Comparative Mutational Analysis and the Glycosylation Patterns of a Peruvian Isolated Avian Influenza A Virus H5N1: Exploring Possible Viral Spillover Events Within One Health Approach
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Molecular Detection
2.3. Whole-Genome Sequencing
2.4. Data Sets
2.5. Mutational Analysis and Genotype Identification
2.6. Prediction of Potential N-Glycosylation Sites in HA and NA Influenza A H5N1 Viruses
3. Results
3.1. Mutational Manual Analysis
3.2. FluMut Mutational Analysis
3.3. Genotype Identification
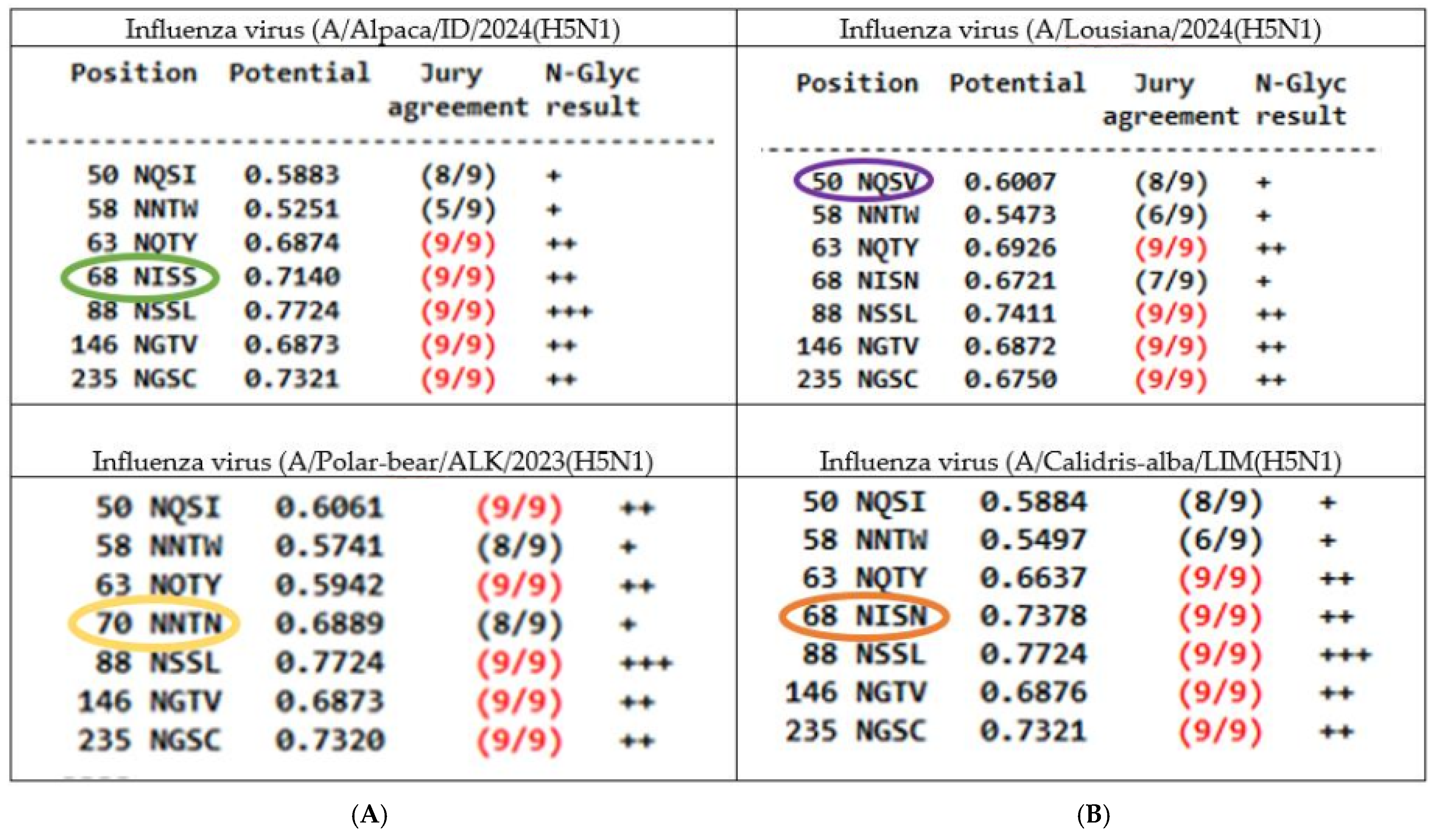
3.4. Glycosylation Patterns
3.4.1. N-Linked Glycosylations (NLG) in the HA of Influenza H5N1 Viruses
3.4.2. N-Linked Glycosylations in the NA of Influenza H5N1 Viruses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duriez, O.; Sassi, Y.; Le Gall-Ladevèze, C.; Giraud, L.; Straughan, R.; Dauverné, L.; Terras, A.; Boulinier, T.; Choquet, R.; Van De Wiele, A.; et al. Highly pathogenic avian influenza affects vultures’ movements and breeding output. Curr. Biol. 2023, 33, 3766–3774.e3. [Google Scholar] [CrossRef] [PubMed]
- Caliendo, V.; Martin, B.B.; Fouchier, R.A.M.; Verdaat, H.; Engelsma, M.; Beerens, N.; Slaterus, R. Highly Pathogenic Avian Influenza Contributes to the Population Decline of the Peregrine Falcon (Falco peregrinus) in The Netherlands. Viruses 2024, 17, 24. [Google Scholar] [CrossRef] [PubMed]
- Giacinti, J.A.; Signore, A.V.; Jones, M.E.; Bourque, L.; Lair, S.; Jardine, C.; Stevens, B.; Bollinger, T.; Goldsmith, D.; British Columbia Wildlife AIV Surveillance Program (BC WASP); et al. Avian influenza viruses in wild birds in Canada following incursions of highly pathogenic H5N1 virus from Eurasia in 2021–2022. mBio 2024, 15, e0320323. [Google Scholar] [CrossRef] [PubMed]
- Banyard, A.C.; Bennison, A.; Byrne, A.M.P.; Reid, S.M.; Lynton-Jenkins, J.G.; Mollett, B.; De Silva, D.; Peers-Dent, J.; Finlayson, K.; Hall, R.; et al. Detection and spread of high pathogenicity avian influenza virus H5N1 in the Antarctic Region. Nat. Commun. 2024, 15, 7433. [Google Scholar] [CrossRef]
- Uhart, M.M.; Vanstreels, R.E.T.; Nelson, M.I.; Olivera, V.; Campagna, J.; Zavattieri, V.; Lemey, P.; Campagna, C.; Falabella, V.; Rimondi, A. Epidemiological data of an influenza A/H5N1 outbreak in elephant seals in Argentina indicates mammal-to-mammal transmission. Nat. Commun. 2024, 15, 9516. [Google Scholar] [CrossRef]
- Tomás, G.; Marandino, A.; Panzera, Y.; Rodríguez, S.; Wallau, G.L.; Dezordi, F.Z.; Pérez, R.; Bassetti, L.; Negro, R.; Williman, J.; et al. Highly pathogenic avian influenza H5N1 virus infections in pinnipeds and seabirds in Uruguay: Implications for bird–mammal transmission in South America. Virus Evol. 2024, 10, veae031. [Google Scholar] [CrossRef]
