Experimental Release of Orphaned Wild Felids into a Tropical Rainforest in Southwestern Costa Rica
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
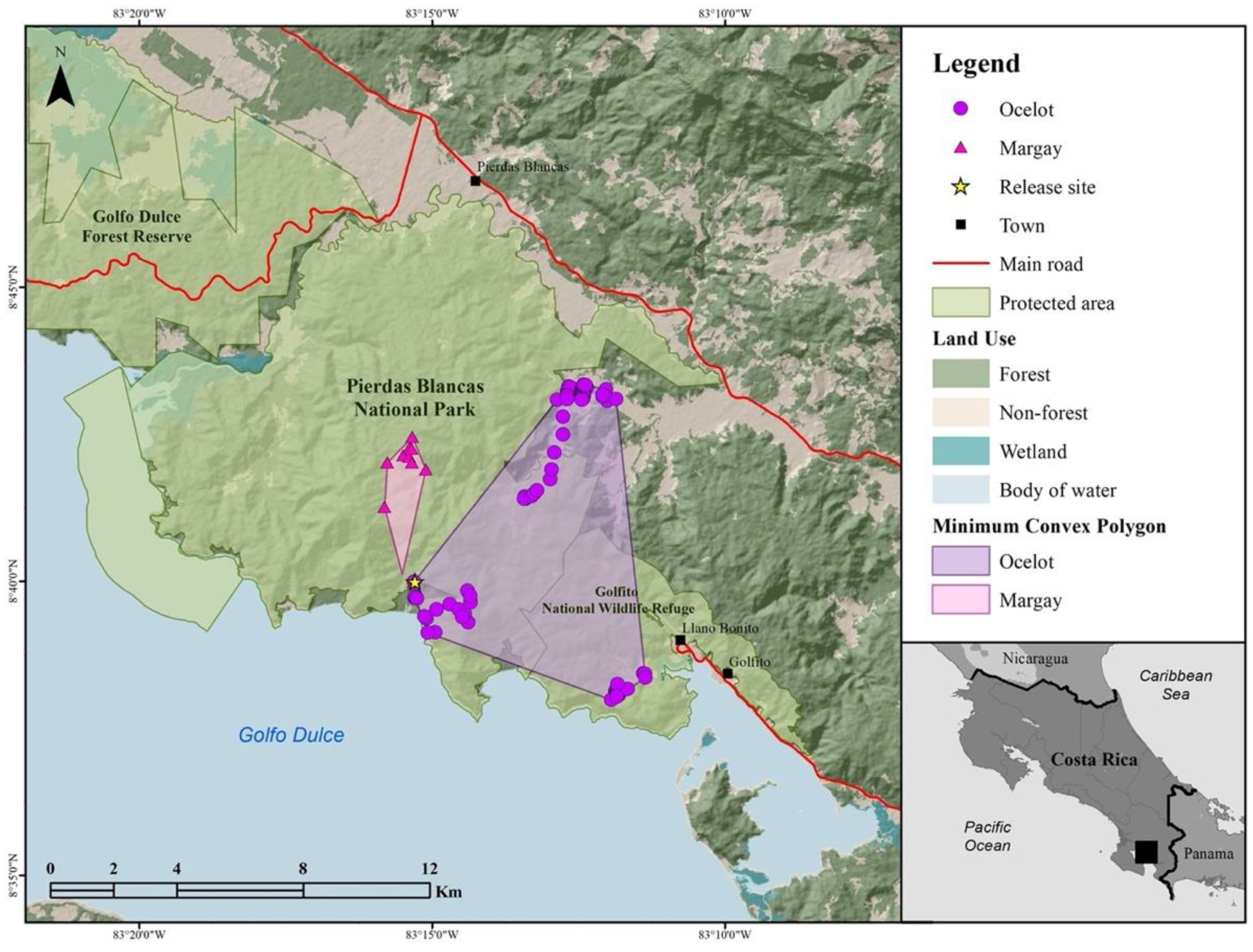
2.1. Rehabilitation and Release Location Sites and Management
2.2. Pre-Release Preparation
2.3. Release Protocol
2.4. Post-Release Monitoring
3. Results
3.1. Preliminary Wildlife Assessment
3.2. Case 1; Ocelot ♂
3.3. Case 2; Margay ♀
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Biomass Class | Scientific Name | Common Name | Weight Class | Ocelot | Margay |
|---|---|---|---|---|---|
| Bird | Patagioenas flavirostris | Red-billed pigeon | 200–500 g | x | |
| Quiscalus mexicanus | Great-tailed grackle | 100–200 g | x | x | |
| Psittacara finschi | Crimson-fronted parakeet | 100–200 g | x | x | |
| Crax rubra | Great curassow | 1–5 kg | x | ||
| Ortalis cinereiceps | Grey-headed chachalaca | 500–1000 g | x | x | |
| Penelope purpurascens | Crested guan | 1–5 kg | x | ||
| Ardea alba | Great egret | 500–1000 g | x | ||
| Pitangus sulphuratus | Flycatcher | <100 g | x | ||
| Columbina inca | Inca dove | <100 g | x | ||
| Momotus lessonii | Lesson’s motmot | <100 g | x | ||
| Glaucidium brasilianum | Ferruginous pygmy owl | <100 g | x | ||
| Turdus grayi | Clay-colored robin | <100 g | x | ||
| Amazona autumnalis | Red-lored amazon | 200–500 g | x | ||
| Brotogeri jugularis | Orange-chinned parakeet | <100 g | x | ||
| Nyctidromus albicollis | Common Pauraque | <100 g | x | ||
| Melanerpes hoffmannii | Hoffmann’s woodpecker | <100 g | x | ||
| Tyrannus melancholicus | Tropical kingbird | <100 g | x | ||
| Psarocolius montezuma | Montezuma oropendola | 200–500 g | x | ||
| Pteroglossus torquatus | collared aracari | 200–500 g | |||
| Columba livia | Common pigeons | 200–500 g | x | x | |
| Zenaida asiatica | White-winged dove | 100–200 g | x | x | |
| Coragyps athratus | Black vulture | 1–5 kg | x | ||
| Dendrocygna autumnalis | black-bellied whistling duck | 500–1000 g | x | ||
