Expression of Cell-Cycle Regulatory Proteins pRb, Cyclin D1, and p53 Is Not Associated with Recurrence Rates of Equine Sarcoids
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Selection and Study Design
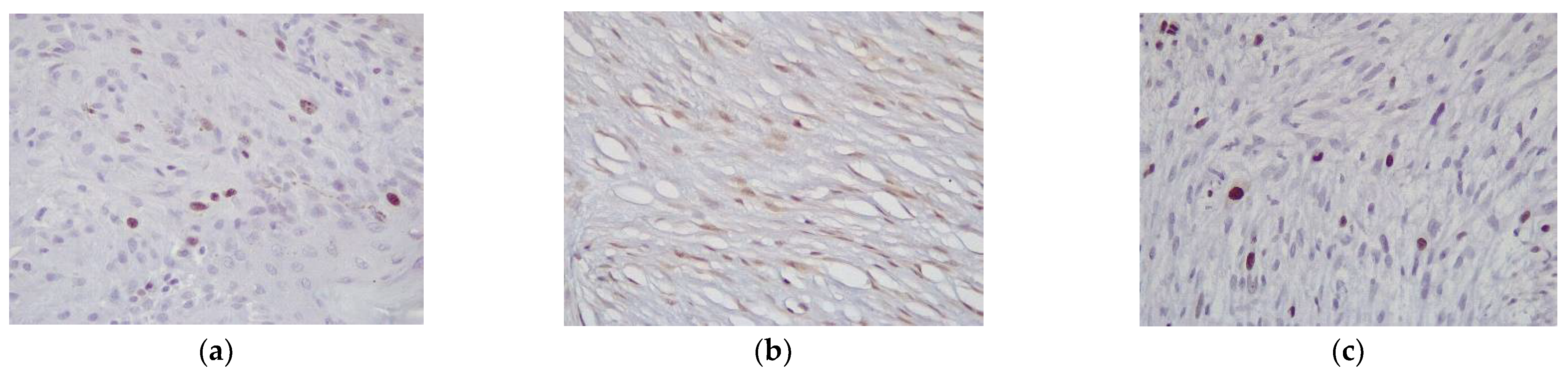
2.2. Immunohistochemistry
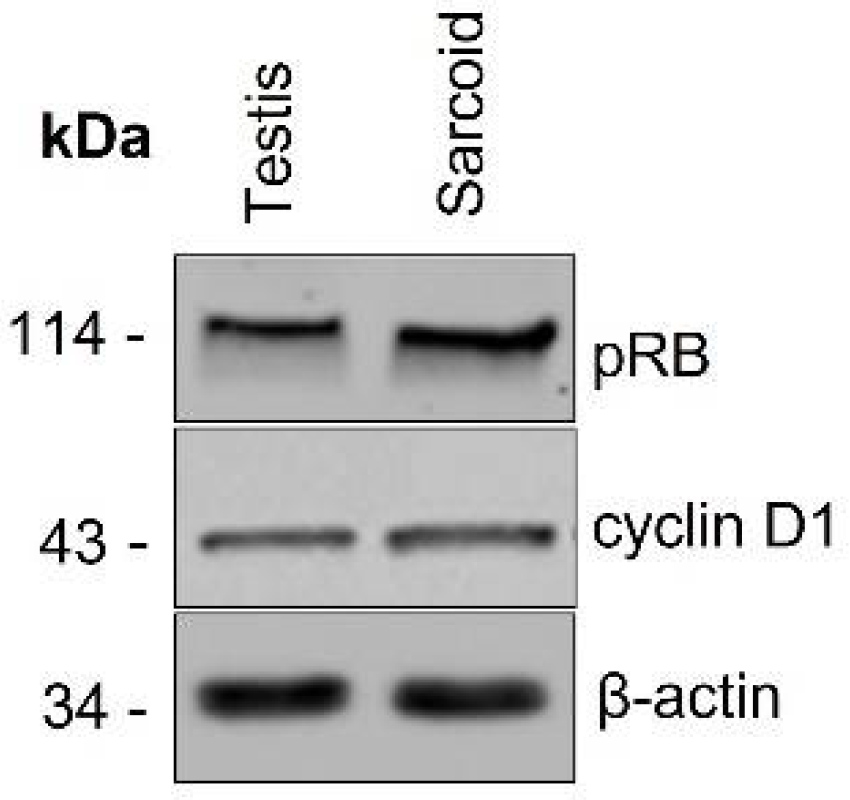
2.3. Western Blotting
2.4. Follow-Up
2.5. Statistical Analysis
3. Results
3.1. Population Characteristics
3.2. Western Blotting and Immunohistochemistry
3.3. Follow-Up
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Knowles, E.J.; Tremaine, W.H.; Pearson, G.R.; Mair, T.S. A Database Survey of Equine Tumours in the United Kingdom. Equine Vet. J. 2016, 48, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, P.A.; Wobeser, B.; Martin, L.E.R.; Dennis, M.M.; Duncan, C.G. Cutaneous Neoplastic Lesions of Equids in the Central United States and Canada: 3351 Biopsy Specimens from 3272 Equids (2000–2010). J. Am. Vet. Med. Assoc. 2013, 242, 99–104. [Google Scholar] [CrossRef]
- Wobeser, B.K.; Davies, J.L.; Hill, J.E.; Jackson, M.L.; Kidney, B.A.; Mayer, M.N.; Townsend, H.G.G.; Allen, A.L. Epidemiology of Equine Sarcoids in Horses in Western Canada. Can. Vet. J. 2010, 51, 1103–1108. [Google Scholar]
- Bergvall, K.E. Sarcoids. Vet. Clin. N. Am. Equine Pract. 2013, 29, 657–671. [Google Scholar] [CrossRef] [PubMed]
- Knottenbelt, D.C. Sarcoids. In Clinical Equine Oncology; Knottenbelt, D.C., Patterson-Kane, J., Snalune, K.L., Eds.; Elsevier: Toronto, ON, Canada, 2015; pp. 203–229. [Google Scholar]
- Chambers, G.; Ellsmore, V.A.; O’Brien, P.M.; Reid, S.W.J.; Love, S.; Campo, M.S.; Nasir, L. Association of Bovine Papillomavirus with the Equine Sarcoid. J. Gen. Virol. 2003, 84, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Finlay, M.; Yuan, Z.; Morgan, I.M.; Campo, M.S.; Nasir, L. Equine Sarcoids: Bovine Papillomavirus Type 1 Transformed Fibroblasts Are Sensitive to Cisplatin and UVB Induced Apoptosis and Show Aberrant Expression of P53. Vet. Res. 2012, 43, 81. [Google Scholar] [CrossRef]
- Schafer, K.A. The Cell Cycle: A Review. Vet. Pathol. 1998, 478, 461–478. [Google Scholar] [CrossRef]