- Ulloa, M.; Fernández, A.; Ariyama, N.; Colom-Rivero, A.; Rivera, C.; Nuñez, P.; Sanhueza, P.; Johow, M.; Araya, H.; Torres, J.C.; et al. Mass mortality event in South American sea lions (Otaria flavescens) correlated to highly pathogenic avian influenza (HPAI) H5N1 outbreak in Chile. Veter Q. 2023, 43, 1–10. [Google Scholar] [CrossRef]
- Oguzie, J.U.; Marushchak, L.V.; Shittu, I.; Lednicky, J.A.; Miller, A.L.; Hao, H.; Nelson, M.I.; Gray, G.C. Avian Influenza A(H5N1) Virus among Dairy Cattle, Texas, USA. Emerg. Infect. Dis. 2024, 30, 1425–1429. [Google Scholar] [CrossRef]
- Nguyen, T.Q.; Hutter, C.; Markin, A.; Thomas, M.; Lantz, K.; Lea Killian, M.; Janzen, G.M.; Vijendran, S.; Wagle, S.; Inderski, B.; et al. Emergence and interstate spread of highly pathogenic avian influenza A(H5N1) in dairy cattle. biorxiv 2024. [Google Scholar] [CrossRef]
- Bordes, L.; Vreman, S.; Heutink, R.; Roose, M.; Venema, S.; Pritz-Verschuren, S.B.E.; Rijks, J.M.; Gonzales, J.L.; Germeraad, E.A.; Engelsma, M.; et al. Highly Pathogenic Avian Influenza H5N1 Virus Infections in Wild Red Foxes (Vulpes vulpes) Show Neurotropism and Adaptive Virus Mutations. Microbiol. Spectr. 2023, 11, e0286722. [Google Scholar] [CrossRef]
- Barry, K.T.; Tate, M.D. Flu on the Brain: Identification of Highly Pathogenic Influenza in the Brains of Wild Carnivores in The Netherlands. Pathogens 2023, 12, 1111. [Google Scholar] [CrossRef] [PubMed]
- Chothe, S.K.; Srinivas, S.; Misra, S.; Nallipogu, N.C.; Gilbride, E.; LaBella, L.; Mukherjee, S.; Gauthier, C.H.; Pecoraro, H.L.; Webb, B.T.; et al. Marked neurotropism and potential adaptation of H5N1 clade 2.3.4.4.b virus in naturally infected domestic cats. Emerg. Microbes Infect. 2024, 14, 2440498. [Google Scholar] [CrossRef] [PubMed]
- Ly, H. Highly pathogenic avian influenza H5N1 virus infection of companion animals. Virulence 2023, 15, 2289780. [Google Scholar] [CrossRef] [PubMed]
- Pulit-Penaloza, J.A.; Brock, N.; Belser, J.A.; Sun, X.; Pappas, C.; Kieran, T.J.; Thakur, P.B.; Zeng, H.; Cui, D.; Frederick, J.; et al. Highly pathogenic avian influenza A(H5N1) virus of clade 2.3.4.4b isolated from a human case in Chile causes fatal disease and transmits between co-housed ferrets. Emerg. Microbes Infect. 2024, 13, 2332667. [Google Scholar] [CrossRef]
- Duarte, P.M.; El-Nakeep, S.; Shayestegan, F.; Tazerji, S.S.; Malik, Y.S.; Roncada, P.; Tilocca, B.; Gharieb, R.; Hogan, U.; Ahmadi, H.; et al. Addressing the recent transmission of H5N1 to new animal species and humans, warning of the risks and its relevance in One-Health. Ger. J. Microbiol. 2024, 4, 39–53. [Google Scholar] [CrossRef]
