Anemia in Elderly Patients: Contribution of Renal Aging and Chronic Kidney Disease
Abstract
1. Introduction
2. Aging Kidney
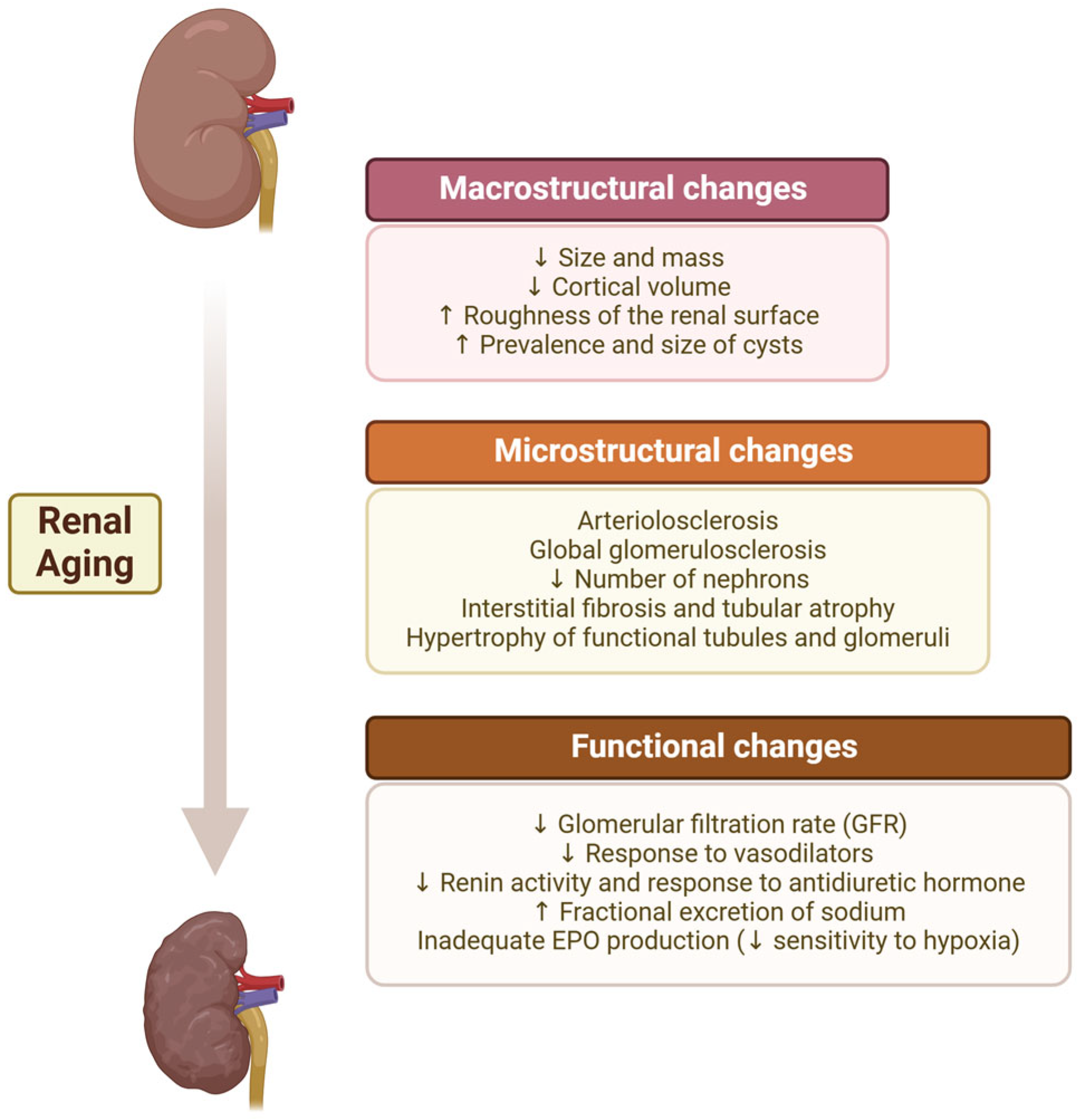
2.1. Anatomical Changes
2.1.1. Macrostructural Changes
2.1.2. Microstructural CHANGES
Nephrosclerosis
Nephron Hypertrophy
Number of Nephrons
Glomerular Filtration Rate per Nephron (snGFR)
2.2. Functional Changes
2.2.1. Physiological Decline in GFR
2.2.2. Hormonal Changes
3. Chronic Kidney Disease
3.1. Definition and Classification
3.2. Etiology and Risk Factors
3.3. Prevalence in the Elderly
3.4. Calculation of GFR in the Elderly
3.5. Anemia—A Complication of CKD
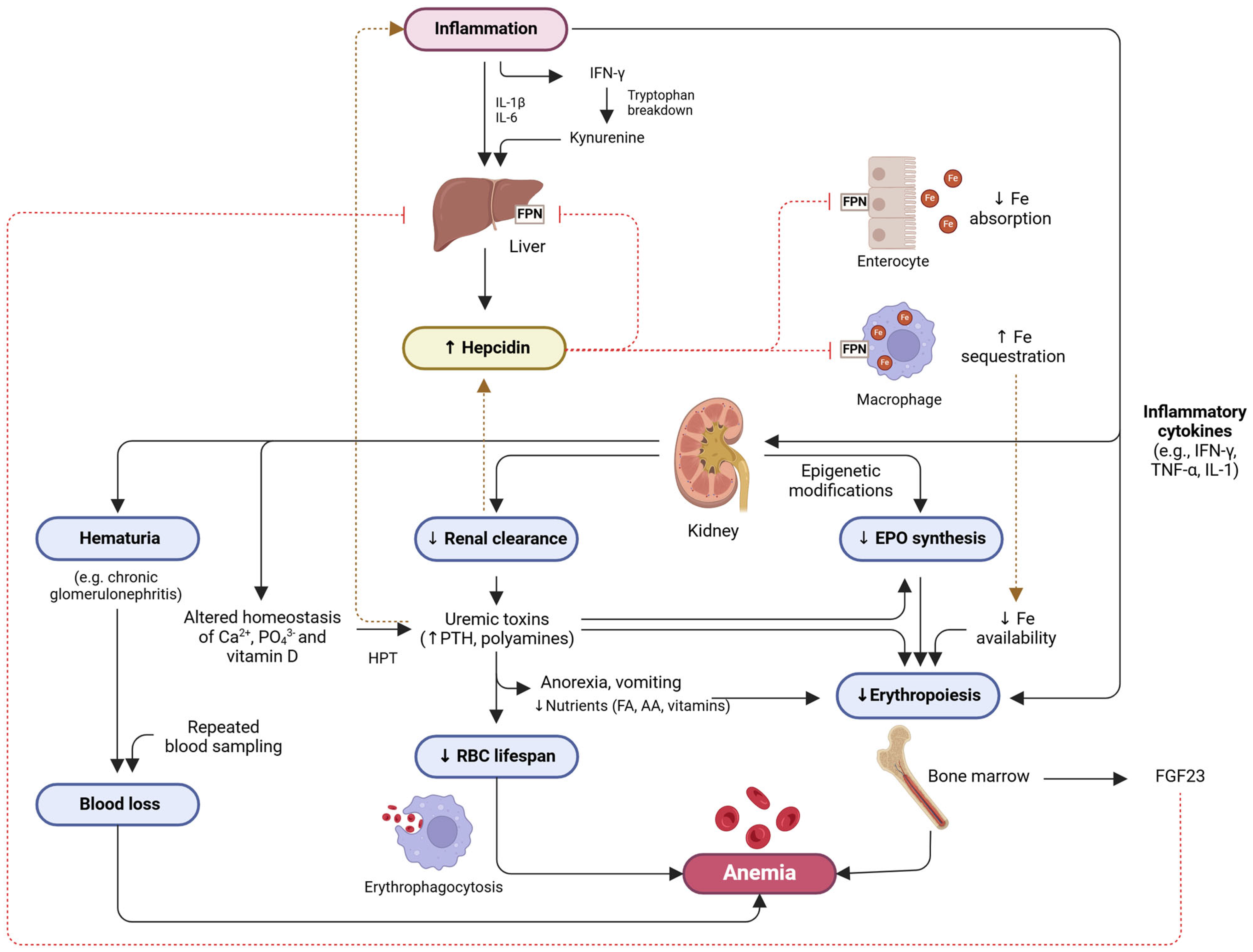
4. CKD Anemia
4.1. Multifactorial Nature
4.2. Normal Erythropoiesis Versus Pathophysiology of CKD Anemia
4.3. Diagnosis
4.4. Application of Artificial Intelligence in Managing Patients with CKD-Anemia
5. Anemia in the Elderly
5.1. Definition
5.2. Adverse Health Effects
5.3. Prevalence
5.4. Etiology
5.4.1. Nutritional Deficiency Anemia
5.4.2. Anemia Resulting from Chronic Diseases
5.4.3. Anemia of Unknown Cases
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lote, C.J. Principles of Renal Physiology; Springer: New York, NY, USA, 2012. [Google Scholar]
- Mahmoud, T.; Borgi, L. The Interplay Between Nutrition, Metabolic, and Endocrine Disorders in Chronic Kidney Disease. Semin. Nephrol. 2021, 41, 180–188. [Google Scholar] [CrossRef]
- Hommos, M.S.; Glassock, R.J.; Rule, A.D. Structural and Functional Changes in Human Kidneys with Healthy Aging. J. Am. Soc. Nephrol. 2017, 28, 2838–2844. [Google Scholar] [CrossRef]
- Farinha, A.; Robalo Nunes, A.; Mairos, J.; Fonseca, C. Anemia in Chronic Kidney Disease: The State of the Art. Acta Med. Port. 2022, 35, 758–764. [Google Scholar] [CrossRef]
- Shiferaw, W.S.; Akalu, T.Y.; Aynalem, Y.A. Risk Factors for Anemia in Patients with Chronic Renal Failure: A Systematic Review and Meta-Analysis. Ethiop. J. Health Sci. 2020, 30, 829–842. [Google Scholar] [CrossRef] [PubMed]
- Sobamowo, H.; Prabhakar, S.S. The Kidney in Aging: Physiological Changes and Pathological Implications. Prog. Mol. Biol. Transl. Sci. 2017, 146, 303–340. [Google Scholar] [CrossRef] [PubMed]