| Anas platyrhynchos | Domestic duck | 1–5 kg | x | ||
| Reptile | Trachemys scripta | Pond slider | 200–500 g | x | |
| Iguana iguana | Green iguana | 1–5 kg | x | x | |
| Mammal | Sciurus variegatoides | Variegated squirrels | 200–500 g | x | x |
| Caluromys derbianus | Woolly opossum | 200–500 g | x | x | |
| Odocoileus virginianus | White-tailed deer | 1–5 kg | x | ||
| Didelphys marsupialis | Common opossum | 1–5 kg | x | x | |
| Procyon lotor | Raccoon | 1–5 kg | x | x | |
| Nasua narica | Coati | 1–5 kg | x | x | |
| Cuniculis paca | Paca | 1–5 kg | x | ||
| Dasyprocta puntacta | Agoui | 1–5 kg | x | ||
| Cavia porcellus | Guinea pig | 500–1000 g | x | x | |
| Rattus rattus | Common rat | 100–200 g | x | x | |
| Ratus norvegicus | Brown rat | 200–500 g | x | x | |
| Mus musculus | Common mouse | <100 g | x | x |
References
- Ripple, W.; Estes, J.; Beschta, R.; Wilmers, C.; Ritchie, E.G.; Hebblewhite, M.; Berger, J.; Bodil, E.; Letnic, M.; Nelson, P.; et al. Status and ecological effects of the world’s largest carnivores. Science 2014, 343, 1241484. [Google Scholar] [CrossRef] [PubMed]
- Ceballos, G.; Ehrlich, P.R.; Soberón, J.; Salazar, I.; Fay, J.P. Global mammal conservation: What must we manage? Science 2005, 309, 603–606. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.; Ralls, K.; Reading, R.P.; Scott, M.; Estes, J. Biological and technical considerations of carnivore translocation: A review. Anim. Conserv. 1999, 2, 59–68. [Google Scholar] [CrossRef]
- Briers-Louw, W.D.; Verschueren, S.; Leslie, A. Big cats return to Majete Wildlife Reserve, Malawi: Evaluating reintroduction success. Afr. J. Wildl. Res. 2019, 49, 34–50. [Google Scholar] [CrossRef]
- International Union for Conservation of Nature. Guidelines for Reintroductions and Other Conservation Translocations. Available online: https://portals.iucn.org/library/sites/library/files/documents/2013-009.pdf (accessed on 15 May 2022).
