Implication of Netrin-1 Gain of Expression in Canine Nodal Lymphoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Selection
2.2. Bioinformatic Comparison between Human and Canine Netrin-1
2.3. Detection of Netrin-1 Expression by Immunohistochemistry
2.4. Quantification of Intracellular Netrin-1 by Flow Cytometry
2.5. Quantification of Netrin-1 Expression by RT-qPCR
2.6. Lymphoma Cell Cultures and Cell Death Assays
2.7. Statistical Analyses
3. Results
3.1. Netrin-1 Expression in Normal Lymph Nodes and in Nodal Lymphomas
3.1.1. Netrin-1 Expression in Normal Lymph Nodes
3.1.2. Netrin-1 Expression in Nodal Lymphomas
3.2. Netrin-1 Quantification in Normal Lymph Node and in Nodal Lymphoma
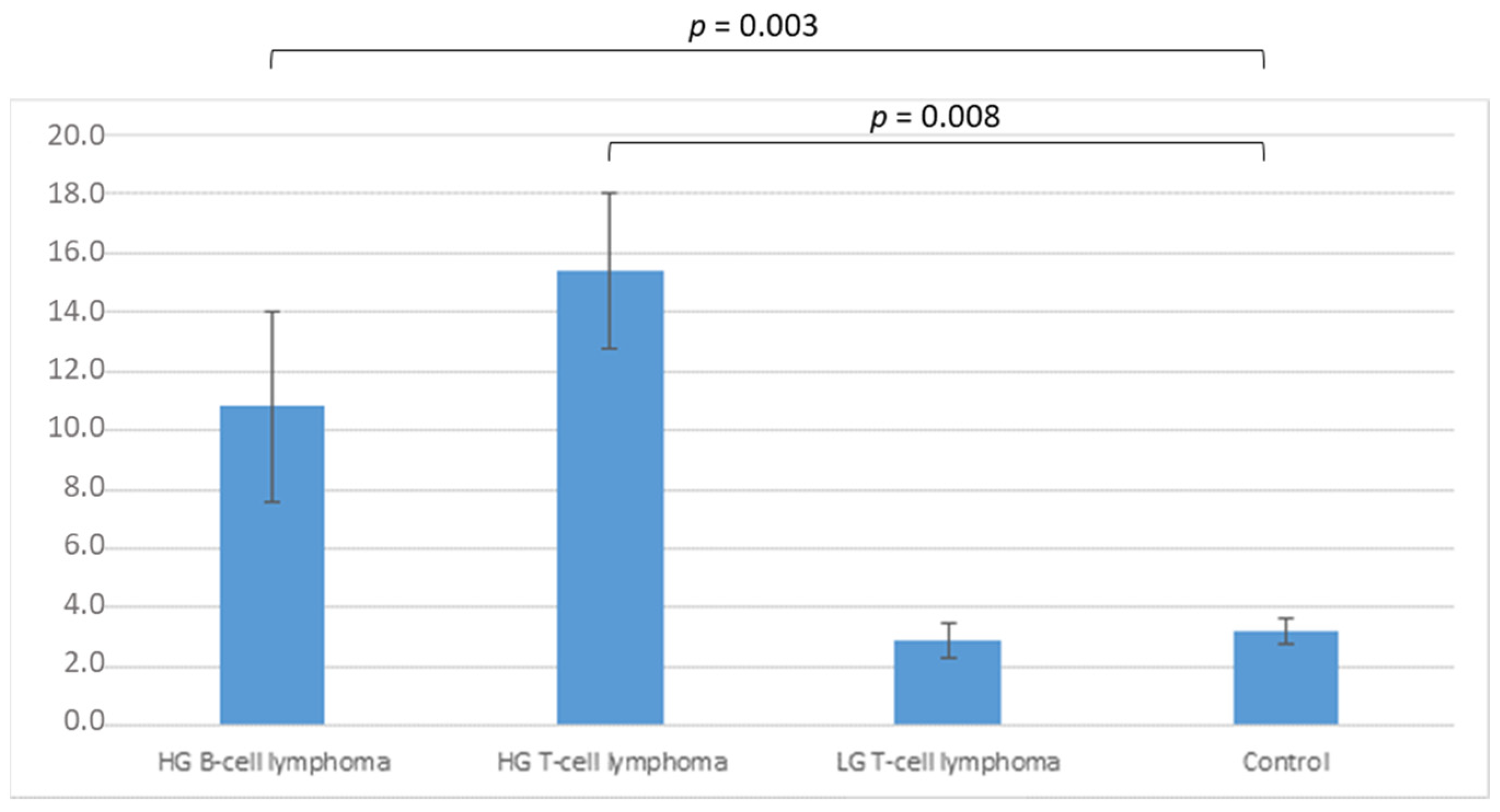
3.2.1. Flow Cytometry Analysis
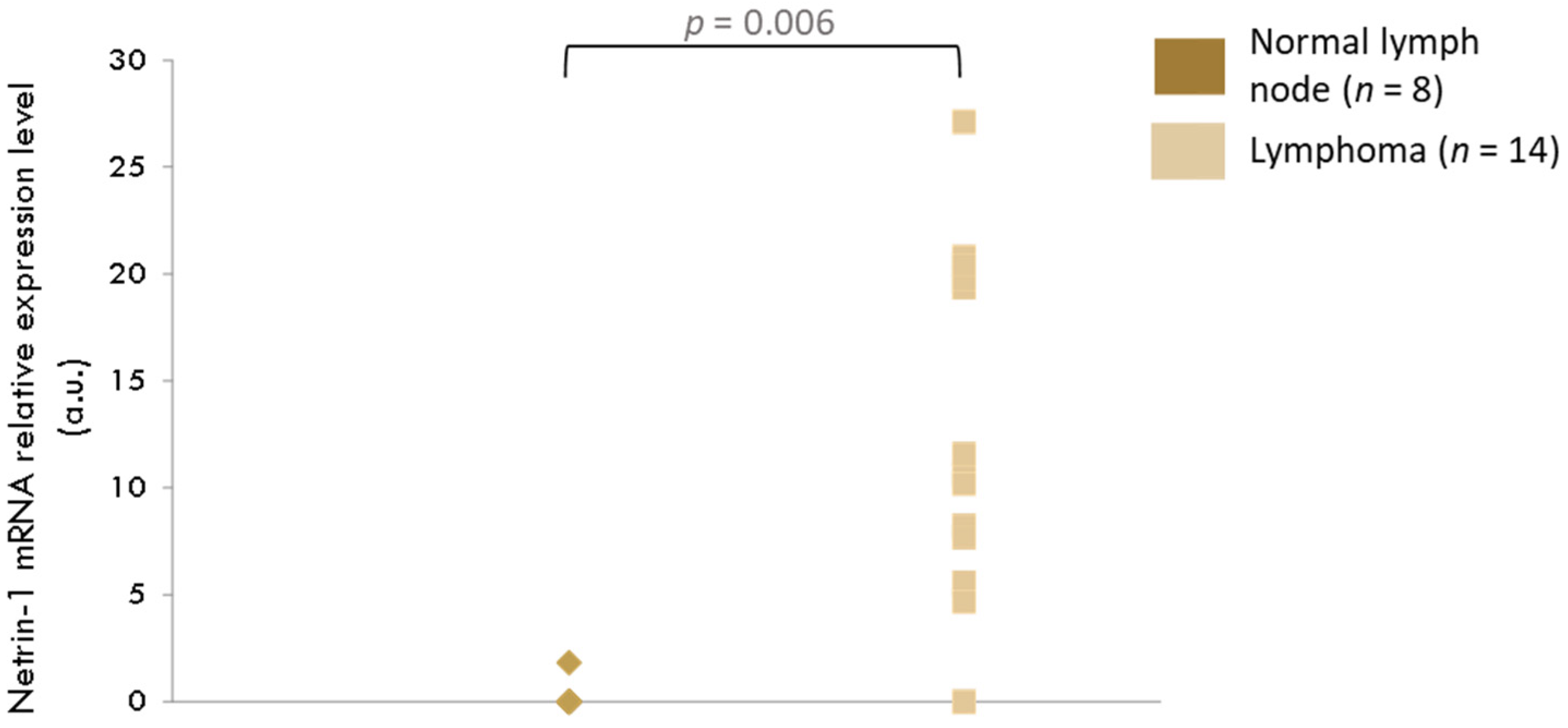
3.2.2. RT-qPCR Analysis
3.3. In Vitro effect of Netrin-1 on Lymphoma Cells
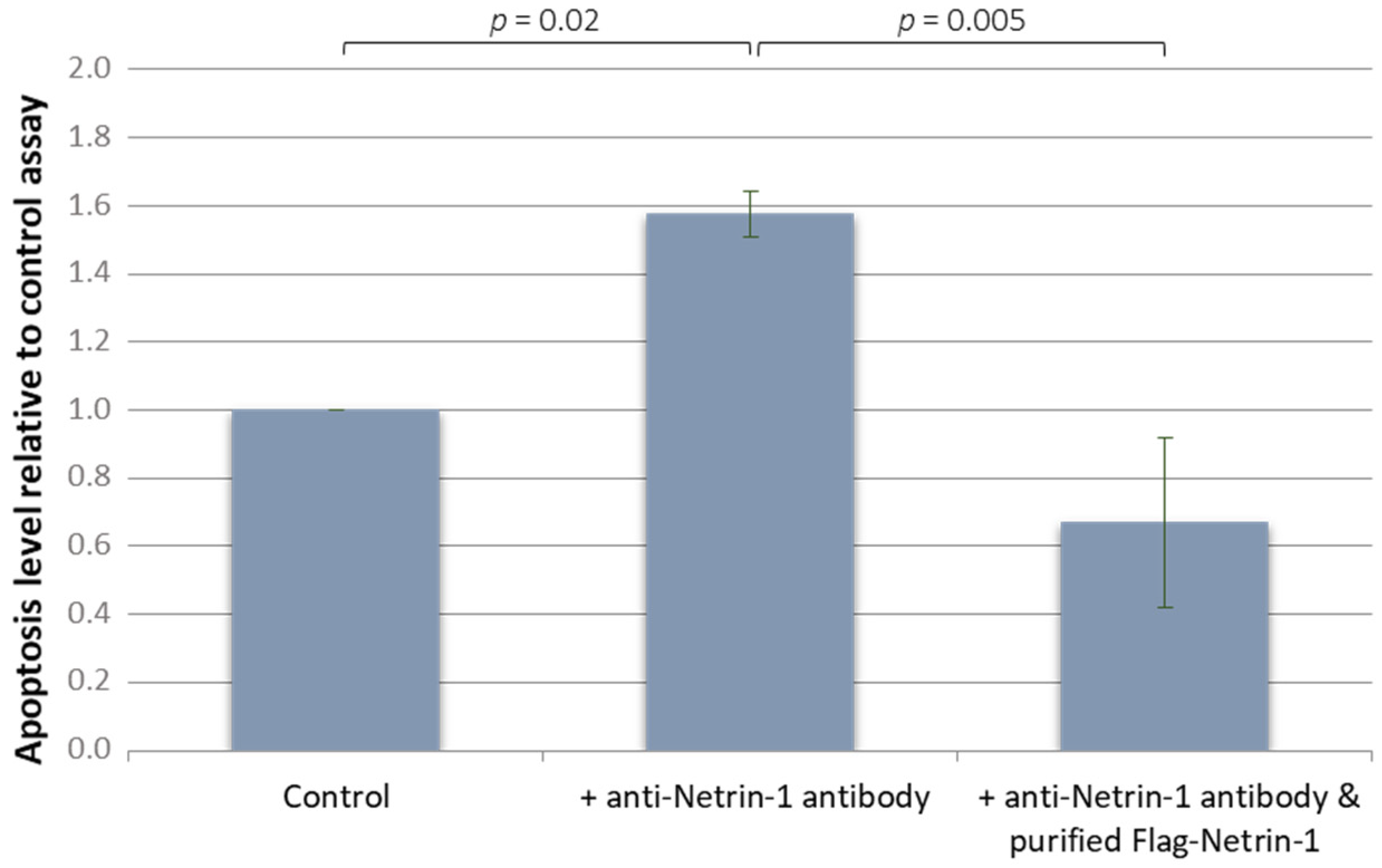
3.3.1. Effect on Lymphoma Cell Line
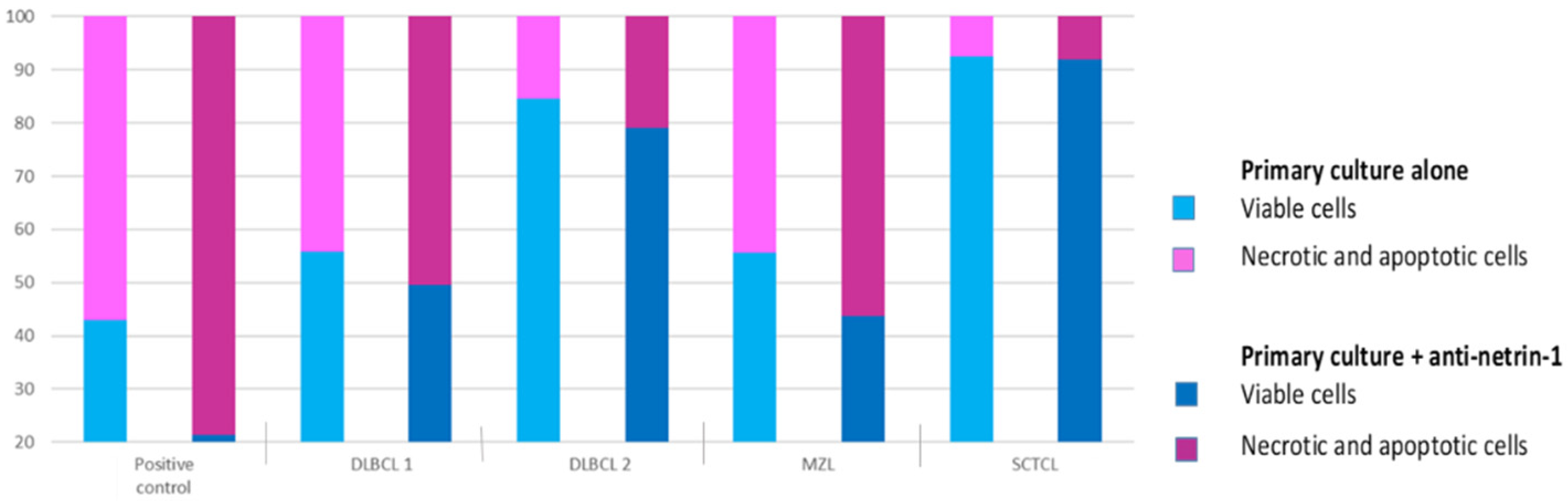
3.3.2. Effect on Primary Canine Nodal Lymphoma Cell
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moore, S.W.; Tessier-Lavigne, M.; Kennedy, T.E. Netrins and their receptors. Adv. Exp. Med. Biol. 2007, 621, 17–31. [Google Scholar] [PubMed]
- Mehlen, P.; Furne, C. Netrin-1: When a neuronal guidance cue turns out to be a regulator of tumorigenesis. Cell Mol. Life Sci. 2005, 62, 2599. [Google Scholar] [CrossRef] [PubMed]
- Bradford, D.; Cole, S.J.; Cooper, H.M. Netrin-1: Diversity in development. Int. J. Biochem. Cell Biol. 2009, 41, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Mehlen, P.; Rabizadeh, S.; Snipas, S.J.; Assa-Munt, N.; Salvesen, G.S.; Bredesen, D.E. The DCC gene product induces apoptosis by a mechanism requiring receptor proteolysis. Nature 1998, 395, 801–804. [Google Scholar] [CrossRef]
