Stability of microRNAs in Canine Serum—A Prerequisite for Use as Biomarkers in Tumour Diagnostics
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Dogs and Sampling
2.2. RNA Isolation
2.3. Quantification of miRNA by ddPCR
2.4. Statistical Analysis
3. Results
3.1. miRNA Values at Time Point 0
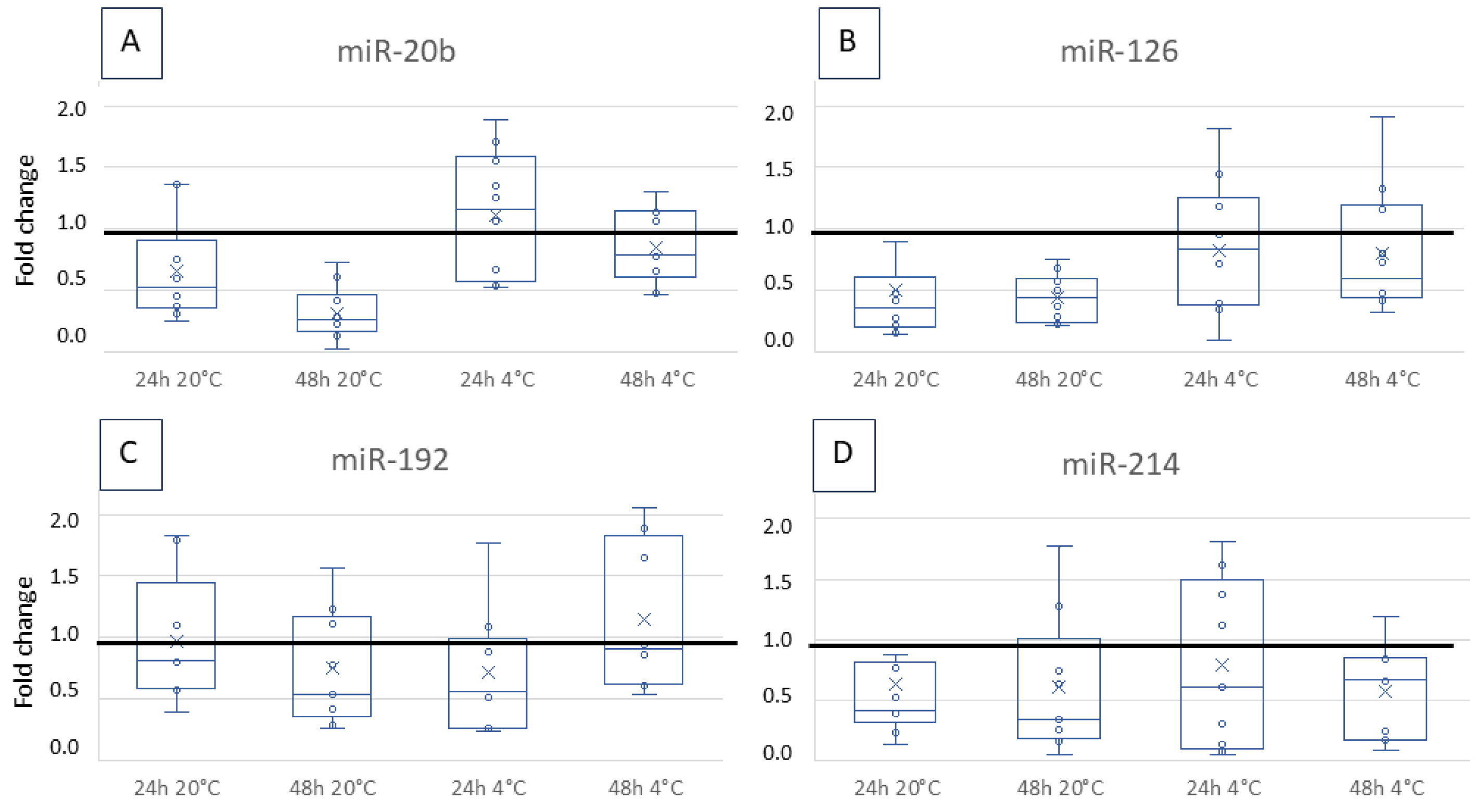
3.2. Fold Change over Storage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ddPCR | digital droplet PCR |
| miRNA | microRNA |
| miR | microRNA |
References
- Aupperle-Lellbach, H.; Kehl, A.; de Brot, S.; van der Weyden, L. Clinical Use of Molecular Biomarkers in Canine and Feline Oncology: Current and Future. Vet. Sci. 2024, 11, 199. [Google Scholar] [CrossRef] [PubMed]
- Kehl, A.; Aupperle-Lellbach, H.; de Brot, S.; van der Weyden, L. Review of Molecular Technologies for Investigating Canine Cancer. Animals 2024, 14, 769. [Google Scholar] [CrossRef] [PubMed]
- Sohel, M.M.H. Circulating microRNAs as biomarkers in cancer diagnosis. Life Sci. 2020, 248, 117473. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Ambros, V. The functions of animal microRNAs. Nature 2004, 431, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, N.; Iguchi, H.; Ochiya, T. Circulating microRNA in body fluid: A new potential biomarker for cancer diagnosis and prognosis. Cancer Sci. 2010, 101, 2087–2092. [Google Scholar] [CrossRef]
- Shuai, Y.; Zhang, X.; Lavrijssen, B.D.A.; Ikram, M.A.; Ruiter, R.; Stricker, B.; Ghanbari, M. Dysregulation of plasma circulating microRNAs in all-cause and cause-specific cancers: The Rotterdam Study. Biomark. Res. 2024, 12, 83. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Millen, J.-C.; Ramos, R.I.; Linehan, J.A.; Wilson, T.G.; Hoon, D.S.B.; Bustos, M.A. Cell-free and extracellular vesicle microRNAs with clinical utility for solid tumors. Mol. Oncol. 2024. [Google Scholar] [CrossRef]
- Joos, D.; Leipig-Rudolph, M.; Weber, K. Tumour-specific microRNA expression pattern in canine intestinal T-cell-lymphomas. Vet. Comp. Oncol. 2020, 18, 502–508. [Google Scholar] [CrossRef]
- Kehl, A.; Valkai, M.; van de Weyer, A.-L.; Brockmann, M.; Steiger, K.; Schusser, B.; Aupperle-Lellbach, H. miRNA Profiles of Canine Intestinal Carcinomas, Lymphomas and Enteritis Analysed by Digital Droplet PCR from FFPE Material. Vet. Sci. 2023, 10, 125. [Google Scholar] [CrossRef]
- Grimes, J.A.; Robinson, K.R.; Bullington, A.-C.M.; Schmiedt, J.M. Identification of serum microRNAs with differential expression between dogs with splenic masses and healthy dogs with histologically normal spleens. Am. J. Vet. Res. 2021, 82, 659–666. [Google Scholar] [CrossRef]
