Angiotensin II Blood Serum Levels in Piglets, after Intra-Dermal or Intra-Muscular Vaccination against PRRSV
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Farm
2.3. Health History and Pre-Trial Period
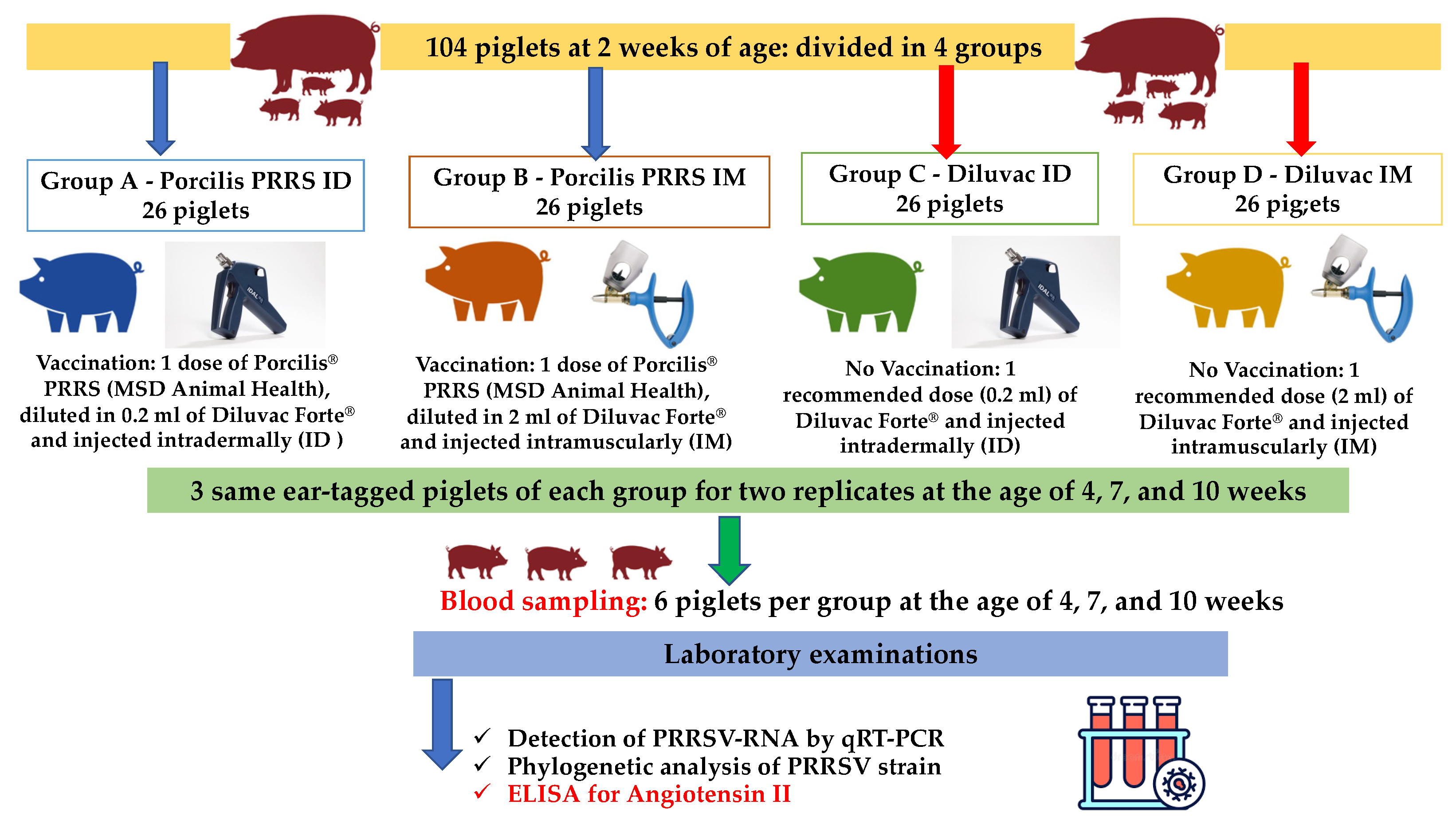
2.4. Study Design
2.5. Sampling/Examinations
2.6. Statistical Analysis
3. Results
3.1. PCR Testing
3.2. Angiotensin II Results
3.3. Correlation between PRRSV Viral Load and Ang II
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Papatsiros, V.G. Porcine Herd Health Management Practices for the Control of PRRSV Infection. In A Bird’s-Eye View of Veterinary Medicine; Perez-Marin, C.C., Ed.; In-Tech: Rijeka, Croatia, 2012. [Google Scholar]
- Zimmerman, J.J.; Benfield, D.A.; Dee, S.A.; Murtaugh, M.P.; Stadejek, T.; Stevenson, G.W.; Torremorell, M. Porcine Reproductive and Respiratory Syndrome. In Diseases of Swine, 10th ed.; John Willey & Sons: Iowa, IA, USA, 2012. [Google Scholar]
- Papatsiros, V.G.; Alexopoulos, C.; Kritas, S.K.; Koptopoulos, G.; Nauwynck, H.J.; Pensaert, M.B.; Kyriakis, S.C. Long-term administration of a commercial porcine reproductive and respiratory syndrome virus (PRRSV)-inactivated vaccine in PRRSV-endemically infected sows. J. Vet. Med. B 2006, 53, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Linhares, D.C.; Johnson, C.; Morrison, R.B. Correction: Economic Analysis of Vaccination Strategies for PRRS Control. PLoS ONE 2016, 11, 0150444. [Google Scholar] [CrossRef]
- Charerntantanakul, W. Porcine reproductive and respiratory syndrome virus vaccines: Immunogenicity, efficacy, and safety aspects. World J. Virol. 2012, 1, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Nan, Y.; Wu, C.; Gu, G.; Sun, W.; Zhang, Y.J.; Zhou, E.M. Improved Vaccine against PRRSV: Current Progress and Future Perspective. Front. Microbiol. 2017, 8, 1635. [Google Scholar] [CrossRef] [PubMed]
- Romani, N.; Flacher, V.; Tripp, C.H.; Sparber, F.; Ebner, S.; Stoitzner, P. Targeting skin dendritic cells to improve intradermal vaccination. Curr. Top. Microbiol. Immunol. 2012, 351, 113–138. [Google Scholar] [CrossRef] [PubMed]
- Teunissen, M.; Haniffa, M.; Collin, M. Insight, into the immunobiology of human skin and functional specialization of skin dendritic cell subsets to innovate intradermal vaccination design. Curr. Top. Microbiol. Immunol. 2012, 351, 25–76. [Google Scholar] [CrossRef] [PubMed]
- Mikulska-Skupien, E.; Szweda, W.; Procajlo, Z.; Platt-Samoraj, A. Indices of nonspecific cellular immune response in pigs after intradermal vaccination with deleted Aujeszky’s disease vaccine and after experimental infection. Bull. Vet. Inst. Pulawy 2004, 48, 347–354. [Google Scholar]