- Núñez, J.-F.M. The normal ageing kidney—Morphology and physiology. Rev. Clin. Gerontol. 2008, 18, 175–197. [Google Scholar] [CrossRef]
- Denic, A.; Glassock, R.J.; Rule, A.D. Structural and Functional Changes with the Aging Kidney. Adv. Chronic Kidney Dis. 2016, 23, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, E.C.; Vrtiska, T.J.; Lieske, J.C.; Dillon, J.J.; Stegall, M.D.; Li, X.; Bergstralh, E.J.; Rule, A.D. Prevalence of renal artery and kidney abnormalities by computed tomography among healthy adults. Clin. J. Am. Soc. Nephrol. 2010, 5, 431–438. [Google Scholar] [CrossRef]
- Wang, X.; Vrtiska, T.J.; Avula, R.T.; Walters, L.R.; Chakkera, H.A.; Kremers, W.K.; Lerman, L.O.; Rule, A.D. Age, kidney function, and risk factors associate differently with cortical and medullary volumes of the kidney. Kidney Int. 2014, 85, 677–685. [Google Scholar] [CrossRef]
- Rule, A.D.; Sasiwimonphan, K.; Lieske, J.C.; Keddis, M.T.; Torres, V.E.; Vrtiska, T.J. Characteristics of renal cystic and solid lesions based on contrast-enhanced computed tomography of potential kidney donors. Am. J. Kidney Dis. 2012, 59, 611–618. [Google Scholar] [CrossRef]
- Elsherbiny, H.E.; Alexander, M.P.; Kremers, W.K.; Park, W.D.; Poggio, E.D.; Prieto, M.; Lieske, J.C.; Rule, A.D. Nephron hypertrophy and glomerulosclerosis and their association with kidney function and risk factors among living kidney donors. Clin. J. Am. Soc. Nephrol. 2014, 9, 1892–1902. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.C.; Busque, S.; Workeneh, B.; Ho, B.; Derby, G.; Blouch, K.L.; Sommer, F.G.; Edwards, B.; Myers, B.D. Effects of aging on glomerular function and number in living kidney donors. Kidney Int. 2010, 78, 686–692. [Google Scholar] [CrossRef]
- Nyengaard, J.R.; Bendtsen, T.F. Glomerular number and size in relation to age, kidney weight, and body surface in normal man. Anat. Rec. 1992, 232, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Denic, A.; Lieske, J.C.; Chakkera, H.A.; Poggio, E.D.; Alexander, M.P.; Singh, P.; Kremers, W.K.; Lerman, L.O.; Rule, A.D. The Substantial Loss of Nephrons in Healthy Human Kidneys with Aging. J. Am. Soc. Nephrol. 2017, 28, 313–320. [Google Scholar] [CrossRef]
