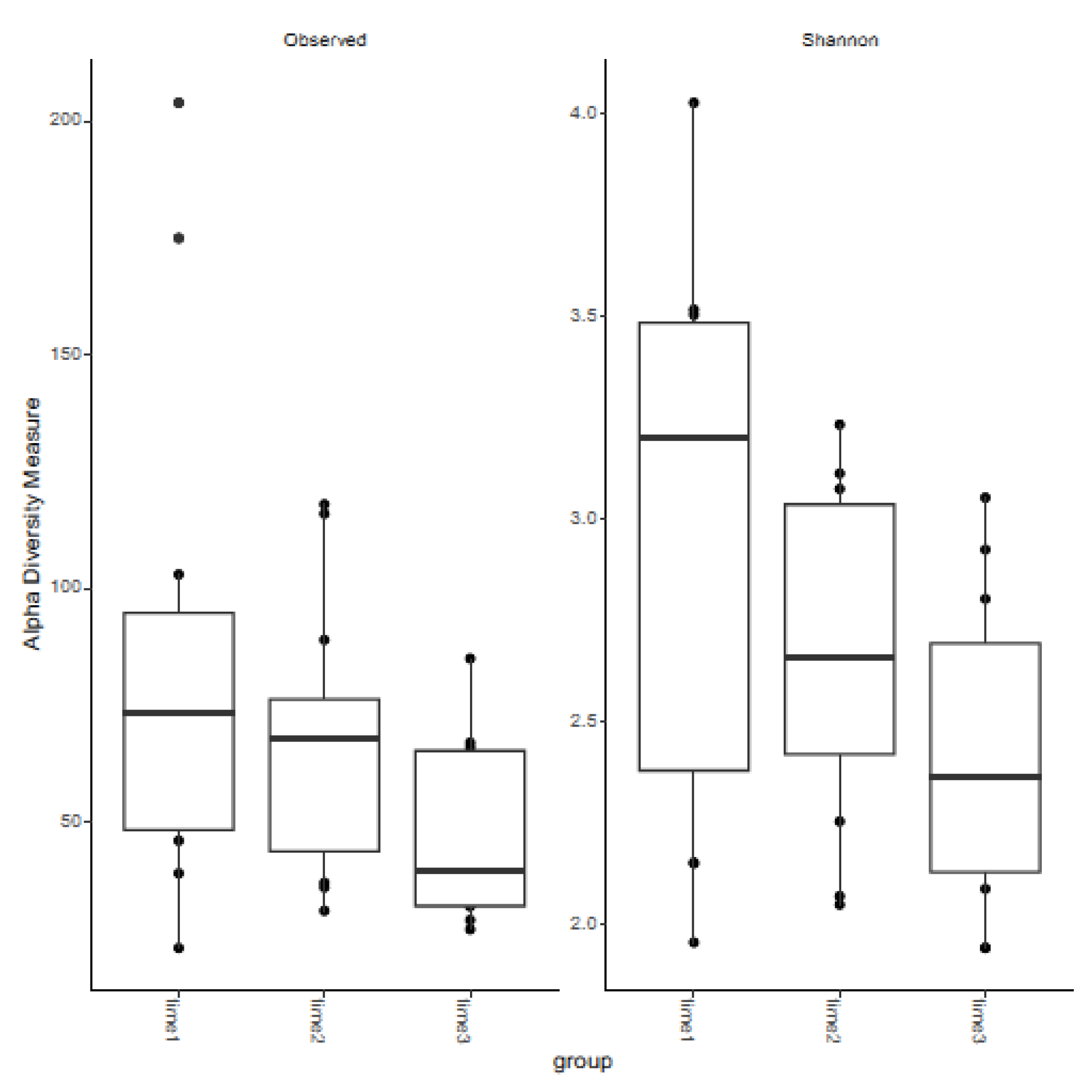
3.2. Measures of Alpha Diversity
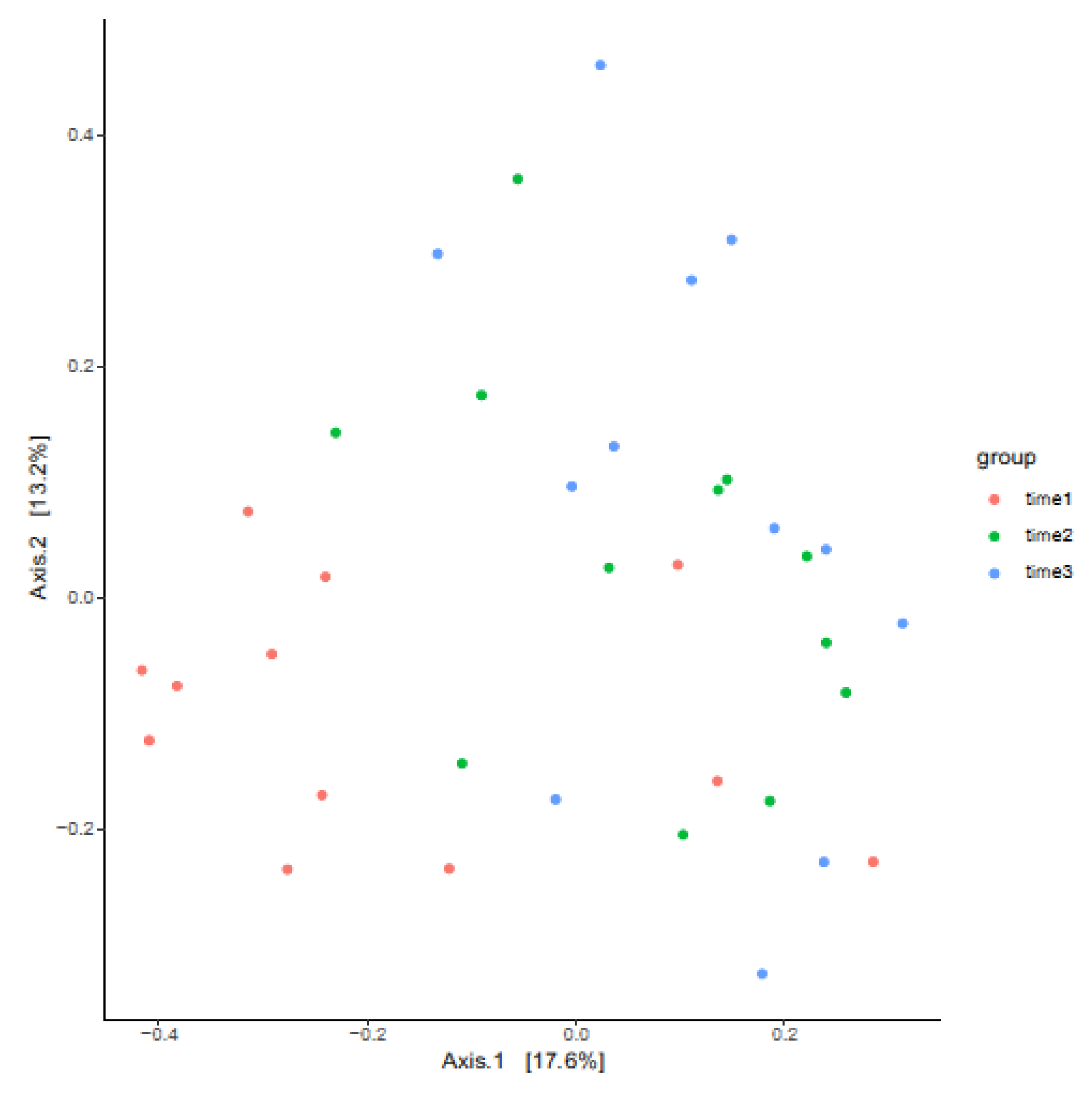
3.3. Measures of Beta Diversity
3.4. Breeding Trial Following LGC Probiotic Application
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hinrichs, K.; Cummings, M.R.; Sertich, P.L.; Kenney, R.M. Clinical significance of aerobic bacterial flora of the uterus, vagina, vestibule, and clitoral fossa of clinically normal mares. J. Am. Vet. Med. Assoc. 1988, 193, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Mohammadsadegh, M.; Salehi, T.Z.; Ghasemzadeh-Nava, H.; Bokaie, S. The important clitoral isolated bacteria of Iranian problem mares. Afr. J. Microbiol. Res. 2012, 6, 2882–2887. [Google Scholar] [CrossRef]
- Sirota, I.; Zarek, S.M.; Segars, J.H. Potential influence of the microbiome on infertility and assisted reproductive technology. Semin. Reprod. Med. 2014, 32, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Beckers, K.F.; Gomes, V.C.L.; Crissman, K.R.; Liu, C.C.; Schulz, C.J.; Childers, G.W.; Sones, J.L. Metagenetic analysis of the pregnant microbiome in horses. Animals 2023, 13, 1999. [Google Scholar] [CrossRef]
- Hemberg, E.; Niazi, A.; Guo, Y.; Debnar, V.J.; Vincze, B.; Morrell, J.M.; Kutvolgyi, G. Microbial profiling of amniotic fluid, umbilical blood and placenta of the foaling mare. Animals 2023, 13, 2029. [Google Scholar] [CrossRef]
- van Heule, M.; Monteiro, H.; Bazzazan, A.; Scoggin, K.; Rolston, M.; El-Sheikh Ali, H.; Weimer, B.C.; Ball, B.; Daels, P.; Dini, P. Characterization of the equine placental microbial population in healthy pregnancies. Theriogenology 2023, 206, 60–70. [Google Scholar] [CrossRef]
- Barba, M.; Martínez-Boví, R.; Quereda, J.J.; Mocé, M.L.; Plaza-Dávila, M.; Jiménez-Trigos, E.; Gomez-Martin, A.; Gonzales-Torres, P.; Carbonetto, B.; Garcia-Rosello, E. Vaginal microbiota is stable throughout the estrous cycle in Arabian mares. Animals 2020, 10, 2020. [Google Scholar] [CrossRef]
- Gil-Miranda, A.; Caddey, B.; Orellana-Guerrero, D.; Smith, H.; Samper, J.C.; Gomez, D.E. Vaginal and uterine microbiota of healthy maiden mares during estrus. Vet. Sci. 2024, 11, 323. [Google Scholar] [CrossRef]
- Jones, E. Characterization of the Equine Microbiome During Late Gestation and the Early Postpartum Period, and at Various Times During the Estrous Cycle in Mares Being Bred with Raw or Extended Semen. Master’s Thesis, Kansas State University, Manhattan, KS, USA, 2019. [Google Scholar]