- Martelli, P.; Cordioli, P.; Alborali, L.G.; Gozio, S.; De Angelis, E.; Ferrari, L.; Lombardi, G.; Borghetti, P. Protection and immune response in pigs intradermally vaccinated against porcine reproductive and respiratory syndrome (PRRS) and subsequently exposed to a heterologous European (Italian cluster) field strain. Vaccine 2007, 25, 3400–3408. [Google Scholar] [CrossRef]
- Martelli, P.; Gozio, S.; Ferrari, L.; Rosina, S.; De Angelis, E.; Quintavalla, C.; Bottarelli, E.; Borghetti, P. Efficacy of a modified live porcine reproductive and respiratory syndrome virus (PRRSV) vaccine in pigs naturally exposed to a heterologous European (Italian cluster) field strain: Clinical protection and cell-mediated immunity. Vaccine 2009, 27, 3788–3799. [Google Scholar] [CrossRef]
- Baker, S.R.; Mondaca, E.; Polson, D.; Dee, S.A. Evaluation of a needle-free injection device to prevent hematogenous transmission of porcine reproductive and respiratory syndrome virus. J. Swine Health Prod. 2012, 20, 123–128. [Google Scholar]
- Stadler, J.; Naderer, L.; Beffort, L.; Ritzmann, M.; Emrich, D.; Hermanns, W.; Fiebig, K.; Saalmüller, A.; Gerner, W.; Glatthaar-Saalmüller, B.; et al. Safety and immune responses after intradermal application of Porcilis PRRS in either the neck or the perianal region. PLoS ONE 2018, 13, e0203560. [Google Scholar] [CrossRef] [PubMed]
- Madapong, A.; Saeng-Chuto, K.; Chaikhumwang, P.; Tantituvanont, A.; Saardrak, K.; Pedrazuela Sanz, R.; Miranda Alvarez, J.; Nilubol, D. Immune response and protective efficacy of intramuscular and intradermal vaccination with porcine repro- ductive and respiratory syndrome virus 1 (PRRSV-1) modified live vaccine against highly pathogenic PRRSV-2 (HP-PRRSV-2) challenge, either alone or in combination with of PRRSV-1. Vet. Microbiol. 2020, 244, 108655. [Google Scholar] [CrossRef] [PubMed]
- Sno, M.; Cox, E.; Holtslag, H.; Nell, T.; Pel, S.; Segers, R.; Fachinger, V.; Witvliet, M. Efficacy and safety of a new intradermal PCV2 vaccine in pigs. Trials Vaccinol. 2016, 5, 24–31. [Google Scholar] [CrossRef]
- Ellegaard, B.; Korsgarrd, J.; Nielsen, G.B. Comparison of intradermal and intramuscular porcine circovirus type 2 vaccination methods concerning labor, production parameters, and antimicrobial treatments: A randomized field study in a Danish finishing herd. J. Swine Health Prod. 2021, 29, 129–132. [Google Scholar]
- Tassis, P.D.; Papatsiros, V.G.; Nell, T.; Maes, D.; Alexopoulos, C.; Kyriakis, S.C.; Tzika, E.D. Clinical evaluation of intradermal vaccination against porcine enzootic pneumonia (Mycoplasma hyopneumoniae). Vet. Rec. 2012, 170, 261. [Google Scholar] [CrossRef]
- Lee, S.I.; Jeong, C.G.; Ul Salam Mattoo, S.; Nazki, S.; Prasad Aganja, R.; Kim, S.C.; Khatun, A.; Oh, Y.; Noh, S.H.; Lee, S.M.; et al. Protective immunity induced by concurrent intradermal injection of porcine circovirus type 2 and Mycoplasma hyopneumoniae inactivated vaccines in pigs. Vaccine 2021, 39, 6691–6699. [Google Scholar] [CrossRef] [PubMed]