- Altamura, G.; Corteggio, A.; Nasir, L.; Yuan, Z.Q.; Roperto, F.; Borzacchiello, G. Analysis of Activated Platelet-Derived Growth Factor β Receptor and Ras-MAP Kinase Pathway in Equine Sarcoid Fibroblasts. BioMed Res. Int. 2013, 2013, 283985. [Google Scholar] [CrossRef]
- Borzacchiello, G.; Mogavero, S.; De Vita, G.; Roperto, S.; Della Salda, L.; Roperto, F. Activated Platelet-Derived Growth Factor Beta Receptor Expression, PI3K-AKT Pathway Molecular Analysis, and Transforming Signals in Equine Sarcoids. Vet. Pathol. 2009, 46, 589–597. [Google Scholar] [CrossRef]
- Nasir, L.; McFarlane, S.T.; Reid, S.W.J. Mutational Status of the Tumour Suppressor Gene (P53) in Donkey Sarcoid Tumours. Vet. J. 1999, 157, 99–101. [Google Scholar] [CrossRef]
- Bucher, K.; Szalai, G.; Marti, E.; Griot-Wenk, M.E.; Lazary, S.; Pauli, U. Tumour Suppressor Gene P53 in the Horse: Identification, Cloning, Sequencing and a Possible Role in the Pathogenesis of Equine Sarcoid. Res. Vet. Sci. 1996, 61, 114–119. [Google Scholar] [CrossRef]
- Martens, A.; De Moor, A.; Demeulemeester, J.; Ducatelle, R. Histopathological Characteristics of Five Clinical Types of Equine Sarcoid. Res. Vet. Sci. 2000, 69, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Nixon, C.; Chambers, G.; Ellsmore, V.; Campo, M.S.; Burr, P.; Argyle, D.J.; Reid, S.W.J.; Nasir, L. Expression of Cell Cycle Associated Proteins Cyclin A, CDK-2, P27 Kip1 and P53 in Equine Sarcoids. Cancer Lett. 2005, 221, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Tomita, Y.; Ogawa, T.; Jin, Z.; Shirasawa, H. Genus Specific Features of Bovine Papillomavirus E6, E7, E5 and E8 Proteins. Virus Res. 2007, 124, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Narechania, A.; Terai, M.; Chen, Z.; DeSalle, R.; Burk, R.D. Lack of the Canonical PRB-Binding Domain in the E7 ORF of Artiodactyl Papillomaviruses Is Associated with the Development of Fibropapillomas. J. Gen. Virol. 2004, 85, 1243–1250. [Google Scholar] [CrossRef]
- Tura, G.; Savini, F.; Gallina, L.; La Ragione, R.M.; Durham, A.E.; Mazzeschi, M.; Lauriola, M.; Avallone, G.; Sarli, G.; Brunetti, B.; et al. Fibroblast-Associated Protein-α Expression and BPV Nucleic Acid Distribution in Equine Sarcoids. Vet. Pathol. 2021, 58, 1–7. [Google Scholar] [CrossRef]
