Oviduct Epithelial Cell-Derived Extracellular Vesicles Improve Porcine Trophoblast Outgrowth
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Oocyte Collection and In Vitro Maturation (IVM)
2.3. Collection of Porcine Oviductal Fluid and Epithelial Cells
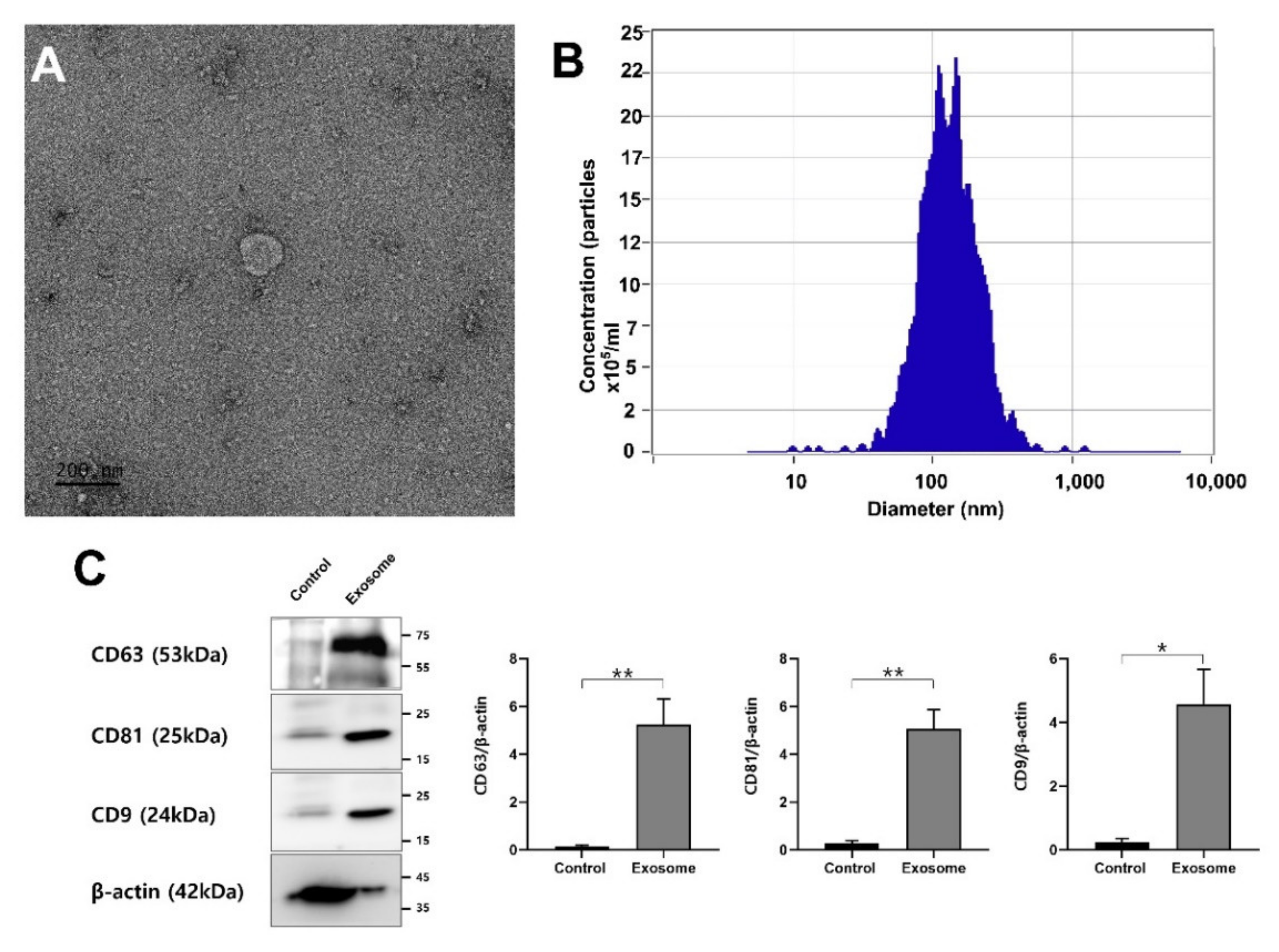
2.4. Isolation, Purification and Characterization of EVs
2.5. ZetaView Nanoparticle Tracking Analysis (NTA)
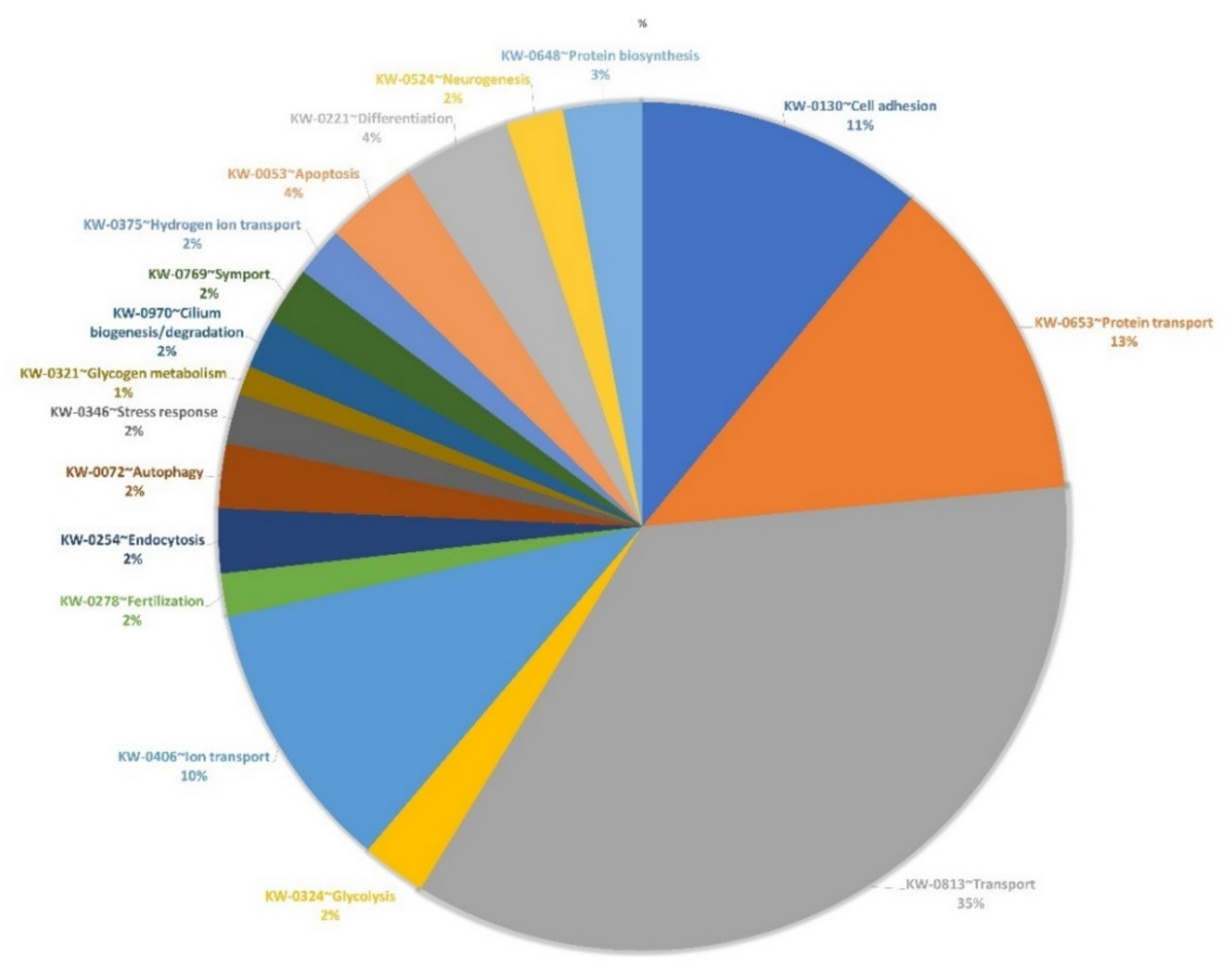
2.6. Proteomic Analysis of OEC-EVs
2.7. Western Blot
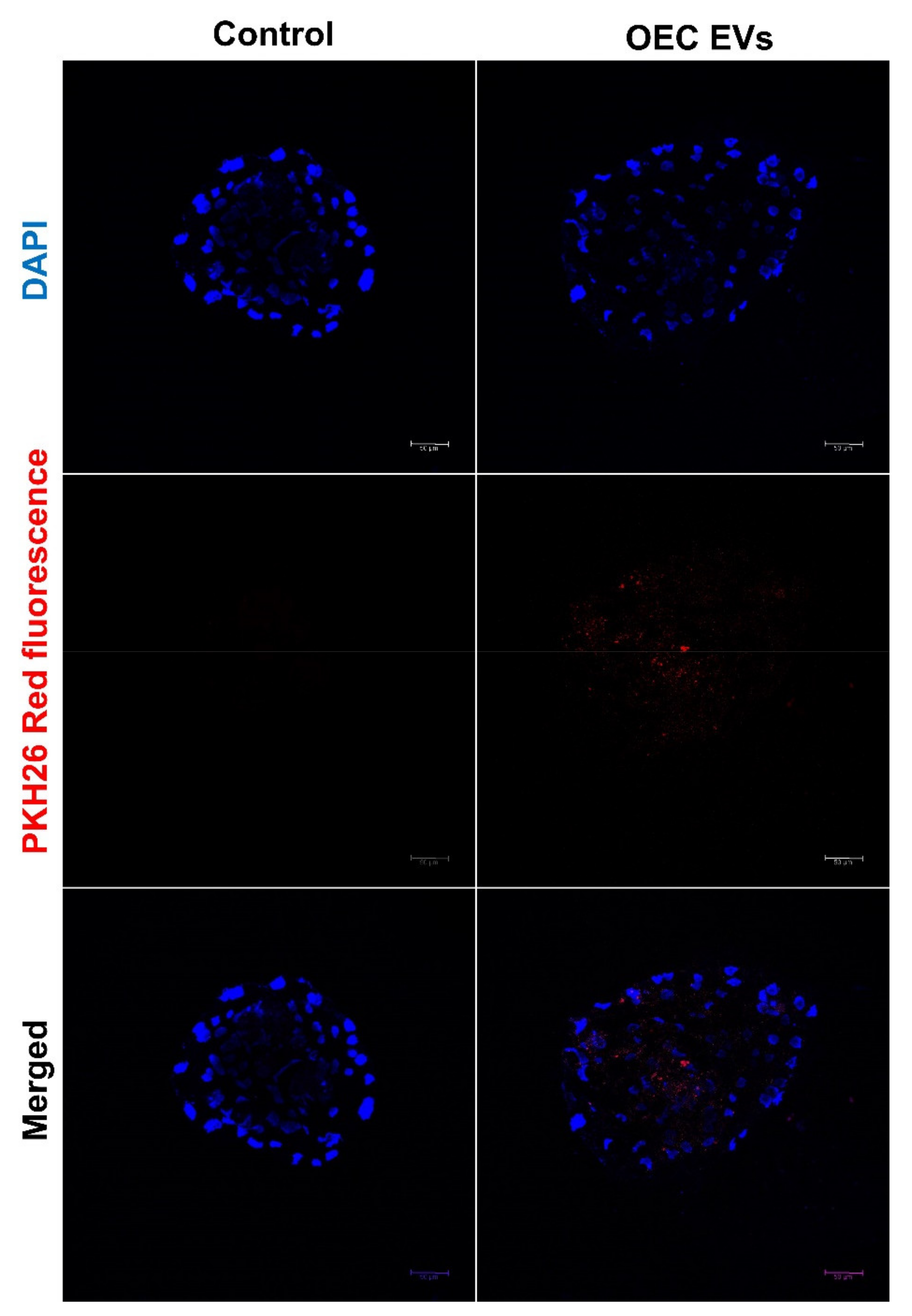
2.8. Uptake of OEC-EVs by the Preimplantation Embryos
2.9. Parthenogenetic Activation of Oocytes and Culture
2.10. Preparation of ICR Mouse Feeder Cells
2.11. Quantitative Real-Time PCR (q-PCR)
2.12. Statistical Analysis
3. Results
3.1. EV Isolation, Characterization and Proteomics
3.2. EV Uptake by the Cultured Embryos
3.3. Effect of OEC EVs on Parthenogenetic Embryo Development
3.4. Effect of OEC EVs on Blastocyst ICM/TE Cell Number
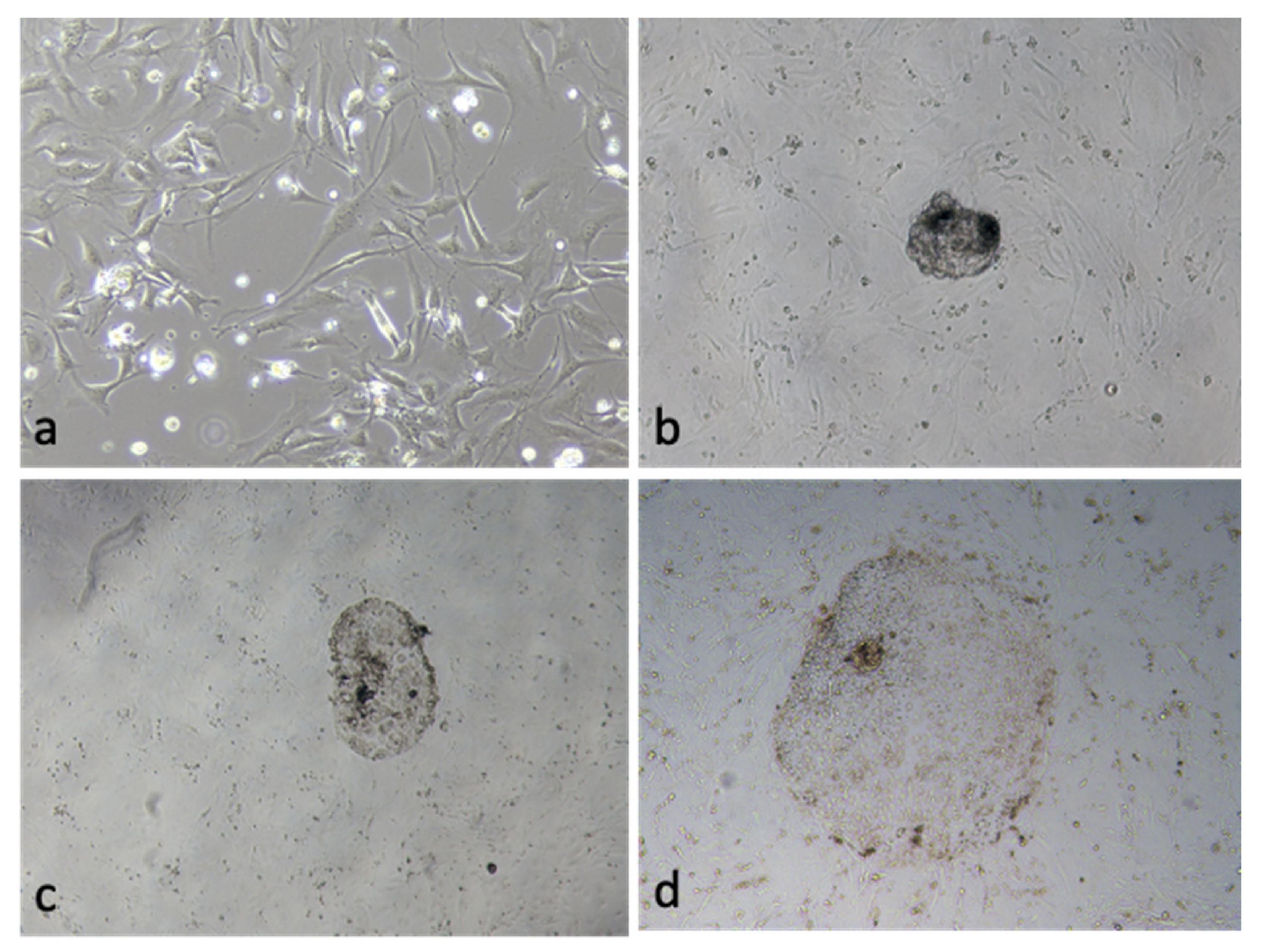
3.5. Effect of OEC EVs on Blastocyst Attachment
3.6. Gene Expression of the OEC EVs Treatment Blastocyst
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shiue, Y.L.; Yang, J.R.; Liao, Y.J.; Kuo, T.Y.; Liao, C.H.; Kang, C.H.; Tai, C.; Anderson, G.B.; Chen, L.R. Derivation of porcine pluripotent stem cells for biomedical research. Theriogenology 2016, 86, 176–181. [Google Scholar] [CrossRef]
- Ezashi, T.; Yuan, Y.; Roberts, R.M. Pluripotent Stem Cells from Domesticated Mammals. Annu. Rev. Anim. Biosci. 2016, 4, 223–253. [Google Scholar] [CrossRef]
- Brevini, T.; Pennarossa, G.; Maffei, S.; Gandolfi, F. Pluripotency network in porcine embryos and derived cell lines. Reprod. Domest. Anim. Zuchthyg. 2012, 47 (Suppl. S4), 86–91. [Google Scholar] [CrossRef]