- Pileri, E.; Mateu, E. Review on the transmission porcine reproductive and respiratory syndrome virus between pigs and farms and impact on vaccination. Vet. Res. 2016, 47, 108. [Google Scholar] [CrossRef]
- Madapong, A.; Saeng-Chuto, K.; Tantituvanont, A.; Nilubol, D. Safety of PRRSV-2 MLV vaccines administrated via the intramuscular or intradermal route and evaluation of PRRSV transmission upon needle-free and needle delivery. Sci. Rep. 2021, 11, 23107. [Google Scholar] [CrossRef]
- Cannon, J.E. Pork quality audit: A review of the factors influencing pork quality 1. J. Muscle Foods 1995, 6, 369–402. [Google Scholar] [CrossRef]
- Chappell, M.C. Biochemical evaluation of the renin-angiotensin system: The good, bad, and absolute? Am. J. Physiol. Heart Circ. Physiol. 2016, 310, 137–152. [Google Scholar] [CrossRef]
- Kobori, H.; Katsurada, A.; Miyata, K.; Ohashi, N.; Satou, R.; Saito, T.; Hagiwara, Y.; Miyashita, K.; Navar, L.G. Determination of plasma and urinary angiotensinogen levels in rodents by newly developed ELISA. Am. J. Physiol. Renal Physiol. 2008, 294, 1257–1263. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, J.L.; Sigmund, C.D. Minireview: Overview of the renin-angiotensin system-an endocrine and paracrine system. Endocrinology 2003, 144, 2179–2183. [Google Scholar] [CrossRef] [PubMed]
- Campbell, D.J. Circulating and tissue angiotensin systems. J. Clin. Investig. 1987, 79, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Johnston, C.I. Franz Volhard Lecture. Renin-angiotensin system: A dual tissue and hormonal system for cardiovascular control. J. Hypertens. Suppl. Off. J. Int. Soc. Hypertens. 1992, 10, 13–26. [Google Scholar]
- Wen, H.; Gwathmey, J.K.; Xie, L.H. Oxidative stress-mediated effects of angiotensin II in the cardiovascular system. World J. Hypertens. 2012, 2, 34–44. [Google Scholar] [CrossRef]
- Phillips, M.I.; Speakman, E.A.; Kimura, B. Levels of angiotensin and molecular biology of the tissue renin angiotensin systems. Regul. Pept. 1993, 43, 1–20. [Google Scholar] [CrossRef]
- Vinson, G.P.; Ho, M.M.; Puddefoot, J.R. The distribution of angiotensin II type 1 receptors, and the tissue renin-angiotensin systems. Mol. Med. Today 1995, 1, 35–39. [Google Scholar] [CrossRef]
- Hernández, J.S.; Barreto-Torres, G.; Kuznetsov, A.V.; Khuchua, Z.; Javadov, S. Crosstalk between AMPK activation and angiotensin II-induced hypertrophy in cardiomyocytes: The role of mitochondria. J. Cell Mol. Med. 2014, 18, 709–720. [Google Scholar] [CrossRef]