- Noronha, I.L.; Santa-Catharina, G.P.; Andrade, L.; Coelho, V.A.; Jacob-Filho, W.; Elias, R.M. Glomerular filtration in the aging population. Front. Med. 2022, 9, 769329. [Google Scholar] [CrossRef] [PubMed]
- Bertram, J.F.; Douglas-Denton, R.N.; Diouf, B.; Hughson, M.D.; Hoy, W.E. Human nephron number: Implications for health and disease. Pediatr. Nephrol. 2011, 26, 1529–1533. [Google Scholar] [CrossRef]
- Luyckx, V.A.; Brenner, B.M. Birth weight, malnutrition and kidney-associated outcomes--a global concern. Nat. Rev. Nephrol. 2015, 11, 135–149. [Google Scholar] [CrossRef]
- Denic, A.; Mathew, J.; Lerman, L.O.; Lieske, J.C.; Larson, J.J.; Alexander, M.P.; Poggio, E.; Glassock, R.J.; Rule, A.D. Single-Nephron Glomerular Filtration Rate in Healthy Adults. N. Engl. J. Med. 2017, 376, 2349–2357. [Google Scholar] [CrossRef]
- Lindeman, R.D.; Tobin, J.; Shock, N.W. Longitudinal studies on the rate of decline in renal function with age. J. Am. Geriatr. Soc. 1985, 33, 278–285. [Google Scholar] [CrossRef]
- Rodríguez-Puyol, D. The aging kidney. Kidney Int. 1998, 54, 2247–2265. [Google Scholar] [CrossRef]
- Zhou, X.J.; Rakheja, D.; Yu, X.; Saxena, R.; Vaziri, N.D.; Silva, F.G. The aging kidney. Kidney Int. 2008, 74, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Musso, C.G.; Oreopoulos, D.G. Aging and physiological changes of the kidneys including changes in glomerular filtration rate. Nephron Physiol. 2011, 119 (Suppl. S1), 1–5. [Google Scholar] [CrossRef] [PubMed]
- Stauder, R.; Valent, P.; Theurl, I. Anemia at older age: Etiologies, clinical implications, and management. Blood 2018, 131, 505–514. [Google Scholar] [CrossRef]
- Musso, C.G.; Musso, C.A.; Joseph, H.; De Miguel, R.; Rendo, P.; Gonzalez, E.; Algranati, L.; dos Ramos Farias, E. Plasma erythropoietin levels in the oldest old. Int. Urol. Nephrol. 2004, 36, 259–262. [Google Scholar] [CrossRef]
- Santos-Silva, A.; Costa, E.; Alves, R. Chronic Kidney Disease. In Biomarkers of Cardiometabolic Risk, Inflammation and Disease; Palavra, F., Reis, F., Marado, D., Sena, A., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 95–111. [Google Scholar]