- Brevini, T.A.; Vassena, R.; Francisci, C.; Gandolfi, F. Role of adenosine triphosphate, active mitochondria, and microtubules in the acquisition of developmental competence of parthenogenetically activated pig oocytes. Biol. Reprod. 2005, 72, 1218–1223. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Son, H.Y.; Kim, S.; Lee, G.S.; Park, C.H.; Kang, S.K.; Lee, B.C.; Hwang, W.S.; Lee, C.K. Isolation and initial culture of porcine inner cell masses derived from in vitro-produced blastocysts. Zygote 2007, 15, 55–63. [Google Scholar] [CrossRef]
- Miyoshi, K.; Taguchi, Y.; Sendai, Y.; Hoshi, H.; Sato, E. Establishment of a porcine cell line from in vitro-produced blastocysts and transfer of the cells into enucleated oocytes. Biol. Reprod. 2000, 62, 1640–1646. [Google Scholar] [CrossRef][Green Version]
- Yang, H.; Shi, L.; Wang, B.A.; Liang, D.; Zhong, C.; Liu, W.; Nie, Y.; Liu, J.; Zhao, J.; Gao, X.; et al. Generation of genetically modified mice by oocyte injection of androgenetic haploid embryonic stem cells. Cell 2012, 149, 605–617. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, M.; Hou, D.; Han, X.; Jin, Y.; Zhao, L.; Nie, X.; Zhou, X.; Yun, T.; Zhao, Y.; et al. Karyotype characterization of in vivo- and in vitro-derived porcine parthenogenetic cell lines. PLoS ONE 2014, 9, e97974. [Google Scholar] [CrossRef]
- Peñalosa-Ruiz, G.; Bright, A.R.; Mulder, K.W.; Veenstra, G.J.C. The interplay of chromatin and transcription factors during cell fate transitions in development and reprogramming. Biochim. Biophys. Acta Gene Regul. Mech. 2019, 1862, 194407. [Google Scholar] [CrossRef]
- Meehan, R.R.; Thomson, J.P.; Lentini, A.; Nestor, C.E.; Pennings, S. DNA methylation as a genomic marker of exposure to chemical and environmental agents. Curr. Opin. Chem. Biol. 2018, 45, 48–56. [Google Scholar] [CrossRef]
- Bradley, J.; Swann, K. Mitochondria and lipid metabolism in mammalian oocytes and early embryos. Int. J. Dev. Biol. 2019, 63, 93–103. [Google Scholar] [CrossRef]
