Characterization and Pathogenicity of Equine Herpesvirus Type 8 Using In-Vitro and In-Vivo Models
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Virus, Cells, and Mice
2.2. Viral Inoculation of Cells
2.3. Determination of TCID50
2.4. Immunofluorescence Detection of EHV-8 Antibody
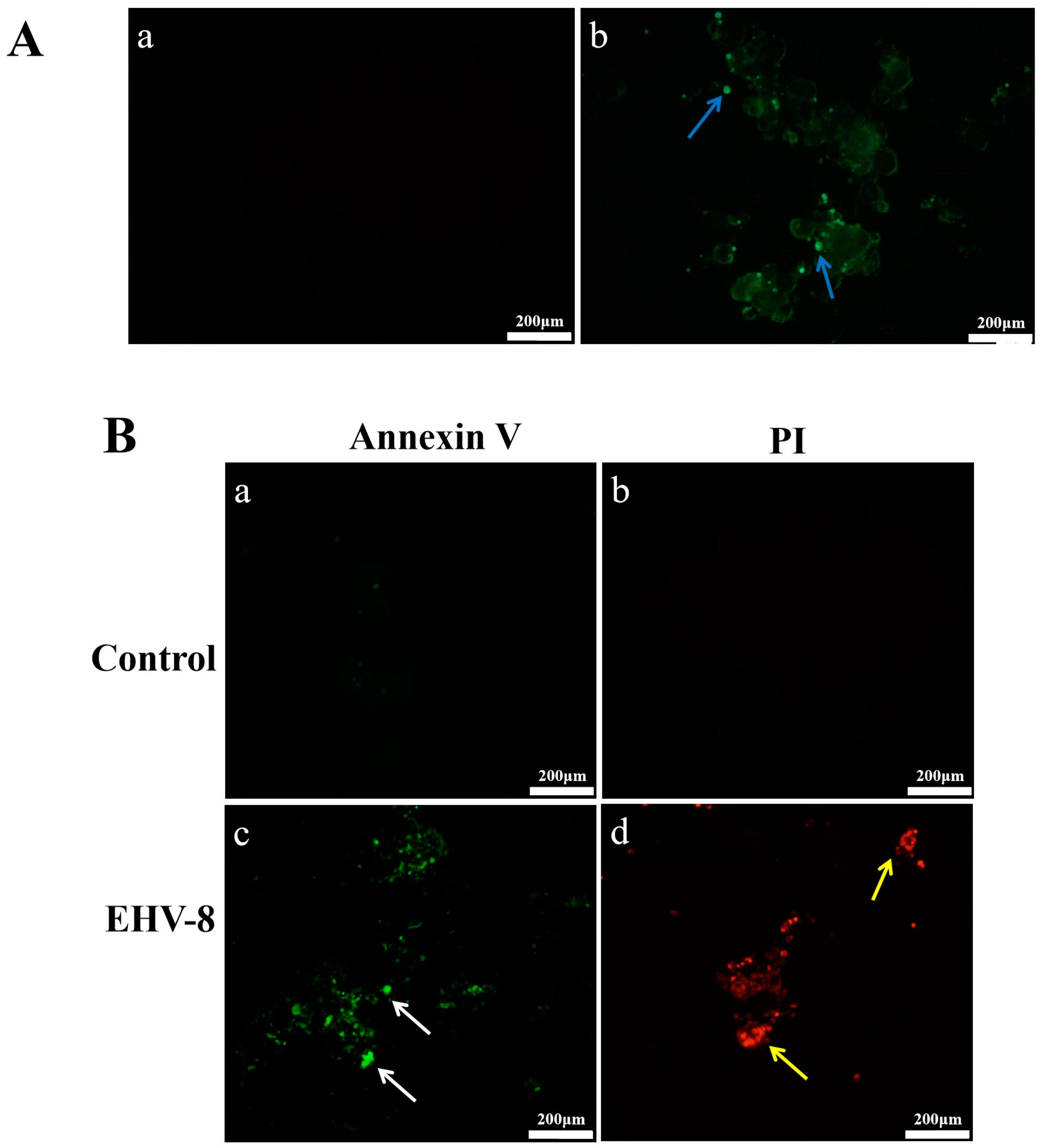
2.5. Apoptosis Detection
2.6. Virus Infection and Sampling of Mice
Lung index = (Body weight (g) Lung weight (g)) × 100
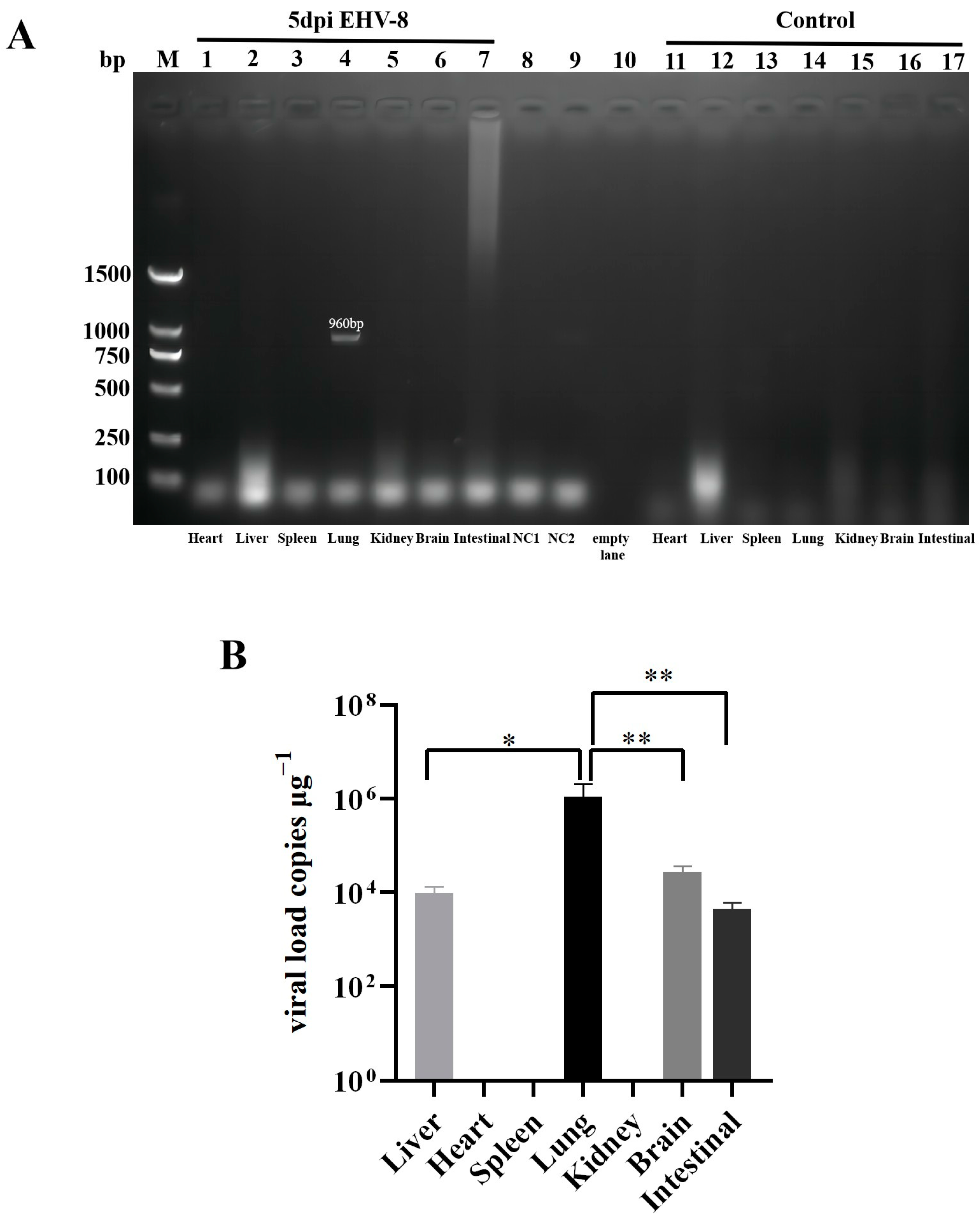
2.7. Determination of Viral Load in Major Organs of Mice
2.8. Serological Detection of EHV-8 Antibodies
2.9. Cytokine Detection
2.10. Data and Analytics
3. Results
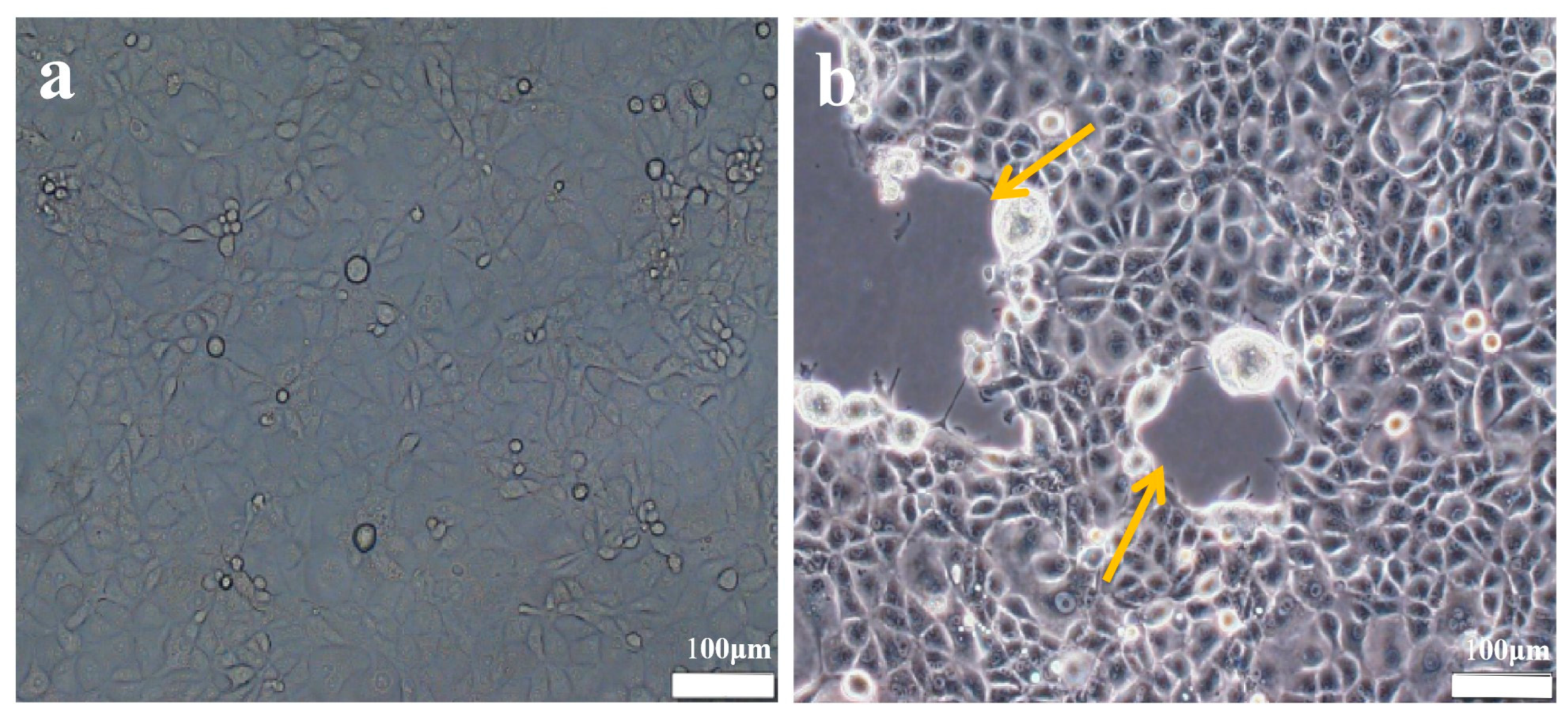
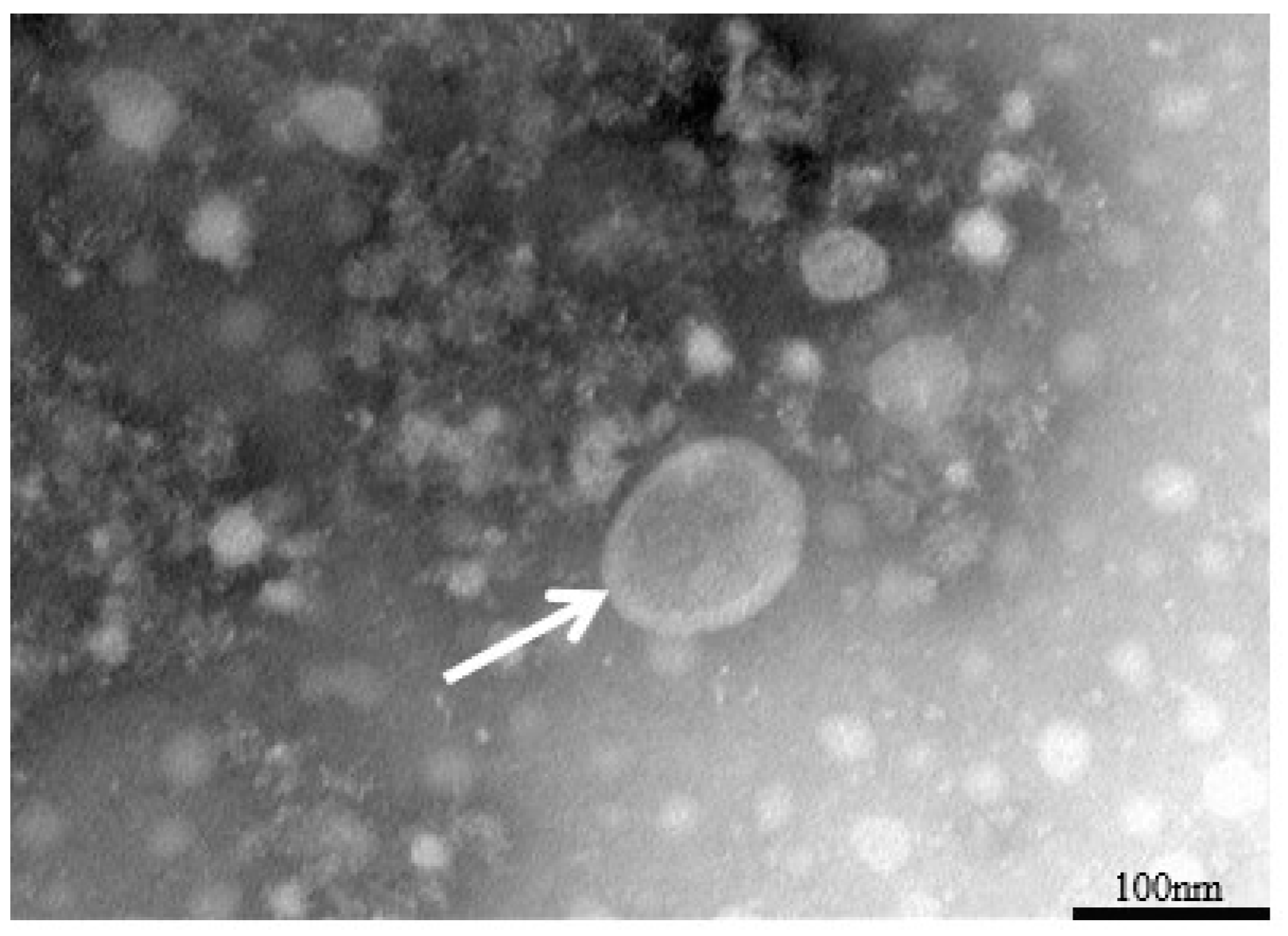
3.1. Identification of the Virus
3.2. Viral Titer Measurement
3.3. Immunofluorescence Assay
3.4. Clinical and Pathological Changes in Mice
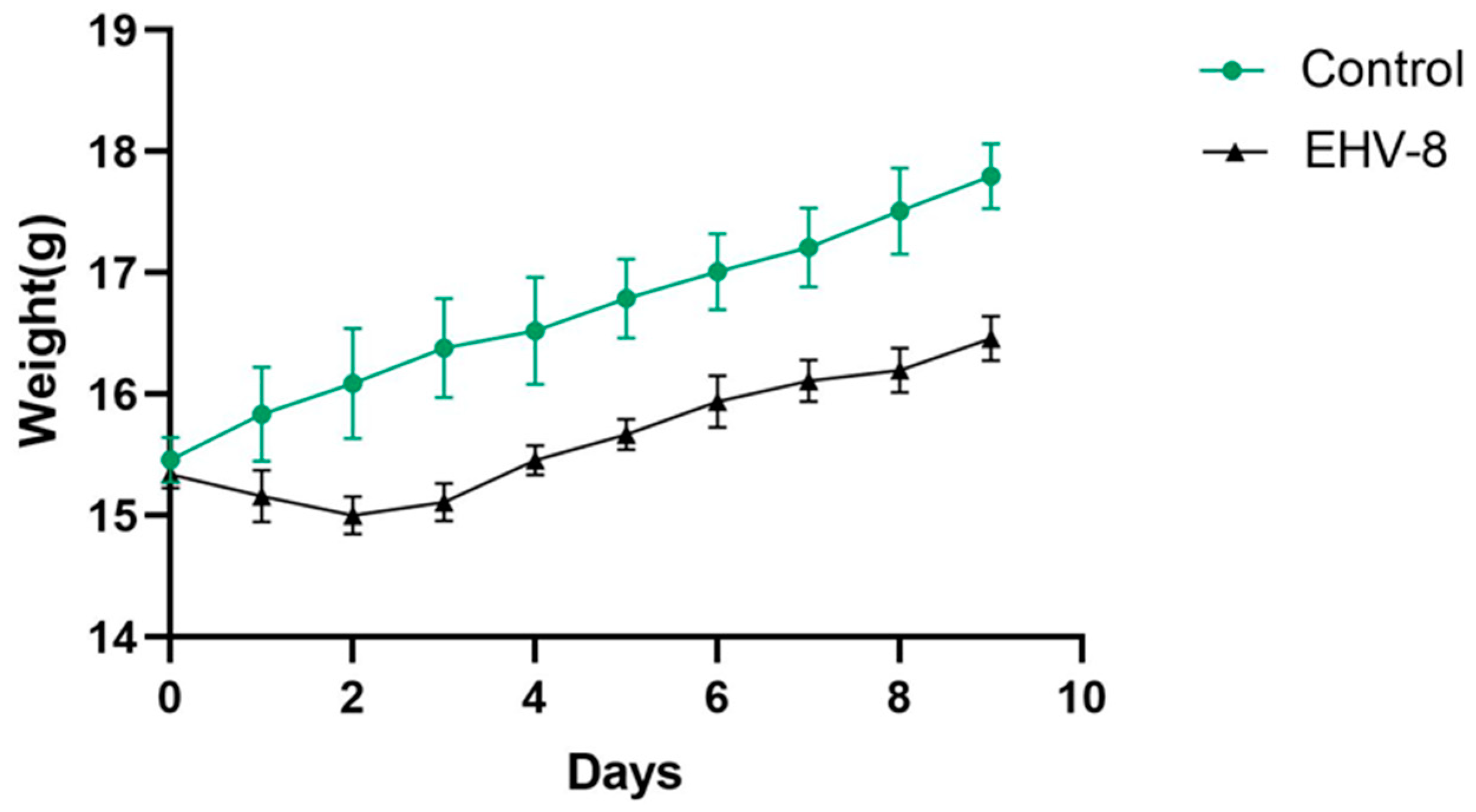