- Kareiva, P.; Marvier, M. Conservation Science, Balancing the Needs of People and Nature; Roberts and Company Publishers Inc.: Denver, CO, USA, 2015; p. 310. [Google Scholar]
- Page, S.K.; Parker, D.M.; Peinke, D.M.; Davies-Mostert, H.T. Assessing the potential threat landscape of a proposed reintroduction site for carnivores. PLoS ONE 2015, 10, e0122782. [Google Scholar]
- Cope, H.R.; McArthur, C.; Dickman, C.R.; Newsome, T.M.; Gray, R.; Herbert, C.A. A systematic review of factors affecting wildlife survival during rehabilitation and release. PLoS ONE 2022, 17, e0265514. [Google Scholar] [CrossRef]
- Mullineaux, E. Veterinary treatment and rehabilitation of indigenous wildlife. J. Small Anim. Pract. 2014, 55, 293–300. [Google Scholar] [CrossRef]
- Hanson, M.; Hollingshead, N.; Schuler, K.; Siemer, W.F.; Martin, P.; Bunting, E.M. Species, causes, and outcomes of wildlife rehabilitation in New York State. PLoS ONE 2021, 16, e0257675. [Google Scholar] [CrossRef]
- Pyke, G.H.; Szabo, J.K. Conservation and the 4 Rs, which are rescue, rehabilitation, release, and research. Conserv. Biol. 2018, 32, 50–59. [Google Scholar] [CrossRef]
- Walker, E.H.; Verschueren, S.; SchmidtKuntzel, A.; Marker, L. Recommendations for the rehabilitation and release of wild-born, captive-raised cheetahs: The importance of pre- and post-release management for optimizing survival. Oryx 2022, 56, 495–504. [Google Scholar] [CrossRef]
- LifeLynx. Lessons Learned from Past Reintroduction and Translocation Efforts with an Emphasis on Carnivores. Available online: https://www.lifelynx.eu/wp-content/uploads/2018/10/Lessons-Carnivore-Reintroduction-Efforts-Final-Version-4.0-2018.pdf (accessed on 15 May 2022).
- Griffith, B.; Scott, M.; Carpenter, W.; Reed, C. Translocation as a species conservation tool: Status and strategy. Science 1989, 245, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Reading, P.; Clark, T. Carnivore Reintroduction: An Interdisciplinary Examination. In Carnivore Behaviour, Ecology and Evolution; Gittleman, J.G., Ed.; Comstock Cornell: New York, NY, USA, 1996; pp. 296–336. [Google Scholar]
- Hayward, M.; Kerley, G.; Adendorff, J.; Moolman, L.; O’Brien, J.; Sholto-Douglas, A.; Bissett, C.; Moolman, L.; Bean, P.; Fogarty, A.; et al. The reintroduction of large carnivores to the Eastern Cape, South Africa: An assessment. Oryx 2007, 41, 205–214. [Google Scholar] [CrossRef]
- Jule, K.R.; Leaver, L.; Lea, S. The effects of captive experience on reintroduction survival in carnivores: A review and analysis. Biol. Conserv. 2008, 141, 355–363. [Google Scholar] [CrossRef]
- Breitenmoser, U.; Breitenmoser-Wursten, C.; Capt, C. Re-introduction and present status of the lynx (Lynx lynx) in Switzerland. Hystrix 1998, 10, 17–30. [Google Scholar]
- Gasparini-Morato, R.; Sartorello, L.; Rampim, L.; Fragoso, C.; May, J.; Teles, P.; Haberfeld, M.; Cunha de Paula, R.; Morato, R. Is reintroduction a tool for the conservation of the jaguar Panthera onca? A case study in the Brazilian Pantanal. Oryx 2021, 55, 461–465. [Google Scholar] [CrossRef]
- Boza, M. National Parks of Costa Rica; Northwestern University Press: Chicago, IL, USA, 1992; p. 91. [Google Scholar]
- Montalvo, V.; Sáenz-Bolaños, C.; Alfaro, L.; Cruz, J.C.; Guimaraes-Rodrigues, F.; Carrillo, E.; Fuller, T. Seasonal use of waterholes and pathways by macrofauna in the dry forest of Costa Rica. J. Trop. Ecol. 2019, 35, 68–73. [Google Scholar] [CrossRef]
- Deem, L.; Karesh, W. The Jaguar Health Program Manual; Field Veterinary Program; Wildlife Conservation Society: New York, NY, USA, 2005; Available online: https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.462.6735&rep=rep1&type=pdf (accessed on 8 March 2015).
