Climate Change Influences the Spread of African Swine Fever Virus
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data
2.1.1. Presence Data
2.1.2. Climatic Variables
2.2. Modelling Approach and Evaluation
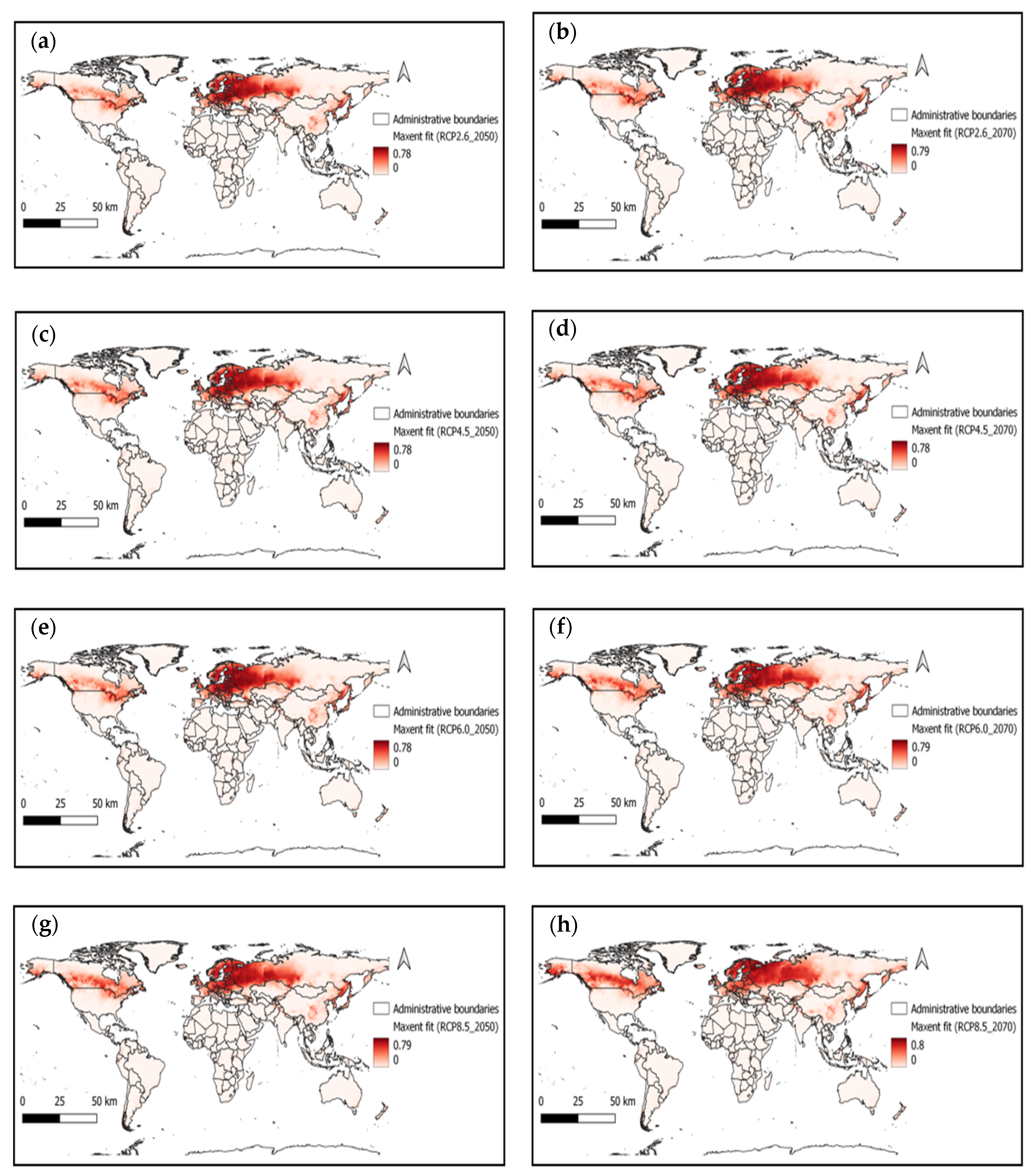
3. Results and Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alamo, T.; Reina, D.G.; Millán Gata, P.; Preciado, V.M.; Giordano, G. Data-Driven Methods for Present and Future Pandemics: Monitoring, Modelling and Managing. Annu. Rev. Control 2021, 52, 448–464. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Cordón, P.J.; Montoya, M.; Reis, A.L.; Dixon, L.K. African Swine Fever: A Re-Emerging Viral Disease Threatening the Global Pig Industry. Vet. J. 2018, 233, 41–48. [Google Scholar] [CrossRef]
- Galindo, I.; Alonso, C. African Swine Fever Virus: A Review. Viruses 2017, 9, 103. [Google Scholar] [CrossRef]
- Plavšic, B.; Rozstalnyy, A.; Park, J.Y.; Guberti, V.; Depner, K.R.; Torres, G. Strategic Challenges to Global Control of African Swine Fever. In Proceedings of the General Sessions on the World Assembly of the Delegates of the OIE, Paris, France, 26–31 May 2019; Volume 33, pp. 26–31. [Google Scholar]
- Ezanno, P.; Picault, S.; Bareille, S.; Beaunée, G.; Boender, G.J.; Dankwa, E.A.; Deslandes, F.; Donnelly, C.A.; Hagenaars, T.J.; Hayes, S.; et al. The African Swine Fever Modelling Challenge: Model Comparison and Lessons Learnt. Epidemics 2022, 40, 100615. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Park, B.; Kang, H.E. Control Measures to African Swine Fever Outbreak: Active Response in South Korea, Preparation for the Future, and Cooperation. J. Vet. Sci. 2021, 22, e13. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, H.; Schulz, K.; Conraths, F.J.; Sauter-Louis, C. A Review of Environmental Risk Factors for African Swine Fever in European Wild Boar. Animals 2021, 11, 2692. [Google Scholar] [CrossRef]