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’Callaghan, C.A.; Lasserson, D.S.; Hobbs, F.D. Global Prevalence of Chronic Kidney Disease—A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0158765. [Google Scholar] [CrossRef]
- Ammirati, A.L. Chronic Kidney Disease. Rev. Assoc. Med. Bras. 2020, 66 (Suppl. S1), s03–s09. [Google Scholar] [CrossRef]
- Stevens, P.E.; Ahmed, S.B.; Carrero, J.J.; Foster, B.; Francis, A.; Hall, R.K.; Herrington, W.G.; Hill, G.; Inker, L.A.; Kazancıoğlu, R.; et al. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef]
- Miller, W.G.; Bruns, D.E.; Hortin, G.L.; Sandberg, S.; Aakre, K.M.; McQueen, M.J.; Itoh, Y.; Lieske, J.C.; Seccombe, D.W.; Jones, G.; et al. Current issues in measurement and reporting of urinary albumin excretion. Clin. Chem. 2009, 55, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Keane, W.F.; Eknoyan, G. Proteinuria, albuminuria, risk, assessment, detection, elimination (PARADE): A position paper of the National Kidney Foundation. Am. J. Kidney Dis. 1999, 33, 1004–1010. [Google Scholar] [CrossRef]
- Levey, A.S.; Coresh, J.; Tighiouart, H.; Greene, T.; Inker, L.A. Measured and estimated glomerular filtration rate: Current status and future directions. Nat. Rev. Nephrol. 2020, 16, 51–64. [Google Scholar] [CrossRef]
- Foundation, N.K. Chronic Kidney Disease (CKD). Available online: https://www.kidney.org/kidney-topics/chronic-kidney-disease-ckd (accessed on 8 January 2025).
- Mayo Clinic. Chronic Kidney Disease. Diseases & Conditions. Available online: https://www.mayoclinic.org/diseases-conditions/chronic-kidney-disease/symptoms-causes/syc-20354521 (accessed on 8 January 2025).
- Centers for Disease Control and Prevention (CDC). Risk Factors for Chronic Kidney Disease. Available online: https://www.cdc.gov/kidney-disease/risk-factors/index.html (accessed on 10 January 2025).
- Fund, A.K. Risk Factors for Kidney Disease. Available online: https://www.kidneyfund.org/all-about-kidneys/risk-factors#risk-factors-for-kidney-disease (accessed on 8 January 2025).