- Gavressea, T.; Kalogeras, K.T.; Koliou, G.A.; Zagouri, F.; Lazaridis, G.; Gogas, H.; Tsigaridas, K.; Koutras, A.; Petraki, K.; Markopoulos, C.; et al. The Prognostic Value of the Immunohistochemical Expression of Phosphorylated RB and P16 Proteins in Association with Cyclin D1 and the P53 Pathway in a Large Cohort of Patients with Breast Cancer Treated with Taxane-Based Adjuvant Chemotherapy. Anticancer Res. 2017, 37, 2947–2957. [Google Scholar] [CrossRef]
- Kusume, T.; Tsuda, H.; Kawabata, M.; Inoue, T. The P16-Cyclin D1/CDK4-PRb Pathway and Clinical Outcome in Epithelial Ovarian Cancer 1. Clin. Cancer Res. 1999, 5, 4152–4157. [Google Scholar]
- Nicolás, I.; Saco, A.; Barnadas, E.; Marimon, L.; Rakislova, N.; Fusté, P.; Rovirosa, A.; Gaba, L.; Buñesch, L.; Gil-Ibañez, B.; et al. Prognostic Implications of Genotyping and P16 Immunostaining in HPV-Positive Tumors of the Uterine Cervix. Mod. Pathol. 2020, 33, 128–137. [Google Scholar] [CrossRef]
- Karpathiou, G.; Monaya, A.; Forest, F.; Froudarakis, M.; Casteillo, F.; Marc Dumollard, J.; Prades, J.M.; Peoc’h, M. P16 and P53 Expression Status in Head and Neck Squamous Cell Carcinoma: A Correlation with Histological, Histoprognostic and Clinical Parameters. Pathology 2016, 48, 341–348. [Google Scholar] [CrossRef]
- Jiromaru, R.; Yamamoto, H.; Yasumatsu, R.; Hongo, T.; Nozaki, Y.; Nakano, T.; Hashimoto, K.; Nakagawa, T.; Oda, Y. P16 Overexpression and Rb Loss Correlate with High-risk HPV Infection in Oropharyngeal Squamous Cell Carcinoma. Histopathology 2021, 79, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Huang, C.C.; Otterson, G.A.; Leon, M.E.; Tang, Y.; Shilo, K.; Villalona, M.A. Altered P16 INK4 and RB1 Expressions Are Associated with Poor Prognosis in Patients with Nonsmall Cell Lung Cancer. J. Oncol. 2012, 2012, 957437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plath, M.; Broglie, M.A.; Förbs, D.; Stoeckli, S.J.; Jochum, W. Prognostic Significance of Cell Cycle-Associated Proteins P16, PRB, Cyclin D1 and P53 in Resected Oropharyngeal Carcinoma. J. Otolaryngol. Head Neck Surg. 2018, 47, 53. [Google Scholar] [CrossRef] [PubMed]
