Biosecurity Implications, Transmission Routes and Modes of Economically Important Diseases in Domestic Fowl and Turkey
Simple Summary
Abstract
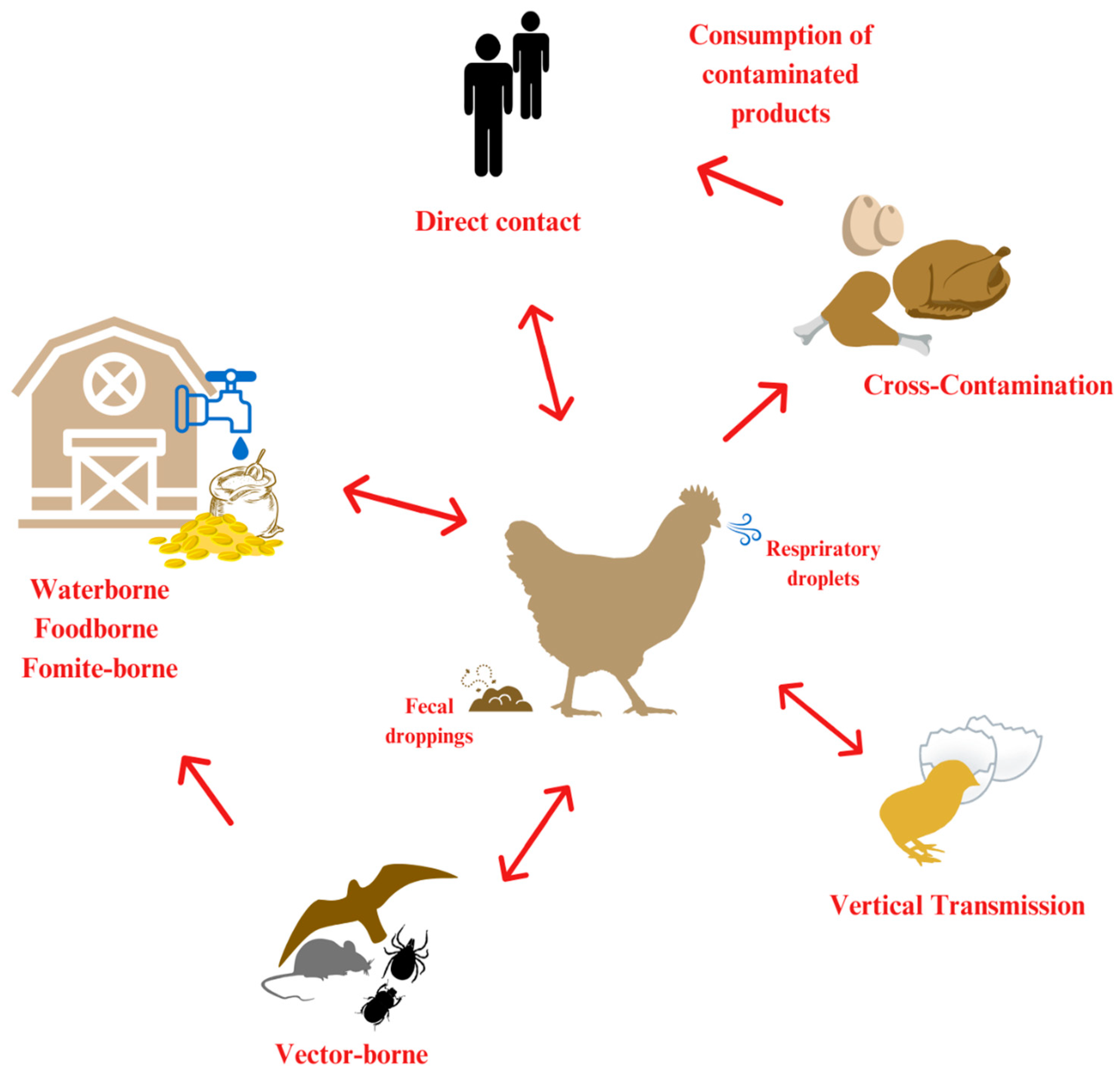
1. Introduction
2. Pathogens
2.1. Viral Pathogens
2.2. Bacterial Pathogens
2.3. Parasitic Pathogens
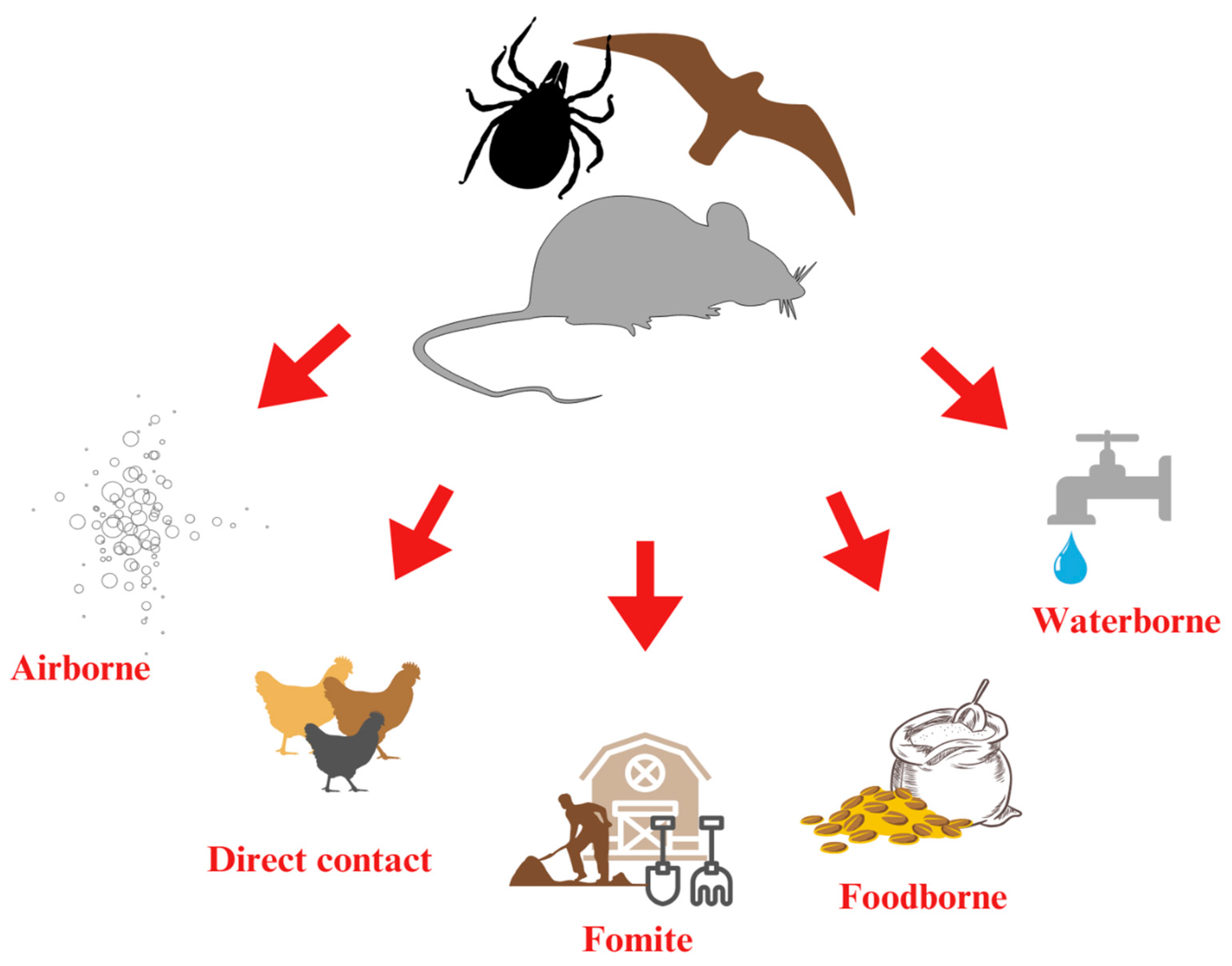
3. Airborne
| Airborne | Distance | Survival Time | Transmission Route | Additional Data |
|---|---|---|---|---|
| Avian Influenza (AI) | Potentially hundreds of miles [101] | 4 °C: more than 900 days 20 °C: 226–293 days 30 °C: 51–58 days | Carried by respiratory droplets and dust particles; risk increases during bird migration [39,100] | |
| Newcastle Disease (ND) | Limited in poor ventilation [109] | −20 °C: At least 6 months in the bone marrow and muscle of slaughtered chickens. 4 °C: survives over a year 20–25 °C: 30–90 days | No data | Poorly ventilated environments increase transmission risk [109] |
| Infectious Laryngotracheitis (ILT) | hundreds of meters under optimal conditions [27] | several months in dry dust | No data | |
| Escherichia coli | 800 m outdoors [104] | 6 min airborne, 9.6 h on surfaces [103] | No data | Higher concentrations indoors; survival depends on environmental conditions [104] |
| Pasteurella multocida | No data | 45 min [47] | No data | |
| Staphylococcus aureus | No data | No data | Remains viable in settled dust for months [105,106] | |
| Marek’s Disease (MD) | No data | 20–25 °C: MDV remains infectious for at least several months. 4 °C: The virus can survive and remain infectious for years [110] | The virus spreads through feather dust and dander [102] | Survival is reduced in humid environments [102] |
| Avian metapneumovirus (aMPV) | No data | Weeks at 4 °C, 4 weeks at 20 °C, 2 days at 37 °C, and 6 h at 50 °C [47] | Only contact spread has been confirmed | |
| Mycoplasma synoviae (MS) | several km | 9 days on synthetic hair, indicating its ability to persist in airborne particles [108] | Lateral transmission occurs readily by direct contact, via the respiratory tract | Infection may also occur as a result of environmental contamina- tion or fomites [47] |
| Mycoplasma gallisepticum (MG) | 4 days on feathers, 6 h in the air 3 days on human hair MG isolates can survive inside the human nose for up to 1 day | Airborne transmission via respiratory and conjunctival routes |
4. Fomite
| Fomite | Survival Time | Additional Data |
|---|---|---|
| Avian encephalomyelitis virus (AEV) | several weeks [47] | No data |
| Avian Influenza (AI) | 5 days at 24 °C; 8 weeks at 4 °C [41] | Feces contamination spreads AI; survival depends on temperature [41] |
| Avian pneumovirus (APV) | APV can survive in turkey litter for up to 60 days at −12 °C [127] | No data |
| Avian reovirus (ARV) | In experimental study, 10 days on eggshells when organic material was present >over 10 days—on feathers, wood shavings, and chicken feed only 2 days on wood and 4 days on paper and cotton [128] | No data |
| Chicken anemia virus (CAV) | Various organs and rectal contents for up to 35 days post-infection [55] | Via fecal-oral route, infected feather follicle epithelium, and possibly via the respiratory tract [55] |
| Infectious Bronchitis Virus (IBV) | weeks in feces during winter [47] | High resilience in feces |
| Gumboro Disease (IBD) | 16 days in feces; >122 days in poultry houses [129] | Resistant to disinfectants; tolerates wide pH range |
| Marek’s Disease (MD) | 8 months on feathers [110,114] | The virus survives well in environmental reservoirs [110,114] |
| Newcastle Disease (ND) | weeks in litter [40,47] | Longer survival at low temperatures [40] |
| Clostridium perfringens | Up to 48 h on surfaces [120] | Forms spores that persist on surfaces [119] |
| Escherichia coli | >28 days on stainless steel [103] | No data |
| Mycoplasma gallisepticum | 2–4 days on feathers, feed, or water [115] | Transmitted via contaminated equipment or water [115] |
| Mycoplasma gallisepticum PG31 | 4 days in feed and 2 days in tap water with 1% culture suspension [115] | No data |
| Mycoplasma synoviae | 2 to 3 days on feathers [115] | No data |
| Pasteurella multocida | Variable; persistent in organic material [47] | No data |
| Salmonella enterica | Up to 291 days in manure dust [116] | No data |
| Eimeria | Coccidiosis oocysts survive a few hours [125] | No data |
5. Waterborne
| Waterborne | Survival Time | Additional Data |
|---|---|---|
| Avian Influenza (AI) | 21 days at 20 °C | Persistence influenced by pH and Temperature [142] |
| Avian reovirus (ARV) | 10 Weeks [128] | No data |
| Infectious Bronchitis Virus (IBV) | 52 days [132] | No data |
| Newcastle Disease (ND) | several days [47] | Stable at neutral/alkaline pH [131] |
| Clostridium perfringens | Months [141] | No data |
| Escherichia coli | Weeks [62,137] | No data |
| Mycoplasma gallisepticum | 1–10 days [143] | Persistence is favored in nutrient-rich environments |
| Pasteurella multocida | 14 days at 4 °C; 49 days at 37 °C [138] | Survival increases with increasing temperature |
| Salmonella Enteritidis | No data | Biofilm formation in water lines allows extended survival, especially in warm conditions (27–30 °C) [135,144] |
6. Vector-Borne
| Vector-Borne | Survival Time | Additional Data |
|---|---|---|
| Avian Influenza (AI) | No data | Transmitted via wild birds and rodents [39] |
| Avian metapneumovirus (aMPV) | No data | Migratory birds significantly contribute to the spread [127] |
| Fowlpox virus (FWPV) | No data | Transmitted via biting insects such as mosquitoes and mites [160] |
| Infectious Laryngotracheitis (ILT) | Darkling beetles harboring ILT for up to 42 days [47] | No Data |
| Escherichia coli | 6–12 days in vectors [152] | Spread via beetles and rodents [162] |
| Pasteurella multocida | 64–300 days in mites [155] | Mites serve as long-term reservoirs of the pathogen [155] |
| Salmonella enterica | Up to 4 months in mites (Dermanyssus gallinae) [156] | Mites act as vectors; wild birds contribute to contamination through feces and physical contact [156] |
| Salmonella Typhimurium | 28 days in beetles’ feces; 45 days in non-living Beetles [163] | Transmitted by darkling beetles (Alphitobius diaperinus) [163] |
7. Vertical Transmission
| Vertical Transmission | Type of Vertical Transmission |
|---|---|
| Avian encephalomyelitis virus (AEV) | Transovarian transmission [54] |
| Avian influenza | There is some evidence of vertical transmission; however, infected eggs are unlikely to hatch successfully [39] |
| Avian reovirus (ARV) | Transovarial and transovum transmission [174] |
| Chicken anemia virus (CAV) | Transovarian transmission [55] |
| Egg drop syndrome 76 (EDS 76) | Transovarian transmission [61] |
| Fowl adenovirus (FAdV) | Transovarian transmission [175] |
| Campylobacter jejuni | Potential but rare vertical transmission; more often transmitted via fecal-oral routes [169,170] |
| Escherichia coli | Transovarial and transovum transmission [62] |
| Mycoplasma gallisepticum | Transovarial transmission [167] |
| Mycoplasma synoviae | Transovarian transmission (infection rate is low) [65,167] |
| Salmonella enteritidis | Transovarial transmission [32,143] |
| Salmonella infantis | Transovarial and transovum transmission [168] |
8. On-Farm Biosecurity and Disinfectants
9. Role of Social Media in Countering Misinformation
10. Discussion
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AEV | Avian encephalomyelitis virus |
| AI | Avian influenza |
| aMPV | Avian metapneumovirus |
| APEC | Avian Pathogenic Escherichia coli |
| APV | Avian pneumovirus |
| ART | Avian rhinotracheitis |
| ARV | Avian Reovirus |
| CAV | Chicken anemia virus |
