Distribution of Coronavirus Receptors in the Swine Respiratory and Intestinal Tract
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. The Study
2.2. Sample Collection
2.3. Quantitative PCR
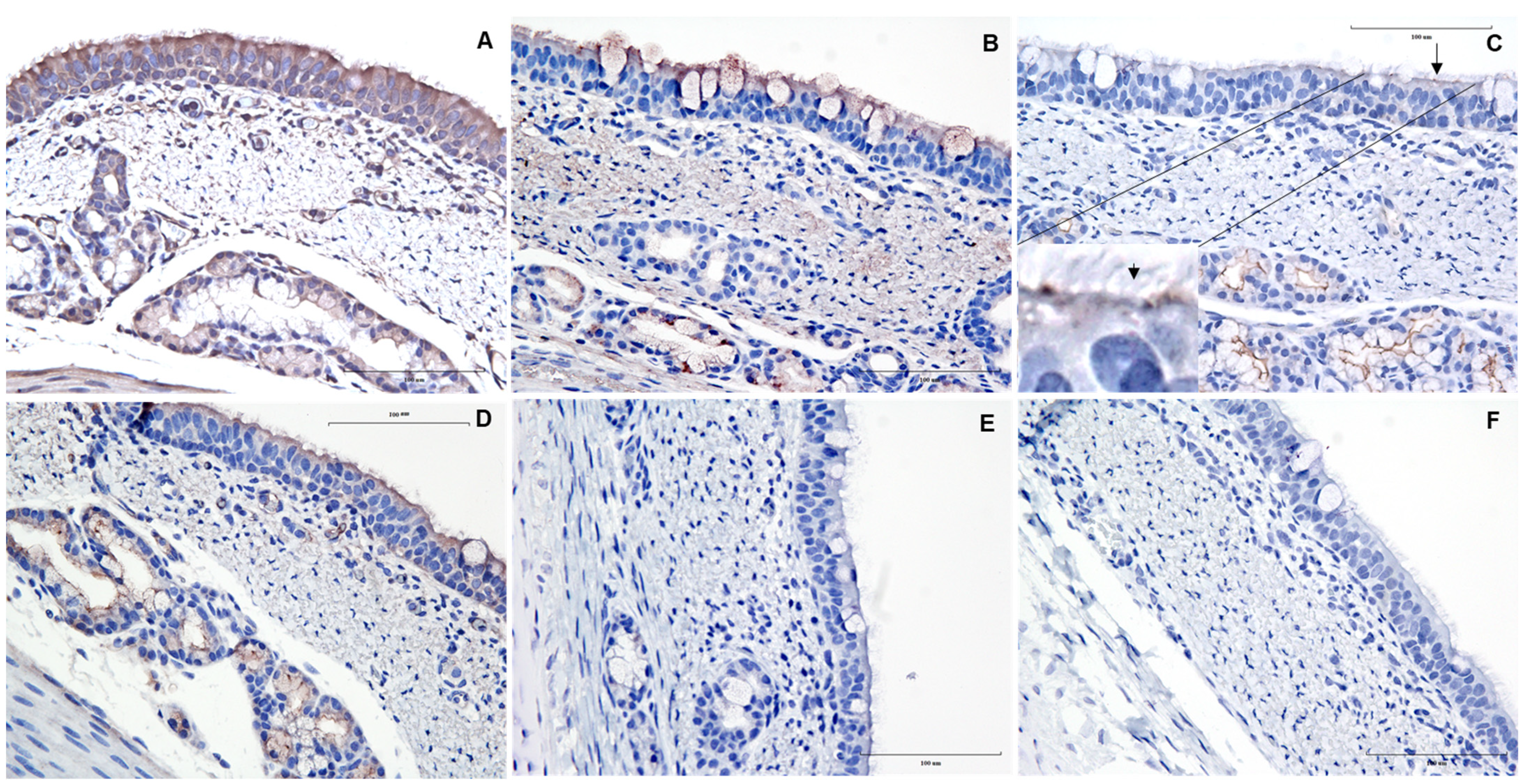
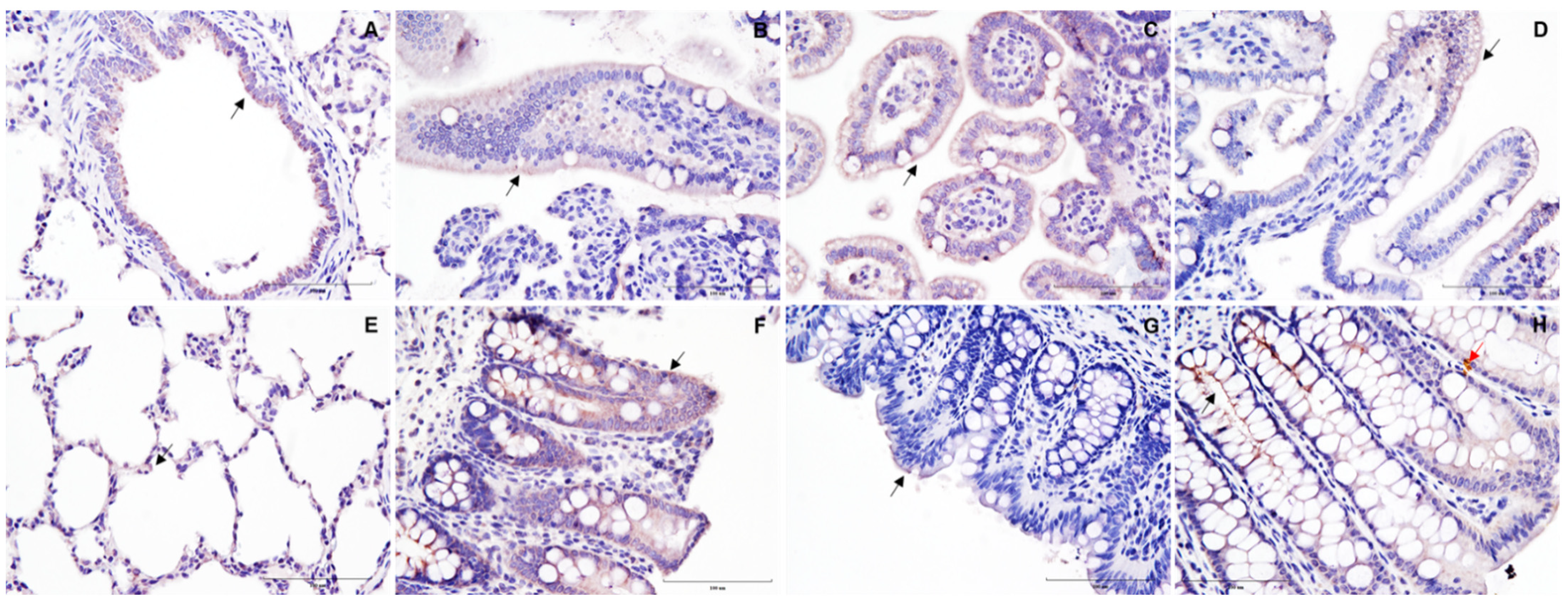
2.4. Immunohistochemistry
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fehr, A.R.; Perlman, S. Coronaviruses: An Overview of Their Replication and Pathogenesis. In Coronaviruses: Methods and Protocols; Springer: New York, NY, USA, 2015; Volume 226, pp. 1–23. ISBN 9781493924387. [Google Scholar]
- Woo, P.C.; Lau, S.K.; Huang, Y.; Yuen, K.Y. Coronavirus Diversity, Phylogeny and Interspecies Jumping. Exp. Biol. Med. 2009, 234, 1117–1127. [Google Scholar] [CrossRef] [PubMed]
- Millet, J.K.; Whittaker, G.R. Host Cell Proteases: Critical Determinants of Coronavirus Tropism and Pathogenesis. Virus Res. 2015, 202, 120–134. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Vlasova, A.N.; Kenney, S.P.; Saif, L.J. Emerging and Re-Emerging Coronaviruses in Pigs. Curr. Opin. Virol. 2019, 34, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Zhang, Y.; Li, Y.; Xia, L.; Guo, Y.; Zhou, Q. Structural Basis for the Recognition of SARS-CoV-2 by Full-Length Human ACE2. Science 2020, 367, 1444–1448. [Google Scholar] [CrossRef] [PubMed]
- Delmas, B.; Gelfi, J.; L’Haridon, R.; Vogel, L.K.; Sjöström, H.; Norén, O.; Laude, H. Aminopeptidase N Is a Major Receptor for the Enteropathogenic Coronavirus TGEV. Nature 1992, 357, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.K.; Jiang, G.S.; Holmes, K.V. Receptor for Mouse Hepatitis Virus Is a Member of the Carcinoembryonic Antigen Family of Glycoproteins. Proc. Natl. Acad. Sci. USA 1991, 88, 5533–5536. [Google Scholar] [CrossRef] [PubMed]
- Raj, V.S.; Mou, H.; Smits, S.L.; Dekkers, D.H.W.; Müller, M.A.; Dijkman, R.; Muth, D.; Demmers, J.A.A.; Zaki, A.; Fouchier, R.A.M.; et al. Dipeptidyl Peptidase 4 Is a Functional Receptor for the Emerging Human Coronavirus-EMC. Nature 2013, 495, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Tikellis, C.; Thomas, M.C. Angiotensin-Converting Enzyme 2 (ACE2) Is a Key Modulator of the Renin Angiotensin System in Health and Disease. Int. J. Pept. 2012, 2012, 256294. [Google Scholar] [CrossRef] [PubMed]