- Richardson, M.A.; Gupta, A.; O’Brien, L.A.; Berg, D.T.; Gerlitz, B.; Syed, S.; Sharma, G.R.; Cramer, M.S.; Heuer, J.G.; Galbreath, E.J.; et al. Treatment of sepsis-induced acquired protein C deficiency reverses Angiotensin-converting enzyme-2 inhibition and decreases pulmonary inflammatory response. J. Pharmacol. Exp. Ther. 2008, 325, 17–26. [Google Scholar] [CrossRef]
- Marchesi, C.; Paradis, P.; Schiffrin, E.L. Role of the renin-angiotensin system in vascular inflammation. Trends Pharmacol. Sci. 2008, 29, 367–374. [Google Scholar] [CrossRef]
- Hagiwara, S.; Iwasaka, H.; Matumoto, S.; Hidaka, S.; Noguchi, T. Effects of an angiotensin-converting enzyme inhibitor on the inflammatory response in in vivo and in vitro models. Crit. Care Med. 2009, 37, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zagariya, A.; Ibarra-Sunga, O.; Gidea, C.; Ang, E.; Deshmukh, S.; Chaudhary, G.; Baraboutis, J.; Filippatos, G.; Uhal, B.D. Angiotensin II induces apoptosis in human and rat alveolar epithelial cells. Am. J. Physiol. 1999, 276, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Gao, F.; Sun, B.; Hao, J.; Liu, Z. Angiotensin-converting enzyme 2 inhibits apoptosis of pulmonary endothelial cells during acute lung injury through suppressing SMAD2 phosphorylation. Cell Physiol. Biochem. 2015, 35, 2203–2212. [Google Scholar] [CrossRef]
- Gómez-Laguna, J.; Salguero, F.J.; Pallarés, F.J.; Carrasco, L. Immunopathogenesis of porcine reproductive and respiratory syndrome in the respiratory tract of pigs. Vet. J. 2013, 195, 148–155. [Google Scholar] [CrossRef]
- Morgan, S.B.; Frossard, J.P.; Pallares, F.J.; Gough, J.; Stadejek, T.; Graham, S.P.; Steinbach, F.; Drew, T.W.; Salguero, F.J. Pathology and virus distribution in the lung and lymphoid tissues of pigs experimentally inoculated with three distinct Type 1 PRRS virus isolates of varying pathogenicity. Transbound. Emerg. Dis. 2016, 63, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Costers, S.; Lefebvre, D.J.; Delputte, P.L.; Nauwynck, H.J. Porcine reproductive and respiratory syndrome virus modulates apoptosis during replication in alveolar macrophages. Arch. Virol. 2008, 153, 1453–1465. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Gómez, I.M.; Barranco, I.; Amarilla, S.P.; García-Nicolás, O.; Salguero, F.J.; Carrasco, L.; Gómez-Laguna, J. Activation of extrinsic-and Daxx-mediated pathways in lymphoid tissue of PRRSV-infected pigs. Vet. Microbiol. 2014, 172, 186–194. [Google Scholar] [CrossRef]
- Labarque, G.; Van Gucht, S.; Nauwynck, H.; Van Reeth, K.; Pensaert, M. Apoptosis in the lungs of pigs infected with porcine reproductive and respiratory syndrome virus and associations with the production of apoptogenic cytokines. Vet. Res. 2003, 34, 249–260. [Google Scholar] [CrossRef]