- Collard, T.J.; Urban, B.C.; Patsos, H.A.; Hague, A.; Townsend, P.A.; Paraskeva, C.; Williams, A.C. The Retinoblastoma Protein (Rb) as an Anti-Apoptotic Factor: Expression of Rb Is Required for the Antiapoptotic Function of BAG-1 Protein in Colorectal Tumour Cells. Cell Death Dis. 2012, 3, e408–e409. [Google Scholar] [CrossRef]
- Lu, J.-W.; Lin, Y.-M.; Chang, J.-G.; Yeh, K.-T.; Chen, R.-M.; Tsai, J.J.P.; Su, W.-W.; Hu, R.-M. Clinical Implications of Deregulated CDK4 and Cyclin D1 Expression in Patients with Human Hepatocellular Carcinoma. Med. Oncol. 2013, 30, 379. [Google Scholar] [CrossRef]
- Cheng, G.; Zhang, L.; Lv, W.; Dong, C.; Wang, Y.; Zhang, J. Overexpression of Cyclin D1 in Meningioma Is Associated with Malignancy Grade and Causes Abnormalities in Apoptosis, Invasion and Cell Cycle Progression. Med. Oncol. 2015, 32, 439. [Google Scholar] [CrossRef]
- Song, J.Y.; Song, L.; Herrera, A.F.; Venkataraman, G.; Murata-collins, J.L.; Bedell, V.H.; Chen, Y.Y.; Kim, Y.S.; Tadros, R.; Nathwani, B.N.; et al. Cyclin D1 Expression in Peripheral T-Cell Lymphomas. Mod. Pathol. 2016, 29, 1306–1312. [Google Scholar] [CrossRef]
- Zamboni, C.; Brocca, G.; Ferraresso, S.; Ferro, S.; Sammarco, A.; Dal Corso, C.; Iussich, S.; de Andres, P.J.; Martìnez de Merlo, E.M.; Cavicchioli, L.; et al. Cyclin D1 Immunohistochemical Expression and Somatic Mutations in Canine Oral Melanoma. Vet. Comp. Oncol. 2019, 18, 231–238. [Google Scholar] [CrossRef]
- Araldi, R.P.; Mazzuchelli-de-Souza, J.; Modolo, D.G.; Souza, E.B.D.; Melo, T.C.D.; Spadacci-Morena, D.D.; Magnelli, R.F.; Carvalho, M.A.C.R.D.; de Sá Júnior, P.L.; Carvalho, R.F.D.; et al. Mutagenic Potential of Bos Taurus Papillomavirus Type 1 E6 Recombinant Protein: First Description. BioMed Res. Int. 2015, 2015, 806361. [Google Scholar] [CrossRef]
- Haspeslagh, M.; Vlaminck, L.E.M.; Martens, A.M. Treatment of Sarcoids in Equids: 230 Cases (2008–2013). J. Am. Vet. Med. Assoc. 2016, 249, 311–318. [Google Scholar] [CrossRef]

| Protein | Score | Percent (%) | Fibroblastic n(%) | Mixed n (%) | Nodular n (%) | Occult n (%) | Verrucous n (%) | p Value |
|---|---|---|---|---|---|---|---|---|
| pRb | low (score 1) | 51% | 5 (55.6) | 1 (20.0) | 16 (55.2) | 1 (33.3) | 5 (55.6) | 0.618 |