| CIA | Chicken infectious anemia |
| EDS 76 | Egg drop syndrome 76 |
| FAdV | Fowl adenovirus |
| FAO | Food and Agriculture Organization of the United Nations |
| FT | Fowl Typhoid |
| FWPV | Fowlpox virus |
| HP | Hydrogen Peroxide |
| HPAI | Highly Pathogenic Avian Influenza |
| IBD | Infectious Bursal Disease |
| IBV | Infectious Bronchitis Virus |
| ILT | Infectious Laryngotracheitis |
| LPAI | Low Pathogenic Avian Influenza |
| MD MDV | Marek’s Disease Marek’s Disease Virus |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| MS | Mycoplasma synoviae |
| MT | Metric Tons |
| NDV | Newcastle disease virus |
| OECD | Organization for Economic Co-operation and Development |
| PCV | Packed cell volume |
| PD QAC | Pullorum disease Quaternary ammonium compounds |
| SDF | Surface Decontamination Foam |
| SHS | Swollen head syndrome |
| TRT | Turkey rhinotracheitis |
References
- Attia, Y.A.; Rahman, M.T.; Hossain, M.J.; Basiouni, S.; Khafaga, A.F.; Shehata, A.A.; Hafez, H.M. Poultry Production and Sustainability in Developing Countries under the COVID-19 Crisis: Lessons Learned. Animals 2022, 12, 644. [Google Scholar] [CrossRef] [PubMed]
- Castro, F.L.S.; Chai, L.; Arango, J.; Owens, C.M.; Smith, P.A.; Reichelt, S.; DuBois, C.; Menconi, A. Poultry Industry Paradigms: Connecting the Dots. J. Appl. Poult. Res. 2023, 32, 100310. [Google Scholar] [CrossRef]
- Connolly, G.; Clark, C.M.; Campbell, R.E.; Byers, A.W.; Reed, J.B.; Campbell, W.W. Poultry Consumption and Human Health: How Much Is Really Known? A Systematically Searched Scoping Review and Research Perspective. Adv. Nutr. 2022, 13, 2115–2124. [Google Scholar] [CrossRef] [PubMed]
- Mottet, A.; Tempio, G. Global Poultry Production: Current State and Future Outlook and Challenges. Worlds Poult. Sci. J. 2017, 73, 245–256. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the UN. FAO Agribusiness Handbook; FAO: Rome, Italy, 2012. [Google Scholar]
- Kennedy, D. B Vitamins and the Brain: Mechanisms, Dose and Efficacy—A Review. Nutrients 2016, 8, 68. [Google Scholar] [CrossRef]
- Marangoni, F.; Corsello, G.; Cricelli, C.; Ferrara, N.; Ghiselli, A.; Lucchin, L.; Poli, A. Role of Poultry Meat in a Balanced Diet Aimed at Maintaining Health and Wellbeing: An Italian Consensus Document. Food Nutr. Res. 2015, 59, 27606. [Google Scholar] [CrossRef]
- Bist, R.B.; Bist, K.; Poudel, S.; Subedi, D.; Yang, X.; Paneru, B.; Mani, S.; Wang, D.; Chai, L. Sustainable Poultry Farming Practices: A Critical Review of Current Strategies and Future Prospects. Poult. Sci. 2024, 103, 104295. [Google Scholar] [CrossRef]
- OECD; Food and Agriculture Organization of the United Nations. OECD-FAO Agricultural Outlook 2024–2033; OECD: Paris, France, 2024; ISBN 978-92-64-72259-0. [Google Scholar]
- Gržinić, G.; Piotrowicz-Cieślak, A.; Klimkowicz-Pawlas, A.; Górny, R.L.; Ławniczek-Wałczyk, A.; Piechowicz, L.; Olkowska, E.; Potrykus, M.; Tankiewicz, M.; Krupka, M.; et al. Intensive Poultry Farming: A Review of the Impact on the Environment and Human Health. Sci. Total Environ. 2023, 858, 160014. [Google Scholar] [CrossRef]
- Zamani, O.; Bittmann, T.; Ortega, D.L. The Effect of Avian Influenza Outbreaks on Retail Price Premiums in the United States Poultry Market. Poult. Sci. 2024, 103, 104102. [Google Scholar] [CrossRef]
- Kovács, L.; Klaucke, C.R.; Farkas, M.; Bakony, M.; Jurkovich, V.; Könyves, L. The Correlation between On-Farm Biosecurity and Animal Welfare Indices in Large-Scale Turkey Production. Poult. Sci. 2025, 104, 104598. [Google Scholar] [CrossRef]
- Alarcón, L.V.; Allepuz, A.; Mateu, E. Biosecurity in Pig Farms: A Review. Porc. Health Manag. 2021, 7, 5. [Google Scholar] [CrossRef]
- Scott, A.B.; Singh, M.; Groves, P.; Hernandez-Jover, M.; Barnes, B.; Glass, K.; Moloney, B.; Black, A.; Toribio, J.-A. Biosecurity Practices on Australian Commercial Layer and Meat Chicken Farms: Performance and Perceptions of Farmers. PLoS ONE 2018, 13, e0195582. [Google Scholar] [CrossRef] [PubMed]
- Chantziaras, I.; Boyen, F.; Callens, B.; Dewulf, J. Correlation between Veterinary Antimicrobial Use and Antimicrobial Resistance in Food-Producing Animals: A Report on Seven Countries. J. Antimicrob. Chemother. 2014, 69, 827–834. [Google Scholar] [CrossRef]
- Treiber, F.M.; Beranek-Knauer, H. Antimicrobial Residues in Food from Animal Origin—A Review of the Literature Focusing on Products Collected in Stores and Markets Worldwide. Antibiotics 2021, 10, 534. [Google Scholar] [CrossRef] [PubMed]
- Huber, N.; Andraud, M.; Sassu, E.L.; Prigge, C.; Zoche-Golob, V.; Käsbohrer, A.; D’Angelantonio, D.; Viltrop, A.; Żmudzki, J.; Jones, H.; et al. What Is a Biosecurity Measure? A Definition Proposal for Animal Production and Linked Processing Operations. One Health 2022, 15, 100433. [Google Scholar] [CrossRef] [PubMed]
- Grace, D.; Knight-Jones, T.J.D.; Melaku, A.; Alders, R.; Jemberu, W.T. The Public Health Importance and Management of Infectious Poultry Diseases in Smallholder Systems in Africa. Foods 2024, 13, 411. [Google Scholar] [CrossRef]
- Dewulf, J.; Van Immerseel, F. Biosecurity in Animal Production and Veterinary Medicine: From Principles to Practice; CAB International: Oxford, UK, 2020; ISBN 978-1-78924-568-4. [Google Scholar]
- Islam, A.; Rahman, M.Z.; Hassan, M.M.; Epstein, J.H.; Klaassen, M. Farm Biosecurity Practices Affecting Avian Influenza Virus Circulation in Commercial Chicken Farms in Bangladesh. One Health 2024, 18, 100681. [Google Scholar] [CrossRef]
- Vastolo, A.; Serrapica, F.; Cavallini, D.; Fusaro, I.; Atzori, A.S.; Todaro, M. Editorial: Alternative and Novel Livestock Feed: Reducing Environmental Impact. Front. Vet. Sci. 2024, 11, 1441905. [Google Scholar] [CrossRef]
- Ayala, A.J.; Yabsley, M.J.; Hernandez, S.M. A Review of Pathogen Transmission at the Backyard Chicken-Wild Bird Interface. Front. Vet. Sci. 2020, 7, 539925. [Google Scholar] [CrossRef]
- van Seventer, J.M.; Hochberg, N.S. Principles of Infectious Diseases: Transmission, Diagnosis, Prevention, and Control. In International Encyclopedia of Public Health, 2nd ed.; Quah, S.R., Ed.; Academic Press: Oxford, UK, 2017; pp. 22–39. ISBN 978-0-12-803708-9. [Google Scholar]
- Simancas-Racines, A.; Cadena-Ullauri, S.; Guevara-Ramírez, P.; Zambrano, A.K.; Simancas-Racines, D. Avian Influenza: Strategies to Manage an Outbreak. Pathogens 2023, 12, 610. [Google Scholar] [CrossRef]
- Rasheed, M. A Review of Current Knowledge on Avian Newcastle Infection in Commercial Poultry in the Kingdome of Saudi Arabia. Open Vet. J. 2024, 14, 12. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.A.; Grace, D.; Kock, R.; Alonso, S.; Rushton, J.; Said, M.Y.; McKeever, D.; Mutua, F.; Young, J.; McDermott, J.; et al. Zoonosis Emergence Linked to Agricultural Intensification and Environmental Change. Proc. Natl. Acad. Sci. USA 2013, 110, 8399–8404. [Google Scholar] [CrossRef] [PubMed]
- Gowthaman, V.; Kumar, S.; Koul, M.; Dave, U.; Murthy, T.R.G.K.; Munuswamy, P.; Tiwari, R.; Karthik, K.; Dhama, K.; Michalak, I.; et al. Infectious Laryngotracheitis: Etiology, Epidemiology, Pathobiology, and Advances in Diagnosis and Control—A Comprehensive Review. Vet. Q. 2020, 40, 140–161. [Google Scholar] [CrossRef] [PubMed]
- Gerba, C.P. Chapter 22—Environmentally Transmitted Pathogens. In Environmental Microbiology, 2nd ed.; Maier, R.M., Pepper, I.L., Gerba, C.P., Eds.; Academic Press: San Diego, CA, USA, 2009; pp. 445–484. ISBN 978-0-12-370519-8. [Google Scholar]
- Amaral, L.D. Drinking Water as a Risk Factor to Poultry Health. Rev. Bras. Ciênc. Avícola 2004, 6, 191–199. [Google Scholar] [CrossRef]
- van der Kolk, J.H. Role for Migratory Domestic Poultry and/or Wild Birds in the Global Spread of Avian Influenza? Vet. Q. 2019, 39, 161–167. [Google Scholar] [CrossRef]
