Clinical and Pathological Diagnosis of Hereditary Gastrointestinal Polyposis in Jack Russell Terriers
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Clinical Case Series Study
2.1.1. Patient Enrollment and Case Information
2.1.2. Clinical Examination
2.1.3. Treatment
2.2. Pathological Study
2.2.1. Case and Sample Information
2.2.2. Histopathological Analysis
2.2.3. Statistical Analysis
2.3. Genotyping Assays
3. Results
3.1. Clinical Case Series Study
3.1.1. Clinical Signs
3.1.2. Hematologic Abnormality
3.1.3. Location and Number of GI Polyps
3.1.4. Prognosis and Recurrence
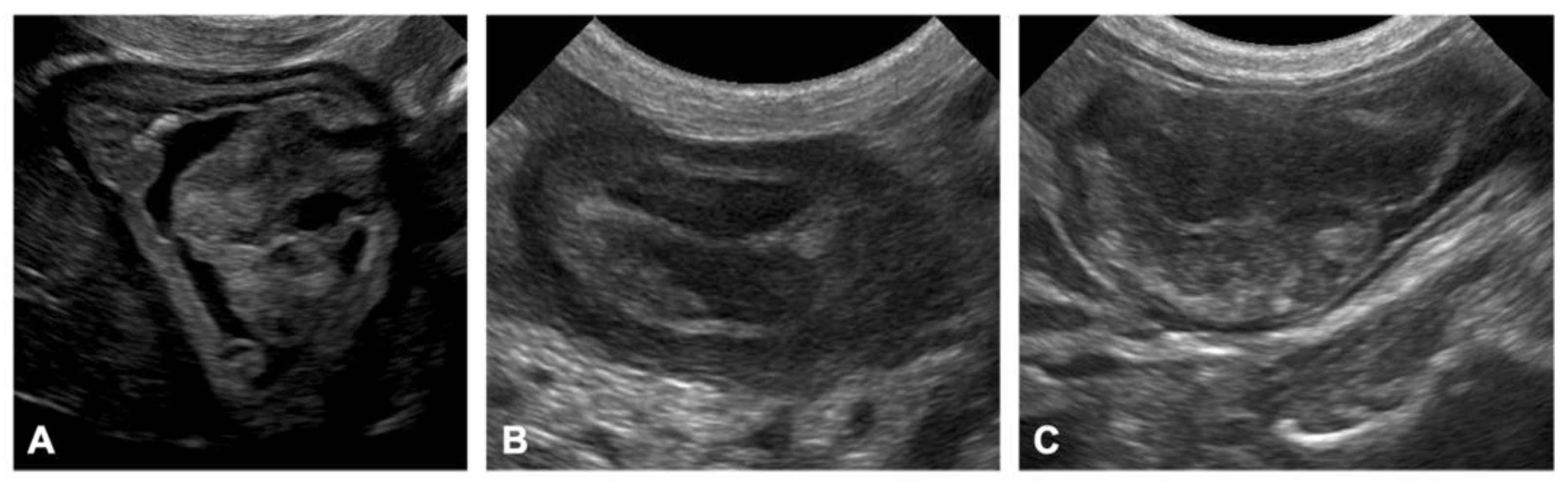
3.1.5. Diagnostic Imaging
Radiography
Ultrasonography
CT Scan
Endoscopy
Diagnostic Imaging of Invasive and Metastatic Tumors
3.1.6. Treatment
3.2. Pathological Studies
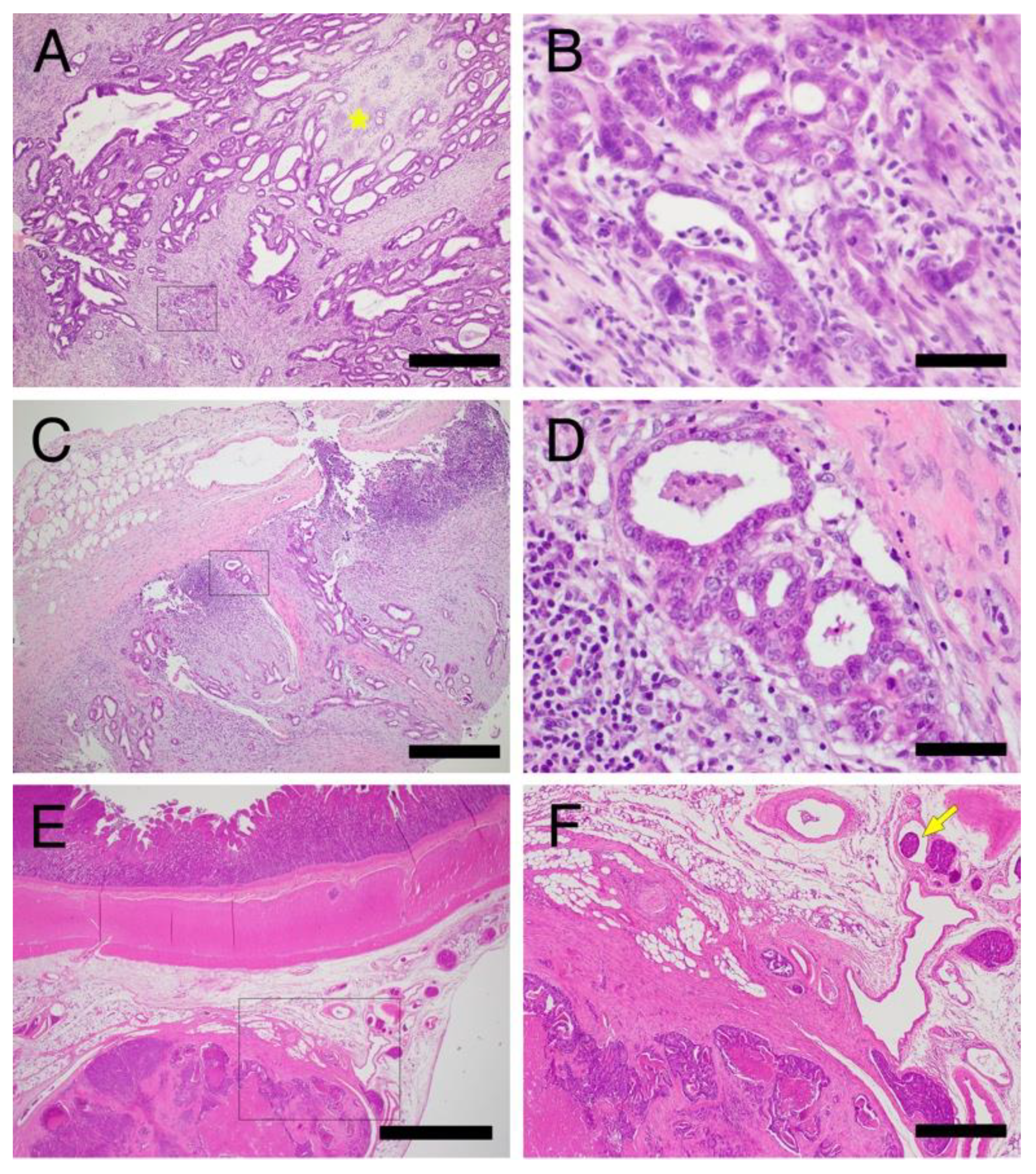
3.2.1. Histopathological Features of Hereditary GI Tumors in JRTs with the Germline APC Variant
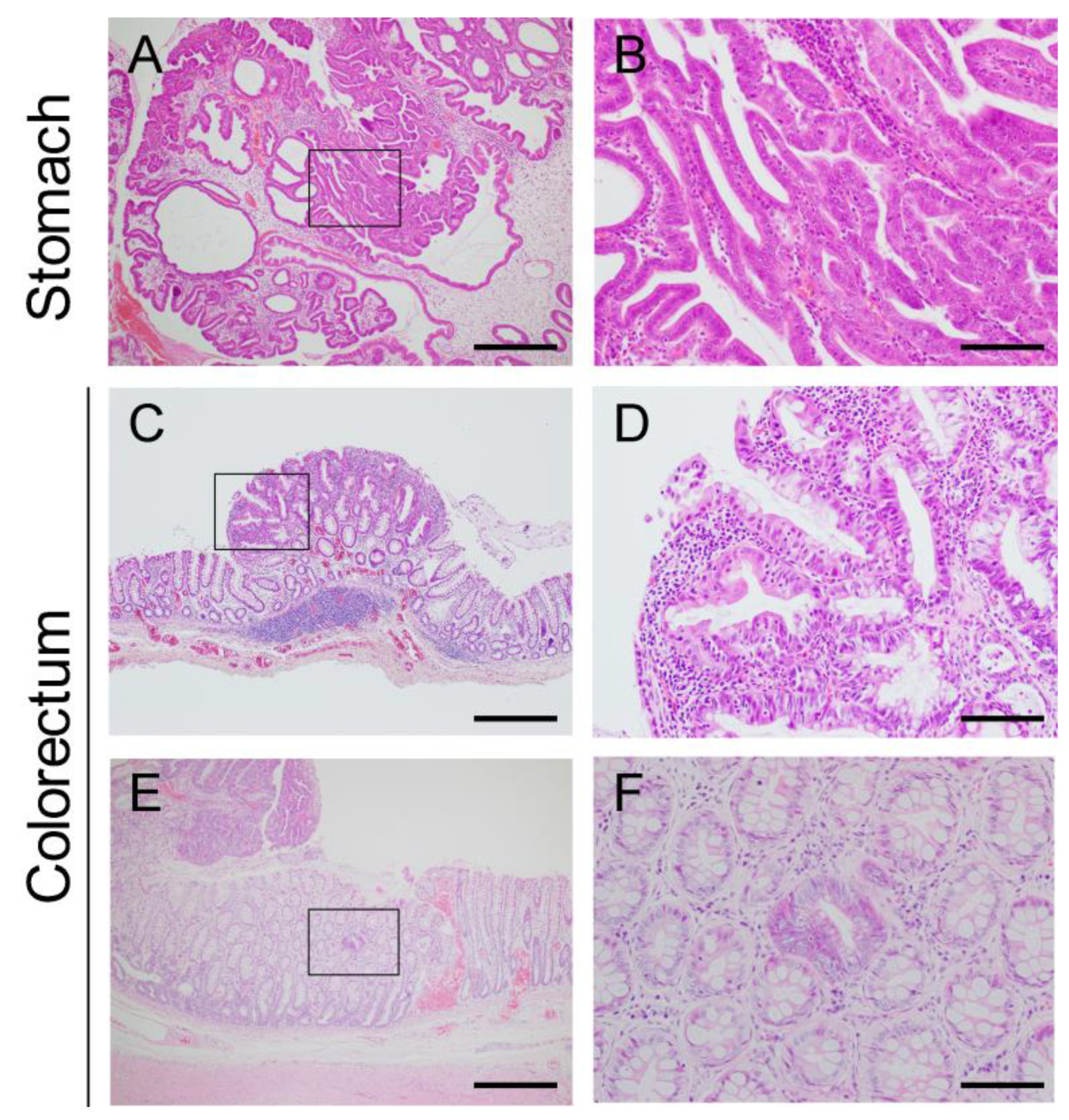
Stomach
Colorectum
Small Intestine
3.2.2. Microscopic Intramucosal Lesions in the Colorectum