- Arakawa, H. Netrin-1 and its receptors in tumorigenesis. Nat. Rev. Cancer 2004, 4, 978–987. [Google Scholar] [CrossRef]
- Castets, M.; Coissieux, M.-M.; Delloye-Bourgeois, C.; Bernard, L.; Delcros, J.-G.; Bernet, A.; Laudet, V.; Mehlen, P. Inhibition of endothelial cell apoptosis by netrin-1 during angiogenesis. Dev. Cell 2009, 16, 614–620. [Google Scholar] [CrossRef]
- Castets, M.; Mehlen, P. Netrin-1 role in angiogenesis: To be or not to be a pro-angiogenic factor? Cell Cycle 2010, 9, 1466–1471. [Google Scholar] [CrossRef]
- Barallobre, M.J.; Pascual, M.; Del Río, J.A.; Soriano, E. The Netrin family of guidance factors: Emphasis on Netrin-1 signalling. Brain Res. Brain Res. Rev. 2005, 49, 22–47. [Google Scholar] [CrossRef]
- Bredesen, D.E.; Mehlen, P.; Rabizadeh, S. Receptors that mediate cellular dependence. Cell Death Differ. 2005, 12, 1031–1043. [Google Scholar] [CrossRef]
- Mehlen, P.; Bredesen, D.E. The dependence receptor hypothesis. Apoptosis 2004, 9, 37–49. [Google Scholar] [CrossRef]
- Mehlen, P.; Mazelin, L. The dependence receptors DCC and UNC5H as a link between neuronal guidance and survival. Biol. Cell 2003, 95, 425–436. [Google Scholar] [CrossRef]
- Llambi, F.; Causeret, F.; Bloch-Gallego, E.; Mehlen, P. Netrin-1 acts as a survival factor via its receptors UNC5H and DCC. EMBO J. 2001, 20, 2715–2722. [Google Scholar] [CrossRef] [PubMed]
- Castets, M.; Broutier, L.; Molin, Y.; Brevet, M.; Chazot, G.; Gadot, N.; Paquet, A.; Mazelin, L.; Jarrosson-Wuilleme, L.; Scoazec, J.-Y.; et al. DCC constrains tumour progression via its dependence receptor activity. Nature 2011, 482, 534–537. [Google Scholar] [CrossRef] [PubMed]
- Krimpenfort, P.; Song, J.Y.; Proost, N.; Zevenhoven, J.; Jonkers, J.; Berns, A. Deleted in colorectal carcinoma suppresses metastasis in p53-deficient mammary tumours. Nature 2012, 482, 538–541. [Google Scholar] [CrossRef] [PubMed]