- Heishima, K.; Ichikawa, Y.; Yoshida, K.; Iwasaki, R.; Sakai, H.; Nakagawa, T.; Tanaka, Y.; Hoshino, Y.; Okamura, Y.; Murakami, M.; et al. Circulating microRNA-214 and -126 as potential biomarkers for canine neoplastic disease. Sci. Rep. 2017, 7, 2301. [Google Scholar] [CrossRef] [PubMed]
- Dirksen, K.; Verzijl, T.; Grinwis, G.C.; Favier, R.P.; Penning, L.C.; Burgener, I.A.; van der Laan, L.J.; Fieten, H.; Spee, B. Use of Serum MicroRNAs as Biomarker for Hepatobiliary Diseases in Dogs. J. Vet. Intern. Med. 2016, 30, 1816–1823. [Google Scholar] [CrossRef] [PubMed]
- Oosthuyzen, W.; ten Berg, P.W.L.; Francis, B.; Campbell, S.; Macklin, V.; Milne, E.; Gow, A.G.; Fisher, C.; Mellanby, R.J.; Dear, J.W. Sensitivity and specificity of microRNA-122 for liver disease in dogs. J. Vet. Intern. Med. 2018, 32, 1637–1644. [Google Scholar] [CrossRef] [PubMed]
- Heishima, K.; Mori, T.; Ichikawa, Y.; Sakai, H.; Kuranaga, Y.; Nakagawa, T.; Tanaka, Y.; Okamura, Y.; Masuzawa, M.; Sugito, N.; et al. MicroRNA-214 and MicroRNA-126 Are Potential Biomarkers for Malignant Endothelial Proliferative Diseases. Int. J. Mol. Sci. 2015, 16, 25377–25391. [Google Scholar] [CrossRef]
- Köberle, V.; Pleli, T.; Schmithals, C.; Augusto Alonso, E.; Haupenthal, J.; Bönig, H.; Peveling-Oberhag, J.; Biondi, R.M.; Zeuzem, S.; Kronenberger, B.; et al. Differential stability of cell-free circulating microRNAs: Implications for their utilization as biomarkers. PLoS ONE 2013, 8, e75184. [Google Scholar] [CrossRef]
- Yamada, A.; Cox, M.A.; Gaffney, K.A.; Moreland, A.; Boland, C.R.; Goel, A. Technical factors involved in the measurement of circulating microRNA biomarkers for the detection of colorectal neoplasia. PLoS ONE 2014, 9, e112481. [Google Scholar] [CrossRef]
- Kupec, T.; Bleilevens, A.; Iborra, S.; Najjari, L.; Wittenborn, J.; Maurer, J.; Stickeler, E. Stability of circulating microRNAs in serum. PLoS ONE 2022, 17, e0268958. [Google Scholar] [CrossRef]
- Matias-Garcia, P.R.; Wilson, R.; Mussack, V.; Reischl, E.; Waldenberger, M.; Gieger, C.; Anton, G.; Peters, A.; Kuehn-Steven, A. Impact of long-term storage and freeze-thawing on eight circulating microRNAs in plasma samples. PLoS ONE 2020, 15, e0227648. [Google Scholar] [CrossRef]
- Glinge, C.; Clauss, S.; Boddum, K.; Jabbari, R.; Jabbari, J.; Risgaard, B.; Tomsits, P.; Hildebrand, B.; Kääb, S.; Wakili, R.; et al. Stability of Circulating Blood-Based MicroRNAs—Pre-Analytic Methodological Considerations. PLoS ONE 2017, 12, e0167969. [Google Scholar] [CrossRef]