- De La Rocque, S. Climate Change: Impact on the Epidemiology and Control of Animal Diseases. OIE Rev. Sci. Tech. 2008, 27, 303–308. [Google Scholar]
- Abbass, K.; Qasim, M.Z.; Song, H.; Murshed, M.; Mahmood, H.; Younis, I. A Review of the Global Climate Change Impacts, Adaptation, and Sustainable Mitigation Measures. Environ. Sci. Pollut. Res. 2022, 29, 42539–42559. [Google Scholar] [CrossRef]
- WHO. Climate Change and Health Fact Sheets on Sustainable Development Goals: Health Targets; WHO: Geneva, Switzerland, 2018; Volume 143. [Google Scholar]
- Patz, J.A.; Olson, S.H. Climate Change and Health: Global to Local Influences on Disease Risk. Ann. Trop. Med. Parasitol. 2006, 100, 535–549. [Google Scholar] [CrossRef]
- Yue, X.L.; Gao, Q.X. Contributions of Natural Systems and Human Activity to Greenhouse Gas Emissions. Adv. Clim. Change Res. 2018, 9, 243–252. [Google Scholar] [CrossRef]
- Masson-Delmotte, V.; Zhai, P.; Chen, Y.; Goldfarb, L.; Gomis, M.I.; Matthews, J.B.R.; Berger, S.; Huang, M.; Yelekçi, O.; Yu, R.; et al. (Eds.) Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021. [Google Scholar]
- Caroprese, M.; Bradford, B.J.; Rhoads, R.P. Impact of Climate Change on Immune Responses in Agricultural Animals. Front. Vet. Sci. 2021, 8, 844. [Google Scholar] [CrossRef] [PubMed]
- Watts, N.; Amann, M.; Arnell, N.; Ayeb-Karlsson, S.; Beagley, J.; Belesova, K.; Boykoff, M.; Byass, P.; Cai, W.; Campbell-Lendrum, D.; et al. The 2020 Report of The Lancet Countdown on Health and Climate Change: Responding to Converging Crises. Lancet 2021, 397, 129–170. [Google Scholar] [CrossRef]
- Fawzy, S.; Osman, A.I.; Doran, J.; Rooney, D.W. Strategies for Mitigation of Climate Change: A Review. Environ. Chem. Lett. 2020, 18, 2069–2094. [Google Scholar] [CrossRef]
- IPCC Climate Change: A Threat to Human Wellbeing and Health of the Planet. 2022. Available online: https://www.ipcc.ch/2022/02/28/pr-wgii-ar6 (accessed on 31 August 2022).