- Vinhas, J.; Aires, I.; Batista, C.; Branco, P.; Brandão, J.; Nogueira, R.; Raposo, J.F.; Rodrigues, E. RENA Study: Cross-Sectional Study to Evaluate CKD Prevalence in Portugal. Nephron 2020, 144, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Mallappallil, M.; Friedman, E.A.; Delano, B.G.; McFarlane, S.I.; Salifu, M.O. Chronic kidney disease in the elderly: Evaluation and management. Clin. Pract. 2014, 11, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Coresh, J.; Astor, B.C.; Greene, T.; Eknoyan, G.; Levey, A.S. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am. J. Kidney Dis. 2003, 41, 1–12. [Google Scholar] [CrossRef] [PubMed]
- NIH. CKD in the General Population; NIH: Bethesda, MD, USA, 2022.
- Kovesdy, C.P. Epidemiology of chronic kidney disease: An update 2022. Kidney Int. Suppl. 2022, 12, 7–11. [Google Scholar] [CrossRef]
- Raman, M.; Middleton, R.J.; Kalra, P.A.; Green, D. Estimating renal function in old people: An in-depth review. Int. Urol. Nephrol. 2017, 49, 1979–1988. [Google Scholar] [CrossRef]
- Cockcroft, D.W.; Gault, M.H. Prediction of creatinine clearance from serum creatinine. Nephron 1976, 16, 31–41. [Google Scholar] [CrossRef]
- Delanaye, P.; Cavalier, E.; Pottel, H.; Stehlé, T. New and old GFR equations: A European perspective. Clin. Kidney J. 2023, 16, 1375–1383. [Google Scholar] [CrossRef]
- Levey, A.S.; Bosch, J.P.; Lewis, J.B.; Greene, T.; Rogers, N.; Roth, D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann. Intern. Med. 1999, 130, 461–470. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Schaeffner, E.S.; Ebert, N.; Delanaye, P.; Frei, U.; Gaedeke, J.; Jakob, O.; Kuhlmann, M.K.; Schuchardt, M.; Tölle, M.; Ziebig, R.; et al. Two novel equations to estimate kidney function in persons aged 70 years or older. Ann. Intern. Med. 2012, 157, 471–481. [Google Scholar] [CrossRef]
- Foundation, N.K. eGFR Calculator. Available online: https://www.kidney.org/professionals/kdoqi/gfr_calculator (accessed on 8 January 2025).
- Foundation, N.K. NKF and ASN Release New Way to Diagnose Kidney Diseases. Available online: https://www.kidney.org/news/nkf-and-asn-release-new-way-to-diagnose-kidney-diseases (accessed on 8 January 2025).
- Lee, S.; Lee, G.H.; Kim, H.; Yang, H.S.; Hur, M. Application of the European Kidney Function Consortium Equation to Estimate Glomerular Filtration Rate: A Comparison Study of the CKiD and CKD-EPI Equations Using the Korea National Health and Nutrition Examination Survey (KNHANES 2008–2021). Medicina 2024, 60, 612. [Google Scholar] [CrossRef]
- Foundation, N.K. CKD-EPI Creatinine Equation (2021). Available online: https://www.kidney.org/professionals/kdoqi/gfr_calculator/formula (accessed on 8 January 2025).
- Foundation, N.K. CKD-EPI Creatinine-Cystatin Equation (2021). Available online: https://www.kidney.org/content/ckd-epi-creatinine-cystatin-equation-2021 (accessed on 8 January 2025).