- Alcântara-Neto, A.S.; Schmaltz, L.; Caldas, E.; Blache, M.C.; Mermillod, P.; Almiñana, C. Porcine oviductal extracellular vesicles interact with gametes and regulate sperm motility and survival. Theriogenology 2020, 155, 240–255. [Google Scholar] [CrossRef]
- Harris, E.A.; Stephens, K.K.; Winuthayanon, W. Extracellular Vesicles and the Oviduct Function. Int. J. Mol. Sci. 2020, 21, 8280. [Google Scholar] [CrossRef]
- Liu, C.; Yin, H.; Jiang, H.; Du, X.; Wang, C.; Liu, Y.; Li, Y.; Yang, Z. Extracellular Vesicles Derived from Mesenchymal Stem Cells Recover Fertility of Premature Ovarian Insufficiency Mice and the Effects on their Offspring. Cell Transplant. 2020, 29, 963689720923575. [Google Scholar] [CrossRef]
- de Almeida Monteiro Melo Ferraz, M.; Fujihara, M.; Nagashima, J.B.; Noonan, M.J.; Inoue-Murayama, M.; Songsasen, N. Follicular extracellular vesicles enhance meiotic resumption of domestic cat vitrified oocytes. Sci. Rep. 2020, 10, 8619. [Google Scholar] [CrossRef]
- Lee, S.H.; Saadeldin, I.M. Exosomes as a Potential Tool for Supporting Canine Oocyte Development. Animals 2020, 10, 1971. [Google Scholar] [CrossRef]
- Matsuno, Y.; Kanke, T.; Maruyama, N.; Fujii, W.; Naito, K.; Sugiura, K. Characterization of mRNA profiles of the exosome-like vesicles in porcine follicular fluid. PLoS ONE 2019, 14, e0217760. [Google Scholar] [CrossRef]
- da Silveira, J.C.; Andrade, G.M.; Simas, R.C.; Martins-Júnior, H.A.; Eberlin, M.N.; Smith, L.C.; Perecin, F.; Meirelles, F.V. Lipid profile of extracellular vesicles and their relationship with bovine oocyte developmental competence: New players in intra follicular cell communication. Theriogenology 2021, 174, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.-K.; Qamar, A.-Y.; Tanga, B.-M.; Bang, S.; Seong, G.; Fang, X.; Kim, G.; Edirisinghe, S.-L.; De Zoysa, M.; Kang, D.-H.; et al. Modified Spirulina maxima Pectin Nanoparticles Improve the Developmental Competence of In Vitro Matured Porcine Oocytes. Animals 2021, 11, 2483. [Google Scholar] [CrossRef] [PubMed]