3.2.3. Endoscopic Biopsy
3.2.4. Comparison of Hereditary and Sporadic GI Tumors in Dogs
Depth of Tumor Invasion
Histological Type
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jasperson, K.W.; Tuohy, T.M.; Neklason, D.W.; Burt, R.W. Hereditary and familial colon cancer. Gastroenterology 2010, 138, 2044–2058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshizaki, K.; Hirata, A.; Nishii, N.; Kawabe, M.; Goto, M.; Mori, T.; Sakai, H. Familial adenomatous polyposis in dogs: Hereditary gastrointestinal polyposis in Jack Russell Terriers with germline APC mutations. Carcinogenesis 2021, 42, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Head, K.W.; Cullen, J.M.; Dubielzig, R.R. Histological Classification of Tumors of the Alimentary System of Domestic Animals; Armed Forces Institute of Pathology: Washington, DC, USA, 2003; Volume 10, p. 257. [Google Scholar]
- Munday, J.S.; Löhr, C.V.; Kiupel, M. Tumors of the Alimentary Tract. In Tumors in Domestic Animals, 5th ed.; Meuten, D.J., Ed.; Willey & Sons: Hoboken, NJ, USA, 2016; pp. 499–601. [Google Scholar]
- Seim-Wikse, T.; Jorundsson, E.; Nodtvedt, A.; Grotmol, T.; Bjornvad, C.R.; Kristensen, A.T.; Skancke, E. Breed predisposition to canine gastric carcinoma--a study based on the Norwegian canine cancer register. Acta Vet. Scand. 2013, 55, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohmi, A.; Ohno, K.; Chambers, J.K.; Uchida, K.; Nakagawa, T.; Tomiyasu, H.; Tsujimoto, H. Clinical and histopathological features and prognosis of gastrointestinal adenocarcinomas in Jack Russell Terriers. J. Vet. Med. Sci. 2021, 83, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Nibe, K.; Chambers, J.K.; Uneyama, M.; Nakashima, K.; Ohno, K.; Tsujimoto, H.; Uchida, K.; Nakayama, H. A histopathological study on spontaneous gastrointestinal epithelial tumors in dogs. J. Toxicol. Pathol. 2020, 33, 105–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- JAPAN KENNEL CLUB. Number of Registered Dogs by Breeds. Available online: https://www.jkc.or.jp/ (accessed on 1 August 2022).