- Fox, C.S.; Matsushita, K.; Woodward, M.; Bilo, H.J.; Chalmers, J.; Heerspink, H.J.; Lee, B.J.; Perkins, R.M.; Rossing, P.; Sairenchi, T.; et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: A meta-analysis. Lancet 2012, 380, 1662–1673. [Google Scholar] [CrossRef] [PubMed]
- Bello, A.K.; Alrukhaimi, M.; Ashuntantang, G.E.; Basnet, S.; Rotter, R.C.; Douthat, W.G.; Kazancioglu, R.; Köttgen, A.; Nangaku, M.; Powe, N.R.; et al. Complications of chronic kidney disease: Current state, knowledge gaps, and strategy for action. Kidney Int. Suppl. 2017, 7, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Farrington, D.K.; Sang, Y.; Grams, M.E.; Ballew, S.H.; Dunning, S.; Stempniewicz, N.; Coresh, J. Anemia Prevalence, Type, and Associated Risks in a Cohort of 5.0 Million Insured Patients in the United States by Level of Kidney Function. Am. J. Kidney Dis. 2023, 81, 201–209.e1. [Google Scholar] [CrossRef]
- Cumming, R.G.; Mitchell, P.; Craig, J.C.; Knight, J.F. Renal impairment and anaemia in a population-based study of older people. Intern. Med. J. 2004, 34, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Astor, B.C.; Muntner, P.; Levin, A.; Eustace, J.A.; Coresh, J. Association of kidney function with anemia: The Third National Health and Nutrition Examination Survey (1988–1994). Arch. Intern. Med. 2002, 162, 1401–1408. [Google Scholar] [CrossRef]
- Bowling, C.B.; Inker, L.A.; Gutiérrez, O.M.; Allman, R.M.; Warnock, D.G.; McClellan, W.; Muntner, P. Age-specific associations of reduced estimated glomerular filtration rate with concurrent chronic kidney disease complications. Clin. J. Am. Soc. Nephrol. 2011, 6, 2822–2828. [Google Scholar] [CrossRef]
- Dmitrieva, O.; de Lusignan, S.; Macdougall, I.C.; Gallagher, H.; Tomson, C.; Harris, K.; Desombre, T.; Goldsmith, D. Association of anaemia in primary care patients with chronic kidney disease: Cross sectional study of quality improvement in chronic kidney disease (QICKD) trial data. BMC Nephrol. 2013, 14, 24. [Google Scholar] [CrossRef]
- Ku, E.; Del Vecchio, L.; Eckardt, K.U.; Haase, V.H.; Johansen, K.L.; Nangaku, M.; Tangri, N.; Waikar, S.S.; Więcek, A.; Cheung, M.; et al. Novel anemia therapies in chronic kidney disease: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2023, 104, 655–680. [Google Scholar] [CrossRef]
- Babitt, J.L.; Lin, H.Y. Mechanisms of anemia in CKD. J. Am. Soc. Nephrol. 2012, 23, 1631–1634. [Google Scholar] [CrossRef]
- Macdougall, I.C. Chapter 13—Development of Recombinant Erythropoietin and Erythropoietin Analogs. In Textbook of Nephro-Endocrinology, 2nd ed.; Singh, A.K., Williams, G.H., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 217–232. [Google Scholar]
- Kunadharaju, R.; Silberstein, P. Erythropoiesis. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Singh, A.K. Chapter 12—Erythropoiesis: The Roles of Erythropoietin and Iron. In Textbook of Nephro-Endocrinology, 2nd ed.; Singh, A.K., Williams, G.H., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 207–215. [Google Scholar]
- Zadrazil, J.; Horak, P. Pathophysiology of anemia in chronic kidney diseases: A review. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2015, 159, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Haase, V.H. Regulation of erythropoiesis by hypoxia-inducible factors. Blood Rev. 2013, 27, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Haase, V.H. Hypoxic regulation of erythropoiesis and iron metabolism. Am. J. Physiol. Renal Physiol. 2010, 299, F1–F13. [Google Scholar] [CrossRef]
- Tomosugi, N.; Kawabata, H.; Wakatabe, R.; Higuchi, M.; Yamaya, H.; Umehara, H.; Ishikawa, I. Detection of serum hepcidin in renal failure and inflammation by using ProteinChip System. Blood 2006, 108, 1381–1387. [Google Scholar] [CrossRef]
- Nemeth, E.; Ganz, T. Regulation of iron metabolism by hepcidin. Annu. Rev. Nutr. 2006, 26, 323–342. [Google Scholar] [CrossRef]
- Pietrangelo, A. Hemochromatosis: An endocrine liver disease. Hepatology 2007, 46, 1291–1301. [Google Scholar] [CrossRef]