- Pugh, G. The Role of Microbial Diversity in Female Reproductive Tract Health: An Analysis of the Microbiome’s Influence on Pregnancy Outcomes in Postparturient Mares. Master’s Thesis, Eastern Kentucky University, Richmond, KY, USA, 2018. [Google Scholar]
- Heil, B.A.; van Heule, M.; Thompson, S.K.; Kearns, T.A.; Beckers, K.F.; Oberhaus, E.L.; King, G.; Daels, P.; Dini, P.; Sones, J.L. Metagenomic characterization of the equine endometrial microbiome during anestrus. J. Equine Vet. Sci. 2023, 125, 104718. [Google Scholar] [CrossRef]
- Heil, B.A.; van Heule, M.; Thompson, S.K.; Kearns, T.A.; Oberhaus, E.L.; King, G.; Daels, P.; Dini, P.; Sones, J.L. Effect of sampling method on detection of the equine uterine microbiome during estrus. Vet. Sci. 2023, 10, 644. [Google Scholar] [CrossRef]
- Holyoak, G.R.; Premathilake, H.U.; Lyman, C.C.; Sones, J.L.; Gunn, A.; Wieneke, X.; DeSilva, U. The healthy equine uterus harbors a distinct core microbiome plus a rich and diverse microbiome that varies with geographical location. Sci. Rep. 2022, 12, 14790. [Google Scholar] [CrossRef] [PubMed]
- Holyoak, G.R.; Lyman, C.C.; Wieneke, X.; DeSilvab, U. The equine endometrial microbiome. Clin. Theriogenol. 2018, 10, 273–278. [Google Scholar]
- Krekeler, N.; Legione, A.; Perriam, W.; Finan, S.; Heil, B.A.; Burden, C.A.; McKinnon, A.O.M.; Marth, C.D.A. Association of the uterine microbiome to mare fertility. J. Equine Vet. Sci. 2023, 125, 104724. [Google Scholar] [CrossRef]
- Sathe, S.; Leiken, A.; Plummer, P. Metagenomic sequencing of the uterine microbial environment during estrus and early pregnancy in mares. Clin. Theriogenol. 2017, 9, 453. [Google Scholar]
- Schnobrich, M.; Atwood, K.; Barr, B.; Bradecamp, E.A.; Scoggin, C.F. Next Generation DNA Sequencing, culture and cytology results in 10 clinically normal mares. Clin. Theriogenol. 2017, 9, 443. [Google Scholar]
- Thomson, P.; Pareja, J.; Núñez, A.; Santibáñez, R.; Castro, R. Characterization of microbial communities and predicted metabolic pathways in the uterus of healthy mares. Open Vet. J. 2022, 12, 797–805. [Google Scholar] [CrossRef]
- Virendra, A.; Gulavane, S.U.; Ahmed, Z.A.; Reddy, R.; Chaudhari, R.J.; Gaikwad, S.M.; Shelar, R.R.; Ingole, S.D.; Thorat, D.D.; Kahnam, A.; et al. Metagenomic analysis unravels novel taxonomic differences in the uterine microbiome between healthy mares and mares with endometritis. Vet. Med. Sci. 2024, 10, e1369. [Google Scholar] [CrossRef]
- Ferris, R.A. Therapeutics for infectious endometritis: A clinical perspective. Rev. Bras. Reprod. Anim. 2017, 4, 175–179. [Google Scholar]
- Ricketts, S. The treatment of equine endometritis in studfarm practice. Pferdeheilkun 2007, 15, 588–593. [Google Scholar] [CrossRef]