- Cocciolo, G.; Circella, E.; Pugliese, N.; Lupini, C.; Mescolini, G.; Catelli, E.; Borchert-Stuhlträger, M.; Zoller, H.; Thomas, E.; Camarda, A. Evidence of Vector Borne Transmission of Salmonella enterica Enterica Serovar Gallinarum and Fowl Typhoid Disease Mediated by the Poultry Red Mite, Dermanyssus gallinae (De Geer, 1778). Parasit. Vectors 2020, 13, 513. [Google Scholar] [CrossRef]
- Shaji, S.; Selvaraj, R.K.; Shanmugasundaram, R. Salmonella Infection in Poultry: A Review on the Pathogen and Control Strategies. Microorganisms 2023, 11, 2814. [Google Scholar] [CrossRef]
- Brown Jordan, A.; Sookhoo, J.; Blake, L.; Crooks, P.; Mohammed, Z.; Molawatti-Bisnath, J.; Carrington, C.V.F.; Oura, C.A.L. Serological Evidence for Eight Globally Important Poultry Viruses in Trinidad & Tobago. Prev. Vet. Med. 2018, 149, 75–81. [Google Scholar] [CrossRef]
- Okinda, C.; Nyalala, I.; Korohou, T.; Okinda, C.; Wang, J.; Achieng, T.; Wamalwa, P.; Mang, T.; Shen, M. A Review on Computer Vision Systems in Monitoring of Poultry: A Welfare Perspective. Artif. Intell. Agric. 2020, 4, 184–208. [Google Scholar] [CrossRef]
- Gentile, N.; Carrasquer, F.; Marco-Fuertes, A.; Marin, C. Backyard Poultry: Exploring Non-Intensive Production Systems. Poult. Sci. 2024, 103, 103284. [Google Scholar] [CrossRef]
- Zhou, Z.; Shen, B.; Bi, D. Chapter 30—Management of Pathogens in Poultry. In Animal Agriculture; Bazer, F.W., Lamb, G.C., Wu, G., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 515–530. ISBN 978-0-12-817052-6. [Google Scholar]
- Sheen, J.K.; Rasambainarivo, F.; Saad-Roy, C.M.; Grenfell, B.T.; Metcalf, C.J.E. Markets as Drivers of Selection for Highly Virulent Poultry Pathogens. Nat. Commun. 2024, 15, 605. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zeng, X.; Cui, P.; Yan, C.; Chen, H. Alarming Situation of Emerging H5 and H7 Avian Influenza and Effective Control Strategies. Emerg. Microbes Infect. 2023, 12, 2155072. [Google Scholar] [CrossRef] [PubMed]
- Blagodatski, A.; Trutneva, K.; Glazova, O.; Mityaeva, O.; Shevkova, L.; Kegeles, E.; Onyanov, N.; Fede, K.; Maznina, A.; Khavina, E.; et al. Avian Influenza in Wild Birds and Poultry: Dissemination Pathways, Monitoring Methods, and Virus Ecology. Pathogens 2021, 10, 630. [Google Scholar] [CrossRef] [PubMed]
- Absalón, A.E.; Cortés-Espinosa, D.V.; Lucio, E.; Miller, P.J.; Afonso, C.L. Epidemiology, Control, and Prevention of Newcastle Disease in Endemic Regions: Latin America. Trop. Anim. Health Prod. 2019, 51, 1033–1048. [Google Scholar] [CrossRef]
- Kurmi, B.; Murugkar, H.V.; Nagarajan, S.; Tosh, C.; Dubey, S.C.; Kumar, M. Survivability of Highly Pathogenic Avian Influenza H5N1 Virus in Poultry Faeces at Different Temperatures. Indian J. Virol. 2013, 24, 272–277. [Google Scholar] [CrossRef]
- Zhang, D.; Ding, Z.; Xu, X. Pathologic Mechanisms of the Newcastle Disease Virus. Viruses 2023, 15, 864. [Google Scholar] [CrossRef]
- Bello, M.B.; Yusoff, K.; Ideris, A.; Hair-Bejo, M.; Peeters, B.P.H.; Omar, A.R. Diagnostic and Vaccination Approaches for Newcastle Disease Virus in Poultry: The Current and Emerging Perspectives. BioMed Res. Int. 2018, 2018, 7278459. [Google Scholar] [CrossRef]
- Rafique, S.; Jabeen, Z.; Pervaiz, T.; Rashid, F.; Luo, S.; Xie, L.; Xie, Z. Avian Infectious Bronchitis Virus (AIBV) Review by Continent. Front. Cell. Infect. Microbiol. 2024, 14, 1325346. [Google Scholar] [CrossRef]
- Franzo, G.; Tucciarone, C.M.; Moreno, A.; Legnardi, M.; Massi, P.; Tosi, G.; Trogu, T.; Ceruti, R.; Pesente, P.; Ortali, G.; et al. Phylodynamic Analysis and Evaluation of the Balance between Anthropic and Environmental Factors Affecting IBV Spreading among Italian Poultry Farms. Sci. Rep. 2020, 10, 7289. [Google Scholar] [CrossRef]
- Khan, R.S.A.; Sajid, S.; Habib, M.; Ali, W.; Salah-ud-Din Shah, M.; Sarfraz, M. History of Gumboro (Infectious Bursal Disease) in Pakistan. Saudi Pharm. J. 2017, 25, 453–459. [Google Scholar] [CrossRef]
- Diseases of Poultry, 1st ed.; Swayne, D.E., Boulianne, M., Logue, C.M., McDougald, L.R., Nair, V., Suarez, D.L., Wit, S., Grimes, T., Johnson, D., Kromm, M., et al., Eds.; Wiley: Hoboken, NJ, USA, 2020; ISBN 978-1-119-37116-8. [Google Scholar]
- Pajić, M.; Knežević, S.; Djurdjević, B.; Polaček, V.; Todorović, D.; Petrović, T.; Lazić, S. Diagnosis of Infectious Laryngotracheitis Outbreaks on Layer Hen and Broiler Breeder Farms in Vojvodina, Serbia. Animals 2022, 12, 3551. [Google Scholar] [CrossRef] [PubMed]
- Boodhoo, N.; Gurung, A.; Sharif, S.; Behboudi, S. Marek’s Disease in Chickens: A Review with Focus on Immunology. Vet. Res. 2016, 47, 119. [Google Scholar] [CrossRef] [PubMed]
- Luqman, M.; Duhan, N.; Temeeyasen, G.; Selim, M.; Jangra, S.; Mor, S.K. Geographical Expansion of Avian Metapneumovirus Subtype B: First Detection and Molecular Characterization of Avian Metapneumovirus Subtype B in US Poultry. Viruses 2024, 16, 508. [Google Scholar] [CrossRef] [PubMed]
- Kaboudi, K.; Lachheb, J. Avian Metapneumovirus Infection in Turkeys: A Review on Turkey Rhinotracheitis. J. Appl. Poult. Res. 2021, 30, 100211. [Google Scholar] [CrossRef]
- Escobar-Alfonso, S.; Alvarez-Mira, D.M.; Beltran-Leon, M.; Ramirez-Nieto, G.; Gomez, A.P. Avian Metapneumovirus Subtype B Circulation in Poultry and Wild Birds of Colombia. Pathogens 2024, 13, 882. [Google Scholar] [CrossRef]
- Tannock, G.A.; Shafren, D.R. Avian Encephalomyelitis: A Review. Avian Pathol. 1994, 23, 603–620. [Google Scholar] [CrossRef]
- Zhang, F.; Luo, Y.; Wei, Q.; Xiong, L.; Xie, Q.; Tan, J.; Wu, C.; Li, N.; Kang, Z. Research Note: Pathogenetic Characteristics of Avian Encephalomyelitis Virus in Guangdong and Jiangxi Provinces, China. Poult. Sci. 2024, 103, 103264. [Google Scholar] [CrossRef]
- Fatoba, A.J.; Adeleke, M.A. Chicken Anemia Virus: A Deadly Pathogen of Poultry. Acta Virol. 2019, 63, 19–25. [Google Scholar] [CrossRef]
- Choi, Y.-R.; Kim, S.-W.; Shang, K.; Park, J.-Y.; Zhang, J.; Jang, H.-K.; Wei, B.; Cha, S.-Y.; Kang, M. Avian Reoviruses From Wild Birds Exhibit Pathogenicity to Specific Pathogen Free Chickens by Footpad Route. Front. Vet. Sci. 2022, 9, 844903. [Google Scholar] [CrossRef]
- Ishag, H.Z.A.; Terab, A.M.A.; El Tigani-Asil, E.T.A.; Bensalah, O.K.; Khalil, N.A.H.; Khalafalla, A.I.; Al Hammadi, Z.M.A.H.; Shah, A.A.M.; Al Muhairi, S.S.M. Pathology and Molecular Epidemiology of Fowl Adenovirus Serotype 4 Outbreaks in Broiler Chicken in Abu Dhabi Emirate, UAE. Vet. Sci. 2022, 9, 154. [Google Scholar] [CrossRef]
- Cizmecigil, U.Y.; Umar, S.; Yilmaz, A.; Bayraktar, E.; Turan, N.; Tali, B.; Aydin, O.; Tali, H.E.; Yaramanoglu, M.; Yilmaz, S.G.; et al. Characterisation of Fowl Adenovirus (FAdV-8b) Strain Concerning the Geographic Analysis and Pathological Lesions Associated with Inclusion Body Hepatitis in Broiler Flocks in Turkey. J. Vet. Res. 2020, 64, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Yu, G.; Niu, Y.; Cai, Y.; Liu, S. Airborne Transmission of a Serotype 4 Fowl Adenovirus in Chickens. Viruses 2019, 11, 262. [Google Scholar] [CrossRef]
- Mazaheri, A.; Prusas, C.; Voß, M.; Hess, M. Vertical Transmission of Fowl Adenovirus Serotype 4 Investigated in Specified Pathogen-Free Birds after Experimental Infection. Eur. Poult. Sci. 2003, 67, 6–10. [Google Scholar] [CrossRef]
- McFerran, J.B. Egg Drop Syndrome, 1976 (EDS’76). Vet. Q. 1979, 1, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.; Zhang, L.; Adhikari, P.; Evans, J.D.; Ramachandran, R. Avian Pathogenic Escherichia coli (APEC) in Broiler Breeders: An Overview. Pathogens 2023, 12, 1280. [Google Scholar] [CrossRef] [PubMed]
- Kromann, S.; Jensen, H.E. In Vivo Models of Escherichia coli Infection in Poultry. Acta Vet. Scand. 2022, 64, 33. [Google Scholar] [CrossRef]