- Silkes, R.S.; Gannon, W. Animal Care and Use Committee of The American Society of Mammologists. Guidelines of the American Society of Mammalogists for the use of wild mammals in research. J. Mammal. 2011, 92, 235–253. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015; Available online: http://www.R-project.org/ (accessed on 15 May 2022).
- Calenge, C. The package adehabitat for the R software: Tool for the analysis of space and habitat use by animals. Ecol. Model. 2006, 197, 516–519. [Google Scholar] [CrossRef]
- Houser, A.; Gusset, M.; Bragg, C.J.; Boast, L.K.; Somers, M.J. Pre-release hunting training and post-release monitoring are key components in the rehabilitation of orphaned large felids. S. Afr. J. Wildl. Res. 2011, 41, 11–20. [Google Scholar] [CrossRef]
- Grogan, A.; Kelly, A. A review of RSPCA research into wildlife rehabilitation. Vet. Rec. 2013, 172, 211–215. [Google Scholar] [CrossRef]
- Weber, S.; Weisel, S. Rescate, rehabilitación y liberación de felinos silvestres, en Costa Rica. In Rescate de Fauna en el Neotrópico, Iniciativas y Perspectivas; Drews, C., Ed.; Editorial Universidad Nacional: Heredia, Costa Rica, 1999; pp. 373–392. [Google Scholar]
- Dillon, A.; Kelly, M.J. Ocelot home range, overlap and density: Comparing radio telemetry with camera trapping. J. Zoo 2008, 275, 391–398. [Google Scholar] [CrossRef]
- Oliveira, T.G.; Pereira, J. Intraguild predation and interspecific killing as structuring forces of carnivoran communities in South America. J. Mammal. Evol. 2014, 21, 427–436. [Google Scholar] [CrossRef]
- Massara, L.; Paschoal, R.; Bailey, L.; Doherty, L.F.; Hirsch, P.; Chiarello, G.A. Factors influencing ocelot occupancy in Brazilian Atlantic Forest reserves. Biotropica 2018, 50, 125–134. [Google Scholar] [CrossRef]
- Khan, K.; Mohammed, R.S. Captive ocelots at Trinidad’s Emperor Valley Zoo: Retrospective and suggested management. Living World J. Trinidad Tobago Field Nat. 2015, 2015, 52–56. [Google Scholar]
- Linnell, J.; Breitenmoser, U.; Breitenmoser-Würsten, C.; Odden, J.; Arx, M. Recovery of Eurasian lynx in Europe: What part has reintroduction played? In Reintroduction of Top-Order Predators; Hayward, M., Somers, M., Eds.; John Wiley & Sons, Incorporated: Hoboken, NJ, USA, 2009; pp. 72–91. [Google Scholar]
- Dickens, M.J.; Delehanty, D.J.; Romero, L.M. Stress: An inevitable component of animal translocation. Biol. Conserv. 2010, 143, 1329–1341. [Google Scholar] [CrossRef]
- Wemmer, C.; Sunquist, M.E. Felid reintroductions: Economic and energetic considerations. In Proceedings of the 5th International Snow Leopard Symposium, Srinagar, India, 13–15 October 1986; Freeman, H., Ed.; Wildlife Institute India: Delhi, India, 1988; pp. 193–205. [Google Scholar]