- EFSA Panel on Animal Health and Animal Welfare (AHAW); ECDC; Alvarez, J.; Boklund, A.; Dippel, S.; Dórea, F.; Figuerola, J.; Herskin, M.S.; Michel, V.; Miranda Chueca, M.Á.; et al. Preparedness, prevention and control related to zoonotic avian influenza. EFSA J. 2025, 23, e9191. [Google Scholar] [CrossRef]
- Dholakia, V.; Quantrill, J.L.; Richardson, S.; Pankaew, N.; Brown, M.D.; Yang, J.; Capelastegui, F.; Masonou, T.; Case, K.M.; Aejian, J.; et al. Polymerase mutations underlie early adaptation of H5N1 influenza virus to dairy cattle and other mammals. bioRxiv 2025. [Google Scholar] [CrossRef]
- Lin, T.-H.; Zhu, X.; Wang, S.; Zhang, D.; McBride, R.; Yu, W.; Babarinde, S.; Paulson, J.C.; Wilson, I.A. A single mutation in bovine influenza H5N1 hemagglutinin switches specificity to human receptors. Science 1979, 386, 1128–1134. [Google Scholar] [CrossRef]
- Xiao, Y.; Sheng, Z.-M.; Williams, S.L.; Taubenberger, J.K. Two complete 1918 influenza A/H1N1 pandemic virus genomes characterized by next-generation sequencing using RNA isolated from formalin-fixed, paraffin-embedded autopsy lung tissue samples along with evidence of secondary bacterial co-infection. mBio 2024, 15, e0321823. [Google Scholar] [CrossRef]
- Sealy, J.E.; Peacock, T.P.; Sadeyen, J.-R.; Chang, P.; Everest, H.J.; Bhat, S.; Iqbal, M. Adsorptive mutation and N-linked glycosylation modulate influenza virus antigenicity and fitness. Emerg. Microbes Infect. 2020, 9, 2622–2631. [Google Scholar] [CrossRef]
- Sun, Y.; Zhu, Y.; Zhang, P.; Sheng, S.; Guan, Z.; Cong, Y. Hemagglutinin glycosylation pattern-specific effects: Implications for the fitness of H9.4.2.5-branched H9N2 avian influenza viruses. Emerg. Microbes Infect. 2024, 13, 2364736. [Google Scholar] [CrossRef] [PubMed]
- Bao, D.; Xue, R.; Zhang, M.; Lu, C.; Ma, T.; Ren, C.; Zhang, T.; Yang, J.; Teng, Q.; Li, X.; et al. N-Linked Glycosylation Plays an Important Role in Budding of Neuraminidase Protein and Virulence of Influenza Viruses. J. Virol. 2021, 95, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.; Jang, Y.H.; Bin Kwon, S.; Lee, C.M.; Han, G.; Seong, B.L. Glycosylation of hemagglutinin and neuraminidase of influenza a virus as signature for ecological spillover and adaptation among influenza reservoirs. Viruses 2018, 10, 183. [Google Scholar] [CrossRef] [PubMed]
- Fouchier, R.A.M.; Bestebroer, T.M.; Herfst, S.; Van Der Kemp, L.; Rimmelzwaan, G.F.; Osterhaus, A.D.M.E. Detection of influenza A viruses from different species by pcr amplification of conserved sequences in the matrix gene. J. Clin. Microbiol. 2000, 38, 4096–4101. [Google Scholar] [CrossRef]
- WHO. WHO Information for the Molecular Detection of Influenza Viruses. 2017, pp. 1–60. Available online: https://cdn.who.int/media/docs/default-source/influenza/molecular-detention-of-influenza-viruses/protocols_influenza_virus_detection_feb_2021.pdf (accessed on 15 April 2025).