- Marouf, S.; Khalf, M.A.; Alorabi, M.; El-Shehawi, A.M.; El-Tahan, A.M.; El-Hack, M.E.A.; El-Saadony, M.T.; Salem, H.M. Mycoplasma gallisepticum: A Devastating Organism for the Poultry Industry in Egypt. Poult. Sci. 2022, 101, 101658. [Google Scholar] [CrossRef]
- Yadav, J.P.; Tomar, P.; Singh, Y.; Khurana, S.K. Insights on Mycoplasma gallisepticum and Mycoplasma synoviae Infection in Poultry: A Systematic Review. Anim. Biotechnol. 2022, 33, 1711–1720. [Google Scholar] [CrossRef]
- Shivaprasad, H.L. Fowl Typhoid and Pullorum Disease. Rev. Sci. Tech. Int. Off. Epizoot. 2000, 19, 405–424. [Google Scholar] [CrossRef]
- Ngongolo, K. Awareness and Perceptions of Poultry Keepers about the Prevalence of Fowl Typhoid in Chickens Kept in Dodoma, Tanzania. BMC Vet. Res. 2024, 20, 453. [Google Scholar] [CrossRef]
- Lamichhane, B.; Mawad, A.M.M.; Saleh, M.; Kelley, W.G.; Harrington, P.J.; Lovestad, C.W.; Amezcua, J.; Sarhan, M.M.; El Zowalaty, M.E.; Ramadan, H.; et al. Salmonellosis: An Overview of Epidemiology, Pathogenesis, and Innovative Approaches to Mitigate the Antimicrobial Resistant Infections. Antibiotics 2024, 13, 76. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S. Pathogenicity of Salmonella Enteritidis in Poultry. Int. J. Food Microbiol. 1994, 21, 89–105. [Google Scholar] [CrossRef] [PubMed]
- Wales, A.D.; Davies, R.H. A Critical Review of Salmonella Typhimurium Infection in Laying Hens. Avian Pathol. 2011, 40, 429–436. [Google Scholar] [CrossRef]
- Mughini-Gras, L.; Van Hoek, A.H.A.M.; Cuperus, T.; Dam-Deisz, C.; Van Overbeek, W.; Van Den Beld, M.; Wit, B.; Rapallini, M.; Wullings, B.; Franz, E.; et al. Prevalence, Risk Factors and Genetic Traits of Salmonella Infantis in Dutch Broiler Flocks. Vet. Microbiol. 2021, 258, 109120. [Google Scholar] [CrossRef] [PubMed]
- Mbuthia, P.G.; Njagi, L.W.; Nyaga, P.N.; Bebora, L.C.; Minga, U.; Kamundia, J.; Olsen, J.E. Pasteurella multocida in Scavenging Family Chickens and Ducks: Carrier Status, Age Susceptibility and Transmission between Species. Avian Pathol. 2008, 37, 51–57. [Google Scholar] [CrossRef]
- Al Hakeem, W.G.; Fathima, S.; Shanmugasundaram, R.; Selvaraj, R.K. Campylobacter jejuni in Poultry: Pathogenesis and Control Strategies. Microorganisms 2022, 10, 2134. [Google Scholar] [CrossRef]
- Haag, A.F.; Fitzgerald, J.R.; Penadés, J.R. Staphylococcus aureus in Animals. Microbiol. Spectr. 2019, 7, 731–746. [Google Scholar] [CrossRef]
- Tolba, O.; Loughrey, A.; Goldsmith, C.E.; Millar, B.C.; Rooney, P.J.; Moore, J.E. Survival of Epidemic Strains of Healthcare (HA-MRSA) and Community-Associated (CA-MRSA) Meticillin-Resistant Staphylococcus aureus (MRSA) in River-, Sea- and Swimming Pool Water. Int. J. Hyg. Environ. Health 2008, 211, 398–402. [Google Scholar] [CrossRef]
- Crespo-Piazuelo, D.; Lawlor, P.G. Livestock-Associated Methicillin-Resistant Staphylococcus aureus (LA-MRSA) Prevalence in Humans in Close Contact with Animals and Measures to Reduce on-Farm Colonisation. Ir. Vet. J. 2021, 74, 21. [Google Scholar] [CrossRef]
- Abreu, R.; Semedo-Lemsaddek, T.; Cunha, E.; Tavares, L.; Oliveira, M. Antimicrobial Drug Resistance in Poultry Production: Current Status and Innovative Strategies for Bacterial Control. Microorganisms 2023, 11, 953. [Google Scholar] [CrossRef]
- Silva, A.P.; Cooper, G.; Blakey, J.; Jerry, C.; Shivaprasad, H.L.; Stoute, S. Retrospective Summary of Erysipelothrix rhusiopathiae Diagnosed in Avian Species in California (2000–19). Avian Dis. 2020, 64, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Bobrek, K.; Gaweł, A.; Mazurkiewicz, M. Infections with Erysipelothrix rhusiopathiae in Poultry Flocks. Worlds Poult. Sci. J. 2013, 69, 803–812. [Google Scholar] [CrossRef]
- Cooper, K.K.; Songer, J.G.; Uzal, F.A. Diagnosing Clostridial Enteric Disease in Poultry. J. Vet. Diagn. Investig. 2013, 25, 314–327. [Google Scholar] [CrossRef]
- Antonissen, G.; Van Immerseel, F.; Pasmans, F.; Ducatelle, R.; Haesebrouck, F.; Timbermont, L.; Verlinden, M.; Janssens, G.P.J.; Eeckhaut, V.; Eeckhout, M.; et al. The Mycotoxin Deoxynivalenol Predisposes for the Development of Clostridium Perfringens-Induced Necrotic Enteritis in Broiler Chickens. PLoS ONE 2014, 9, e108775. [Google Scholar] [CrossRef] [PubMed]
- Pietruska, A.; Bortoluzzi, C.; Hauck, R. A Meta-Analysis of the Effect of Eimeria spp. and/or Clostridium perfringens Infection on the Microbiota of Broiler Chickens. Poult. Sci. 2023, 102, 102652. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Elbestawy, A.R.; El-Shall, N.A.; Saad, A.M.; Salem, H.M.; El-Tahan, A.M.; Khafaga, A.F.; Taha, A.E.; AbuQamar, S.F.; et al. Necrotic Enteritis in Broiler Chickens: Disease Characteristics and Prevention Using Organic Antibiotic Alternatives—A Comprehensive Review. Poult. Sci. 2022, 101, 101590. [Google Scholar] [CrossRef]
- Noormohammadi, A.H.; Markham, P.F.; Whithear, K.G.; Walker, I.D.; Gurevich, V.A.; Ley, D.H.; Browning, G.F. Mycoplasma synoviae Has Two Distinct Phase-Variable Major Membrane Antigens, One of Which Is a Putative Hemagglutinin. Infect. Immun. 1997, 65, 2542–2547. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, N.; Hébert, G.; Pelletier, M.C.; Cai, H.Y.; Brochu-Morin, M.-E.; Vaillancourt, J.-P. Prevalence of Mycoplasma Synoviae and Its Impact on Productivity in Commercial Poultry Farms in Quebec, Canada. Avian Dis. 2021, 65, 546–552. [Google Scholar] [CrossRef]
- Refisa, N.; Rebuma, T. Review on Importance of Ectoparasites in Backyard Chicken Production Systems in Rural Ethiopia. Int. J. Med. Parasitol. Epidemiol. Sci. 2024, 5, 125–132. [Google Scholar] [CrossRef]
- Murillo, A.C.; Mullens, B.A. A Review of the Biology, Ecology, and Control of the Northern Fowl Mite, Ornithonyssus sylviarum (Acari: Macronyssidae). Vet. Parasitol. 2017, 246, 30–37. [Google Scholar] [CrossRef]
- Hwang, E.T. Management of the Poultry Red Mite Dermanyssus gallinae with Physical Control Methods by Inorganic Material and Future Perspectives. Poult. Sci. 2023, 102, 102772. [Google Scholar] [CrossRef] [PubMed]
- Beer, L.C.; Petrone-Garcia, V.M.; Graham, B.D.; Hargis, B.M.; Tellez-Isaias, G.; Vuong, C.N. Histomonosis in Poultry: A Comprehensive Review. Front. Vet. Sci. 2022, 9, 880738. [Google Scholar] [CrossRef] [PubMed]
- Mitra, T.; Kidane, F.A.; Hess, M.; Liebhart, D. Unravelling the Immunity of Poultry Against the Extracellular Protozoan Parasite Histomonas meleagridis Is a Cornerstone for Vaccine Development: A Review. Front. Immunol. 2018, 9, 2518. [Google Scholar] [CrossRef]
- El-Shall, N.A.; Abd El-Hack, M.E.; Albaqami, N.M.; Khafaga, A.F.; Taha, A.E.; Swelum, A.A.; El-Saadony, M.T.; Salem, H.M.; El-Tahan, A.M.; AbuQamar, S.F.; et al. Phytochemical Control of Poultry Coccidiosis: A Review. Poult. Sci. 2022, 101, 101542. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Prather, K.A.; Sznitman, J.; Jimenez, J.L.; Lakdawala, S.S.; Tufekci, Z.; Marr, L.C. Airborne Transmission of Respiratory Viruses. Science 2021, 373, eabd9149. [Google Scholar] [CrossRef]
- De Rooij, M.M.T.; Hoek, G.; Schmitt, H.; Janse, I.; Swart, A.; Maassen, C.B.M.; Schalk, M.; Heederik, D.J.J.; Wouters, I.M. Insights into Livestock-Related Microbial Concentrations in Air at Residential Level in a Livestock Dense Area. Environ. Sci. Technol. 2019, 53, 7746–7758. [Google Scholar] [CrossRef]
- Chinivasagam, H.N.; Tran, T.; Maddock, L.; Gale, A.; Blackall, P.J. Mechanically Ventilated Broiler Sheds: A Possible Source of Aerosolized Salmonella, Campylobacter, and Escherichia coli. Appl. Environ. Microbiol. 2009, 75, 7417–7425. [Google Scholar] [CrossRef]