3.4.1. Effect of EHV-8 Infection on Body Weight in Mice
3.4.2. Effect of EHV-8 Infection on Lung Indices in Mice
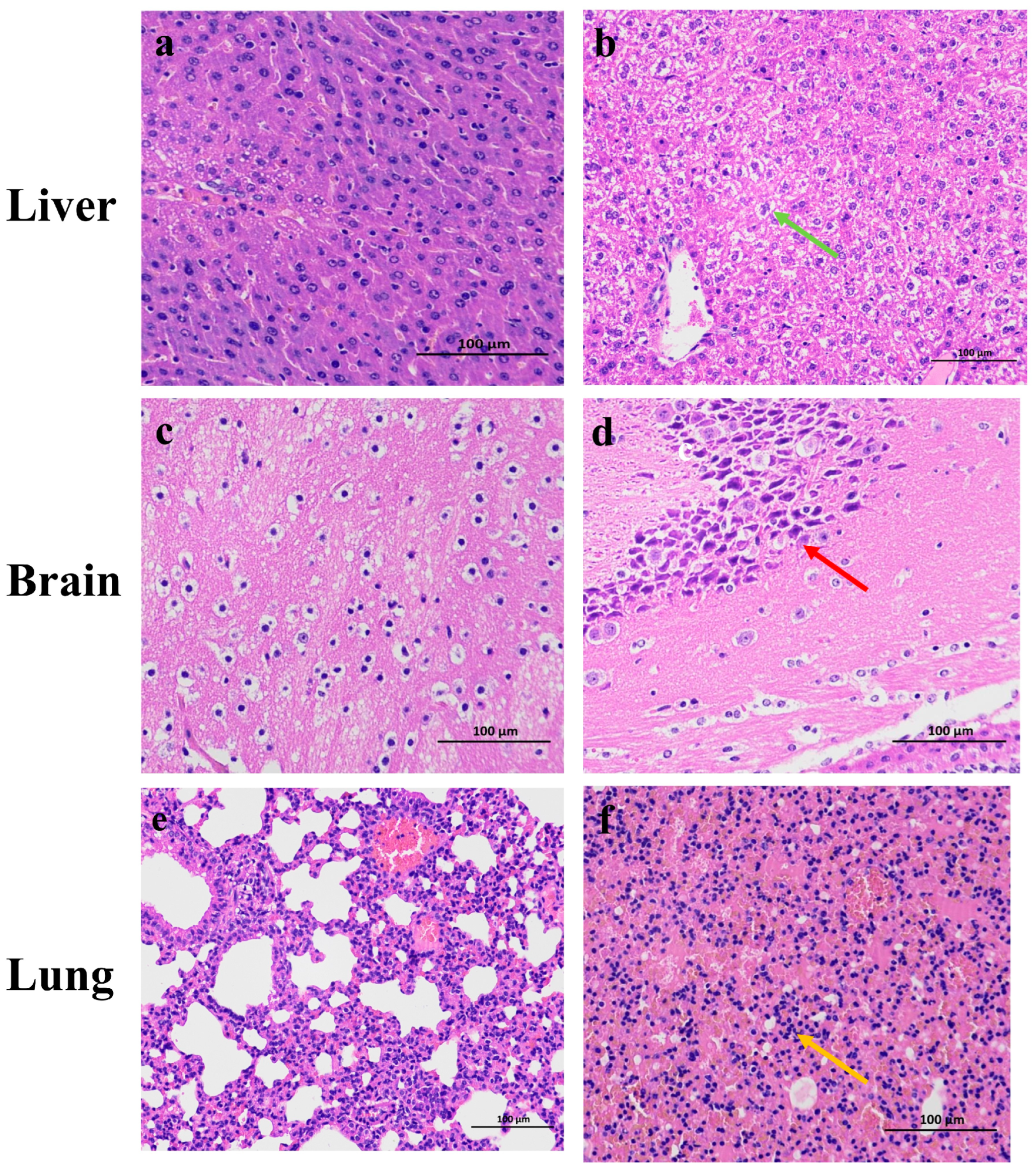
3.4.3. Pathological Observations of EHV-8 Infection on Mouse Organs
3.4.4. Distribution of EHV-8 Infected Mice in Different Organs and at Different Times of the Day
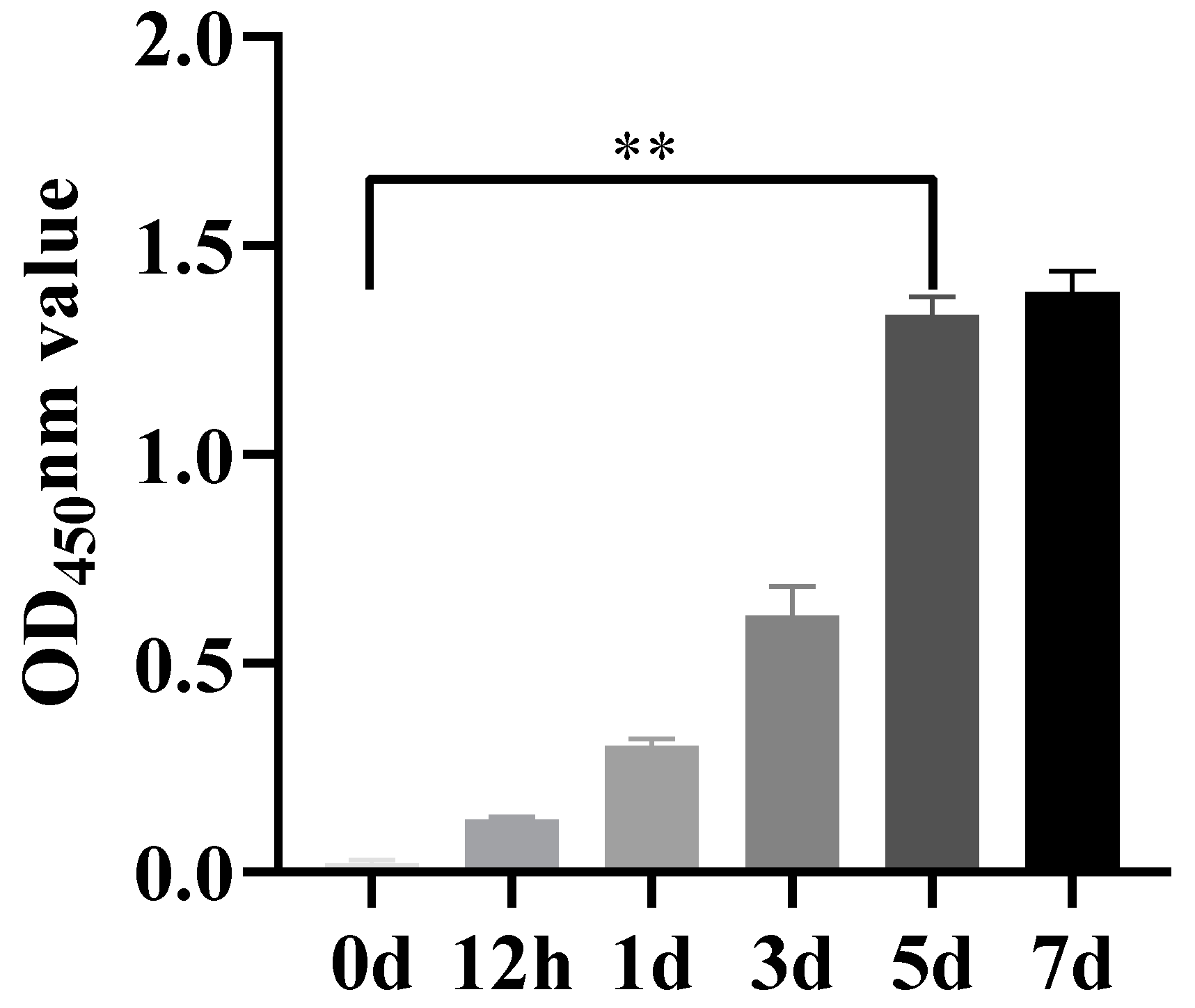
3.5. Temporal Dynamics of Anti-EHV-8 Immune Response
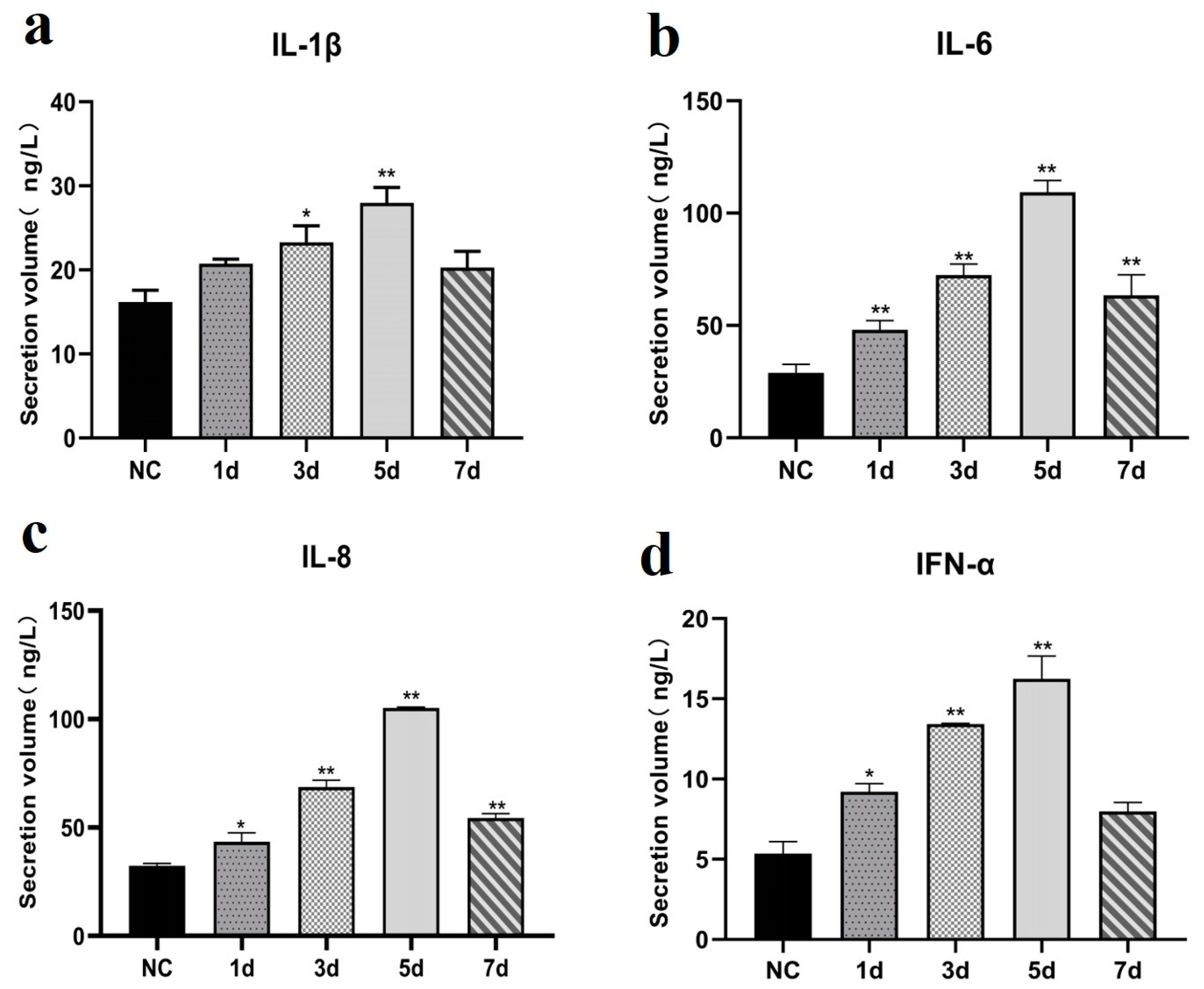
3.6. Cytokine Expression Patterns Following EHV-8 Infection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El Brini, Z.; Cullinane, A.; Garvey, M.; Fassi Fihri, O.; Fellahi, S.; Amraoui, F.; Loutfi, C.; Sebbar, G.; Paillot, R.; Piro, M. First Molecular and Phylogenetic Characterization of Equine Herpesvirus-1 (EHV-1) and Equine Herpesvirus-4 (EHV-4) in Morocco. Animals 2025, 15, 102. [Google Scholar] [CrossRef] [PubMed]