- Antunes, S.A.; Canziani, M.E. Hepcidin: An important iron metabolism regulator in chronic kidney disease. J. Bras. Nefrol. 2016, 38, 351–355. [Google Scholar] [CrossRef]
- Sagar, P.; Angmo, S.; Sandhir, R.; Rishi, V.; Yadav, H.; Singhal, N.K. Effect of hepcidin antagonists on anemia during inflammatory disorders. Pharmacol. Ther. 2021, 226, 107877. [Google Scholar] [CrossRef] [PubMed]
- Verga Falzacappa, M.V.; Vujic Spasic, M.; Kessler, R.; Stolte, J.; Hentze, M.W.; Muckenthaler, M.U. STAT3 mediates hepatic hepcidin expression and its inflammatory stimulation. Blood 2007, 109, 353–358. [Google Scholar] [CrossRef]
- Higashimoto, Y.; Tanaka, K.; Matsui, T.; Sakaguchi, T.; Yamagishi, S.I.; Motomiya, Y. Fibroblast Growth Factor 23 Contributes to Regulation of Hepcidin/Ferroportin Axis. Austin J. Pharmacol. Ther. 2020, 8, 1118. [Google Scholar] [CrossRef]
- Courbon, G.; Thomas, J.J.; Martinez-Calle, M.; Wang, X.; Spindler, J.; Von Drasek, J.; Hunt-Tobey, B.; Mehta, R.; Isakova, T.; Chang, W.; et al. Bone-derived C-terminal FGF23 cleaved peptides increase iron availability in acute inflammation. Blood 2023, 142, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Kanamori, Y.; Murakami, M.; Sugiyama, M.; Hashimoto, O.; Matsui, T.; Funaba, M. Interleukin-1β (IL-1β) transcriptionally activates hepcidin by inducing CCAAT enhancer-binding protein δ (C/EBPδ) expression in hepatocytes. J. Biol. Chem. 2017, 292, 10275–10287. [Google Scholar] [CrossRef]
- Platten, M.; Nollen, E.A.A.; Röhrig, U.F.; Fallarino, F.; Opitz, C.A. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat. Rev. Drug Discov. 2019, 18, 379–401. [Google Scholar] [CrossRef]
- Weiss, G.; Schroecksnadel, K.; Mattle, V.; Winkler, C.; Konwalinka, G.; Fuchs, D. Possible role of cytokine-induced tryptophan degradation in anaemia of inflammation. Eur. J. Haematol. 2004, 72, 130–134. [Google Scholar] [CrossRef]
- Eleftheriadis, T.; Pissas, G.; Antoniadi, G.; Liakopoulos, V.; Stefanidis, I. Kynurenine, by activating aryl hydrocarbon receptor, decreases erythropoietin and increases hepcidin production in HepG2 cells: A new mechanism for anemia of inflammation. Exp. Hematol. 2016, 44, 60–67.e1. [Google Scholar] [CrossRef]
- Rivkin, M.; Simerzin, A.; Zorde-Khvalevsky, E.; Chai, C.; Yuval, J.B.; Rosenberg, N.; Harari-Steinfeld, R.; Schneider, R.; Amir, G.; Condiotti, R.; et al. Inflammation-Induced Expression and Secretion of MicroRNA 122 Leads to Reduced Blood Levels of Kidney-Derived Erythropoietin and Anemia. Gastroenterology 2016, 151, 999–1010.e3. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Kumagai, N.; Suzuki, N. Alteration of the DNA Methylation Signature of Renal Erythropoietin-Producing Cells Governs the Sensitivity to Drugs Targeting the Hypoxia-Response Pathway in Kidney Disease Progression. Front. Genet. 2019, 10, 1134. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Blanchard, K.L. DNA methylation represses the expression of the human erythropoietin gene by two different mechanisms. Blood 2000, 95, 111–119. [Google Scholar] [CrossRef]
- Cha, H.J. Erythropoiesis: Insights from a genomic perspective. Exp. Mol. Med. 2024, 56, 2099–2104. [Google Scholar] [CrossRef]
- Pasricha, S.R.; Lim, P.J.; Duarte, T.L.; Casu, C.; Oosterhuis, D.; Mleczko-Sanecka, K.; Suciu, M.; Da Silva, A.R.; Al-Hourani, K.; Arezes, J.; et al. Hepcidin is regulated by promoter-associated histone acetylation and HDAC3. Nat. Commun. 2017, 8, 403. [Google Scholar] [CrossRef]
- Sharp, P.A.; Clarkson, R.; Hussain, A.; Weeks, R.J.; Morison, I.M. DNA methylation of hepatic iron sensing genes and the regulation of hepcidin expression. PLoS ONE 2018, 13, e0197863. [Google Scholar] [CrossRef] [PubMed]
- Macdougall, I.C. Anaemia in CKD—Treatment standard. Nephrol. Dial. Transplant. 2023, 39, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Chapter 1: Diagnosis and evaluation of anemia in CKD. Kidney Int. Suppl. 2012, 2, 288–291. [CrossRef]
- CPR 1.2.: Evaluation of Anemia in CKD. Am. J. Kidney Dis. 2006, 47, S28–S32. [CrossRef]