- Saadeldin, I.M.; Kim, S.J.; Choi, Y.B.; Lee, B.C. Improvement of Cloned Embryos Development by Co-Culturing with Parthenotes: A Possible Role of Exosomes/Microvesicles for Embryos Paracrine Communication. Cell. Reprogram. 2014, 16, 223–234. [Google Scholar] [CrossRef]
- Mehdiani, A.; Maier, A.; Pinto, A.; Barth, M.; Akhyari, P.; Lichtenberg, A. An innovative method for exosome quantification and size measurement. J. Vis. Exp. JoVE 2015, e50974. [Google Scholar] [CrossRef]
- Lee, H.; Yun, S.H.; Hyon, J.-y.; Lee, S.-Y.; Yi, Y.-S.; Choi, C.-W.; Jun, S.; Park, E.C.; Kim, S.I. Streptococcus equi-derived extracellular vesicles as a vaccine candidate against Streptococcus equi infection. Vet. Microbiol. 2021, 259, 109165. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Yun, S.H.; Lee, H.; Yi, Y.-S.; Park, E.C.; Kim, W.; Kim, H.-Y.; Lee, J.C.; Kim, G.-H.; Kim, S.I. Analysis of the Extracellular Proteome of Colistin-Resistant Korean Acinetobacter baumannii Strains. ACS Omega 2020, 5, 5713–5720. [Google Scholar] [CrossRef] [PubMed]
- Saadeldin, I.M.; Swelum, A.A.-A.; Elsafadi, M.; Mahmood, A.; Osama, A.; Shikshaky, H.; Alfayez, M.; Alowaimer, A.N.; Magdeldin, S. Thermotolerance and plasticity of camel somatic cells exposed to acute and chronic heat stress. J. Adv. Res. 2020, 22, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef] [PubMed]
- Qamar, A.Y.; Fang, X.; Kim, M.J.; Cho, J. Improved viability and fertility of frozen-thawed dog sperm using adipose-derived mesenchymal stem cells. Sci. Rep. 2020, 10, 7034. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, J.B. Pitfalls associated with lipophilic fluorophore staining of extracellular vesicles for uptake studies. J. Extracell. Vesicles 2019, 8, 1582237. [Google Scholar] [CrossRef]
- Takov, K.; Yellon, D.M.; Davidson, S.M. Confounding factors in vesicle uptake studies using fluorescent lipophilic membrane dyes. J. Extracell. Vesicles 2017, 6, 1388731. [Google Scholar] [CrossRef]