- Worku, A.; Molla, W.; Kenubih, A.; Negussie, H.; Admassu, B.; Ejo, M.; Dagnaw, G.G.; Bitew, A.B.; Fentahun, T.; Getnet, K.; et al. Molecular Detection of Equine Herpesviruses from Field Outbreaks in Donkeys in Northwest Amhara Region, Ethiopia. Vet. Med. Int. 2024, 2024, 9928835. [Google Scholar] [CrossRef] [PubMed]
- Ruan, L.; Li, L.; Yang, R.; You, A.; Khan, M.Z.; Yu, Y.; Chen, L.; Li, Y.; Liu, G.; Wang, C.; et al. Equine Herpesvirus-1 Induced Respiratory Disease in Dezhou Donkey Foals: Case Study from China, 2024. Vet. Sci. 2025, 12, 56. [Google Scholar] [CrossRef] [PubMed]
- Schvartz, G.; Edery, N.; Moss, L.; Hadad, R.; Steinman, A.; Karniely, S. Equid Herpesvirus 8 Isolated from an Adult Donkey in Israel. J. Equine Vet. Sci. 2020, 94, 103247. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, S.; Li, W.; Yu, Y.; Sun, Q.; Chen, W.; Zhou, H.; Wang, C.; Li, L.; Xu, M.; et al. Rutin prevents EqHV-8 induced infection and oxidative stress via Nrf2/HO-1 signaling pathway. Front. Cell. Infect. Microbiol. 