- Punzón-Jiménez, P.; Labarta, E. The impact of the female genital tract microbiome in women health and reproduction: A review. J. Assist. Reprod. Gene 2021, 38, 2519–2541. [Google Scholar] [CrossRef]
- Kikuchi, N.; Iguchi, I.; Hiramune, T. Capsule types of Klebsiella pneumoniae isolated from the genital tract of mares with metritis, extra-genital sites of healthy mares and the genital tract of stallions. Vet. Microbiol. 1987, 15, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Miller, C. Infections and neoplastic conditions of the vulva and perineum. In Current Therapy in Equine Reproduction; Samper, J., Pycock, J., McKinnon, A., Eds.; Saunders Elsevier: St. Louis, MI, USA, 2007; pp. 161–166. [Google Scholar]
- Tiago, G.; Júlio, C.; António, R. Conception rate, uterine infection and embryo quality after artificial insemination and natural breeding with a stallion carrier of Pseudomonas aeruginosa: A case report. Acta Vet. Scand. 2012, 54, 20. [Google Scholar] [CrossRef] [PubMed]
- Anukam, K.C.; Osazuwa, E.; Osemene, G.I.; Ehigiagbe, F.; Bruce, A.W.; Reid, G. Clinical study comparing probiotic Lactobacillus GR-1 and RC-14 with metronidazole vaginal gel to treat symptomatic bacterial vaginosis. Microbes Infect. 2006, 8, 2772–2776. [Google Scholar] [CrossRef] [PubMed]
- Ehrström, S.; Daroczy, K.; Rylander, E.; Samuelsson, C.; Johannesson, U.; Anzén, B.; Påhlson, C. Lactic acid bacteria colonization and clinical outcome after probiotic supplementation in conventionally treated bacterial vaginosis and vulvovaginal candidiasis. Microbes Infect. 2010, 12, 691–699. [Google Scholar] [CrossRef]
- Eriksson, K.; Carlsson, B.; Forsum, U.; Larsson, P.G. A double-blind treatment study of bacterial vaginosis with normal vaginal lactobacilli after an open treatment with vaginal clindamycin ovules. Acta Derm. Venereol. 2005, 85, 42–46. [Google Scholar] [CrossRef]
- Kovachev, S.M.; Vatcheva-Dobrevska, R.S. Local probiotic therapy for vaginal Candida albicans infections. Probiotics Antimicrob. Proteins 2015, 7, 38–44. [Google Scholar] [CrossRef]
- Martinez, R.C.; Franceschini, S.A.; Patta, M.C.; Quintana, S.M.; Candido, R.C.; Ferreira, J.C.; De Martinis, E.C.P.; Reid, G. Improved treatment of vulvovaginal candidiasis with fluconazole plus probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14. Lett. Appl. Microbio 2009, 48, 269–274. [Google Scholar] [CrossRef]
- Mastromarino, P.; Macchia, S.; Meggiorini, L.; Trinchieri, V.; Mosca, L.; Perluigi, M.; Midulla, C. Effectiveness of Lactobacillus-containing vaginal tablets in the treatment of symptomatic bacterial vaginosis. Clin. Microbiol. Infect. 2009, 15, 67–74. [Google Scholar] [CrossRef]