- Bossers, A.; De Rooij, M.M.; Van Schothorst, I.; Velkers, F.C.; Smit, L.A. Detection of Airborne Wild Waterbird-Derived DNA Demonstrates Potential for Transmission of Avian Influenza Virus via Air Inlets into Poultry Houses, the Netherlands, 2021 to 2022. Eurosurveillance 2024, 29, 2400350. [Google Scholar] [CrossRef]
- Koutsoumanis, K.; Allende, A.; Álvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Herman, L.; EFSA Panel on Biological Hazards (BIOHAZ); et al. Transmission of Antimicrobial Resistance (AMR) during Animal Transport. EFSA J. 2022, 20, e07586. [Google Scholar] [CrossRef]
- Tang, J.W. The Effect of Environmental Parameters on the Survival of Airborne Infectious Agents. J. R. Soc. Interface 2009, 6, S737–S746. [Google Scholar] [CrossRef]
- James, J.; Warren, C.; De Silva, D.; Lewis, T.; Grace, K.; Reid, S.; Falchieri, M.; Brown, I.; Banyard, A. The Role of Airborne Particles in the Epidemiology of Clade 2.3.4.4b H5N1 High Pathogenicity Avian Influenza Virus in Commercial Poultry Production Units. Viruses 2023, 15, 1002. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, B.G.; Kim, Y.J.; Shim, J.E.; Yeo, M.-K. Assessment of Airborne Bacteria in the Indoor of Public-Use Facilities Concentrated on Influencing Factors and Opportunistic Pathogenic Bacteria. Air Qual. Atmos. Health 2024, 17, 1725–1738. [Google Scholar] [CrossRef]
- Tellier, R. Review of Aerosol Transmission of Influenza A Virus. Emerg. Infect. Dis. 2006, 12, 1657–1662. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, X.D.; Zhao, Y.; Lin, J.; Purswell, J.L.; Tabler, T.; Voy, B.; Hawkins, S.; Evans, J.D. Modeling Long-Distance Airborne Transmission of Highly Pathogenic Avian Influenza Carried by Dust Particles. Sci. Rep. 2023, 13, 16255. [Google Scholar] [CrossRef] [PubMed]
- Calnek, B.W.; Hitchner, S.B. Survival and Disinfection of Marek’s Disease Virus and the Effectiveness of Filters in Preventing Airborne Dissemination. Poult. Sci. 1973, 52, 35–43. [Google Scholar] [CrossRef]
- Nguyen, X.D.; Zhao, Y.; Evans, J.D.; Lin, J.; Purswell, J.L. Survival of Escherichia coli in Airborne and Settled Poultry Litter Particles. Animals 2022, 12, 284. [Google Scholar] [CrossRef]
- Yao, M.; Gao, Y.; Chai, T.; Cai, Y.; Duan, H. Antibiotic Resistance of Airborne Escherichia coli from Hen House and Rabbitry and Their Spreading to Surroundings. Northwest A F University (Nat. Sci. Ed.) 2007, 8, 60–64. [Google Scholar]
- Kozajda, A.; Jeżak, K.; Kapsa, A. Airborne Staphylococcus Aureus in Different Environments—A Review. Environ. Sci. Pollut. Res. 2019, 26, 34741–34753. [Google Scholar] [CrossRef]
- Tao, C.-W.; Chen, J.-S.; Hsu, B.-M.; Koner, S.; Hung, T.-C.; Wu, H.-M.; Rathod, J. Molecular Evaluation of Traditional Chicken Farm-Associated Bioaerosols for Methicillin-Resistant Staphylococcus Aureus Shedding. Antibiotics 2021, 10, 917. [Google Scholar] [CrossRef]
- Salles, G.B.C.; Pilati, G.V.T.; Muniz, E.C.; De Lima Neto, A.J.; Vogt, J.R.; Dahmer, M.; Savi, B.P.; Padilha, D.A.; Fongaro, G. Trends and Challenges in the Surveillance and Control of Avian Metapneumovirus. Viruses 2023, 15, 1960. [Google Scholar] [CrossRef]
- Abolnik, C.; Gouws, J. Extended Survival Times of Mycoplasma gallisepticum and Mycoplasma Synoviae on Kanekalon Synthetic Hair Fibres. Poult. Sci. 2014, 93, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Ather, B.; Mirza, T.M.; Edemekong, P.F. Airborne Precautions. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Calnek, B.W.; Witter, R.L. Marek’s Disease—A Model for Herpesvirus Oncology. CRC Crit. Rev. Microbiol. 1985, 12, 293–320. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.; Smith, R.L.; Pongolini, S.; Bolzoni, L. Modelling Farm-to-Farm Disease Transmission through Personnel Movements: From Visits to Contacts, and Back. Sci. Rep. 2017, 7, 2375. [Google Scholar] [CrossRef]
- Kraay, A.N.M.; Hayashi, M.A.L.; Hernandez-Ceron, N.; Spicknall, I.H.; Eisenberg, M.C.; Meza, R.; Eisenberg, J.N.S. Fomite-Mediated Transmission as a Sufficient Pathway: A Comparative Analysis across Three Viral Pathogens. BMC Infect. Dis. 2018, 18, 540. [Google Scholar] [CrossRef]
- Wagari, A. A Review on Infectious Bursal Disease in Poultry. Health Econ. Outcome Res. Open Access 2021, 7, 167. [Google Scholar]
- Couteaudier, M.; Courvoisier, K.; Trapp-Fragnet, L.; Denesvre, C.; Vautherot, J.-F. Keratinocytes Derived from Chicken Embryonic Stem Cells Support Marek’s Disease Virus Infection: A Highly Differentiated Cell Model to Study Viral Replication and Morphogenesis. Virol. J. 2016, 13, 7. [Google Scholar] [CrossRef]
- Christensen, N.H.; Yavari, C.A.; McBain, A.J.; Bradbury, J.M. Investigations into the Survival of Mycoplasma Gallisepticum, Mycoplasma Synoviae and Mycoplasma Iowae on Materials Found in the Poultry House Environment. Avian Pathol. 1994, 23, 127–143. [Google Scholar] [CrossRef]
- Oni, R.A.; Sharma, M.; Buchanan, R.L. Survival of Salmonella enterica in Dried Turkey Manure and Persistence on Spinach Leaves. J. Food Prot. 2015, 78, 1791–1799. [Google Scholar] [CrossRef]
- Newell, D.G.; Fearnley, C. Sources of Campylobacter Colonization in Broiler Chickens. Appl. Environ. Microbiol. 2003, 69, 4343–4351. [Google Scholar] [CrossRef]
- Smith, S.; Meade, J.; Gibbons, J.; McGill, K.; Bolton, D.; Whyte, P. The Impact of Environmental Conditions on Campylobacter jejuni Survival in Broiler Faeces and Litter. Infect. Ecol. Epidemiol. 2016, 6, 31685. [Google Scholar] [CrossRef] [PubMed]
- Fancher, C.A.; Zhang, L.; Kiess, A.S.; Adhikari, P.A.; Dinh, T.T.N.; Sukumaran, A.T. Avian Pathogenic Escherichia coli and Clostridium Perfringens: Challenges in No Antibiotics Ever Broiler Production and Potential Solutions. Microorganisms 2020, 8, 1533. [Google Scholar] [CrossRef]
- Alzubeidi, Y.S.; Udompijitkul, P.; Talukdar, P.K.; Sarker, M.R. Inactivation of Clostridium perfringens Spores Adhered onto Stainless Steel Surface by Agents Used in a Clean-in-Place Procedure. Int. J. Food Microbiol. 2018, 277, 26–33. [Google Scholar] [CrossRef] [PubMed]
- McDougald, L.R. Blackhead Disease (Histomoniasis) in Poultry: A Critical Review. Avian Dis. 2005, 49, 462–476. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, A.; Gauly, M.; Abel, H.; Daş, G.; Humburg, J.; Weiss, A.T.A.; Breves, G.; Rautenschlein, S. Pathobiology of Heterakis Gallinarum Mono-Infection and Co-Infection with Histomonas meleagridis in Layer Chickens. Avian Pathol. 2011, 40, 277–287. [Google Scholar] [CrossRef]
- Fudge, C. Understanding Histomoniasis Progression and Transmission in Turkeys. Master’s Thesis, Faculty of North Carolina State University, Raleigh, NC, USA, 2022. [Google Scholar]
- Mesa-Pineda, C.; Navarro-Ruíz, J.L.; López-Osorio, S.; Chaparro-Gutiérrez, J.J.; Gómez-Osorio, L.M. Chicken Coccidiosis: From the Parasite Lifecycle to Control of the Disease. Front. Vet. Sci. 2021, 8, 787653. [Google Scholar] [CrossRef]
- Zhao, D.; Suo, J.; Liang, L.; Liang, R.; Zhou, R.; Ding, J.; Liu, X.; Suo, X.; Zhang, S.; Tang, X. Innovative Prevention and Control of Coccidiosis: Targeting Sporogony for New Control Agent Development. Poult. Sci. 2024, 103, 104246. [Google Scholar] [CrossRef]
- Chen, B.L.; Bradley, M.A. Temperature and Humidity Effects on Off-Host Survival of the Northern Fowl Mite (Acari: Macronyssidae) and the Chicken Body Louse (Phthiraptera: Menoponidae). J. Econ. Entomol. 2014, 101, 637–646. [Google Scholar] [CrossRef]
- Velayudhan, B.T.; Lopes, V.C.; Noll, S.L.; Halvorson, D.A.; Nagaraja, K.V. Avian Pneumovirus and Its Survival in Poultry Litter. Avian Dis. 2003, 47, 764–768. [Google Scholar] [CrossRef]
- Savage, C.E.; Jones, R.C. The Survival of Avian Reoviruses on Materials Associated with the Poultry House Environment. Avian Pathol. 2003, 32, 417–423. [Google Scholar] [CrossRef]