- Landazabal-Castillo, S.; Suarez-Agüero, D.; Alva-Alvarez, L.; Mamani-Zapana, E.; Mayta-Huatuco, E. Highly pathogenic avian influenza A virus subtype H5N1 (clade 2.3.4.4b) isolated from a natural protected area in Peru. Microbiol. Resour. Announc. 2024, 13, e0041724. [Google Scholar] [CrossRef]
- Madeira, F.; Madhusoodanan, N.; Lee, J.; Eusebi, A.; Niewielska, A.; Tivey, A.R.N.; Lopez, R.; Butcher, S. The EMBL-EBI Job Dispatcher sequence analysis tools framework in 2024. Nucleic Acids Res. 2024, 52, W521–W525. [Google Scholar] [CrossRef]
- GNU Affero. FluMut. 2024. Available online: https://github.com/izsvenezie-virology/FluMut (accessed on 20 January 2025).
- Philippine Genome Center. FluSurver in GISAID. 2024. Available online: https://gisaid.org/database-features/flusurver-mutations-app/ (accessed on 21 February 2024).
- Youk, S.; Torchetti, M.K.; Lantz, K.; Lenoch, J.B.; Killian, M.L.; Leyson, C.; Bevins, S.N.; Dilione, K.; Ip, H.S.; Stallknecht, D.E.; et al. H5N1 highly pathogenic avian influenza clade 2.3.4.4b in wild and domestic birds: Introductions into the United States and reassortments, December 2021–April 2022. Virology 2023, 587, 109860. [Google Scholar] [CrossRef]
- Gupta, R.; Brunak, S. Prediction of glycosylation across the human proteome and the correlation to protein function. Pac. Symp. Biocomput. 2002, 7, 310–322. [Google Scholar]
- Boeijen, M. Glycosylation of the Influenza A Virus Hemagglutinin Protein. 2013. Available online: https://studenttheses.uu.nl/bitstream/handle/20.500.12932/14431/glycosylation%20of%20the%20influenza%20A%20virus%20hemagglutinin%20protein1.pdf?sequence=1 (accessed on 15 April 2025).
- Lambertucci, S.A.; Santangeli, A.; Plaza, P.I. The threat of avian influenza H5N1 looms over global biodiversity. Nat. Rev. Biodivers. 2025, 1, 7–9. [Google Scholar] [CrossRef]
- Ahamad, M.I.; Yao, Z.; Ren, L.; Zhang, C.; Li, T.; Lu, H.; Mehmood, M.S.; Rehman, A.; Adil, M.; Lu, S.; et al. Impact of heavy metals on aquatic life and human health: A case study of River Ravi Pakistan. Front. Mar. Sci. 2024, 11, 1374835. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Zhang, K.; Shi, J.; Gao, Y.; Zheng, J.; He, J.; Zhang, J.; Song, Y.; Zhang, R.; et al. Association between heavy metals exposure and persistent infections: The mediating role of immune function. Front. Public. Health 2024, 12, 1367644. [Google Scholar] [CrossRef] [PubMed]
- Glaser, L.; Stevens, J.; Zamarin, D.; Wilson, I.A.; García-Sastre, A.; Tumpey, T.M.; Basler, C.F.; Taubenberger, J.K.; Palese, P. A Single Amino Acid Substitution in 1918 Influenza Virus Hemagglutinin Changes Receptor Binding Specificity. J. Virol. 2005, 79, 11533–11536. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Ibrahim, M.S.; Ellakany, H.F.; Kawashita, N.; Mizuike, R.; Hiramatsu, H.; Sriwilaijaroen, N.; Takagi, T.; Suzuki, Y.; Ikuta, K. Acquisition of Human-Type Receptor Binding Specificity by New H5N1 Influenza Virus Sublineages during Their Emergence in Birds in Egypt. PLOS Pathog. 2011, 7, e1002068. [Google Scholar] [CrossRef] [PubMed]