- Oerlemans, E.F.M.; Bellen, G.; Claes, I.; Henkens, T.; Allonsius, C.N.; Wittouck, S.; van den Broek, M.F.L.; Wuyts, S.; Kiekens, F.; Donders, G.G.G.; et al. Impact of a lactobacilli-containing gel on vulvovaginal candidosis and the vaginal microbiome. Sci. Rep. 2020, 10, 7976. [Google Scholar] [CrossRef]
- Vicariotto, F.; Mogna, L.; Del Piano, M. Effectiveness of the two microorganisms Lactobacillus fermentum LF15 and Lactobacillus plantarum LP01, formulated in slow-release vaginal tablets, in women affected by bacterial vaginosis: A pilot study. J. Clin. Gastroenterol. 2014, 48, 106–112. [Google Scholar] [CrossRef]
- Fraga, M.; Perelmuter, K.; Delucchi, L.; Cidade, E.; Zunino, P. Vaginal lactic acid bacteria in the mare: Evaluation of the probiotic potential of native Lactobacillus spp. and Enterococcus spp. strains. Antonie Leeuwenhoek 2008, 93, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.A.; Castañares, M.; Mouguelar, H.; Valenciano, J.A.; Pellegrino, M.S. Isolation of lactic acid bacteria from the reproductive tract of mares as potentially beneficial strains to prevent equine endometritis. Vet. Res. Commun. 2024, 48, 1353–1366. [Google Scholar] [CrossRef] [PubMed]
- Ametaj, B.N.; Iqbal, S.; Selami, F.; Odhiambo, J.F.; Wang, Y.; Gänzle, M.G.; Dunn, S.M.; Zebeli, Q. Intravaginal administration of lactic acid bacteria modulated the incidence of purulent vaginal discharges, plasma haptoglobin concentrations, and milk production in dairy cows. Res. Vet. Sci. 2014, 96, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Odhiambo, J.F.; Farooq, U.; Lam, T.; Dunn, S.M.; Ametaj, B.N. Intravaginal probiotics modulated metabolic status and improved milk production and composition of transition dairy cows. J. Anim. Sci. 2016, 94, 760–770. [Google Scholar] [CrossRef]
- Deng, Q.; Odhiambo, J.F.; Farooq, U.; Lam, T.; Dunn, S.M.; Ametaj, B.N. Intravaginal lactic Acid bacteria modulated local and systemic immune responses and lowered the incidence of uterine infections in periparturient dairy cows. PLoS ONE 2014, 10, e0124167. [Google Scholar] [CrossRef]
- Overbeck, W.; Witte, T.S.; Heuwieser, W. Comparison of three diagnostic methods to identify subclinical endometritis in mares. Theriogenology 2011, 75, 1311–1318. [Google Scholar] [CrossRef]