- Giardiello, F.M.; Burt, R.W.; Järvinin, H.J.; Offerhaus, G.J. Familial adenomatous polyposis. In WHO Classification of Tumours of the Digestive System, 4th ed.; Bosman, F.T., Carneiro, F., Hruban, R.H., Theise, N.D., Eds.; World Health Organization classification of tumours; World Health Organization: Lyon, France, 2010; pp. 147–151. [Google Scholar]
- Yoshizaki, K.; Hirata, A.; Matsushita, H.; Sakaguchi, M.; Yoneji, W.; Owaki, K.; Sakai, H. Molecular epidemiological study of germline APC variant associated with hereditary gastrointestinal polyposis in dogs: Current frequency in Jack Russell Terriers in Japan and breed distribution. BMC Vet. Res. 2022, 18, 230. [Google Scholar] [CrossRef] [PubMed]
- Yoshizaki, K.; Hirata, A.; Matsushita, H.; Nishii, N.; Kawabe, M.; Mori, T.; Sakai, H. PCR-based genotyping assays to detect germline APC variant associated with hereditary gastrointestinal polyposis in Jack Russell terriers. BMC Vet. Res. 2021, 17, 32. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Byrd, D.R.; Edge, S.B.; Greene, F.L. AJCC Cancer Staging Manual; Springer International Publishing AG: Cham, Switzerland, 2016. [Google Scholar]
- Brierley, J.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours; John Wiley & Sons: Chichester, UK, 2017. [Google Scholar]
- Amorim, I.; Taulescu, M.A.; Day, M.J.; Catoi, C.; Reis, C.A.; Carneiro, F.; Gartner, F. Canine Gastric Pathology: A Review. J. Comp. Pathol. 2016, 154, 9–37. [Google Scholar] [CrossRef] [PubMed]
- Paoloni, M.C.; Penninck, D.G.; Moore, A.S. Ultrasonographic and clinicopathologic findings in 21 dogs with intestinal adenocarcinoma. Vet. Radiol. Ultrasound 2002, 43, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Swann, H.M.; Holt, D.E. Canine gastric adenocarcinoma and leiomyosarcoma: A retrospective study of 21 cases (1986–1999) and literature review. J. Am. Anim. Hosp. Assoc. 2002, 38, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Vail, D.M.; Thamm, D.H.; Liptak, J. Cancer of the Gastrointestinal Tract. In Withrow and MacEwen’s Small Animal Clinical Oncology, 6th ed.; Liptak, J.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 432–491. [Google Scholar]
- Kim, H.S.; Woo, D.K.; Bae, S.I.; Kim, Y.I.; Kim, W.H. Microsatellite instability in the adenoma-carcinoma sequence of the stomach. Lab. Investig. 2000, 80, 57–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donner, J.; Anderson, H.; Davison, S.; Hughes, A.M.; Bouirmane, J.; Lindqvist, J.; Lytle, K.M.; Ganesan, B.; Ottka, C.; Ruotanen, P.; et al. Frequency and distribution of 152 genetic disease variants in over 100,000 mixed breed and purebred dogs. PLoS Genet. 2018, 14, e1007361. [Google Scholar] [CrossRef] [PubMed]
- Giger, U.; Sargan, D.R.; McNiel, E.A. Breed-specific Hereditary Diseases and Genetic Screening. In The Dog and Its Genome; Ostrander, E.A., Giger, U., Lindblad-Toh, K., Eds.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2006; pp. 249–289. [Google Scholar]
- Rokhsar, J.L.; Canino, J.; Raj, K.; Yuhnke, S.; Slutsky, J.; Giger, U. Web resource on available DNA variant tests for hereditary diseases and genetic predispositions in dogs and cats: An Update. Hum. Genet. 2021, 140, 1505–1515. [Google Scholar] [CrossRef] [PubMed]