- Scanes, C.G. Human Activity and Habitat Loss: Destruction, Fragmentation, and Degradation. In Animals and Human Society; Academic Press: Cambridge, MA, USA, 2017; pp. 451–482. ISBN 9780128052471. [Google Scholar]
- Bellard, C.; Bertelsmeier, C.; Leadley, P.; Thuiller, W.; Courchamp, F. Impacts of Climate Change on the Future of Biodiversity. Ecol. Lett. 2012, 15, 365–377. [Google Scholar] [CrossRef]
- Baker, R.E.; Mahmud, A.S.; Miller, I.F.; Rajeev, M.; Rasambainarivo, F.; Rice, B.L.; Takahashi, S.; Tatem, A.J.; Wagner, C.E.; Wang, L.F.; et al. Infectious Disease in an Era of Global Change. Nat. Rev. Microbiol. 2021, 20, 193–205. [Google Scholar] [CrossRef]
- Lafferty, K.D. The Ecology of Climate Change and Infectious Diseases. Ecology 2009, 90, 888–900. [Google Scholar] [CrossRef]
- Johnson, P.T.J.; Townsend, A.R.; Cleveland, C.C.; Glibert, P.M.; Howarth, R.W.; Mckenzie, V.J.; Rejmankova, E.; Ward, M.H. Linking Environmental Nutrient Enrichment and Disease Emergence in Humans and Wildlife. Ecol. Appl. 2010, 20, 16–29. [Google Scholar] [CrossRef]
- Gupta, S.; Rouse, B.T.; Sarangi, P.P. Did Climate Change Influence the Emergence, Transmission, and Expression of the COVID-19 Pandemic? Front. Med. 2021, 8, 769208. [Google Scholar] [CrossRef]
- Epstein, P.R. Chikungunya Fever Resurgence and Global Warming. Am. J. Trop. Med. Hyg. 2007, 76, 403–404. [Google Scholar] [CrossRef]
- Magiri, R.; Muzandu, K.; Gitau, G.; Choongo, K.; Iji, P. Impact of Climate Change on Animal Health, Emerging and Re-Emerging Diseases in Africa. In African Handbook of Climate Change Adaptation; Springer: Cham, Switzerland, 2021; pp. 1835–1851. [Google Scholar]
- Smolinski, M.S.; Hamburg, M.A.; Lederberg, J. Emerging Microbial Threats to Health in the 21st Century; National Academy of Sciences: Washington, DC, USA, 2003; Volume 398. [Google Scholar]
- Glud, H.A.; George, S.; Skovgaard, K.; Larsen, L.E. Zoonotic and Reverse Zoonotic Transmission of Viruses between Humans and Pigs. APMIS 2021, 129, 675–693. [Google Scholar] [CrossRef]
- Mishra, J.; Mishra, P.; Arora, N.K. Linkages between Environmental Issues and Zoonotic Diseases: With Reference to COVID-19 Pandemic. Environ. Sustain. 2021, 4, 455–467. [Google Scholar] [CrossRef]
- Patz, J.A.; Olson, S.H. Malaria Risk and Temperature: Influences from Global Climate Change and Local Land Use Practices. Proc. Natl. Acad. Sci. USA 2006, 103, 5635–5636. [Google Scholar] [CrossRef] [PubMed]
- Qazi, A.W.; Saqib, Z.; Zaman-ul-Haq, M. Trends in Species Distribution Modelling in Context of Rare and Endemic Plants: A Systematic Review. Ecol. Processes 2022, 11, 1–11. [Google Scholar] [CrossRef]
- Yackulic, C.B.; Chandler, R.; Zipkin, E.F.; Royle, J.A.; Nichols, J.D.; Campbell Grant, E.H.; Veran, S. Presence-Only Modelling Using MAXENT: When Can We Trust the Inferences? Methods Ecol. Evol. 2013, 4, 236–243. [Google Scholar] [CrossRef]