- Kozich, J.J.; Westcott, S.L.; Baxter, N.T.; Highlander, S.K.; Schloss, P.D. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl. Environ. Microbiol. 2013, 79, 5112–5120. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Bernabeu, A.; Lledo, B.; Díaz, M.C.; Lozano, F.M.; Ruiz, V.; Fuentes, A.; Lopez-Pineda, A.; Moliner, B.; Castillo, J.C.; Ortiz, J.A.; et al. Effect of the vaginal microbiome on the pregnancy rate in women receiving assisted reproductive treatment. J. Assist. Reprod. Genet. 2019, 36, 2111–2119. [Google Scholar] [CrossRef]
- Deka, N.; Hassan, S.; Seghal Kiran, G.; Selvin, J. Insights into the role of vaginal microbiome in women’s health. J. Basic Microbiol. 2021, 61, 1071–1084. [Google Scholar] [CrossRef]
- Franasiak, J.M.; Werner, M.D.; Juneau, C.R.; Tao, X.; Landis, J.; Zhan, Y.; Treff, N.R.; Scott, R.T. Endometrial microbiome at the time of embryo transfer: Next-generation sequencing of the 16S ribosomal subunit. J. Assist. Reprod. Genet. 2016, 33, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Hernandes, C.; Silveira, P.; Rodrigues Sereia, A.F.; Christoff, A.P.; Mendes, H.; Valter de Oliveira, L.F.; Podgaec, S. Microbiome profile of deep endometriosis patients: Comparison of vaginal fluid, endometrium and lesion. Diagnostics 2020, 10, 163. [Google Scholar] [CrossRef] [PubMed]
- Koedooder, R.; Singer, M.; Schoenmakers, S.; Savelkoul, P.H.M.; Morré, S.A.; de Jonge, J.D.; Poort, L.; Cuypers, W.J.S.S.; Beckers, N.G.M.; Broekmans, F.J.M.; et al. The vaginal microbiome as a predictor for outcome of in vitro fertilization with or without intracytoplasmic sperm injection: A prospective study. Hum. Reprod. 2019, 34, 1042–1054. [Google Scholar] [CrossRef] [PubMed]
- Kyono, K.; Hashimoto, T.; Kikuchi, S.; Nagai, Y.; Sakuraba, Y. A pilot study and case reports on endometrial microbiota and pregnancy outcome: An analysis using 16S rRNA gene sequencing among IVF patients, and trial therapeutic intervention for dysbiotic endometrium. Reprod. Med. Biol. 2019, 18, 72–82. [Google Scholar] [CrossRef]
- Liu, Y.; Ko, E.Y.; Wong, K.K.; Chen, X.; Cheung, W.C.; Law, T.S.; Chung, J.P.; Tsui, S.K.; Li, T.; Chim, S.S. Endometrial microbiota in infertile women with and without chronic endometritis as diagnosed using a quantitative and reference range-based method. Fertil. Steril. 2019, 112, 707–717. [Google Scholar] [CrossRef]
- Moore, D.E.; Soules, M.R.; Klein, N.A.; Fujimoto, V.Y.; Agnew, K.J.; Eschenbach, D.A. Bacteria in the transfer catheter tip influence the live-birth rate after in vitro fertilization. Fertil. Steril. 2000, 74, 1118–1124. [Google Scholar] [CrossRef]