2024, 14, 1386462. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Wang, T.; Ren, H.; Liu, W.; Li, Y.; Wang, C.; Li, L. Characterizing the Pathogenesis and Immune Response of Equine Herpesvirus 8 Infection in Lung of Mice. Animals 2022, 12, 2495. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, M.; Kimbara, S.; Ichise, H.; Ishikawa, N.; Nishihara, Y.; Nishio, M.; Sekiya, I. Cleaning methods for biosafety cabinet to eliminate residual mycoplasmas, viruses, and endotoxins after changeover. Regen. Ther. 2025, 28, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Broeckl, C.V.; Hiereth, S.; Straubinger, R.K. A comparative study evaluating three line immunoassays available for serodiagnosis of equine Lyme borreliosis: Detection of Borrelia burgdorferi sensu lato-specific antibodies in serum samples of vaccinated and non-vaccinated horses. PLoS ONE 2024, 19, e0316170. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Xu, L.; Xu, G.; Xu, Y.; Liu, H.; Hu, Y.; Ye, X.; Huang, Q.; Tang, C.; Duan, N.; et al. Fei-yan-qing-hua decoction exerts an anti-inflammatory role during influenza by inhibiting the infiltration of macrophages and neutrophils through NF-κB and p38 MAPK pathways. J. Ethnopharmacol. 2025, 337, 118846. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Qi, L.; Zhang, J.; Xu, D.; Zhang, J.; Zhao, X.; Liu, W. Establishment of TaqMan Real-time Fluorescence Quantitative PCR Detection Method for Equine Herpesvirus Type 8. Chin. Vet. J. 2023, 59, 24–28. [Google Scholar]
- Zhang, J. Epidemiological Survey of Equine Herpesvirus in Large-Scale Donkey Farms and Preparation of gD Protein Polyantibodies of EHV-8 Isolates; Liaocheng University: Liaocheng, China, 2022. [Google Scholar]