- Röhrborn, D.; Wronkowitz, N.; Eckel, J. DPP4 in Diabetes. Front. Immunol. 2015, 6, 386. [Google Scholar] [CrossRef] [PubMed]
- Danielsen, E.M.; Hansen, G.H.; Niels-Christiansen, L.L. Localization and Biosynthesis of Aminopeptidase N in Pig Fetal Small Intestine. Gastroenterology 1995, 109, 1039–1050. [Google Scholar] [CrossRef]
- Te, N.; Vergara-Alert, J.; Lehmbecker, A.; Pérez, M.; Haagmans, B.L.; Baumgärtner, W.; Bensaid, A.; Segalés, J. Co-Localization of Middle East Respiratory Syndrome Coronavirus (MERS-CoV) and Dipeptidyl Peptidase-4 in the Respiratory Tract and Lymphoid Tissues of Pigs and Llamas. Transbound. Emerg. Dis. 2019, 66, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Olivares, M.; Neyrinck, A.M.; Pötgens, S.A.; Beaumont, M.; Salazar, N.; Cani, P.D.; Bindels, L.B.; Delzenne, N.M. The DPP-4 Inhibitor Vildagliptin Impacts the Gut Microbiota and Prevents Disruption of Intestinal Homeostasis Induced by a Western Diet in Mice. Diabetologia 2018, 61, 1838–1848. [Google Scholar] [CrossRef] [PubMed]
- Färber, I.; Krüger, J.; Rocha, C.; Armando, F.; von Köckritz-Blickwede, M.; Pöhlmann, S.; Braun, A.; Baumgärtner, W.; Runft, S.; Krüger, N. Investigations on SARS-CoV-2 Susceptibility of Domestic and Wild Animals Using Primary Cell Culture Models Derived from the Upper and Lower Respiratory Tract. Viruses 2022, 14, 828. [Google Scholar] [CrossRef] [PubMed]
- Lean, F.Z.X.; Núñez, A.; Spiro, S.; Priestnall, S.L.; Vreman, S.; Bailey, D.; James, J.; Wrigglesworth, E.; Suarez-Bonnet, A.; Conceicao, C.; et al. Differential Susceptibility of SARS-CoV-2 in Animals: Evidence of ACE2 Host Receptor Distribution in Companion Animals, Livestock and Wildlife by Immunohistochemical Characterisation. Transbound. Emerg. Dis. 2022, 69, 2275–2286. [Google Scholar] [CrossRef] [PubMed]