- Abumaghaid, M.M.; Abdelazim, A.M.; Belali, T.M.; Alhujaily, M.; Saadeldin, I.M. Shuttle Transfer of mRNA Transcripts via Extracellular Vesicles from Male Reproductive Tract Cells to the Cumulus–Oocyte Complex in Rabbits (Oryctolagus cuniculus). Front. Vet. Sci. 2022, 9, 816080. [Google Scholar] [CrossRef]
- Cho, J.; Kim, G.; Qamar, A.Y.; Fang, X.; Roy, P.K.; Tanga, B.M.; Bang, S.; Kim, J.K.; Galli, C.; Perota, A.; et al. Improved efficiencies in the generation of multigene-modified pigs by recloning and using sows as the recipient. Zygote 2021, 30, 103–110. [Google Scholar] [CrossRef]
- Yang, L.; Church, G.; Zhao, H.Y.; Huang, L.; Gao, Y.; Wei, H.J.; Yang, G. Porcine germline genome engineering. Proc. Natl. Acad. Sci. USA 2021, 118, e2004836117. [Google Scholar] [CrossRef]
- Qu, P.; Zhao, J.; Hu, H.; Cao, W.; Zhang, Y.; Qi, J.; Meng, B.; Zhao, J.; Liu, S.; Ding, C.; et al. Loss of Renewal of Extracellular Vesicles: Harmful Effects on Embryo Development in vitro. Int. J. Nanomed. 2022, 17, 2301–2318. [Google Scholar] [CrossRef] [PubMed]
- Ashok, D.; Sood, T.J.; Sah, S.; Palta, P.; Mukesh, M.; Chauhan, M.S.; Manik, R.S.; Singla, S.K. Buffalo SCNT embryos exhibit abnormal gene expression of ERK/MAPK pathway and DNA methylation. Reprod. Domest. Anim. Zuchthyg. 2018, 53, 1247–1252. [Google Scholar] [CrossRef]
- Cuthbert, J.M.; Russell, S.J.; Polejaeva, I.A.; Meng, Q.; White, K.L.; Benninghoff, A.D. Comparing mRNA and sncRNA profiles during the maternal-to-embryonic transition in bovine IVF and scNT embryos. Biol. Reprod. 2021, 105, 1401–1415. [Google Scholar] [CrossRef] [PubMed]
- Villata, S.; Canta, M.; Cauda, V. EVs and Bioengineering: From Cellular Products to Engineered Nanomachines. Int. J. Mol. Sci. 2020, 21, 6048. [Google Scholar] [CrossRef]
- Vader, P.; Mol, E.A.; Pasterkamp, G.; Schiffelers, R.M. Extracellular vesicles for drug delivery. Adv. Drug Deliv. Rev. 2016, 106, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy, L.A.; Pink, R.C.; Carter, D.R. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles 2014, 3, 24641. [Google Scholar] [CrossRef] [PubMed]