- Lutz, M.M.; Dunagan, M.M.; Kurebayashi, Y.; Takimoto, T. Key role of the influenza a virus pa gene segment in the emergence of pandemic viruses. Viruses 2020, 12, 365. [Google Scholar] [CrossRef]
- Arai, Y.; Kawashita, N.; Hotta, K.; Hoang, P.V.M.; Nguyen, H.L.K.; Nguyen, T.C.; Vuong, C.D.; Le, T.T.; Le, M.T.Q.; Soda, K.; et al. Multiple polymerase gene mutations for human adaptation occurring in Asian H5N1 influenza virus clinical isolates. Sci. Rep. 2018, 8, 13066. [Google Scholar] [CrossRef]
- Song, J.; Xu, J.; Shi, J.; Li, Y.; Chen, H. Synergistic Effect of S224P and N383D Substitutions in the PA of H5N1 Avian Influenza Virus Contributes to Mammalian Adaptation. Sci. Rep. 2015, 5, 10510. [Google Scholar] [CrossRef]
- Conenello, G.M.; Zamarin, D.; Perrone, L.A.; Tumpey, T.; Palese, P. A single mutation in the PB1-F2 of H5N1 (HK/97) and 1918 influenza a viruses contributes to increased virulence. PLOS Pathog. 2007, 3, e141. [Google Scholar] [CrossRef]
- Varga, Z.T.; Palese, P. The influenza A virus protein PB1-F2. Virulence 2011, 2, 542–546. [Google Scholar] [CrossRef]
- Chakrabarti, A.K.; Pasricha, G. An insight into the PB1F2 protein and its multifunctional role in enhancing the pathogenicity of the influenza A viruses. Virology 2013, 440, 97–104. [Google Scholar] [CrossRef]
- Roubidoux, E.K.; Sano, K.; McMahon, M.; Carreño, J.M.; Capuano, C.; Jiang, K.; Simon, V.; van Bakel, H.; Wilson, P.; Krammer, F. Novel Epitopes of the Influenza Virus N1 Neuraminidase Targeted by Human Monoclonal Antibodies. J. Virol. 2022, 96, e0033222. [Google Scholar] [CrossRef]
- Anoma, S.; Bhattarakosol, P.; Kowitdamrong, E. Characteristics and evolution of hemagglutinin and neuraminidase genes of Influenza A(H3N2) viruses in Thailand during 2015 to 2018. PeerJ 2024, 12, e17523. [Google Scholar] [CrossRef] [PubMed]
- Schulze, I.T. Effects of Glycosylation on the Properties and Functions of Influenza Virus Hemagglutinin. J. Infect. Dis. 1997, 176, S24–S28. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhou, Y.; Yan, H.; An, Q.; Liang, C.; Liu, L.; Qian, J. Molecular Markers and Mechanisms of Influenza A Virus Cross-Species Transmission and New Host Adaptation. Viruses 2024, 16, 883. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-C.; Chen, J.-R.; Tseng, Y.-C.; Hsu, C.-H.; Hung, Y.-F.; Chen, S.-W.; Chen, C.-M.; Khoo, K.-H.; Cheng, T.-J.; Cheng, Y.-S.E.; et al. Glycans on influenza hemagglutinin affect receptor binding and immune response. Proc. Natl. Acad. Sci. USA 2009, 106, 18137–18142. [Google Scholar] [CrossRef]
- Wang, N.; Lu, W.; Yan, L.; Liu, M.; Che, F.; Wang, Y.; Yang, C.; Lv, M.; Cheng, J.; Sun, Q.; et al. Epidemiological and genetic characterization of the influenza A (H1N1) virus in Hangzhou City in 2023. Front. Public. Health 2024, 12, 1464435. [Google Scholar] [CrossRef]
- Chen, W.; Ma, T.; Liu, S.; Zhong, Y.; Yu, H.; Shu, J.; Wang, X.; Li, Z. N-Glycan Profiles of Neuraminidase from Avian Influenza Viruses. Viruses 2024, 16, 190. [Google Scholar] [CrossRef]