- Wang, R.; Zagariya, A.; Ang, E.; Ibarra-Sunga, O.; Uhal, B.D. Fas-induced apoptosis of alveolar epithelial cells requires ANG II generation and receptor interaction. Am. J. Physiol. 1999, 277, 1245–1250. [Google Scholar] [CrossRef]
- Lee, S.M.; Kleiboeker, S.B. Porcine reproductive and respiratory syndrome virus induces apoptosis through a mitochondria-mediated pathway. Virology 2007, 365, 419–434. [Google Scholar] [CrossRef]
- Chang, H.W.; Jeng, C.R.; Lin, C.M.; Liu, J.J.; Chang, C.C.; Tsai, Y.C.; Chia, M.Y.; Pang, V.F. The involvement of Fas/FasL interaction in porcine circovirus type 2 and porcine reproductive and respiratory syndrome virus co-inoculation-associated lymphocyte apoptosis in vitro. Vet Microbiol. 2007, 122, 72–82. [Google Scholar] [CrossRef]
- Dezsö, B.; Jacobsen, J.; Poulsen, K. Evidence for the presence of angiotensins in normal, unstimulated alveolar macrophages and monocytes. J. Hypertens. 1989, 7, 5–11. [Google Scholar] [CrossRef]
- Jones, A.; Woods, D.R. Skeletal muscle RAS and exercise performance. Int. J. Biochem. Cell Biol. 2003, 35, 855–866. [Google Scholar] [CrossRef]
- Dietze, G.J.; Henriksen, E.J. Angiotensin-converting enzyme in skeletal muscle: Sentinel of blood pressure control and glucose homeostasis. J. Renin Angiotensin Aldosterone Syst. 2008, 9, 75–88. [Google Scholar] [CrossRef] [Green Version]
- Alves, C.R.; Alves, G.B.; Pereira, A.C.; Trombetta, I.C.; Dias, R.G.; Mota, G.F.; Fernandes, T.; Krieger, J.E.; Negrão, C.E.; Oliveira, E.M. Vascular reactivity and ACE activity response to exercise training are modulated by the +9/−9 bradykinin B₂ receptor gene functional polymorphism. Physiol. Genomics 2013, 45, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, D.; Huber-Abel, F.A.; Graber, F.; Hoppeler, H.; Fluck, M. The angiotensin converting enzyme insertion/deletion polymorphism alters the response of muscle energy supply lines to exercise. Eur. J. Appl. Physiol. 2013, 113, 1719–1729. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, D.; Brogioli, M.; Maier, T.; White, A.; Waldron, S.; Rittweger, J.; Toigo, M.; Wettstein, J.; Laczko, E.; Flück, M. The angiotensin-converting enzyme insertion/deletion polymorphism modifies exercise-induced muscle metabolism. PLoS ONE 2016, 11, e0149046. [Google Scholar] [CrossRef] [PubMed]
- Lurchachaiwong, W.; Payungporn, S.; Srisatidnarakul, U.; Mungkundar, C.; Theamboonlers, A.; Poovorawan, Y. Rapid detection and strain identification of porcine reproductive and respiratory syndrome virus (PRRSV) by real-time RT-PCR. Lett. Appl. Microbiol. 2008, 46, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R. Interpretation of the Correlation Coefficient: A Basic Review. J. Diagn. Med. Sonogr. 1990, 6, 35–39. [Google Scholar] [CrossRef]