- Eisele, J.; McClaran, J.K.; Runge, J.J.; Holt, D.E.; Culp, W.T.; Liu, S.; Long, F.; Bergman, P.J. Evaluation of risk factors for morbidity and mortality after pylorectomy and gastroduodenostomy in dogs. Vet. Surg. 2010, 39, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Cantatore, M.; Jimeno Sandoval, J.C.; Das, S.; Sesana, A.; Charlesworth, T.; Ryan, T.; Morello, E.M.; Gobbetti, M.; Cinti, F.; Rossanese, M. Submucosal resection via a transanal approach for treatment of epithelial rectal tumors—A multicenter study. Vet. Surg. 2022, 51, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Shida, T.; Maruo, T.; Suga, K.; Kawamura, H.; Takeda, H.; Sugiyama, H.; Ishikawa, T.; Inoue, A.; Yamada, T.; Ito, T.; et al. Rectal Mucosal Pull-Through Surgical Technique for Canine Rectal Multiple Tumor. Jpn. J. Vet. Anesth. Surg. 2008, 39, 11–16. [Google Scholar] [CrossRef]
- Luna, S.P.; Basílio, A.C.; Steagall, P.V.; Machado, L.P.; Moutinho, F.Q.; Takahira, R.K.; Brandão, C.V. Evaluation of adverse effects of long-term oral administration of carprofen, etodolac, flunixin meglumine, ketoprofen, and meloxicam in dogs. Am. J. Vet. Res. 2007, 68, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Mabry, K.; Hill, T.; Tolbert, M.K. Prevalence of gastrointestinal lesions in dogs chronically treated with nonsteroidal anti-inflammatory drugs. J. Vet. Intern. Med. 2021, 35, 853–859. [Google Scholar] [CrossRef] [PubMed]




| Case No. | Sex | Onset Age | Number and Size of Lesions on the Finding of Clinical Imaging * | Treatment | Other Lesion and Symptoms | Survival Time (Days) | Age at Death | Cause of Death | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stomach | Duodenum | Ileum and Jejunum | Colorectum | Surgical Resection | Treatment | |||||||||||||
| Number | Size † (mm) | Number | Size † (mm) | Number | Size † (mm) | Number | Size † (mm) | Age at Sugery | Procedure (Tumor Location ‡) | NSAIDs | Tyrosine Kinase Inhibitor | |||||||
| A | F | 3y2m | 5 | 6.2–15.0 | 1 # | 15 | 9 | 4.4–20.0 | 3 | unmea-surable | 3y4m | Local resection (C) | Carprofen | Toceranib | Epidermal cyst Seizures | 1160 | 6y4m | Systemic metastasis |
| 4y7m | End to end anastomosis (I/J) | Mammary adenoma | ||||||||||||||||
| B | M | 3y3m | 1 | 7 | 1 # | 7.2–15 | 7 | 4.0–34.9 | 11 | 11.2–23.0 | 4y2m | Pull-through (C) | Carprofen | Toceranib | 498 | 4y7m | Intestinal perforation | |
| C | F | 3y10m | 0 | 0 | 4 | 6.2 | 5 | 32.4 | 3y11m | Pull-through (C) | (-) | Epidermal cyst | 684+ (alive) | |||||
| D | F | 4y1m | 7 | 7.6–38.7 | 0 | 0 | 0 | 4y3m | Local resection (S) | Carprofen | 155+ (alive) | |||||||
| E | F | 4y7m | 2 | 0 | 2 | 1 | 4y7m | Local resection (C) | (-) | Lost of | ||||||||
| 5y4m | Pull-through (C) | follow up | ||||||||||||||||
| F | F | 4y7m | 3 | 7.0–13.0 | 0 | 0 | 3 | 9.0–11.0 | 5y | Local resection (C) | Carprofen | Epidermal cyst Seizures | 1039 | 7y8m | Under postmortem examination | |||
| 5y4m | Local resection (S) | Grapiprant | ||||||||||||||||
| 6y1m | Pull-through (C) | Firocoxib | ||||||||||||||||
| 6y10m | Local resection (S) (D) | |||||||||||||||||