- Bernet, A.; Mazelin, L.; Coissieux, M.; Gadot, N.; Ackerman, S.L.; Scoazec, J.; Mehlen, P. Inactivation of the UNC5C Netrin-1 receptor is associated with tumor progression in colorectal malignancies. Gastroenterology 2007, 133, 1840–1848. [Google Scholar] [CrossRef]
- Broutier, L.; Creveaux, M.; Vial, J.; Tortereau, A.; Delcros, J.; Chazot, G.; McCaron, M.J.; Léon, S.; Pangault, C.; Gadot, N.; et al. Targeting netrin-1/DCC interaction in diffuse large B-cell and mantle cell lymphomas. EMBO Mol. Med. 2016, 8, 96–104. [Google Scholar] [CrossRef]
- Valli, V.E.; Bienzle, D.; Meuten, D.J. Tumors of the Hemolymphatic System. In Tumors in Domestic Animals; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 203–321. [Google Scholar]
- Valli, V.E.; Myint, M.S.; Barthel, A.; Bienzle, D.; Caswell, J.; Colbatzky, F.; Durham, A.; Ehrhart, E.J.; Johnson, Y.; Jones, C.; et al. Classification of canine malignant lymphomas according to the World Health Organization criteria. Vet. Pathol. 2011, 48, 198–211. [Google Scholar] [CrossRef]
- Ponce, F.; Marchal, T.; Magnol, J.P.; Turinelli, V.; Ledieu, D.; Bonnefont-Rebeix, C.; Pastor, M.; Delignette, M.L.; Fournel-Fleury, C.A. Morphological study of 608 cases of canine malignant lymphoma in France with a focus on comparative similarities between canine and human lymphoma morphology. Vet. Pathol. 2010, 47, 414–433. [Google Scholar] [CrossRef]
- Breen, M.; Modiano, J.F. Evolutionarily conserved cytogenetic changes in hematological malignancies of dogs and humans--man and his best friend share more than companionship. Chromosome Res. 2008, 16, 145–154. [Google Scholar] [CrossRef]