- Temple, D.; Jiménez, M.; Escribano, D.; Martín-Valls, G.; Díaz, I.; Manteca, X. Welfare Benefits of Intradermal Vaccination of Piglets. Animals 2020, 10, 1898. [Google Scholar] [CrossRef]
- Mitragotri, S. Immunization without needles. Nat. Rev. Immunol. 2005, 5, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Mitragotri, S. Current status and future prospects of needle-free liquid jet injectors. Nat. Rev. Drug Discov. 2006, 5, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Temple, D.; Escribano, D.; Jiménez, M.; Mainau, E.; Cerón, J.J.; Manteca, X. Effect of the needle-free “intra dermal application of liquids” vaccination on the welfare of pregnant sows. Porc. Health Manag. 2017, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Bangert, C.; Brunner, P.M.; Stingl, G. Immune functions of the skin. Clin. Dermatol. 2011, 29, 360–376. [Google Scholar] [CrossRef]
- Göller, M.; Knöppel, H.P.; Fiebig, K.; Kemper, N. Intradermal vaccine application: Effects on suckling behaviour. In Proceedings of the 24th International Pig Veterinary Society Congress, Dublin, Ireland, 7–10 June 2016; p. 625. [Google Scholar]
- Sur, J.H.; Doster, A.R.; Osorio, F.A. Apoptosis induced in vivo during acute infection by porcine reproductive and respiratory syndrome virus. Vet. Pathol. 1998, 35, 506–514. [Google Scholar] [CrossRef]
- He, Y.; Wang, G.; Liu, Y.; Shi, W.; Han, Z.; Wu, J.; Jiang, C.; Wang, S.; Hu, S.; Wen, H.; et al. Characterization of thymus atrophy in piglets infected with highly pathogenic porcine reproductive and respiratory syndrome virus. Vet. Microbiol. 2012, 160, 455–462. [Google Scholar] [CrossRef]
- Karniychuk, U.U.; Saha, D.; Geldhof, M.; Vanhee, M.; Cornillie, P.; Van den Broeck, W.; Nauwynck, H.J. Porcine reproductive and respiratory syndrome virus (PRRSV) causes apoptosis during its replication in fetal implantation sites. Microb. Pathog. 2011, 51, 194–202. [Google Scholar] [CrossRef]
- Maragkakis, G.; Korou, L.M.; Chaintoutis, S.C.; Christodoulopoulos, G.; Dovas, C.I.; Perrea, D.; Athanasiou, L.V.; Konstantopoulos, P.; Maes, D.; Papatsiros, V.G. Investigation of Fas (APO-1)-Related Apoptosis in Piglets Intradermally or Intramuscularly Vaccinated with a Commercial PRRSV MLV. Viral Immunol. 2022, 35, 129–137. [Google Scholar] [CrossRef]
- Ferrari, L.; Martelli, P.; Saleri, R.; De Angelis, E.; Cavalli, V.; Bresaola, M.; Benetti, M.; Borghetti, P. Lymphocyte activation as cytokine gene expression and secretion is related to the porcine reproductive and respiratory syndrome virus (PRRSV) isolate after in vitro homologous and heterologous recall of peripheral blood mononuclear cells (PBMC) from pigs vaccinated and exposed to natural infection. Vet. Immunol. Immunopathol. 2013, 151, 193–206. [Google Scholar] [CrossRef]
| Groups | Blood Sampling/Age | ||