- Kimura, H.; Shibata, M.; Kuzushima, K.; Nishikawa, K.; Nishiyama, Y.; Morishima, T. Detection and direct typing of herpes simplex virus by polymerase chain reaction. Med. Microbiol. Immunol. 1990, 179, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.; Love, D.N.; Whalley, J.M. Comparison of the pathogenesis of acute equine herpesvirus 1 (EHV-1) infection in the horse and the mouse model: A review. Vet. Microbiol. 1999, 68, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Gosztonyi, G.; Borchers, K.; Ludwig, H. Pathogenesis of equine herpesvirus-1 infection in the mouse model. Apmis 2010, 117, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Tong, P.; Pan, J.; Dang, Y.; Yang, E.; Jia, C.; Duan, R.; Tian, S.; Palidan, N.; Kuang, L.; Wang, C.; et al. First identification and isolation of equine herpesvirus type 1 in aborted fetal lung tissues of donkeys. Virol. J. 2024, 21, 117. [Google Scholar] [CrossRef]
- Heinrich, P.C.; Behrmann, I.; Haan, S.; Hermanns, H.M.; Müller-Newen, G.; Schaper, F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 2003, 374, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Krumm, B.; Xiang, Y.; Deng, J. Structural biology of the IL-1 superfamily: Key cytokines in the regulation of immune and inflammatory responses. Protein Sci. 2014, 23, 526–538. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Yao, X.; Fu, Y.; Wang, Y. Interferon-α and its effects on cancer cell apoptosis (Review). Oncol. Lett. 2022, 24, 235. [Google Scholar] [CrossRef]

| Name | Sequences |
|---|---|
| PCR-Forward primer (F) | 5′-TGTGAAAAATTCA AACGT-3′ |
| PCR-Reverse primer (R) | 5′-GAAGGTGCTGTTGCTTTTGCTGG-3′ |
| Quantitative PCR-F | 5′-GCGAACCCTCTGGAACGAAA-3′ |
| Quantitative PCR-R | 5′-TGGCTATCACGTCTCCCAGG-3′ |
| Quantitative PCR-P | 5′-ACTCTTGACGAGCGAGTTGCGGCGA-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, Y.; Xu, D.; Si, W.; Zhang, Y.; Khan, M.Z.; Zhao, X.; Liu, W. Characterization and Pathogenicity of Equine Herpesvirus Type 8 Using In-Vitro and In-Vivo Models. Vet. Sci. 2025, 12, 367. https://doi.org/10.3390/vetsci12040367
Ji Y, Xu D, Si W, Zhang Y, Khan MZ, Zhao X, Liu W. Characterization and Pathogenicity of Equine Herpesvirus Type 8 Using In-Vitro and In-Vivo Models. Veterinary Sciences. 2025; 12(4):367. https://doi.org/10.3390/vetsci12040367
Chicago/Turabian StyleJi, Yanfei, Dandan Xu, Wenxuan Si, Yu Zhang, Muhammad Zahoor Khan, Xia Zhao, and Wenqiang Liu. 2025. "Characterization and Pathogenicity of Equine Herpesvirus Type 8 Using In-Vitro and In-Vivo Models" Veterinary Sciences 12, no. 4: 367. https://doi.org/10.3390/vetsci12040367
APA StyleJi, Y., Xu, D., Si, W., Zhang, Y., Khan, M. Z., Zhao, X., & Liu, W. (2025). Characterization and Pathogenicity of Equine Herpesvirus Type 8 Using In-Vitro and In-Vivo Models. Veterinary Sciences, 12(4), 367. https://doi.org/10.3390/vetsci12040367