- Kopsco, H.L.; Smith, R.L.; Halsey, S.J. A Scoping Review of Species Distribution Modeling Methods for Tick Vectors. Front. Ecol. Evol. 2022, 10, 462. [Google Scholar] [CrossRef]
- Lee, D.S.; Choi, W.I.; Nam, Y.; Park, Y.S. Predicting Potential Occurrence of Pine Wilt Disease Based on Environmental Factors in South Korea Using Machine Learning Algorithms. Ecol. Inform. 2021, 64, 101378. [Google Scholar] [CrossRef]
- Gao, H.; Ma, J. Spatial Distribution and Risk Areas of Foot and Mouth Disease in Mainland China. Prev. Vet. Med. 2021, 189, 105311. [Google Scholar] [CrossRef]
- Alkhamis, M.A.; VanderWaal, K. Spatial and Temporal Epidemiology of Lumpy Skin Disease in the Middle East, 2012–2015. Front. Vet. Sci. 2016, 3, 19. [Google Scholar] [CrossRef]
- Ikegami, M.; Jenkins, T.A.R. Estimate Global Risks of a Forest Disease under Current and Future Climates Using Species Distribution Model and Simple Thermal Model—Pine Wilt Disease as a Model Case. For. Ecol. Manag. 2018, 409, 343–352. [Google Scholar] [CrossRef]
- Al Ruheili, A.M.; Boluwade, A.; Al Subhi, A.M. Assessing the Impact of Climate Change on the Distribution of Lime (16SRII-b) and Alfalfa (16srii-d) Phytoplasma Disease Using Maxent. Plants 2021, 10, 460. [Google Scholar] [CrossRef]
- Narouei-Khandan, H.A.; Worner, S.P.; Viljanen, S.L.H.; Van Bruggen, A.H.C.; Balestra, G.M.; Jones, E. The Potential Global Climate Suitability of Kiwifruit Bacterial Canker Disease (Pseudomonas Syringae Pv. Actinidiae (Psa)) Using Three Modelling Approaches: CLIMEX, Maxent and Multimodel Framework. Climate 2022, 10, 14. [Google Scholar] [CrossRef]
- Pramanik, M.; Singh, P.; Dhiman, R.C. Identification of Bio-Climatic Determinants and Potential Risk Areas for Kyasanur Forest Disease in Southern India Using MaxEnt Modelling Approach. BMC Infect. Dis. 2021, 21, 1226. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Peng, W.; Liu, X.; He, G.; Cai, Y. Spatiotemporal Dynamics and Factors Driving the Distributions of Pine Wilt Disease-Damaged Forests in China. Forests 2022, 13, 261. [Google Scholar] [CrossRef]
- Musolin, D.L.; Nielsen, A.L.; Mazzoni, V.; Hwang, J.H.; Kim, S.-H.; Yoon, S.; Jung, S.; Kim, D.H.; Lee, W.-H. Evaluation of Spatial Distribution of Three Major Leptocorisa (Hemiptera: Alydidae) Pests Using MaxEnt Model. Insects 2022, 13, 750. [Google Scholar] [CrossRef]
- Mora, C.; McKenzie, T.; Gaw, I.M.; Dean, J.M.; von Hammerstein, H.; Knudson, T.A.; Setter, R.O.; Smith, C.Z.; Webster, K.M.; Patz, J.A.; et al. Over Half of Known Human Pathogenic Diseases Can Be Aggravated by Climate Change. Nat. Clim. Chang. 2022, 12, 869–875. [Google Scholar] [CrossRef]
- Neves, S.F.; Silva, M.C.F.; Miranda, J.M.; Stilwell, G.; Cortez, P.P. Predictive Models of Dairy Cow Thermal State: A Review from a Technological Perspective. Vet. Sci. 2022, 9, 416. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum Entropy Modeling of Species Geographic Distributions. Int. J. Glob. Environ. Issues 2006, 6, 231–252. [Google Scholar] [CrossRef]