- Moreno, I.; Codoñer, F.M.; Vilella, F.; Valbuena, D.; Martinez-Blanch, J.F.; Jimenez-Almazán, J.; Alonso, R.; Alama, P.; Remohi, J.; Pellicer, A.; et al. Evidence that the endometrial microbiota has an effect on implantation success or failure. Am. J. Obstet. Gynecol. 2016, 215, 684–703. [Google Scholar] [CrossRef]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.K.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA 2011, 108, 4680–4687. [Google Scholar] [CrossRef]
- Tao, X.; Franasiak, J.M.; Zhan, Y.; Scott, R.T.; Rajchel, J.; Bedard, J.; Newby, R.; Scott, R.T.; Treff, N.R.; Chu, T. Characterizing the endometrial microbiome by analyzing the ultra-low bacteria from embryo transfer catheter tips in IVF cycles: Next generation sequencing (NGS) analysis of the 16S ribosomal gene. Hum. Microbiome J. 2017, 3, 15–21. [Google Scholar] [CrossRef]
- Venneri, M.A.; Franceschini, E.; Sciarra, F.; Rosato, E.; D’Ettorre, G.; Lenzi, A. Human genital tracts microbiota: Dysbiosis crucial for infertility. J. Endocrinol. Investig. 2022, 45, 1151–1160. [Google Scholar] [CrossRef]
- Vitale, S.G.; Ferrari, F.; Ciebiera, M.; Zgliczyńska, M.; Rapisarda, A.M.C.; Vecchio, G.M.; Pino, A.; Angelico, G.; Knafel, A.; Riemma, G.; et al. The role of genital tract microbiome in fertility: A systematic review. Int. J. Mol. Sci. 2022, 23, 180. [Google Scholar] [CrossRef] [PubMed]
- Cummins, C.; Carrington, S.; Fitzpatrick, E.; Duggan, V. Ascending placentitis in the mare: A review. Ir. Vet. J. 2008, 61, 307. [Google Scholar] [CrossRef]
- LeBlanc, M.M.; Causey, R.C. Clinical and subclinical endometritis in the mare: Both threats to fertility. Reprod. Domest. Anim. 2009, 44, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, M.L. Treatment strategies for mares with placentitis. Theriogenology 2005, 64, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Morris, H.A.L.; McCue, P.M.; Aurich, C. Equine endometritis: A review of challenges and new approaches. Reproduction 2020, 160, R95–R110. [Google Scholar] [CrossRef] [PubMed]
- Larsson, P.G.; Forsum, U. Bacterial vaginosis—A disturbed bacterial flora and treatment enigma. Acta Pathol. Microbiol. Immunol. Scand. 2005, 113, 305–316. [Google Scholar] [CrossRef]
- Chen, X.; Lu, Y.; Chen, T.; Li, R. The female vaginal microbiome in health and bacterial vaginosis. Front. Cell. Infect. Microbiol. 2021, 11, 631972. [Google Scholar] [CrossRef]
- Aroutcheva, A.A.; Simoes, J.A.; Faro, S. Antimicrobial protein produced by vaginal Lactobacillus acidophilus that inhibits Gardnerella vaginalis. Infect. Dis. Obstet. Gynecol. 2001, 9, 309583. [Google Scholar] [CrossRef]