- Valli, V.E.; Kass, P.H.; San Myint, M.; Scott, F. Canine lymphomas: Association of classification type, disease stage, tumor subtype, mitotic rate, and treatment with survival. Vet. Pathol. 2013, 50, 738–748. [Google Scholar] [CrossRef]
- Wang, L.; Qin, W.; Huo, Y.-J.; Li, X.; Shi, Q.; Rasko, J.E.J.; Janin, A.; Zhao, W.-L. Advances in targeted therapy for malignant lymphoma. Sig. Transduct. Target Ther. 2020, 5, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Fournel-Fleury, C.; Magnol, J.; Bricaire, P.; Marchal, T.; Chabanne, L.; Delverdier, A.; Bryon, P.; Felman, P. Cytohistological and immunological classification of canine malignant lymphomas: Comparison with human non-Hodgkin’s lymphomas. J. Comp. Pathol. 1997, 117, 35–59. [Google Scholar] [CrossRef]
- Bonnefont-Rebeix, C.; Fournel-Fleury, C.; Ponce, F.; Belluco, S.; Watrelot, D.; E Bouteille, S.; Rapiteau, S.; Razanajaona-Doll, D.; Pin, J.-J.; Leroux, C.; et al. Characterization of a novel canine T-cell line established from a spontaneously occurring aggressive T-cell lymphoma with large granular cell morphology. Immunobiology 2016, 221, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Galtier, N.; Gouy, M.; Gautier, C. SEAVIEW and PHYLO_WIN: Two graphic tools for sequence alignment and molecular phylogeny. Comput. Appl. Biosci. 1996, 12, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Fellman, C.L.; Stokes, J.V.; Archer, T.M.; Pinchuk, L.M.; Lunsford, K.V.; Mackin, A.J. Cyclosporine A affects the in vitro expression of T cell activation-related molecules and cytokines in dogs. Vet. Immunol. Immunopathol. 2011, 140, 175–180. [Google Scholar] [CrossRef]
- Brinkhof, B.; Spee, B.; Rothuizen, J.; Penning, L.C. Development and evaluation of canine reference genes for accurate quantification of gene expression. Anal. Biochem. 2006, 356, 36–43. [Google Scholar] [CrossRef]