- She, Y.-M.; Farnsworth, A.; Li, X.; Cyr, T.D. Topological N-glycosylation and site-specific N-glycan sulfation of influenza proteins in the highly expressed H1N1 candidate vaccines. Sci. Rep. 2017, 7, 10232. [Google Scholar] [CrossRef]
- Ilyushina, N.A.; Govorkova, E.A.; Webster, R.G. Detection of amantadine-resistant variants among avian influenza viruses isolated in North America and Asia. Virology 2005, 341, 102–106. [Google Scholar] [CrossRef]
- Kwon, J.J.; Choi, W.-S.; Jeong, J.H.; Kim, E.-H.; Lee, O.-J.; Yoon, S.-W.; Hwang, J.; Webby, R.J.; Govorkova, E.A.; Choi, Y.K.; et al. An I436N substitution confers resistance of influenza A(H1N1)pdm09 viruses to multiple neuraminidase inhibitors without affecting viral fitness. J. Gen. Virol. 2018, 99, 292–302. [Google Scholar] [CrossRef]
- Nuss, J.M.; Whitaker, P.B.; Air, G.M. Identification of critical contact residues in the NC41 epitope of a subtype N9 influenza virus neuraminidase. Proteins Struct. Funct. Bioinform. 1993, 15, 121–132. [Google Scholar] [CrossRef]
- Xu, J.; Luo, Q.; Huang, Y.; Li, J.; Ye, W.; Yan, R.; Zhou, X.; He, Z.; Liu, G.; Zhu, Q. Influenza neuraminidase mutations and resistance to neuraminidase inhibitors. Emerg. Microbes Infect. 2024, 13, 2429627. [Google Scholar] [CrossRef] [PubMed]
- Rabie-Rudsari, M.; Behboudi, E.; Ranjkesh, A.; Kaveh, K.; Razavi-Nikoo, H.; Haghshenas, M.R.; Moradi, A. Molecular identification of neuraminidase gene mutations in influenza A/H1N1 and A/H3N2 isolates of Mazandaran province, north of Iran. J. Glob. Antimicrob. Resist. 2023, 36, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Signore, A.V.; Joseph, T.; Ranadheera, C.; Erdelyan, C.N.G.; Alkie, T.N.; Raj, S.; Pama, L.; Ayilara, I.; Hisanaga, T.; Lung, O.; et al. Neuraminidase reassortment and oseltamivir resistance in clade 2.3.4.4b A(H5N1) viruses circulating among Canadian poultry, 2024. Emerg. Microbes Infect. 2025, 14, 2469643. [Google Scholar] [CrossRef] [PubMed]
- Kadi, H. A Comprehensive Analysis of H5N1 Evolution: Phylogenetic Insights and Emerging Mutations in Turkey’s Avian Influenza Landscape. Preprint 2024. [Google Scholar] [CrossRef]
- Yang, C.-R.; King, C.-C.; Liu, L.-Y.D.; Ku, C.-C. FluConvert and IniFlu: A suite of integrated software to identify novel signatures of emerging influenza viruses with increasing risk. BMC Bioinform. 2020, 21, 316. [Google Scholar] [CrossRef]

| Mutation | Mutation to Highlighting |
|---|---|
| HA:9V, HA:87T, HA:99S/D, HA:225M, HA:248L, HA:259C, HA:277Y, HA:285E, HA:316E, HA:324T, HA:473K, HA:493K, HA:531L | HA:10T, HA:170D, HA:104G, HA:147M, HA:152S, HA:226T, HA:304N, HA:310V, HA:336N/R, HA:520R/N |
| NA:20A/I, NA:216V, NA:217R, NA:223T, NA:284N, NA:308R/K, NA:340Y/F, NA:364N, NA:442I | NA:23V, NA:44N, NA:45H, NA:48T, NA:53V, NA:62I NA:67I, NA:74L, NA:75I, NA:81D; NA:82P, NA:84A, NA:90P, NA:155H, NA:221S, NA:234I, NA:237F, NA:241I, NA:254R, NA:257R, NA:286S, NA:288V, NA:329S, NA:374V, NA:399L, NA:432R, NA:436V |
| M1:55M, M1:125T, M1:191H, M1:218A, M1:236K | |
| M2:12R, M2:21G, M2:28T, M2:52S | M2:27A |