- Breshears, L.M.; Edwards, V.L.; Ravel, J.; Peterson, M.L. Lactobacillus crispatus inhibits growth of Gardnerella vaginalis and Neisseria gonorrhoeae on a porcine vaginal mucosa model. BMC Microbiol. 2015, 15, 276. [Google Scholar] [CrossRef]
- Kim, H.; Kim, Y.; Kang, C.H. In vivo confirmation of the antimicrobial effect of probiotic candidates against Gardnerella vaginalis. Microorganism 2021, 9, 1690. [Google Scholar] [CrossRef]
- Saunders, S.; Bocking, A.; Challis, J.; Reid, G. Effect of Lactobacillus challenge on Gardnerella vaginalis biofilms. Colloids Surf. B Biointerfaces 2007, 55, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Skarin, A.; Sylwan, J. Vaginal lactobacilli inhibiting growth of Gardnerella vaginalis, Mobiluncus and other bacterial species cultured from vaginal content of women with bacterial vaginosis. Acta Pathol. Microbiol. Immunol. Scand. B 1986, 94, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.; Zhu, H.; Zhao, D.; Yang, P.; Gao, F.; Lu, C.; Yin, U.; Kan, S.; Chen, D. Probiotic Lactobacillus sp. strains inhibit growth, adhesion, biofilm formation, and gene expression of bacterial vaginosis-inducing Gardnerella vaginalis. Microorganisms 2021, 9, 728. [Google Scholar] [CrossRef] [PubMed]
- Koirala, R.; Gargari, G.; Arioli, S.; Taverniti, V.; Fiore, W.; Grossi, E.; Anelli, G.M.; Cetin, I.; Guglielmetti, S. Effect of oral consumption of capsules containing Lactobacillus paracasei LPC-S01 on the vaginal microbiota of healthy adult women: A randomized, placebo-controlled, double-blind crossover study. FEMS Microbiol. Ecol. 2020, 96, fiaa084. [Google Scholar] [CrossRef]
- Bisanz, J.E.; Seney, S.; McMillan, A.; Vongsa, R.; Koenig, D.; Wong, L.; Dvoracek, B.; Gloor, G.B.; Sumarah, M.; Ford, B.; et al. A systems biology approach investigating the effect of probiotics on the vaginal microbiome and host responses in a double blind, placebo-controlled clinical trial of post-menopausal women. PLoS ONE 2014, 9, e104511. [Google Scholar] [CrossRef]
- He, Y.; Niu, X.; Wang, B.; Na, R.; Xiao, B.; Yang, H. Evaluation of the Inhibitory Effects of Lactobacillus gasseri and Lactobacillus crispatus on the adhesion of seven common lower genital tract infection-causing pathogens to vaginal epithelial cells. Front. Med. 2020, 7, 284. [Google Scholar] [CrossRef]
- Cobb, C.M.; Kelly, P.J.; Williams, K.B.; Babbar, S.; Angolkar, M.; Derman, R.J. The oral microbiome and adverse pregnancy outcomes. Int. J. Women’s Health 2017, 9, 551–559. [Google Scholar] [CrossRef]
- Hill, G.B. Preterm birth: Associations with genital and possibly oral microflora. Ann. Periodontol. 1998, 3, 222–232. [Google Scholar] [CrossRef]