- Laheri, S.; Ashary, N.; Bhatt, P.; Modi, D. Oviductal glycoprotein 1 (OVGP1) is expressed by endometrial epithelium that regulates receptivity and trophoblast adhesion. J. Assist. Reprod. Genet. 2018, 35, 1419–1429. [Google Scholar] [CrossRef]
- Alcântara-Neto, A.S.; Fernandez-Rufete, M.; Corbin, E.; Tsikis, G.; Uzbekov, R.; Garanina, A.S.; Coy, P.; Almiñana, C.; Mermillod, P. Oviduct fluid extracellular vesicles regulate polyspermy during porcine in vitro fertilisation. Reprod. Fertil. Dev. 2020, 32, 409–418. [Google Scholar] [CrossRef]
- Fang, X.; Tanga, B.M.; Bang, S.; Seong, G.; Saadeldin, I.M.; Lee, S.; Cho, J. Oviduct epithelial cells-derived extracellular vesicles improve preimplantation developmental competence of in vitro produced porcine parthenogenetic and cloned embryos. Mol. Reprod. Dev. 2022, 89, 54–65. [Google Scholar] [CrossRef]
- Bannikova, S.; Zorov, D.B.; Shoeman, R.L.; Tolstonog, G.V.; Traub, P. Stability and association with the cytomatrix of mitochondrial DNA in spontaneously immortalized mouse embryo fibroblasts containing or lacking the intermediate filament protein vimentin. DNA Cell Biol. 2005, 24, 710–735. [Google Scholar] [CrossRef]
- Yoisungnern, T.; Das, J.; Choi, Y.J.; Parnpai, R.; Kim, J.H. Effect of hexavalent chromium-treated sperm on in vitro fertilization and embryo development. Toxicol. Ind. Health 2016, 32, 1700–1710. [Google Scholar] [CrossRef] [PubMed]
- Jaber, M.; Sebban, S.; Buganim, Y. Acquisition of the pluripotent and trophectoderm states in the embryo and during somatic nuclear reprogramming. Curr. Opin. Genet. Dev. 2017, 46, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Hassani, S.N.; Moradi, S.; Taleahmad, S.; Braun, T.; Baharvand, H. Transition of inner cell mass to embryonic stem cells: Mechanisms, facts, and hypotheses. Cell. Mol. Life Sci. CMLS 2019, 76, 873–892. [Google Scholar] [CrossRef]