- Van Vuuren, D.P.; Edmonds, J.A.; Kainuma, M.; Riahi, K.; Weyant, J. A Special Issue on the RCPs. Clim. Chang. 2011, 109, 1. [Google Scholar] [CrossRef]
- Mighell, E.; Ward, M.P. African Swine Fever Spread across Asia, 2018–2019. Transbound. Emerg. Dis. 2021, 68, 2722–2732. [Google Scholar] [CrossRef]
- Sánchez-Cordón, P.J.; Nunez, A.; Neimanis, A.; Wikström-Lassa, E.; Montoya, M.; Crooke, H.; Gavier-Widén, D. African Swine Fever: Disease Dynamics in Wild Boar Experimentally Infected with ASFV Isolates Belonging to Genotype I and II. Viruses 2019, 11, 852. [Google Scholar] [CrossRef]
- Claes, F.; Kuznetsov, D.; Liechti, R.; Von Dobschuetz, S.; Truong, B.D.; Gleizes, A.; Conversa, D.; Colonna, A.; Demaio, E.; Ramazzotto, S.; et al. The EMPRES-i Genetic Module: A Novel Tool Linking Epidemiological Outbreak Information and Genetic Characteristics of Influenza Viruses. Database 2014, 2014, bau008. [Google Scholar] [CrossRef] [PubMed]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-Km Spatial Resolution Climate Surfaces for Global Land Areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Van Etten, J.; Mattiuzzi, M.; Sumner, M.; Greenberg, J.A.; Lamigueiro, O.P.; Bevan, A.; Racine, E.B.; Shortridge, A. Geographic Data Analysis and Modeling: Package “Raster”. R CRAN Proj. 2015, 2.3-40, 1–134. [Google Scholar]
- R Core Team RStudio | Open Source & Professional Software for Data Science Teams—RStudio. RStudio. 2022. Available online: http://www.rstudio.com/ (accessed on 2 April 2022).
- Taylor, K.E.; Stouffer, R.J.; Meehl, G.A. An Overview of CMIP5 and the Experiment Design. Bull. Am. Meteorol. Soc. 2012, 93, 485–498. [Google Scholar] [CrossRef]
- Dodge, Y. The Concise Encyclopedia of Statistics; Springer: New York, NY, USA, 2008; ISBN 978-0-387-31742-7. [Google Scholar]
- Phillips, S.J.; Dudík, M. Modeling of Species Distributions with Maxent: New Extensions and a Comprehensive Evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silander, J.A. A Practical Guide to MaxEnt for Modeling Species’ Distributions: What It Does, and Why Inputs and Settings Matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Cohen, J. A Coefficient of Agreement for Nominal Scales. Educ. Psychol. Meas. 1960, 20, 37–46. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the Accuracy of Species Distribution Models: Prevalence, Kappa and the True Skill Statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Swets, J.A. Measuring the Accuracy of Diagnostic Systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef]
- McHugh, M.L. Interrater Reliability: The Kappa Statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Phillips, S.J. A Brief Tutorial on Maxent. Available online: www.cs.princeton.edu/~schapire/maxent (accessed on 31 August 2022).