- di Teodoro, G.; Valleriani, F.; Puglia, I.; Monaco, F.; di Pancrazio, C.; Luciani, M.; Krasteva, I.; Petrini, A.; Marcacci, M.; D’Alterio, N.; et al. SARS-CoV-2 Replicates in Respiratory Ex Vivo Organ Cultures of Domestic Ruminant Species. Vet. Microbiol. 2021, 252, 108933. [Google Scholar] [CrossRef] [PubMed]
- Bertram, S.; Heurich, A.; Lavender, H.; Gierer, S.; Danisch, S.; Perin, P.; Lucas, J.M.; Nelson, P.S.; Pöhlmann, S.; Soilleux, E.J. Influenza and SARS-Coronavirus Activating Proteases TMPRSS2 and HAT Are Expressed at Multiple Sites in Human Respiratory and Gastrointestinal Tracts. PLoS ONE 2012, 7, e35876. [Google Scholar] [CrossRef]
- Zhou, C.J.; Liu, B.; Zhu, K.X.; Zhang, Q.H.; Zhang, T.G.; Xu, W.H.; Wang, H.B.; Yu, W.H.; Qu, Y.D.; Wang, H.J.; et al. The Different Expression of Carcinoembryonic Antigen-Related Cell Adhesion Molecule 1 (CEACAM1) and Possible Roles in Gastric Carcinomas. Pathol. Res. Pract. 2009, 205, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Castillo, G.; Mora-Díaz, J.C.; Nelli, R.K.; Giménez-Lirola, L.G. Human Air-Liquid-Interface Organotypic Airway Cultures Express Significantly More ACE2 Receptor Protein and Are More Susceptible to HCoV-NL63 Infection than Monolayer Cultures of Primary Respiratory Epithelial Cells. Microbiol. Spectr. 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Masters, P.S. The Molecular Biology of Coronaviruses. Adv. Virus Res. 2006, 66, 193–292. [Google Scholar] [CrossRef] [PubMed]
| Trachea | Bronchiole | Lung | Duodenum | Jejunum | Ileum | Cecum | Colon | Rectum | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Immunohistochemistry | Viruses Showing Affinity to These Receptors | |||||||||
| APN/CD13 | + (2/2) | + (2/2) | + (2/2) | + (3/3) | + (3/3) | + (3/3) | + (3/3) | + (3/3) | + (3/3) | FCoV, CCoV, TGEV, PRCV, PEDV, PDCoV |
| DPP4/CD26 | + (2/2) | + (2/2) | + (2/2) | + (3/3) | + (3/3) | + (3/3) | + (2/3) | + (2/3) | + (2/3) | MERS-CoV |