- Teshager, N. Pathological and Seroprevalence Studies on Infectious Bursal Disease in Chickens In and Around Bahir Dar, North West, Ethiopia. Mater’s Thesis, Addis Ababa University, Addis Ababa, Ethiopia, 2015. [Google Scholar]
- Brown, J.D.; Swayne, D.E.; Cooper, R.J.; Burns, R.E.; Stallknecht, D.E. Persistence of H5 and H7 Avian Influenza Viruses in Water. Avian Diseases 2007, 51, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Tariq, S.; Rabbani, M.; Javeed, A.; Ghafoor, A.; Anees, M.; Najiullah, M.; Hameed, M.; Younus, M.; Nazir, J. Role of Water Chemistry and Stabilizers on the Vero-Cells-Based Infectivity of Newcastle Disease Virus Live Vaccine. J. Appl. Poult. Res. 2018, 27, 103–111. [Google Scholar] [CrossRef]
- Dey, S.; Pathak, D.C.; Ramamurthy, N.; Maity, H.K.; Chellappa, M.M. Infectious Bursal Disease Virus in Chickens: Prevalence, Impact, and Management Strategies. Vet. Med. Res. Rep. 2019, 10, 85–97. [Google Scholar] [CrossRef]
- Yang, R.; Lin, X.; Song, H.; Zhou, H.; Li, S.; Li, X.; Hao, B.; Li, L. Mycoplasma galliscepticum: An Overview. Afr. J. Microbiol. Res. 2024, 18, 54–71. [Google Scholar] [CrossRef]
- Venkitanarayanan, K.; Thakur, S.; Ricke, S.C. Food Safety in Poultry Meat Production; Food Microbiology and Food Safety; Practical Approaches; Springer: Cham, Switzerland, 2019; ISBN 978-3-030-05011-5. [Google Scholar]
- Raut, R.; Kilonzo-Nthenge, A.; Aniume, T.; Basnet, A.; Watkins, S.; Maharjan, P. Impacts of On-Farm Water Sanitation Practices on Microbial Hygiene in Poultry Waterlines and Efficacy of Sodium Hypochlorite-Based Product on Foodborne Pathogens. J. Appl. Poult. Res. 2024, 33, 100425. [Google Scholar] [CrossRef]
- Avian Pathogenic Escherichia coli (APEC): An Overview of Virulence and Pathogenesis Factors, Zoonotic Potential, and Control Strategies—PMC. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC8069529/ (accessed on 11 January 2025).
- Liu, H.; Whitehouse, C.A.; Li, B. Presence and Persistence of Salmonella in Water: The Impact on Microbial Quality of Water and Food Safety. Front. Public Health 2018, 6, 159. [Google Scholar] [CrossRef] [PubMed]
- Thomson, C.M.; Chanter, N.; Wathes, C.M. Survival of Toxigenic Pasteurella Multocida in Aerosols and Aqueous Liquids. Appl. Environ. Microbiol. 1992, 58, 932–936. [Google Scholar] [CrossRef]
- Bronowski, C.; James, C.E.; Winstanley, C. Role of Environmental Survival in Transmission of Campylobacter jejuni. FEMS Microbiol. Lett. 2014, 356, 8–19. [Google Scholar] [CrossRef]
- Ugochukwu, I.C.I.; Samuel, F.; Orakpoghenor, O.; Nwobi, O.C.; Anyaoha, C.O.; Majesty-Alukagberie, L.O.; Ugochukwu, M.O.; Ugochukwu, E.I. Erysipelas, the Opportunistic Zoonotic Disease: History, Epidemiology, Pathology, and Diagnosis—A Review. Comp. Clin. Pathol. 2019, 28, 853–859. [Google Scholar] [CrossRef]
- Trakulchang, S.P.; Kraft, A.A. Survival of Clostridium perfringens in Refrigerated and Frozen Meat and Poultry Items. J. Food Sci. 1977, 42, 518–521. [Google Scholar] [CrossRef]
- Dalziel, A.E.; Delean, S.; Heinrich, S.; Cassey, P. Persistence of Low Pathogenic Influenza A Virus in Water: A Systematic Review and Quantitative Meta-Analysis. PLoS ONE 2016, 11, e0161929. [Google Scholar]
- Yang, Y.; Ricke, S.C.; Tellez, G.; Kwon, Y.M. Quantitative Tracking of Salmonella Enteritidis Transmission Routes Using Barcode-Tagged Isogenic Strains in Chickens: Proof-of-Concept Study. Front. Vet. Sci. 2017, 4, 15. [Google Scholar] [CrossRef] [PubMed]
- Nasr, M.A.F.; Alkhedaide, A.Q.; Ramadan, A.A.I.; Hafez, A.-E.S.E.; Hussein, M.A. Potential Impact of Stocking Density on Growth, Carcass Traits, Indicators of Biochemical and Oxidative Stress and Meat Quality of Different Broiler Breeds. Poult. Sci. 2021, 100, 101442. [Google Scholar] [CrossRef] [PubMed]
- Malik, Y.S.; Arun Prince Milton, A.; Ghatak, S.; Ghosh, S. Role of Birds in Transmitting Zoonotic Pathogens. In Livestock Diseases and Management; Springer: Singapore, 2021; ISBN 978-981-16-4553-2. [Google Scholar]
- Meerburg, B.G.; Kijlstra, A. Role of Rodents in Transmission of Salmonella and Campylobacter. J. Sci. Food Agric. 2007, 87, 2774–2781. [Google Scholar] [CrossRef]
- Leffer, A.M.; Kuttel, J.; Martins, L.M.; Pedroso, A.C.; Astolfi-Ferreira, C.S.; Ferreira, F.; Ferreira, A.J.P. Vectorial Competence of Larvae and Adults of Alphitobius diaperinus in the Transmission of Salmonella Enteritidis in Poultry. Vector Borne Zoonotic Dis. Larchmt. N 2010, 10, 481–487. [Google Scholar] [CrossRef]
- Nolan, L.K.; Barnes, H.J.; Vaillancourt, J.-P.; Abdul-Aziz, T.; Logue, C.M. Colibacillosis. In Diseases of Poultry; Swayne, D.E., Ed.; Wiley: Hoboken, NJ, USA, 2013; pp. 751–805. ISBN 978-0-470-95899-5. [Google Scholar]
- Ou, S.-C. Improved Detection and Control of Infectious Laryngotracheitis Virus on Poultry Farms. Ph.D. Thesis, Auburn University, Auburn, AL, USA, 2010. [Google Scholar]
- Boozer, E. Insecticide Susceptibility of the Adult Darkling Beetle, Alphitobius Diaperinus (Coleoptera: Tenebrionidae): Topical Treatment with Bifenthrin, Imidacloprid, and Spinosad. Master’s Thesis, Auburn University, Athens, GA, USA, 2008. [Google Scholar]
- Mcallister, J.C.; Steelman, C.D.; Skeeles, J.K.; Newberry, L.A.; Gbur, E.E. Reservoir Competence of Alphitobius Diaperinus (Coleoptera: Tenebrionidae) for Escherichia Coli (Eubacteriales: Enterobacteriaceae). J. Med. Entomol. 1996, 33, 983–987. [Google Scholar] [CrossRef]
- McAllister, J.C.; Steelman, C.D.; Newberry, L.A.; Skeeles, J.K. Isolation of Infectious Bursal Disease Virus from the Lesser Mealworm, Alphitobius diaperinus (Panzer). Poult. Sci. 1995, 74, 45–49. [Google Scholar] [CrossRef]
- George, D.R.; Finn, R.D.; Graham, K.M.; Mul, M.F.; Maurer, V.; Moro, C.V.; Sparagano, O.A. Should the Poultry Red Mite Dermanyssus gallinae Be of Wider Concern for Veterinary and Medical Science? Parasit. Vectors 2015, 8, 178. [Google Scholar] [CrossRef]
- Sommer, D.; Heffels-Redmann, U.; Köhler, K.; Lierz, M.; Kaleta, E.F. Role of the poultry red mite (Dermanyssus gallinae) in the transmission of avian influenza A virus. Tierarztl. Prax. Ausg. G Grosstiere Nutztiere 2016, 44, 26–33. [Google Scholar] [CrossRef]
- Schiavone, A.; Pugliese, N.; Otranto, D.; Samarelli, R.; Circella, E.; De Virgilio, C.; Camarda, A. Dermanyssus gallinae: The Long Journey of the Poultry Red Mite to Become a Vector. Parasit. Vectors 2022, 15, 29. [Google Scholar] [CrossRef]
- Schiavone, A.; Pugliese, N.; Siddique, I.; Samarelli, R.; Saleh, M.S.; Lombardi, R.; Circella, E.; Camarda, A. Vertical Transmission of Salmonella enterica Ser. Gallinarum in Dermanyssus gallinae by the Mean of the Baudruche-Based Artificial Feeding Device. Appl. Sci. 2023, 13, 1929. [Google Scholar] [CrossRef]
- Wang, C.; Xu, X.; Yu, H.; Huang, Y.; Li, H.; Wan, Q.; Pan, B. Low-Temperature Storage of the Poultry Red Mite, Dermanyssus gallinae, Facilitates Laboratory Colony Maintenance and Population Growth. Parasitology 2020, 147, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Bansal, N.; Singh, R.; Chaudhary, D.; Mahajan, N.K.; Joshi, V.G.; Maan, S.; Ravishankar, C.; Sahoo, N.; Mor, S.K.; Radzio-Basu, J.; et al. Prevalence of Newcastle Disease Virus in Wild and Migratory Birds in Haryana, India. Avian Dis. 2022, 66, 141–147. [Google Scholar] [CrossRef] [PubMed]
- van der Meer, C.S.; Paulino, P.G.; Jardim, T.H.A.; Senne, N.A.; Araujo, T.R.; Dos Santos Juliano, D.; Massard, C.L.; Peixoto, M.P.; da Costa Angelo, I.; Santos, H.A. Detection and Molecular Characterization of Avipoxvirus in Culex spp. (Culicidae) Captured in Domestic Areas in Rio de Janeiro, Brazil. Sci. Rep. 2022, 12, 13496. [Google Scholar] [CrossRef]