- NCBI Resource Coordinators. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2018, 46, D8–D13. [Google Scholar] [CrossRef]
- Latil, A.; Chêne, L.; Cochant-Priollet, B.; Mangin, P.; Fournier, G.; Berthon, P.; Cussenot, O. Quantification of expression of netrins, slits and their receptors in human prostate tumors. Int. J. Cancer 2003, 103, 306–315. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Bernet, A.; Mehlen, P. Netrin-1 and its dependence receptors: Role in colorectal cancers. Pathol. Biol. 2005, 53, 328–333. [Google Scholar] [CrossRef]
- Ly, N.P.; Komatsuzaki, K.; Fraser, I.P.; Tseng, A.A.; Prodhan, P.; Moore, K.J.; Kinane, T.B. Netrin-1 inhibits leukocyte migration in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2005, 102, 14729–14734. [Google Scholar] [CrossRef]
- Wang, W.; Reeves, W.B.; Ramesh, G. Netrin-1 and kidney injury. I. Netrin-1 protects against ischemia-reperfusion injury of the kidney. Am. J. Physiol. Ren. Physiol. 2008, 294, F739–F747. [Google Scholar] [CrossRef] [PubMed]
- Delloye-Bourgeois, C.; Goldschneider, D.; Paradisi, A.; Therizols, G.; Belin, S.; Hacot, S.; Rosa-Calatrava, M.; Scoazec, J.-Y.; Diaz, J.-J.; Bernet, A.; et al. Nucleolar localization of a netrin-1 isoform enhances tumor cell proliferation. Sci. Signal 2012, 5, ra57. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Verdun, D. Assembly and disassembly of the nucleolus during the cell cycle. Nucleus 2011, 2, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Coqueret, O. New roles for p21 and p27 cell-cycle inhibitors: A function for each cell compartment? Trends Cell Biol. 2003, 13, 65–70. [Google Scholar] [CrossRef]
- Abbas, T.; Dutta, A. p21 in cancer: Intricate networks and multiple activities. Nat. Rev. Cancer 2009, 9, 400–414. [Google Scholar] [CrossRef]