| ACE2 | + ** (2/2) | + (2/2) | + (2/2) | + * (3/3) | + * (3/3) | + * (3/3) | + * (2/3) | − (3/3) | + (2/3) | HCoV-NL63, SARS-CoV, SARS-CoV2 |
| CEACAM1/CD66a | + (2/2) | + (2/2) | + (2/2) | + # (3/3) | + # (3/3) | + # (3/3) | + # (2/3) | + # (2/3) | + # (2/3) | MHV |
| Gene expression (NCBI reference) | Primer sequences | |||||||||
| APN/CD13 (NM_214277.1) | + (2/2) | Not performed | + (2/2) | + (3/3) | + (3/3) | + (3/3) | + (3/3) | + (3/3) | + (3/3) | For Primer 5′-CACGACACAGATGCAGTCTACAGA-3′ Rev Primer 5′-TGTTGAACGTGGCCTTCATG-3′ |
| DPP4/CD26 (NM_214257.1) | + (2/2) | Not performed | + (2/2) | + (3/3) | + (3/3) | + (3/3) | + (1/3) | + (2/3) | + (2/3) | For Primer 5′-ACCAGGACTCTCAGCCCAAA-3′ Rev Primer 5′-ACAAGTAGTGATCCCCTATTAACACAGA-3′ |
| ACE2 (NM_001123070.1) | + (2/2) | Not performed | + (2/2) | + (2/3) | + (2/3) | + (3/3) | − (3/3) | + (1/3) | − (3/3) | For Primer 5′-GGGTGGTGATGGGATTGGTA-3′ Rev Primer 5′-TTGCTTTTTCTTCCTTCGATCTCT-3′ |
| CEACAM1/CD66a (XM_021094420.1) | + (2/2) | Not performed | + (2/2) | + (2/3) | − (3/3) | + (2/3) | + (1/3) | + (3/3) | + (3/3) | For Primer 5′-TGCTCGCAGAGAGGATAAAACTG-3′ Rev Primer 5′-GGCCTCGCACTGATAATTCC-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nelli, R.K.; Roth, J.A.; Gimenez-Lirola, L.G. Distribution of Coronavirus Receptors in the Swine Respiratory and Intestinal Tract. Vet. Sci. 2022, 9, 500. https://doi.org/10.3390/vetsci9090500
Nelli RK, Roth JA, Gimenez-Lirola LG. Distribution of Coronavirus Receptors in the Swine Respiratory and Intestinal Tract. Veterinary Sciences. 2022; 9(9):500. https://doi.org/10.3390/vetsci9090500
Chicago/Turabian StyleNelli, Rahul Kumar, James Allen Roth, and Luis Gabriel Gimenez-Lirola. 2022. "Distribution of Coronavirus Receptors in the Swine Respiratory and Intestinal Tract" Veterinary Sciences 9, no. 9: 500. https://doi.org/10.3390/vetsci9090500
APA StyleNelli, R. K., Roth, J. A., & Gimenez-Lirola, L. G. (2022). Distribution of Coronavirus Receptors in the Swine Respiratory and Intestinal Tract. Veterinary Sciences, 9(9), 500. https://doi.org/10.3390/vetsci9090500