| PA:42V, PA:59K/G, PA:118U, PA:184S, PA:190F, PA:207V, PA:213K, PA:272N, PA:269K, PA:323I, PA:330V, PA:382G, PA:423T, PA:425F, PA:523L, PA:561V, PA:581I, PA:621V, PA:664R, PA:688G | PA:13V, PA:36T, PA:45S, PA:68S, PA:75Q, PA:86I, PA:100I, PA:142E, PA:201I/T, PA:211I, PA:322L/V, PA:336M, PA:348L, PA:351G, PA:354F, PA:388G, PA:399V, PA:404S, PA:459V, PA:465M/T, PA:486M/L, PA:489S, PA:538G, PA:545V, PA:614D/S, PA:626R, PA:655F |
| PA-X:42V, PA-X:52D, PA-X:62T, PA-X:118V, PA-X:122I, PA-X:184N, PA-X:190F, PA-X:207L, | PA-X:20T, PA-X:36D/T, PA-X:68S, PA-X:70V, PA-X:75Q, PA-X:86I, PA-X:142E, PA-X:160E, PA-X:195K, PA-X:211Y, PA-X:250P, |
| PB2:191G, PB2:199T, PB2:292V, PB2:339R, PB2:444G, PB2:451V, PB2:452V, PB2:453S, PB2:472D, PB2:560M, PB2:575V, PB2:639S, PB2:660R, PB2:679S, PB2:683A, PB2:684S, PB2:697M, PB2:711S | PB2:9N, PB2:79G, PB2:152V, PB2:190R, PB2:251K, PB2:255A, PB2:274V, PB2:346A, PB2:353R, PB2:532L, PB2:539V, PB2:596A, PB2:663R, PB2:666I, PB2:667I, PB2:670R, PB2:677K, PB2:680G, PB2:715S |
| PB1:11R, PB1:14V, PB1:51E, PB1:53E, PB1:121N, PB1:147V, PB1:176T, PB1:321I, PB1:339V, PB1:348V, PB1:371D, PB1:383G, PB1:455D, PB1:394S, PB1:431H, PB1:512L, PB1:576M, PB1:584H, PB1:657H, PB1:719I, PB1:739D | PB1:40I, PB1:171A, PB1:211K, PB1:291A, PB1:372I, PB1:384P/T/A, PB1:390G, PB1:533S, PB1:621K, PB1:660I, PB1:738G |
| NS1:66D, NS1:81V, NS1:88H, NS1:129T, NSI:210R, NSI:202T, NS1:217T, NSI:213L, NSI:219E | NSI:36I, NS1:67G/Q, NS1:75G, NS1:76A, NS1:77R, NS1:136M, NS1:193Q, NSI:201Y |
| NEP:27G, NEP:52V, NEP:56Y, NEP:61K, NEP:64T, NSI:76M, NEP:77K, NEP:85Q NEP:81G, NEP:82E | NEP:36V, NEP:60N, NEP:63E, NEP:89T/V |
| PB1-F2:11R/L, PB1-F2:29R, PB1-F2:35L, PB1-F2:41L, PB1-F2:69L, PB1-F2:78R, PB1-F2:79Q, PB1-F2:90I | PB1-F2:39T, PB1-F2:57Y, PB1-F2:73E |
| NP:41V, NP:190A, NP:221K, NP:234S, NP:253V, NP:323S, NP:363I | NP:48R, NP:63T, NP:119T/V, NP:230L, NP:318L, NP:411A, NP:425V |
| Mutation | Mutation to Highlighting |
|---|---|
| HA:242I, HA:504Y | HA:11I, HA:52A, HA:211I, HA:492D, HA:527I |
| NA:71S, NA:321I | NA:6R, NA:10T, NA:70N, NA:405T |
| M1:82S, M1:227T | |
| M2:88N/D | |
| NP:52H, NP:105M, NP:293K, NP:482N | NP:377N |
| PB2:154F, PB2:362G, PB2:441N, PB2:495I, PB2:631L, PB2:649I, PB2:676A | PB2:58A, PB2:109I, PB2:139I, |
| PB1:179I, PB1:587P, PB1:646I | PB1:16D, PB1:154S, PB1:172D, PB1:207R, PB1:215K, PB1:264D, PB1:375N, PB1:378M, PB1:399D, PB1:429R, PB1:430K/E, PB1:515A, PB1:548F, PB1:614D, PB1:694S |
| PB1-F2:30L, PB1-F2:31E, PB1-F2:44R, PB1-F2:46T, PB1-F2:47S, PB1-F2:48R, PB1-F2:50G, PB1-F2:54K, PB1-F2:55I, PB1-F2:66S, PB1-F2:68I, PB1-F2:70G | PB1-F2:4G, PB1-F2:7I/T/M, PB1-F2:8Q, PB1-F2:12S PB1-F2:17S, PB1-F2:18T, PB1-F2:20R, PB1-F2:21R, PB1-F2:22E, PB1:36T, PB1-F2:40G, PB1-F2:42Y, PB1-F2:49A, PB1-F2:56A, PB1-F2:57C/F, PB1-F2:58W, PB1-F2:65R, PB1-F2:75L, PB1-F2:82S, PB1-F2:84S, PB1-F2:90N |
| NS1:7S/L, NS1:21Q, NS1:88C, NS1:189N | NSI:21R, NS1:26K, NSI:53G, NS1:83P, NS1:87S, NS1:116C/S/N, NS1:147I, NS1:226T |
| NEP:7V/S, NEP:31V, NEP:67G/E | |