- WOAH. Fowlpox; WOAH: Paris, France, 2023; Chapter 3.3.10. [Google Scholar]
- World Health Organization. Handbook for Integrated Vector Management; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Domanska-Blicharz, K.; Opolska, J.; Lisowska, A.; Szczotka-Bochniarz, A. Bacterial and Viral Rodent-Borne Infections on Poultry Farms. An Attempt at a Systematic Review. J. Vet. Res. 2023, 67, 1–10. [Google Scholar] [CrossRef]
- Aubree, R.J. The Lesser Mealworm, Alphitobius diaperinus (Panzer), and Its Role in Salmonella Transmission to Poultry. Master’s Thesis, University of Georgia, Athens, GA, USA, 2007. [Google Scholar]
- Shterzer, N.; Rothschild, N.; Sbehat, Y.; Dayan, J.; Eytan, D.; Uni, Z.; Mills, E. Vertical Transmission of Gut Bacteria in Commercial Chickens Is Limited. Anim. Microbiome 2023, 5, 50. [Google Scholar] [CrossRef]
- De Reu, K.; Heyndrick, M.; Grijspeerdt, K.; Rodenburg, B.; Tuyttens, F.; Uyttendaele, M.; Herman, L. Estimation of the Vertical and Horizontal Bacterial Infection of Hen’s Table Eggs. Worlds Poult. Sci. J. 2007, 64, 142–146. [Google Scholar]
- El-Saadony, M.T.; Saad, A.M.; Yang, T.; Salem, H.M.; Korma, S.A.; Ahmed, A.E.; Mosa, W.F.A.; Abd El-Mageed, T.A.; Selim, S.; Al Jaouni, S.K.; et al. Avian Campylobacteriosis, Prevalence, Sources, Hazards, Antibiotic Resistance, Poultry Meat Contamination, and Control Measures: A Comprehensive Review. Poult. Sci. 2023, 102, 102786. [Google Scholar] [CrossRef]
- Mugunthan, S.P.; Kannan, G.; Chandra, H.M.; Paital, B. Infection, Transmission, Pathogenesis and Vaccine Development against Mycoplasma gallisepticum. Vaccines 2023, 11, 469. [Google Scholar] [CrossRef]
- Lublin, A.; Maler, I.; Mechani, S.; Pinto, R.; Sela-Saldinger, S. Survival of Salmonella enterica Serovar Infantis on and within Stored Table Eggs. J. Food Prot. 2015, 78, 287–292. [Google Scholar] [CrossRef]
- Sahin, O.; Kobalka, P.; Zhang, Q. Detection and Survival of Campylobacter in Chicken Eggs. J. Appl. Microbiol. 2003, 95, 1070–1079. [Google Scholar] [CrossRef]
- Callicott, K.A.; Friðriksdóttir, V.; Reiersen, J.; Lowman, R.; Bisaillon, J.-R.; Gunnarsson, E.; Berndtson, E.; Hiett, K.L.; Needleman, D.S.; Stern, N.J. Lack of Evidence for Vertical Transmission of Campylobacter Spp. in Chickens. Appl. Environ. Microbiol. 2006, 72, 5794–5798. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, A.; Horii, W.; Sano, J.; Kodama, T.; Kato, A.; Shibuya, K.; Saitoh, T. Invasion of Chicken Anemia Virus in Specific-Pathogen-Free Chicken Flocks and Its Successful Elimination from the Colony. Vet. Sci. 2024, 11, 329. [Google Scholar] [CrossRef]
- Wu, B.; Xu, Q.; Li, Z.; Wang, Q.; He, D.; Jiang, X.; Cui, Y.; Feng, Q.; Tang, Y.; Diao, Y. Evidence of Vertical Transmission of Fowl Adenovirus 8b in Ducks. Vet. Microbiol. 2023, 286, 109888. [Google Scholar] [CrossRef] [PubMed]
- Kursa, O.; Tomczyk, G.; Sieczkowska, A.; Kostka, S.; Sawicka-Durkalec, A. Mycoplasma gallisepticum and Mycoplasma synoviae in Turkeys in Poland. Pathog. Basel Switz. 2024, 13, 78. [Google Scholar] [CrossRef] [PubMed]
- Farnoushi, Y.; Heller, D.; Lublin, A. Genetic Characterization of Newly Emerging Avian Reovirus Variants in Chickens with Viral Arthritis/Tenosynovitis in Israel. Virology 2024, 589, 109908. [Google Scholar] [CrossRef]
- Grgić, H.; Philippe, C.; Ojkić, D.; Nagy, E. Study of Vertical Transmission of Fowl Adenoviruses. Can. J. Vet. Res. Rev. Can. Rech. Vet. 2006, 70, 230–233. [Google Scholar]
- Sims, L. Biosecurity Guide for Live Poultry Markets; Food and Agriculture Organization of the United Nations: Rome, Italy, 2015; ISBN 978-92-5-108910-1. [Google Scholar]
- Dorea, F.C.; Berghaus, R.; Hofacre, C.; Cole, D.J. Survey of Biosecurity Protocols and Practices Adopted by Growers on Commercial Poultry Farms in Georgia, U.S.A. Avian Dis. 2010, 54, 1007–1015. [Google Scholar] [CrossRef]
- FAO; WHO; UNICEF; UNDP. Trainee Guide: Improving Bio-Security Practices to Control Highly Pathogenic Avian Influenza; FAO: Rome, Italy, 2011. [Google Scholar]
- A Practical Guide for Managing Risk in Poultry Production, 2nd ed.; Owen, R.L., American Association of Avian Pathologists, Eds.; AAAP, Inc.: Jacksonville, FL, USA, 2017; ISBN 978-0-9789163-8-1. [Google Scholar]
- Gelaude, P.; Schlepers, M.; Verlinden, M.; Laanen, M.; Dewulf, J. Biocheck.UGent: A Quantitative Tool to Measure Biosecurity at Broiler Farms and the Relationship with Technical Performances and Antimicrobial Use. Poult. Sci. 2014, 93, 2740–2751. [Google Scholar] [CrossRef]
- Islam, A.; Islam, M.; Dutta, P.; Rahman, M.A.; Al Mamun, A.; Khan, A.D.; Samad, M.A.; Hassan, M.M.; Rahman, M.Z.; Shirin, T. Association of Biosecurity and Hygiene Practices with Avian Influenza A/H5 and A/H9 Virus Infections in Turkey Farms. Front. Vet. Sci. 2024, 11, 1319618. [Google Scholar] [CrossRef]
- Patyk, K.A.; Fields, V.L.; Beam, A.L.; Branan, M.A.; McGuigan, R.E.; Green, A.; Torchetti, M.K.; Lantz, K.; Freifeld, A.; Marshall, K.; et al. Investigation of Risk Factors for Introduction of Highly Pathogenic Avian Influenza H5N1 Infection among Commercial Turkey Operations in the United States, 2022: A Case-Control Study. Front. Vet. Sci. 2023, 10, 1229071. [Google Scholar] [CrossRef]
- McQuiston, J.H.; Garber, L.P.; Porter-Spalding, B.A.; Hahn, J.W.; Pierson, F.W.; Wainwright, S.H.; Senne, D.A.; Brignole, T.J.; Akey, B.L.; Holt, T.J. Evaluation of Risk Factors for the Spread of Low Pathogenicity H7N2 Avian Influenza Virus among Commercial Poultry Farms. J. Am. Vet. Med. Assoc. 2005, 226, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Yegani, M.; Korver, D.R. Factors Affecting Intestinal Health in Poultry. Poult. Sci. 2008, 87, 2052–2063. [Google Scholar] [CrossRef] [PubMed]
- Newell, D.G.; Elvers, K.T.; Dopfer, D.; Hansson, I.; Jones, P.; James, S.; Gittins, J.; Stern, N.J.; Davies, R.; Connerton, I.; et al. Biosecurity-Based Interventions and Strategies to Reduce Campylobacter Spp. on Poultry Farms. Appl. Environ. Microbiol. 2011, 77, 8605–8614. [Google Scholar] [CrossRef] [PubMed]
- Ssematimba, A.; Hagenaars, T.J.; de Jong, M.C.M. Modelling the Wind-Borne Spread of Highly Pathogenic Avian Influenza Virus between Farms. PLoS ONE 2012, 7, e31114. [Google Scholar] [CrossRef]
- Tyski, S.; Bocian, E.; Laudy, A.E. Animal Health Protection—Assessing Antimicrobial Activity of Veterinary Disinfectants and Antiseptics and Their Compliance with European Standards: A Narrative Review. Pol. J. Microbiol. 2024, 73, 413–431. [Google Scholar] [CrossRef]
- Guan, J.; Chan, M.; Brooks, B.W.; Rohonczy, L. Inactivation of Infectious Bursal Disease and Newcastle Disease Viruses at Temperatures Below 0 C Using Chemical Disinfectants. Avian Dis. 2014, 58, 249–254. [Google Scholar] [CrossRef]
- Khalil, M.M.; Alfateeh, N.M.; Kaoud, H.A. Monitoring the Effect of Disinfection Methods on Mycoplasma gallisepticum in Commercial Layer Farms. Int. J. Poult. Sci. 2022, 21, 28–37. [Google Scholar] [CrossRef]
- Ohashi, I.; Kobayashi, S.; Tamamura-Andoh, Y.; Arai, N.; Takamatsu, D. Disinfectant Resistance of Salmonella in in Vitro Contaminated Poultry House Models and Investigation of Efficient Disinfection Methods Using These Models. J. Vet. Med. Sci. 2022, 84, 1633–1644. [Google Scholar] [CrossRef]
- Laban, S.; Khalil, M.R.; Amir, M.; Nagwa, R.; Mona, S. Phenotypic, Genotypic, Multidrug Resistance Genes and Disinfectant Biocidal Effect of Pasteurella Multocida Isolated from Chickens. Assiut Vet. Med. J. 2019, 65, 10–18. [Google Scholar] [CrossRef]
- Beier, R.C.; Byrd, J.A.; Andrews, K.; Caldwell, D.; Crippen, T.L.; Anderson, R.C.; Nisbet, D.J. Disinfectant and Antimicrobial Susceptibility Studies of the Foodborne Pathogen Campylobacter jejuni Isolated from the Litter of Broiler Chicken Houses. Poult. Sci. 2021, 100, 1024–1033. [Google Scholar] [CrossRef]