| high (score 2–3) | 49% | 4 (44.4) | 4 (80.0) | 13 (44.8) | 2 (66.7) | 4 (44.4) | ||
| Cyclin D1 | low (score 1) | 20% | 2 (22.2) | 1 (20.0) | 5 (17.2) | 3 (100.0) | 0 (0.0) | 0.006 * |
| high (score 2–3) | 80% | 7 (77.8) | 4 (80.0) | 24 (82.8) | 0 (0.0) | 9 (100.0) | ||
| Ki67 Median [95% CI] | 5.45 | 7.30 [2.88, 11.25] | 7.01 [2.71, 7.72] | 5.95 [2.24, 20.00] | 1.75 [1.00, 4.20] | 3.45 [2.90, 6.24] | 0.015 * | |
| Ki67 | low | 49% | 4 (44.4) | 2 (40.0) | 10 (34.5) | 3 (100.0) | 8 (88.9) | 0.021 * |
| high | 51% | 5 (55.6) | 3 (60.0) | 19 (65.5) | 0 (0.0) | 1 (11.1) |
| Clinical Type | LR | Median Tume to LR (95% CI) | DNO (Distant) | Median Time to DNO (95% CI) | No Recurrence |
|---|---|---|---|---|---|
| Fibroblastic (8) | 7 | 145 (60–180) | 0 | - | 1 |
| Mixed (2) | 2 | 150 (108-na) | 1 0 | - | 0 |
| Nodular (13) | 0 | - | 1 | - | 12 |
| Occult (2) | 0 | - | 0 | - | 2 |
| Verrucous (5) | 0 | - | 4 | 205 (95-na) 1 | 1 |
| Cell Cycle Protein | Low | High | p Value |
|---|---|---|---|
| pRb | 14 | 16 | 0.189 |
| Cyclin D1 | 8 | 22 | 0.212 |
| Ki67 | 12 | 18 | 0.664 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tura, G.; Brunetti, B.; Brigandì, E.; Rinnovati, R.; Sarli, G.; Avallone, G.; Muscatello, L.V.; La Ragione, R.M.; Durham, A.E.; Bacci, B. Expression of Cell-Cycle Regulatory Proteins pRb, Cyclin D1, and p53 Is Not Associated with Recurrence Rates of Equine Sarcoids. Vet. Sci. 2022, 9, 474. https://doi.org/10.3390/vetsci9090474
Tura G, Brunetti B, Brigandì E, Rinnovati R, Sarli G, Avallone G, Muscatello LV, La Ragione RM, Durham AE, Bacci B. Expression of Cell-Cycle Regulatory Proteins pRb, Cyclin D1, and p53 Is Not Associated with Recurrence Rates of Equine Sarcoids. Veterinary Sciences. 2022; 9(9):474. https://doi.org/10.3390/vetsci9090474
Chicago/Turabian StyleTura, Giorgia, Barbara Brunetti, Elena Brigandì, Riccardo Rinnovati, Giuseppe Sarli, Giancarlo Avallone, Luisa Vera Muscatello, Roberto Marcello La Ragione, Andy E. Durham, and Barbara Bacci. 2022. "Expression of Cell-Cycle Regulatory Proteins pRb, Cyclin D1, and p53 Is Not Associated with Recurrence Rates of Equine Sarcoids" Veterinary Sciences 9, no. 9: 474. https://doi.org/10.3390/vetsci9090474