- Sasaki, H. Mechanisms of trophectoderm fate specification in preimplantation mouse development. Dev. Growth Differ. 2010, 52, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.; Ganguly, A.; Home, P.; Bhattacharya, B.; Ray, S.; Ghosh, A.; Rumi, M.A.K.; Marsh, C.; French, V.A.; Gunewardena, S.; et al. TEAD4 ensures postimplantation development by promoting trophoblast self-renewal: An implication in early human pregnancy loss. Proc. Natl. Acad. Sci. USA 2020, 117, 17864–17875. [Google Scholar] [CrossRef]
- Gourain, V.; Duluc, I.; Domon-Dell, C.; Freund, J.N. A Core Response to the CDX2 Homeoprotein During Development and in Pathologies. Front. Genet. 2021, 12, 744165. [Google Scholar] [CrossRef]
- Saadeldin, I.M.; Tanga, B.M.; Bang, S.; Fang, X.; Yoon, K.-Y.; Lee, S.; Cho, J. The theranostic roles of extracellular vesicles in pregnancy disorders. J. Anim. Reprod. Biotechnol. 2022, 37, 2–12. [Google Scholar] [CrossRef]

| Genes | Primer Sequences (5′→3′) | Product Size (bp) | Accession No. |
|---|---|---|---|
| GAPDH | F: AGAAGGTGGTGAAGCAGG R: AGCTTGACGAAGTGGTCG | 154 | NM_001206359.1 |
| BAX | F: ACTTCCTTCGAGATCGGC R: GGCCACGAAGATGGTCAC | 110 | XM_003127290 |
| Bcl-2 | F: TTCTCTCGTCGCTACCGC R: CCAGTTCACCCCATCCCT | 123 | XM_021099593 |
| POU5F1 | F: GCCAGAAGGGCAAACGAT R: AGGGTGGTGAAGTGAGGG | 154 | NM_001113060 |
| NANOG | F: AGACTTGGAATAGCCAGC R: CCGCAGTACTTTGAAGTC | 151 | NM_001129971 |
| SOX2 | F: TACAGCATGATGCAGGAC R: GAGCTGGTCATGGAGTTG | 128 | NM_001123197 |
| VIM | F: CAGGCTCAGATCCAGGAACA R: GTCGGCAAACTTGGACTTGT | 156 | XM_005668107.3 |
| c-Myc | F: CAACGTCAGCTTCACCAACA R: TGGGCAGCAACTCGAATTTC | 164 | NM_001005154.1 |
| Klf4 | F: CCAAACTACCCACCCTTCCT R: CTAGGGGTGAAGAAGGTGGG | 163 | XM_005660316.3 |
| KRT8 | F: AAGCTGGTGTCTGAGTCCTC R: GAATTGGCTTGGAGTTGGGG | 166 | NM_001159615.1 |
| TEAD4 | F: ATCGGATGAGGGCAAGATGT R: GCCTGATCCTTTAGCTTGGC | 159 | NM_001142666.1 |