- Enelund, L.; Nielsen, L.N.; Cirera, S. Evaluation of microRNA Stability in Plasma and Serum from Healthy Dogs. Microrna 2017, 6, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Kroh, E.M.; Parkin, R.K.; Mitchell, P.S.; Tewari, M. Analysis of circulating microRNA biomarkers in plasma and serum using quantitative reverse transcription-PCR (qRT-PCR). Methods 2010, 50, 298–301. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.S.; Milosevic, D.; Reddi, H.V.; Grebe, S.K.; Algeciras-Schimnich, A. Analysis of circulating microRNA: Preanalytical and analytical challenges. Clin. Chem. 2011, 57, 833–840. [Google Scholar] [CrossRef]
- Mitchell, P.S.; Parkin, R.K.; Kroh, E.M.; Fritz, B.R.; Wyman, S.K.; Pogosova-Agadjanyan, E.L.; Peterson, A.; Noteboom, J.; O’Briant, K.C.; Allen, A.; et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. USA 2008, 105, 10513–10518. [Google Scholar] [CrossRef]
- Grimes, J.A.; Prasad, N.; Levy, S.; Cattley, R.; Lindley, S.; Boothe, H.W.; Henderson, R.A.; Smith, B.F. A comparison of microRNA expression profiles from splenic hemangiosarcoma, splenic nodular hyperplasia, and normal spleens of dogs. BMC Vet. Res. 2016, 12, 272. [Google Scholar] [CrossRef]
- Benning, L.; Robinson, S.; Follo, M.; Heger, L.A.; Stallmann, D.; Duerschmied, D.; Bode, C.; Ahrens, I.; Hortmann, M. Digital PCR for Quantifying Circulating MicroRNAs in Acute Myocardial Infarction and Cardiovascular Disease. J. Vis. Exp. 2018, 137, e57950. [Google Scholar] [CrossRef]
- Vogt, J.; Sheinson, D.; Katavolos, P.; Irimagawa, H.; Tseng, M.; Alatsis, K.R.; Proctor, W.R. Variance component analysis of circulating miR-122 in serum from healthy human volunteers. PLoS ONE 2019, 14, e0220406. [Google Scholar] [CrossRef] [PubMed]
- Cherry, A.D.; Chu, C.P.; Cianciolo, R.E.; Hokamp, J.A.; Jacobson, S.A.; Nabity, M.B. MicroRNA-126 in dogs with immune complex-mediated glomerulonephritis. J. Vet. Intern. Med. 2023, 38, 216–227. [Google Scholar] [CrossRef]
- Lyngby, J.G.; Gòdia, M.; Brogaard, L.; Kristensen, A.T.; Fredholm, M.; Skancke, E.; Morris, J.; Dupont, N.; Salavati Schmitz, S.; Argyle, D.; et al. Association of fecal and serum microRNA profiles with gastrointestinal cancer and chronic inflammatory enteropathy in dogs. J. Vet. Intern. Med. 2022, 36, 1989–2001. [Google Scholar] [CrossRef]
- Ramadan, E.S.; Salem, N.Y.; Emam, I.A.; AbdElKader, N.A.; Farghali, H.A.; Khattab, M.S. MicroRNA-21 expression, serum tumor markers, and immunohistochemistry in canine mammary tumors. Vet. Res. Commun. 2022, 46, 377–388. [Google Scholar] [CrossRef]
- Konstantinidis, A.O.; Pardali, D.; Adamama-Moraitou, K.K.; Gazouli, M.; Dovas, C.I.; Legaki, E.; Brellou, G.D.; Savvas, I.; Jergens, A.E.; Rallis, T.S.; et al. Colonic mucosal and serum expression of microRNAs in canine large intestinal inflammatory bowel disease. BMC Vet. Res. 2020, 16, 69. [Google Scholar] [CrossRef] [PubMed]
- Wissel, M.; Poirier, M.; Satterwhite, C.; Lin, J.; Islam, R.; Zimmer, J.; Khadang, A.; Zemo, J.; Lester, T.; Fjording, M.; et al. Recommendations on qPCR/ddPCR assay validation by GCC. Bioanalysis 2022, 14, 853–863. [Google Scholar] [CrossRef]