- Phillips, S.J.; Anderson, R.P.; Dudík, M.; Schapire, R.E.; Blair, M.E. Opening the Black Box: An Open-Source Release of Maxent. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Tang, X.; Yuan, Y.; Li, X.; Zhang, J. Maximum Entropy Modeling to Predict the Impact of Climate Change on Pine Wilt Disease in China. Front. Plant Sci. 2021, 12, 652500. [Google Scholar] [CrossRef] [PubMed]
- An, R.; Ji, M.; Zhang, S. Global Warming and Obesity: A Systematic Review. Obes. Rev. 2018, 19, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Carlson, C.J.; Albery, G.F.; Merow, C.; Trisos, C.H.; Zipfel, C.M.; Eskew, E.A.; Olival, K.J.; Ross, N.; Bansal, S. Climate Change Increases Cross-Species Viral Transmission Risk. Nature 2022, 607, 555–562. [Google Scholar] [CrossRef]
- Pig333 South Korea Reduces Wild Boar Population to Slow ASF Spread—Swine News—Pig333, Pig to Pork Community. Available online: https://www.pig333.com/latest_swine_news/south-korea-reduces-wild-boar-population-to-slow-asf-spread_17241/ (accessed on 26 April 2021).
- Escobar, L.E. Ecological Niche Modeling: An Introduction for Veterinarians and Epidemiologists. Front. Vet. Sci. 2020, 7, 713. [Google Scholar] [CrossRef]
- Ungur, A.; Cazan, C.D.; Panait, L.C.; Coroian, M.; Cătoi, C. What Is the Real Influence of Climatic and Environmental Factors in the Outbreaks of African Swine Fever? Animals 2022, 12, 781. [Google Scholar] [CrossRef]
- Van Vuuren, D.P.; Edmonds, J.; Kainuma, M.; Riahi, K.; Thomson, A.; Hibbard, K.; Hurtt, G.C.; Kram, T.; Krey, V.; Lamarque, J.F.; et al. The Representative Concentration Pathways: An Overview. Clim. Chang. 2011, 109, 5–31. [Google Scholar] [CrossRef]
- Engering, A.; Hogerwerf, L.; Slingenbergh, J. Pathogen-Host-Environment Interplay and Disease Emergence. Emerg. Microbes Infect. 2013, 2, e5. [Google Scholar] [CrossRef]
- Rakotoarinia, M.R.; Guillaume Blanchet, F.; Gravel, D.; Lapen, D.R.; Leighton, P.A.; Ogden, N.H.; Ludwig, A. Effects of Land Use and Weather on the Presence and Abundance of Mosquito-Borne Disease Vectors in a Urban and Agricultural Landscape in Eastern Ontario, Canada. PLoS ONE 2022, 17, e0262376. [Google Scholar] [CrossRef]
- Podgórski, T.; Borowik, T.; Łyjak, M.; Woźniakowski, G. Spatial Epidemiology of African Swine Fever: Host, Landscape and Anthropogenic Drivers of Disease Occurrence in Wild Boar. Prev. Vet. Med. 2020, 177, 104691. [Google Scholar] [CrossRef]
- Häsler, B.; Gilbert, W.; Jones, B.A.; Pfeiffer, D.U.; Rushton, J.; Otte, M.J. The Economic Value of One Health in Relation to the Mitigation of Zoonotic Disease Risks. In Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2012; Volume 365, pp. 127–151. [Google Scholar]
- Sharma, N.; Dev, J.; Mangla, M.; Wadhwa, V.M.; Mohanty, S.N.; Kakkar, D. A Heterogeneous Ensemble Forecasting Model for Disease Prediction. New Gener. Comput. 2021, 39, 701–715. [Google Scholar] [CrossRef] [PubMed]
- Hayes, B.H.; Andraud, M.; Salazar, L.G.; Rose, N.; Vergne, T. Mechanistic Modelling of African Swine Fever: A Systematic Review. Prev. Vet. Med. 2021, 191, 105358. [Google Scholar] [CrossRef] [PubMed]