- Fitamant, J.; Guenebeaud, C.; Coissieux, M.-M.; Guix, C.; Treilleux, I.; Scoazec, J.-Y.; Bachelot, T.; Bernet, A.; Mehlen, P. Netrin-1 expression confers a selective advantage for tumor cell survival in metastatic breast cancer. Proc. Natl. Acad. Sci. USA 2008, 105, 4850–4855. [Google Scholar] [CrossRef]
- Delloye-Bourgeois, C.; Brambilla, E.; Coissieux, M.-M.; Guenebeaud, C.; Pedeux, R.; Firlej, V.; Cabon, F.; Brambilla, C.; Mehlen, P.; Bernet, A. Interference with netrin-1 and tumor cell death in non-small cell lung cancer. J. Natl. Cancer Inst. 2009, 101, 237–247. [Google Scholar] [CrossRef]
- Delloye-Bourgeois, C.; Fitamant, J.; Paradisi, A.; Cappellen, D.; Douc-Rasy, S.; Raquin, M.-A.; Stupack, D.; Nakagawara, A.; Rousseau, R.; Combaret, V.; et al. Netrin-1 acts as a survival factor for aggressive neuroblastoma. J. Exp. Med. 2009, 206, 833–847. [Google Scholar] [CrossRef]
- Link, B.-C.; Reichelt, U.; Schreiber, M.; Kaifi, J.T.; Wachowiak, R.; Bogoevski, D.; Bubenheim, M.; Cataldegirmen, G.; Gawad, K.A.; Issa, R.; et al. Prognostic implications of netrin-1 expression and its receptors in patients with adenocarcinoma of the pancreas. Ann. Surg. Oncol. 2007, 14, 2591–2599. [Google Scholar] [CrossRef]
- Papanastasiou, A.D.; Pampalakis, G.; Katsaros, D.; Sotiropoulou, G. Netrin-1 overexpression is predictive of ovarian malignancies. Oncotarget 2011, 2, 363–367. [Google Scholar] [CrossRef][Green Version]
- Mehlen, P.; Puisieux, A. Metastasis: A question of life or death. Nat. Rev. Cancer 2006, 6, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Delcros, J.G.; Mehlen, P. Dependence receptors: Life or death choices. Bull. Cancer 2013, 100, 1261–1274. [Google Scholar] [CrossRef] [PubMed]