- Takeuchi, N.; Ekuni, D.; Irie, K.; Furuta, M.; Tomofuji, T.; Morita, M.; Watanabe, T. Relationship between periodontal inflammation and fetal growth in pregnant women: A cross-sectional study. Arch. Gynecol. Obstet. 2012, 287, 951–957. [Google Scholar] [CrossRef]
- Fardini, Y.; Chung, P.; Dumm, R.; Joshi, N.; Han, Y.W. Transmission of diverse oral bacteria to murine placenta: Evidence for the oral microbiome as a potential source of intrauterine infection. Infect. Immun. 2010, 78, 1789–1796. [Google Scholar] [CrossRef]
- Han, Y.W.; Redline, R.W.; Li, M.; Yin, L.; Hill, G.B.; McCormick, T.S. Fusobacterium nucleatum induces premature and term stillbirths in pregnant mice: Implication of oral bacteria in preterm birth. Infect. Immun. 2004, 72, 2272–2279. [Google Scholar] [CrossRef] [PubMed]
- Ginther, O.J. Reproductive Biology of the Mare: Basic and Applied Aspects, 1st ed.; Equiservices Publishing: Cross Plains, WI, USA, 1979. [Google Scholar]
- LeBlanc, M.M. The current status of antibiotic use in equine reproduction. Equine Vet. Edu 2010, 21, 156–167. [Google Scholar] [CrossRef]
| Mare | Time 0 | Time 12 h | Time 48 h |
|---|---|---|---|
| 1 | 0.008 | 0.436 | 0 |
| 2 | 0.150 | 0.052 | 0 |
| 3 | 0.061 | 0.291 | 0 |
| 4 | 0 | 0.100 | 0.001 |
| 5 | 0 | 0.555 | 0 |
| 6 | 0 | 12.085 | 0.011 |
| 7 | 0.042 | 0.877 | 0.025 |
| 8 | 0 | 3.293 | 0 |
| 9 | 0 | 0.868 | 0 |
| 10 | 0.004 | 3.074 | 0 |
| 11 | 0.010 | 0.185 | 0 |
| 12 | 0.034 | 0.678 | 0.219 |
| Mean | 0.026 | 1.874 | 0.022 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herzog, F.; Crissman, K.R.; Beckers, K.F.; Zhou, G.; Liu, C.-C.; Sones, J.L. Lactobacillus Genus Complex Probiotic-Induced Changes on the Equine Clitoral Microbiome. Vet. Sci. 2025, 12, 232. https://doi.org/10.3390/vetsci12030232
Herzog F, Crissman KR, Beckers KF, Zhou G, Liu C-C, Sones JL. Lactobacillus Genus Complex Probiotic-Induced Changes on the Equine Clitoral Microbiome. Veterinary Sciences. 2025; 12(3):232. https://doi.org/10.3390/vetsci12030232
Chicago/Turabian StyleHerzog, Fiona, Kassandra R. Crissman, Kalie F. Beckers, Guoli Zhou, Chin-Chi Liu, and Jenny L. Sones. 2025. "Lactobacillus Genus Complex Probiotic-Induced Changes on the Equine Clitoral Microbiome" Veterinary Sciences 12, no. 3: 232. https://doi.org/10.3390/vetsci12030232
APA StyleHerzog, F., Crissman, K. R., Beckers, K. F., Zhou, G., Liu, C.-C., & Sones, J. L. (2025). Lactobacillus Genus Complex Probiotic-Induced Changes on the Equine Clitoral Microbiome. Veterinary Sciences, 12(3), 232. https://doi.org/10.3390/vetsci12030232