- Denstedt, E.; Porco, A.; Hwang, J.; Nga, N.T.T.; Ngoc, P.T.B.; Chea, S.; Khammavong, K.; Milavong, P.; Sours, S.; Osbjer, K.; et al. Detection of African Swine Fever Virus in Free-Ranging Wild Boar in Southeast Asia. Transbound. Emerg. Dis. 2021, 68, 2669–2675. [Google Scholar] [CrossRef]
- Gervasi, V.; Marcon, A.; Bellini, S.; Guberti, V. Evaluation of the Efficiency of Active and Passive Surveillance in the Detection of African Swine Fever in Wild Boar. Vet. Sci. 2020, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- United Nations. Sustainable Development Goal 13: Climate Action; United Nations: New York, NY, USA, 2019. [Google Scholar]
- WHO. OIE WHO-OIE Operational Framework for Good Governance at the Human-Animal Interface: Bridging WHO and OIE Tools for the Assessment of National Capacities; WHO: Geneva, Switzerland, 2014. [Google Scholar]



| Bioclimatic Variables * | Code | VIF Value | Unit |
|---|---|---|---|
| Annual Mean Temperature | Bio1 | 309.80 | °C |
| Mean Diurnal Range (Mean of monthly (max temp–min temp)) | Bio2 | 72.45 | °C |
| Isothermality (Bio2/Bio7) (×100) | Bio3 | 17.20 | % |
| Temperature Seasonality (standard deviation ×100) | Bio4 | 1435.91 | °C |
| Max Temperature of Warmest Month | Bio5 | Inf | °C |
| Min Temperature of Coldest Month | Bio6 | Inf | °C |
| Temperature Annual Range (Bio5-BIO6) | Bio7 | Inf | °C |
| Mean Temperature of Wettest Quarter | Bio8 | 6.17 | °C |
| Mean Temperature of Driest Quarter | Bio9 | 8.11 | °C |
| Mean Temperature of Warmest Quarter | Bio10 | 937.86 | °C |
| Mean Temperature of Coldest Quarter | Bio11 | 1471.54 | °C |
| Annual Precipitation | Bio12 | 294.04 | mm |
| Precipitation of Wettest Month | Bio13 | 211.56 | mm |
| Precipitation of Driest Month | Bio14 | 65.35 | mm |
| Precipitation Seasonality (Coefficient of Variation) | Bio15 | 77.61 | % |
| Precipitation of Wettest Quarter | Bio16 | 801.18 | mm |
| Precipitation of Driest Quarter | Bio17 | 104.71 | mm |
| Precipitation of Warmest Quarter | Bio18 | 334.52 | mm |
| Precipitation of Coldest Quarter | Bio19 | 29.94 | mm |
| Variable | Percent Contribution | Permutation Importance |
|---|---|---|
| Bio14 | 49.3 | 17 |
| Bio1 | 34 | 29.4 |
| Bio9 | 5.5 | 0.7 |
| Bio12 | 3.1 | 7.8 |
| Bio3 | 2.8 | 3.6 |
| Bio10 | 2.2 | 7.1 |
| Bio2 | 1.9 | 23.8 |
| Bio15 | 0.7 | 5 |
| Bio7 | 0.5 | 5.4 |
| Bio8 | 0.2 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiwari, S.; Dhakal, T.; Kim, T.-S.; Lee, D.-H.; Jang, G.-S.; Oh, Y. Climate Change Influences the Spread of African Swine Fever Virus. Vet. Sci. 2022, 9, 606. https://doi.org/10.3390/vetsci9110606
Tiwari S, Dhakal T, Kim T-S, Lee D-H, Jang G-S, Oh Y. Climate Change Influences the Spread of African Swine Fever Virus. Veterinary Sciences. 2022; 9(11):606. https://doi.org/10.3390/vetsci9110606
Chicago/Turabian StyleTiwari, Shraddha, Thakur Dhakal, Tae-Su Kim, Do-Hun Lee, Gab-Sue Jang, and Yeonsu Oh. 2022. "Climate Change Influences the Spread of African Swine Fever Virus" Veterinary Sciences 9, no. 11: 606. https://doi.org/10.3390/vetsci9110606
APA StyleTiwari, S., Dhakal, T., Kim, T.-S., Lee, D.-H., Jang, G.-S., & Oh, Y. (2022). Climate Change Influences the Spread of African Swine Fever Virus. Veterinary Sciences, 9(11), 606. https://doi.org/10.3390/vetsci9110606