|---|---|---|---|
| 4 Weeks | 7 Weeks | 10 Weeks | |
| Number of PRRSV Positive Samples/Total Samples Average Ct Value (Min–Max) | |||
| Group A | 0/6 | 0/6 | 6/6 |
| (Porcilis PRRS ID) | N/A | N/A | 33.3 (30.5–36.5) |
| Group B | 0/6 | 2/6 | 6/6 |
| (Porcilis PRRS IM) | N/A | 35.2 (32.3–38) | 34.6 (29.5–39.7) |
| Group C | 0/6 | 5/6 | 6/6 |
| (Diluvac ID) | N/A | 34.7 (25.5–40.8) | 29.4 (25.4–32.9) |
| Group D | 0/6 | 5/6 | 6/6 |
| (Diluvac IM) | N/A | 29.2 (24.2–32.2) | 34.4 (25.6–39.0) |
| Group | Time (Weeks) | Mean Angiotensin Concentration (×102 pg/mL) | SE | Median | IQR |
|---|---|---|---|---|---|
| Group A (Porcilis PRRS ID) | 4 | 0.25 | 0.25 | 0.00 | 0.00–0.00 |
| 7 | 0.26 a | 0.21 | 0.06 | 0.00–0.14 | |
| 10 | 0.17 | 0.13 | 0.00 | 0.00–0.17 | |
| Group B (Porcilis PRRS IM) | 4 | 2.97 | 1.86 | 0.90 | 0.09–3.63 |
| 7 | 3.44 b | 2.51 | 1.05 | 0.28–2.31 | |
| 10 | 3.20 | 1.80 | 0.65 | 0.20–6.63 | |
| Group C (Diluvac ID) | 4 | 1.06 | 0.37 | 0.83 | 0.62–1.32 |
| 7 | 0.56 a | 0.42 | 0.08 | 0.03–0.45 | |
| 10 | 1.41 | 0.71 | 0.78 | 0.20–2.03 | |
| Group D (Diluvac IM)) | 4 | 4.13 | 2.30 | 1.14 | 0.17–7.52 |
| 7 | 17.63 c | 7.51 | 11.37 | 4.73–25.14 | |
| 10 | 2.93 | 2.05 | 0.19 | 0.01–3.64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maragkakis, G.; Athanasiou, L.V.; Korou, L.-M.; Chaintoutis, S.C.; Dovas, C.; Perrea, D.N.; Papakonstantinou, G.; Christodoulopoulos, G.; Maes, D.; Papatsiros, V.G. Angiotensin II Blood Serum Levels in Piglets, after Intra-Dermal or Intra-Muscular Vaccination against PRRSV. Vet. Sci. 2022, 9, 496. https://doi.org/10.3390/vetsci9090496
Maragkakis G, Athanasiou LV, Korou L-M, Chaintoutis SC, Dovas C, Perrea DN, Papakonstantinou G, Christodoulopoulos G, Maes D, Papatsiros VG. Angiotensin II Blood Serum Levels in Piglets, after Intra-Dermal or Intra-Muscular Vaccination against PRRSV. Veterinary Sciences. 2022; 9(9):496. https://doi.org/10.3390/vetsci9090496
Chicago/Turabian StyleMaragkakis, Georgios, Labrini V. Athanasiou, Laskarina-Maria Korou, Serafeim C. Chaintoutis, Chrysostomos Dovas, Despina N. Perrea, Georgios Papakonstantinou, Georgios Christodoulopoulos, Dominiek Maes, and Vasileios G. Papatsiros. 2022. "Angiotensin II Blood Serum Levels in Piglets, after Intra-Dermal or Intra-Muscular Vaccination against PRRSV" Veterinary Sciences 9, no. 9: 496. https://doi.org/10.3390/vetsci9090496
APA StyleMaragkakis, G., Athanasiou, L. V., Korou, L.-M., Chaintoutis, S. C., Dovas, C., Perrea, D. N., Papakonstantinou, G., Christodoulopoulos, G., Maes, D., & Papatsiros, V. G. (2022). Angiotensin II Blood Serum Levels in Piglets, after Intra-Dermal or Intra-Muscular Vaccination against PRRSV. Veterinary Sciences, 9(9), 496. https://doi.org/10.3390/vetsci9090496