- Thiébault, K.; Mazelin, L.; Pays, L.; Llambi, F.; Joly, M.-O.; Scoazec, J.-Y.; Saurin, J.-C.; Romeo, G.; Mehlen, P. The netrin-1 receptors UNC5H are putative tumor suppressors controlling cell death commitment. Proc. Natl. Acad. Sci. USA 2003, 100, 4173–4178. [Google Scholar] [CrossRef] [PubMed]

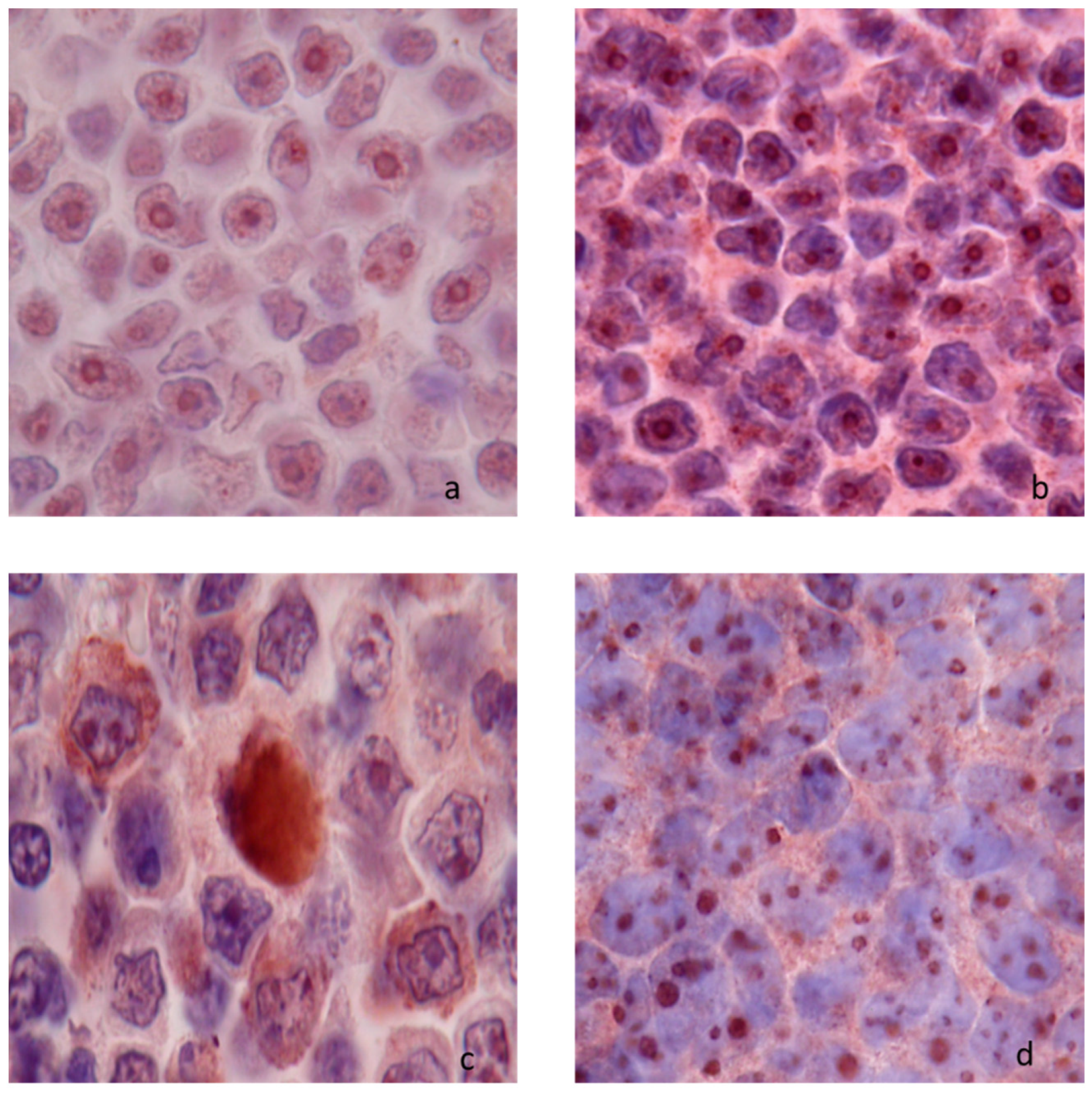
| Semi-Quantitative Level of Expression | Percentage of Immunostained Neoplastic Cells |
|---|---|
| 0 | No expression |
| + | <5% |
| ++ | 5–50% |
| +++ | >50% |
| Primer Sequence (Forward) (5′→3′) | Tm (°C) | Primer Sequence (Reverse) (5′→3′) | Tm (°C) | |
|---|---|---|---|---|
| Netrin-1 | CGAGTGCGTGGGTGATGTAACTGC (24) | 66.1 | GTGGCAATCGCAGGCTTTGCAGGCC (25) | 69.5 |
| Hprt [27] | AGCTTGCTGGTGAAAAGGAC (20) | 56.0 | TTATAGTCAAGGGCATATCC (20) | 56.0 |
| Grade | Morphological Type | n | Nucleolar Expression | Cytoplasmic Expression | ||||
|---|---|---|---|---|---|---|---|---|
| 0 | + | 0 | + | ++ | +++ | |||
| Low-grade | Lymphoplasmacytic | 4 | 0 | 4 | 3 | 0 | 1 | 0 |
| Small cell | 4 | 0 | 4 | 1 | 1 | 2 | 0 | |
| Follicular | 3 | 1 | 2 | 2 | 0 | 1 | 0 | |
| Mantle cell | 1 | 1 | 1 | 0 | 0 | 0 | ||
| Marginal zone | 14 | 2 | 12 | 13 | 0 | 1 | 0 | |
| High-grade | Centroblastic monomorphic | 1 | 0 | 1 | 1 | 0 | 0 | 0 |
| Centroblastic polymorphic | 52 | 5 | 47 | 23 | 16 | 13 | 0 | |
| Immunoblastic | 16 | 2 | 14 | 12 | 1 | 2 | 1 | |
| Burkitt-like | 8 | 2 | 6 | 2 | 1 | 3 | 2 | |
| Small cells NOS | 6 | 3 | 3 | 3 | 1 | 2 | 0 | |
| Grade | n (%) | Nucleolar Expression | Cytoplasmic Expression | ||
|---|---|---|---|---|---|
| 0 | + | 0 | Presence | ||
| Low-grade | 26 (100) | 4 (15.4) | 22 (84.6) | 20 (76.9) | 6 (23.1) |
| High-grade | 83 (100) | 12 (14.4) | 71 (85.6) | 41 (49.4) | 42 (50.6) |
| Grade | Morphological Type | n | Nucleolar Expression | Cytoplasmic Expression | ||||
|---|---|---|---|---|---|---|---|---|
| 0 | + | 0 | + | ++ | +++ | |||