- Fidalgo, S.G.; Longbottom, C.J.; Rjley, T.V. Susceptibility of Erysipelothrix Rhusiopathiae to Antimicrobial Agents and Home Disinfectants. Pathology 2002, 34, 462–465. [Google Scholar] [CrossRef] [PubMed]
- McCrea, B.A.; Macklin, K.S. Effect of Different Cleaning Regimens on Recovery of Clostridium perfringens on Poultry Live Haul Containers. Poult. Sci. 2006, 85, 909–913. [Google Scholar] [CrossRef]
- Bengtong, P.; Thomrongsuwannakij, T.; Chansiripornchai, N. Inactivation of Infectious Bronchitis Virus with Various Kinds of Disinfectants. Thai J. Vet. Med. 2013, 43, 405–409. [Google Scholar] [CrossRef]
- Chambers, A.E.; Dixon, M.M.; Harvey, S.P. Studies of the Suitability of Fowlpox as a Decontamination and Thermal Stability Simulant for Variola Major. Int. J. Microbiol. 2009, 2009, 158749. [Google Scholar] [CrossRef] [PubMed]
- Mor, S.K.; Bekele, A.Z.; Sharafeldin, T.A.; Porter, R.E.; Goyal, S.M. Efficacy of Five Commonly Used Disinfectants Against Turkey Arthritis Reovirus. Avian Dis. 2015, 59, 71–73. [Google Scholar] [CrossRef]
- Eterpi, M.; McDonnell, G.; Thomas, V. Decontamination Efficacy against Mycoplasma. Lett. Appl. Microbiol. 2011, 52, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Lochner, H.; Swenson, R.; Martinson, K. 120 Disseminating Equine Science with Infographics on Social Media. J. Equine Vet. Sci. 2021, 100, 103583. [Google Scholar] [CrossRef]
- Glenn, B.P.; Randel, L.; Zimbelman, R.G.; Culp, A. Strategies for Communicating Animal Science to Policymakers in the United States. Anim. Front. 2015, 5, 13–22. [Google Scholar]
- Liaw, S.-T. Digital Public Health: Quality, Interoperability and Capability Maturity. In Oxford Research Encyclopedia of Global Public Health; Oxford University Press, 2024; ISBN 978-0-19-063236-6. [Google Scholar]
- Cortegiani, A.; Battaglini, D.; Amato, G.; Behr, A.U.; Donadello, K.; Einav, S.; Frigo, M.G.; Fullin, G.; Giannini, A.; Ippolito, M.; et al. Dissemination of Clinical and Scientific Practice through Social Media: A SIAARTI Consensus-Based Document. J. Anesth. Analg. Crit. Care 2024, 4, 21. [Google Scholar] [CrossRef]
- Moran, R.E.; Knesl, O. How Can the Veterinary Profession Tackle Social Media Misinformation? J. Am. Vet. Med. Assoc. 2025, 1, 1–7. [Google Scholar] [CrossRef]
- Delpont, M.; Salazar, L.G.; Dewulf, J.; Zbikowski, A.; Szeleszczuk, P.; Dufay-Lefort, A.-C.; Rousset, N.; Spaans, A.; Amalraj, A.; Tilli, G.; et al. Monitoring Biosecurity in Poultry Production: An Overview of Databases Reporting Biosecurity Compliance from Seven European Countries. Front. Vet. Sci. 2023, 10, 1231377. [Google Scholar] [CrossRef]
- Shekaili, T.A.; Clough, H.; Ganapathy, K.; Baylis, M. Sero-Surveillance and Risk Factors for Avian Influenza and Newcastle Disease Virus in Backyard Poultry in Oman. Prev. Vet. Med. 2015, 122, 145–153. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, T.; Zhang, N.; Li, J.; Wang, Y.; Kulyar, M.F.-A.; Han, Z.; Li, Y. Effect of Stocking Density and Age on Physiological Performance and Dynamic Gut Bacterial and Fungal Communities in Langya Hens. Microb. Cell Factories 2021, 20, 218. [Google Scholar] [CrossRef] [PubMed]
- Abo-Al-Ela, H.G.; El-Kassas, S.; El-Naggar, K.; Abdo, S.E.; Jahejo, A.R.; Al Wakeel, R.A. Stress and Immunity in Poultry: Light Management and Nanotechnology as Effective Immune Enhancers to Fight Stress. Cell Stress Chaperones 2021, 26, 457–472. [Google Scholar] [CrossRef] [PubMed]
- Gurler, T.; Elmer, T.; Cui, Y.; Omer, S.; Riffat, S. Performance Evaluation of a Novel PVT-GSHP Heating System on Energy-Efficient Poultry Houses: Long-Term Monitoring. Int. J. Low-Carbon Technol. 2021, 16, 393–406. [Google Scholar] [CrossRef]
- Gomes, A.V.S.; Quinteiro-Filho, W.M.; Ribeiro, A.; Ferraz-de-Paula, V.; Pinheiro, M.L.; Baskeville, E.; Akamine, A.T.; Astolfi-Ferreira, C.S.; Ferreira, A.J.P.; Palermo-Neto, J. Overcrowding Stress Decreases Macrophage Activity and Increases Salmonella Enteritidis Invasion in Broiler Chickens. Avian Pathol. 2014, 43, 82–90. [Google Scholar] [CrossRef]
- Guo, W.; Lv, C.; Guo, M.; Zhao, Q.; Yin, X.; Zhang, L. Innovative Applications of Artificial Intelligence in Zoonotic Disease Management. Sci. One Health 2023, 2, 100045. [Google Scholar] [CrossRef]

| Pathogen | Effective Disinfectants |
|---|---|
| Avian encephalomyelitis virus (AEV) | 10% isopropyl alcohol and 2% formalin [54] |
| Avian influenza virus (AI) | Virkon (oxidizing agent), Accel (hydrogen peroxide-based), Cresol (3%) and Phenol (5%) [188] |
| Avian metapneumovirus (aMPV) | quaternary ammonia, ethanol, iodophor, phenol derivatives, and sodium hypochlorite [51] |
| Avian reoviruses (ARV) | Quaternary ammonium compounds combined with aldehydes [197] |
| Fowlpox virus (FWPV) | 70% ethanol, 50% isopropyl alcohol, 0.5% sodium hypochlorite, 30% formaldehyde, 10% benzalkonium chloride, a combination of 6.67% cetyltrimethylammonium chloride and 3.33% benzalkonium chloride, or a mixture of 1.75% iodine and 10% polyethylene glycol nonylphenyl ether [196] |
| Infectious bronchitis virus (IBV) | Potassium Peroxymonosulfate (e.g., Virkon S), Sodium Dodecylbenzenesulfonate [195] |
| Infectious bursal disease virus (IBDV) | Virkon, Surface Decontamination Foam (SDF), Bleach (varies in effectiveness) [195] |
| Infectious laryngotracheitis virus (ILT) | Cresol (3%), Phenol (5%), Sodium Hydroxide (1%) [27] |
| Marek’s disease virus (MDV) | Chlorine, Quaternary Ammonium Compounds, Sodium Hydroxide [102] |
| Newcastle disease virus (NDV) | Virkon, Accel, Sodium Hydroxide (1%) [188] |
| Campylobacter jejuni | Benzalkonium Chloride, P-128, Ammonium Chloride-based disinfectants [192] |
| Clostridium perfringens | pressure washing, pressure washing combined with sodium hypochlorite (5%) or quaternary ammonium sprays [194] |
| Escherichia coli | Benzalkonium Chloride, P-128 (ammonium chloride-based) |
| Erysipelothrix rhusiopathiae | Hypochlorite, Sodium Hydroxide, Remove organic matter before application [193] |
| Mycoplasma gallispeticum | Quaternary ammonium compounds, phenolics, and chlorine-based products [198] |
| Mycoplasma (synoviae) | Ethanol and alkaline detergent formulations [198] |
| Pasteurella multocida | combination of glutaraldehyde and quaternary ammonium compounds (QAC) achieved a significant reduction [191] |
| Salmonella spp. | Chlorine Dioxide, Dolomitic Lime, Sodium Hypochlorite [190] |
| Staphylococcus aureus | Quaternary Ammonium Compounds, Sodium Hypochlorite |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovács, L.; Domaföldi, G.; Bertram, P.-C.; Farkas, M.; Könyves, L.P. Biosecurity Implications, Transmission Routes and Modes of Economically Important Diseases in Domestic Fowl and Turkey. Vet. Sci. 2025, 12, 391. https://doi.org/10.3390/vetsci12040391
Kovács L, Domaföldi G, Bertram P-C, Farkas M, Könyves LP. Biosecurity Implications, Transmission Routes and Modes of Economically Important Diseases in Domestic Fowl and Turkey. Veterinary Sciences. 2025; 12(4):391. https://doi.org/10.3390/vetsci12040391
Chicago/Turabian StyleKovács, László, Gerda Domaföldi, Pia-Charlotte Bertram, Máté Farkas, and László Péter Könyves. 2025. "Biosecurity Implications, Transmission Routes and Modes of Economically Important Diseases in Domestic Fowl and Turkey" Veterinary Sciences 12, no. 4: 391. https://doi.org/10.3390/vetsci12040391
APA StyleKovács, L., Domaföldi, G., Bertram, P.-C., Farkas, M., & Könyves, L. P. (2025). Biosecurity Implications, Transmission Routes and Modes of Economically Important Diseases in Domestic Fowl and Turkey. Veterinary Sciences, 12(4), 391. https://doi.org/10.3390/vetsci12040391