| CDX2 | F: GTGTTAAACCCCACCGTCACR: CAACCGCACCTGTCTTTACC | 194 | NM_001278769.1 |
| Groups | No. of Embryos | |||
|---|---|---|---|---|
| Cultured | Cleaved (% ± SEM) | Develop to BL (% ± SEM) | Hatched BL (% ± SEM) | |
| Control | 554 | 472 (85.2 ± 0.6) | 118 (21.3 ± 0.8) | 33 (6.0 ± 0.6) |
| OEC EVs | 573 | 495 (86.4 ± 0.9) | 183 (31.9 ± 0.9) * | 82 (14.3 ± 0.6) * |
| Groups | No. of Cells | ||
|---|---|---|---|
| ICM ± SEM | TE ± SEM | ICM/TE % ± SEM | |
| Control | 8.2 ± 0.5 | 29.7 ± 1.5 | 28.4 ± 2.1 |
| OEC-EVs | 15.3 ± 0.6 * | 33.6 ± 1.2 * | 43.7 ± 2.3 * |
| Groups | No. of Cells | ||
|---|---|---|---|
| Cultured BL | Attachment (% ± SEM) | Outgrowth (% ± SEM) | |
| Control | 65 | 19 (29.2 ± 2.5) | 4 (6.2 ± 1.8) |
| OEC-EVs | 85 | 37 (43.5 ± 2.1) * | 12 (14.1 ± 2.9) * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, X.; Tanga, B.M.; Bang, S.; Seo, C.; Kim, H.; Saadeldin, I.M.; Lee, S.; Cho, J. Oviduct Epithelial Cell-Derived Extracellular Vesicles Improve Porcine Trophoblast Outgrowth. Vet. Sci. 2022, 9, 609. https://doi.org/10.3390/vetsci9110609
Fang X, Tanga BM, Bang S, Seo C, Kim H, Saadeldin IM, Lee S, Cho J. Oviduct Epithelial Cell-Derived Extracellular Vesicles Improve Porcine Trophoblast Outgrowth. Veterinary Sciences. 2022; 9(11):609. https://doi.org/10.3390/vetsci9110609
Chicago/Turabian StyleFang, Xun, Bereket Molla Tanga, Seonggyu Bang, Chaerim Seo, Heyyoung Kim, Islam M. Saadeldin, Sanghoon Lee, and Jongki Cho. 2022. "Oviduct Epithelial Cell-Derived Extracellular Vesicles Improve Porcine Trophoblast Outgrowth" Veterinary Sciences 9, no. 11: 609. https://doi.org/10.3390/vetsci9110609
APA StyleFang, X., Tanga, B. M., Bang, S., Seo, C., Kim, H., Saadeldin, I. M., Lee, S., & Cho, J. (2022). Oviduct Epithelial Cell-Derived Extracellular Vesicles Improve Porcine Trophoblast Outgrowth. Veterinary Sciences, 9(11), 609. https://doi.org/10.3390/vetsci9110609