| Lymphoblastic | 13 * | 4 | 8 | 5 | 2 | 6 | 0 | |
| Low-grade | Pleomorphic small cell | 1 | 1 | 0 | 1 | 0 | 0 | 0 |
| Small clear cell (T-zone) | 13 | 4 | 9 | 6 | 4 | 3 | 0 | |
| High-grade | Pleomorphic mixed | 6 | 1 | 5 | 0 | 4 | 2 | 0 |
| Aggressive large granular cell | 20 * | 9 | 10 | 3 | 3 | 12 | 2 | |
| Plasmacytoid | 2 | 2 | 1 | 1 | 0 | 0 | ||
| Anaplastic | 3 | 1 | 2 | 1 | 0 | 2 | 0 | |
| Immunoblastic | 2 | 1 | 1 | 2 | 0 | 0 | 0 | |
| Grade | n (%) | Nucleolar Expression | Cytoplasmic Expression | ||
|---|---|---|---|---|---|
| 0 | + | 0 | Presence | ||
| Lymphoblastic | 13 * (100) | 4 (33.3) | 8 (66.7) | 5 (38.4) | 8 (61.6) |
| Low-grade | 14 (100) | 5 (35.7) | 9 (64.3) | 7 (50.0) | 7 (50.0) |
| High-grade | 33 * (100) | 14 (43.8) | 18 (56.2) | 7 (21.2) | 26 (78.8) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tortereau, A.; Milhau, N.; Rhumy, E.; Castets, M.; Ponce, F.; Mehlen, P.; Marchal, T. Implication of Netrin-1 Gain of Expression in Canine Nodal Lymphoma. Vet. Sci. 2022, 9, 494. https://doi.org/10.3390/vetsci9090494
Tortereau A, Milhau N, Rhumy E, Castets M, Ponce F, Mehlen P, Marchal T. Implication of Netrin-1 Gain of Expression in Canine Nodal Lymphoma. Veterinary Sciences. 2022; 9(9):494. https://doi.org/10.3390/vetsci9090494
Chicago/Turabian StyleTortereau, Antonin, Nadège Milhau, Elodie Rhumy, Marie Castets, Frédérique Ponce, Patrick Mehlen, and Thierry Marchal. 2022. "Implication of Netrin-1 Gain of Expression in Canine Nodal Lymphoma" Veterinary Sciences 9, no. 9: 494. https://doi.org/10.3390/vetsci9090494
APA StyleTortereau, A., Milhau, N., Rhumy, E., Castets, M., Ponce, F., Mehlen, P., & Marchal, T. (2022). Implication of Netrin-1 Gain of Expression in Canine Nodal Lymphoma. Veterinary Sciences, 9(9), 494. https://doi.org/10.3390/vetsci9090494