- Milliano, J.; Di Stefano, J.; Courtney, P.; Temple-Smith, P.; Coulson, G. Soft-release versus hard-release for reintroduction of an endangered species: An experimental comparison using eastern barred bandicoots (Perameles gunnii). Wildl. Res. 2016, 43, 1–12. [Google Scholar] [CrossRef]
- Hunter, L.T.B. The Behavioural Ecology of Reintroduced Lions and Cheetahs in the Phinda Resource Reserve, KwaZulu-Natal, South Africa. Ph.D. Thesis, University of Pretoria, Pretoria, South Africa, 1998. [Google Scholar]
- Clarke, R.H.; Boulton, R.L.; Clarke, M.F. Translocation of the socially complex black-eared miner Manorina melanotis: A trial using hard and soft release techniques. Pac. Conserv. Biol. 2002, 8, 223–234. [Google Scholar] [CrossRef]


| Ocelot | Margay | |||
|---|---|---|---|---|
| Percent of Total | Prey Items (n) | Percent of Total | Prey Items (n) | |
| Reptile biomass | ||||
| 200–500 g | — | 0 | 0.9 | 6 |
| 1–5 kg | 4.5 | 41 | — | 0 |
| Bird biomass | ||||
| <100 g | — | 0 | 5.2 | 37 |
| 100–200 g | 5.5 | 50 | 10.4 | 74 |
| 200–500 g | 12.7 | 115 | 18.5 | 131 |
| 500–1000 g | 0.3 | 3 | 3.9 | 28 |
| 1–5 kg | 0.9 | 8 | — | 0 |
| Mammal biomass | ||||
| <100 g | 25.6 | 232 | 46.3 | 328 |
| 100–200 g | 29.0 | 263 | 13.0 | 92 |
| 200–500 g | 16.4 | 149 | 1.8 | 13 |
| 500–1000 g | 4.1 | 37 | — | 0 |
| 1–5 kg | 0.9 | 8 | — | 0 |
| Ocelot | Margay | |
|---|---|---|
| Release age (months) | 19 | 22 |
| 100% Minimum Convex Hull (MCH) (km2) | 44.38 | 3.48 |
| Step dispersal distance (km) | 0.484; CI [0.212–0.757] | 1.171; CI [0.379–1.963] |
| Tracking days (n) | 54 | 20 |
| Locations (n) | 93 | 13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montalvo, V.H.; Hagnauer, I.; Cruz-Díaz, J.C.; Morera, B.; Lloyd, K.; Sáenz-Bolaños, C.; Fuller, T.K.; Carrillo, E. Experimental Release of Orphaned Wild Felids into a Tropical Rainforest in Southwestern Costa Rica. Vet. Sci. 2022, 9, 468. https://doi.org/10.3390/vetsci9090468
Montalvo VH, Hagnauer I, Cruz-Díaz JC, Morera B, Lloyd K, Sáenz-Bolaños C, Fuller TK, Carrillo E. Experimental Release of Orphaned Wild Felids into a Tropical Rainforest in Southwestern Costa Rica. Veterinary Sciences. 2022; 9(9):468. https://doi.org/10.3390/vetsci9090468
Chicago/Turabian StyleMontalvo, Víctor H., Isabel Hagnauer, Juan C. Cruz-Díaz, Brayan Morera, Kevin Lloyd, Carolina Sáenz-Bolaños, Todd K. Fuller, and Eduardo Carrillo. 2022. "Experimental Release of Orphaned Wild Felids into a Tropical Rainforest in Southwestern Costa Rica" Veterinary Sciences 9, no. 9: 468. https://doi.org/10.3390/vetsci9090468