| PA:113R, PA:237K/A, PA:272E, PA:277P, PA:479E, PA:558L | PA:57R, PA:219I, PA:432I, PA:497R, |
| PA-X:113R, PA-X:215L, | PA-X:57Q, PA-X:61I, PA-X:85T, PA-X:193S, PA-X:245N |
| Protein | Mutation |
|---|---|
| HA | 110S, 139P, 142E, 143T, 154Q, 157P, 171D, 208K 234Q, 239R |
| NA | 46P, 76A, 78Q, 99I, 100Y, 258I, 289M, 366S, 382E, 418M, 434N |
| M1 | 140A, 144L, 165I, |
| M2 | 18N |
| NP | 136L |
| PB2 | 699K, 741S |
| PB1 | 177E, 478S, 490F, 535I, 536N, 558T, 598P, 609Y, 610C |
| PB1-F2 | No PB1-F2 sequence in the reference genome |
| NS1 | 6I, 18V, 22F, 23S, 24D, 25Q, 27L, 28C, 54I, 60A, 73S, 84V, 94T, 95L, 112A, 114G, 117I, 127R, 137L, 140Q, 146L, 153E, 158G, 161S, 163L, 170T, 180V, 191T, 194V, 197T, 198L, 205S, 206S, 211R, 221K, 224R, 225T |
| NEP (NS2) | 6V, 14M, 22G, 26E, 37S, 40L, 48A, 49V, 68Q, 83V, 86R, 88K, 100M, 111Q |
| PA | 63V, 129I, 212C, 228N, 361K, 536K, 544E, 585L, 586L, 716R |
| PA-X | no PA-X sequence in the reference genome |
| PB2 | 355R, 699K |
| Protein | Mutation |
|---|---|
| PB1 | 59S, 75D |
| PB2 | 334S, 340R, 463V, 464M, 471T, 478I, 590G, 616V |
| NP | 450N |
| M2 | 28I, 51V, 61G |
| M1 | 85S, 87T, 101R, 200V, 230R, 232D |
| HA | 120M, 131L, 199N, 201E, 205N, 226A/T, 336S/N, 527V/I, 549M |
| NA | 8T, 20V/A, 44Y/N, 81T/D, 155Y, 188I, 269M, 287D, 340S, 336S, 338M, 339P, 340S, 395E, 460G |
| NEP | 63A, 64K, 81E, 85H, 89I |
| PA-x | 252R/K |
| PB1-F2 | 11Q, 12L/S |
| NS1 | 44R, 55E, 56T, 59R, 63Q, 70E/G, 71E, 74D, 90L, 111V/A, 118R, 139D, 145I, 166L, 171D, 192V, 204R, 207N, 209D, 213P, 226T |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Landazabal-Castillo, S.; Alva-Alvarez, L.; Suarez-Agϋero, D.; Mamani-Zapana, E.; Mayta-Huatuco, E. Comparative Mutational Analysis and the Glycosylation Patterns of a Peruvian Isolated Avian Influenza A Virus H5N1: Exploring Possible Viral Spillover Events Within One Health Approach. Vet. Sci. 2025, 12, 392. https://doi.org/10.3390/vetsci12040392
Landazabal-Castillo S, Alva-Alvarez L, Suarez-Agϋero D, Mamani-Zapana E, Mayta-Huatuco E. Comparative Mutational Analysis and the Glycosylation Patterns of a Peruvian Isolated Avian Influenza A Virus H5N1: Exploring Possible Viral Spillover Events Within One Health Approach. Veterinary Sciences. 2025; 12(4):392. https://doi.org/10.3390/vetsci12040392
Chicago/Turabian StyleLandazabal-Castillo, Sandra, Lucero Alva-Alvarez, Dilan Suarez-Agϋero, Enrique Mamani-Zapana, and Egma Mayta-Huatuco. 2025. "Comparative Mutational Analysis and the Glycosylation Patterns of a Peruvian Isolated Avian Influenza A Virus H5N1: Exploring Possible Viral Spillover Events Within One Health Approach" Veterinary Sciences 12, no. 4: 392. https://doi.org/10.3390/vetsci12040392
APA StyleLandazabal-Castillo, S., Alva-Alvarez, L., Suarez-Agϋero, D., Mamani-Zapana, E., & Mayta-Huatuco, E. (2025). Comparative Mutational Analysis and the Glycosylation Patterns of a Peruvian Isolated Avian Influenza A Virus H5N1: Exploring Possible Viral Spillover Events Within One Health Approach. Veterinary Sciences, 12(4), 392. https://doi.org/10.3390/vetsci12040392