- Lievens, A.; Jacchia, S.; Kagkli, D.; Savini, C.; Querci, M. Measuring Digital PCR Quality: Performance Parameters and Their Optimization. PLoS ONE 2016, 11, e0153317. [Google Scholar] [CrossRef]
- Farina, N.H.; Wood, M.E.; Perrapato, S.D.; Francklyn, C.S.; Stein, G.S.; Stein, J.L.; Lian, J.B. Standardizing analysis of circulating microRNA: Clinical and biological relevance. J. Cell. Biochem. 2014, 115, 805–811. [Google Scholar] [CrossRef]
- Laprovitera, N.; Grzes, M.; Porcellini, E.; Ferracin, M. Cancer Site-Specific Multiple microRNA Quantification by Droplet Digital PCR. Front. Oncol. 2018, 8, 447. [Google Scholar] [CrossRef] [PubMed]
- Noszczyk-Nowak, A.; Zacharski, M.; Michałek, M. Screening for Circulating MiR-208a and -b in Different Cardiac Arrhythmias of Dogs. J. Vet. Res. 2018, 62, 359–363. [Google Scholar] [CrossRef]
- Campomenosi, P.; Gini, E.; Noonan, D.M.; Poli, A.; D’Antona, P.; Rotolo, N.; Dominioni, L.; Imperatori, A. A comparison between quantitative PCR and droplet digital PCR technologies for circulating microRNA quantification in human lung cancer. BMC Biotechnol. 2016, 16, 60. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ding, Q.; Plant, P.; Basheer, M.; Yang, C.; Tawedrous, E.; Krizova, A.; Boulos, C.; Farag, M.; Cheng, Y.; et al. Droplet digital PCR improves urinary exosomal miRNA detection compared to real-time PCR. Clin. Biochem. 2019, 67, 54–59. [Google Scholar] [CrossRef]
- Rajakumar, T.; Horos, R.; Jehn, J.; Schenz, J.; Muley, T.; Pelea, O.; Hofmann, S.; Kittner, P.; Kahraman, M.; Heuvelman, M.; et al. A blood-based miRNA signature with prognostic value for overall survival in advanced stage non-small cell lung cancer treated with immunotherapy. Npj Precis. Oncol. 2022, 6, 19. [Google Scholar] [CrossRef]

| Study | miRNAs | Species | Material | Storage Condition | Results | Reference |
|---|---|---|---|---|---|---|
| Enelund et al., 2017 | miR-16, 23a, 26a, let7a | canine | serum, plasma, PAXGene | Storage for 24 h | PAXGene showed the highest values. Decline after 24 h in serum and plasma. There is no difference between serum and plasma. | [21] |
| Glinge et al., 2017 | miR-1, 21, 29b | human | serum, plasma, EDTA, LiHep | Blood is stored for 4, 8, 12, 24 and 72 h before making serum 24 h and 9 months storage | There is no difference between EDTA, serum and plasma. EDTA storage has no effect until 72 h. Incubation of serum at RT showed reduction after 24 h. | [20] |
| Köberle et al., 2013 | miR-1, 16, 21, 122, 142 | human | serum | 1-, 3-, 5-, and 24-h RT | Decline for miR-1 and 122. Slight decline for miR-16, 21, 142. | [16] |