| G | M | 6y7m | 4 | 4.9–21.5 | 0 | 0 | 5 | 2.9–18.9 | 6y9m | Pull-through (C) | Firocoxib | 197+ (alive) | ||||||
| H | F | 6y8m | 0 | 1 # | 1 | 1 | (-) | Firocoxib | 818+ (alive) | |||||||||
| I | M | 7y2m | 5 | 1 | 0 | 0 | (-) | (-) | Intracardiac tumor | 109 | 7y5m | Intracardiac mass | ||||||
| J | F | 7y3m | 1 | 20.9 | 0 | 0 | 8 | 39 | 7y5m | Pull-through (C) | Carprofen | Epidermal cyst Mammary adenoma | 213+ (alive) | |||||
| 7y7m | Local resection (S) | |||||||||||||||||
| K | M | 7y3m | 4 | 0 | 0 | 0 | 7y3m | Local resection (S) | Firocoxib | 1303+ (alive) | ||||||||
| 10y2m | Local resection (S) | |||||||||||||||||
| L | F | 8y | 4 | 5.9–29.3 | 0 | 0 | 0 | 8y2m | Full layer resection (S) | Firocoxib | Epidermal cyst | 412+ (alive) | ||||||
| M | F | 11y2m | 3 | 10.6–21.8 | 0 | 0 | 0 | 11y4m | Palliative surgery (S) | Carprofen | Toceranib | Epidermal cyst Adrenal hyperplasia | 140 | 11y6m | Systemic metastasis | |||
| N | F | 11y6m | 3 | 18.0–52.3 | 0 | 2 | 36.7 | 0 | (-) | Grapiprant | 29 | 11y6m | Gastric perforation | |||||
| Total Number | Histopathological Diagnosis | |||
|---|---|---|---|---|
| Hyperplastic Polyp | Adenoma | Adenocarcinoma | ||
| Stomach | 54 | 4 (7.4%) | 19 (35.2%) | 31 (57.4%) |
| Cardia | 4 | 0 | 2 | 2 |
| Corpus | 9 | 0 | 2 | 7 |
| Antrum | 37 | 3 | 13 | 21 |
| Unrecorded | 4 | 1 | 2 | 1 |
| Small intestine | 9 | 0 (0%) | 0 (0%) | 9 (100%) |
| Duodenum | 4 | 0 | 0 | 4 |
| Jejunum and ileum | 5 | 0 | 0 | 5 |
| Large intestine | 70 | 0 (0%) | 1 (1.4%) | 69 (98.6%) |
| Colon | 11 | 0 | 0 | 11 |
| Rectum | 59 | 0 | 1 | 58 |
| Total | Depth of Tumor Invasion | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Stomach | Tis | T1a | T1b | T2 | T3 | T4a | T4b | ||
| JRTs | 20 | 18 | 2 | p < 0.05 | |||||
| Other breeds | 7 | 4 | 2 | 1 | |||||
| Small intestine | Tis | T1a | T1b | T2 | T3 | T4 | |||
| JRTs | 5 | 2 | 2 | 1 | p < 0.01 | ||||
| Other breeds | 14 | 1 | 6 | 7 | |||||
| Large intestine | Tis | T1 | T2 | T3 | T4a | T4b | |||
| JRTs | 53 | 52 | 1 | p < 0.05 | |||||
| Other breeds | 23 | 19 | 1 | 1 | 2 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoneji, W.; Yoshizaki, K.; Hirata, A.; Yoneji, K.; Sakai, H. Clinical and Pathological Diagnosis of Hereditary Gastrointestinal Polyposis in Jack Russell Terriers. Vet. Sci. 2022, 9, 551. https://doi.org/10.3390/vetsci9100551
Yoneji W, Yoshizaki K, Hirata A, Yoneji K, Sakai H. Clinical and Pathological Diagnosis of Hereditary Gastrointestinal Polyposis in Jack Russell Terriers. Veterinary Sciences. 2022; 9(10):551. https://doi.org/10.3390/vetsci9100551
Chicago/Turabian StyleYoneji, Wakana, Kyoko Yoshizaki, Akihiro Hirata, Kensuke Yoneji, and Hiroki Sakai. 2022. "Clinical and Pathological Diagnosis of Hereditary Gastrointestinal Polyposis in Jack Russell Terriers" Veterinary Sciences 9, no. 10: 551. https://doi.org/10.3390/vetsci9100551
APA StyleYoneji, W., Yoshizaki, K., Hirata, A., Yoneji, K., & Sakai, H. (2022). Clinical and Pathological Diagnosis of Hereditary Gastrointestinal Polyposis in Jack Russell Terriers. Veterinary Sciences, 9(10), 551. https://doi.org/10.3390/vetsci9100551