| Kupec et al., 2022 | miR-22, 23, 27b, 28, 99a, 100, 125b, 151a, 192, 193a, 193b, 194, 323b, 361, 1260a | human | serum | 14 days at −80 °C | Slightly lower expression after storage. Differences between miRNAs. | [18] |
| Matias-Garcia et al., 2020 | miR-23a, 24, 30c, 33b, 93, 103a, 124, 191, 451a | human | plasma | Long storage (17 years) at −80 °C | No effect. | [19] |
| McDonald et al., 2011 | miR-15b, 16, 24 | human | serum, plasma | 24, 48, 72 h at 4 °C and −20 °C | Values are higher in plasma than in serum decline during storage. | [23] |
| Mitchell et al., 2008 | miR-15b, 16, 19b, 24 | human | serum, plasma | 24 h at RT | There is no difference between serum and plasma. Minimal effect after 24 h RT. | [24] |
| Yamada et al., 2014 | miR-16, 21, 29a, 125b | human | serum, plasma | 7 days at 4 °C | More miR-16 in plasma than in serum. lower levels in stored sera for all. | [17] |
| No. | Breed | Age | Sex | Body Weight |
|---|---|---|---|---|
| 1 | Old German Herding Dog | 5 years | fc | 20 kg |
| 2 | Old German Herding Dog | 5 years | f | 20 kg |
| 3 | Tornjak | 3 years | f | 50 kg |
| 4 | Tornjak | 3 years | mc | 50 kg |
| 5 | Tibet Terrier | 15 years | mc | 11 kg |
| 6 | Cairn Terrier | 6 years | fc | 9 kg |
| 7 | German Shorthaired Pointer | 1 year | m | 26 kg |
| 8 | Mix | 3 years | fc | 12 kg |
| 9 | Australian Shepherd | 2 years | f | 23 kg |
| 10 | Mix | 7 years | fc | 12 kg |
| Median c/µL | Min.–Max. c/µL | |
|---|---|---|
| miR-20b | 13,016 | 4781–26,041 |
| miR-21 | 63,205 | 27,775–120,206 |
| miR-122 | 919 | 231–2313 |
| miR-126 | 17,331 | 4630–46,382 |
| miR-192 | 7,298 | 3773–17,169 |
| miR-214 | 5,160 | 1698–17,903 |
| miR-222 | 15,063 | 5525–40,313 |
| miR-494 | 556 | 27–965 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kehl, A.; Klein, R.; Steiger, K.; Aupperle-Lellbach, H. Stability of microRNAs in Canine Serum—A Prerequisite for Use as Biomarkers in Tumour Diagnostics. Vet. Sci. 2025, 12, 390. https://doi.org/10.3390/vetsci12040390
Kehl A, Klein R, Steiger K, Aupperle-Lellbach H. Stability of microRNAs in Canine Serum—A Prerequisite for Use as Biomarkers in Tumour Diagnostics. Veterinary Sciences. 2025; 12(4):390. https://doi.org/10.3390/vetsci12040390
Chicago/Turabian StyleKehl, Alexandra, Ruth Klein, Katja Steiger, and Heike Aupperle-Lellbach. 2025. "Stability of microRNAs in Canine Serum—A Prerequisite for Use as Biomarkers in Tumour Diagnostics" Veterinary Sciences 12, no. 4: 390. https://doi.org/10.3390/vetsci12040390
APA StyleKehl, A., Klein, R., Steiger, K., & Aupperle-Lellbach, H. (2025). Stability of microRNAs in Canine Serum—A Prerequisite for Use as Biomarkers in Tumour Diagnostics. Veterinary Sciences, 12(4), 390. https://doi.org/10.3390/vetsci12040390