- Gaweda, A.E.; Brier, M.E. Artificial Intelligence in Medicine in Anemia. In Artificial Intelligence in Medicine; Lidströmer, N., Ashrafian, H., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 1441–1451. [Google Scholar]
- Gaweda, A.E.; Lederer, E.D.; Brier, M.E. Artificial intelligence-guided precision treatment of chronic kidney disease-mineral bone disorder. CPT Pharmacomet. Syst. Pharmacol. 2022, 11, 1305–1315. [Google Scholar] [CrossRef]
- Ohara, T.; Ikeda, H.; Sugitani, Y.; Suito, H.; Huynh, V.Q.H.; Kinomura, M.; Haraguchi, S.; Sakurama, K. Artificial intelligence supported anemia control system (AISACS) to prevent anemia in maintenance hemodialysis patients. Int. J. Med. Sci. 2021, 18, 1831–1839. [Google Scholar] [CrossRef] [PubMed]
- Brier, M.E.; Gaweda, A.E. Artificial intelligence for optimal anemia management in end-stage renal disease. Kidney Int. 2016, 90, 259–261. [Google Scholar] [CrossRef] [PubMed]
- Price, E.A.; Schrier, S.L. Anemia in the elderly: Introduction. Semin. Hematol. 2008, 45, 207–209. [Google Scholar] [CrossRef] [PubMed]
- Ble, A.; Fink, J.C.; Woodman, R.C.; Klausner, M.A.; Windham, B.G.; Guralnik, J.M.; Ferrucci, L. Renal function, erythropoietin, and anemia of older persons: The InCHIANTI study. Arch. Intern. Med. 2005, 165, 2222–2227. [Google Scholar] [CrossRef]
- WHO. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Steensma, D.P.; Tefferi, A. Anemia in the elderly: How should we define it, when does it matter, and what can be done? Mayo Clin. Proc. 2007, 82, 958–966. [Google Scholar] [CrossRef]
- Gonzalez, L.M.B.; Seidl, E.M.F. Aging from the perspective of elderly men. Paidéia 2011, 21, 345–352. [Google Scholar] [CrossRef]
- Beutler, E.; Felitti, V.J.; Koziol, J.A.; Ho, N.J.; Gelbart, T. Penetrance of 845G→A (C282Y) HFE hereditary haemochromatosis mutation in the USA. Lancet 2002, 359, 211–218. [Google Scholar] [CrossRef]
- Beutler, E.; Waalen, J. The definition of anemia: What is the lower limit of normal of the blood hemoglobin concentration? Blood 2006, 107, 1747–1750. [Google Scholar] [CrossRef] [PubMed]
- Plan and Operation of the Third National Health and Nutrition Examination Survey, 1988–1994. Series 1: Programs and Collection Procedures; Vital and Health Statistics, Series 1; National Center for Health Statistics: Hyattsville, MD, USA, 1994; pp. 1–407.
- Tettamanti, M.; Lucca, U.; Gandini, F.; Recchia, A.; Mosconi, P.; Apolone, G.; Nobili, A.; Tallone, M.V.; Detoma, P.; Giacomin, A.; et al. Prevalence, incidence and types of mild anemia in the elderly: The “Health and Anemia” population-based study. Haematologica 2010, 95, 1849–1856. [Google Scholar] [CrossRef]
- Chaves, P.H.; Carlson, M.C.; Ferrucci, L.; Guralnik, J.M.; Semba, R.; Fried, L.P. Association between mild anemia and executive function impairment in community-dwelling older women: The Women’s Health and Aging Study II. J. Am. Geriatr. Soc. 2006, 54, 1429–1435. [Google Scholar] [CrossRef] [PubMed]
- Endres, H.G.; Wedding, U.; Pittrow, D.; Thiem, U.; Trampisch, H.J.; Diehm, C. Prevalence of anemia in elderly patients in primary care: Impact on 5-year mortality risk and differences between men and women. Curr. Med. Res. Opin. 2009, 25, 1143–1158. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Eisenstaedt, R.S.; Ferrucci, L.; Klein, H.G.; Woodman, R.C. Prevalence of anemia in persons 65 years and older in the United States: Evidence for a high rate of unexplained anemia. Blood 2004, 104, 2263–2268. [Google Scholar] [CrossRef]
- Nathavitharana, R.L.; Murray, J.A.; D’Sousa, N.; Sheehan, T.; Frampton, C.M.; Baker, B.W. Anaemia is highly prevalent among unselected internal medicine inpatients and is associated with increased mortality, earlier readmission and more prolonged hospital stay: An observational retrospective cohort study. Intern. Med. J. 2012, 42, 683–691. [Google Scholar] [CrossRef]
- Geisel, T.; Martin, J.; Schulze, B.; Schaefer, R.; Bach, M.; Virgin, G.; Stein, J. An etiologic profile of anemia in 405 geriatric patients. Anemia 2014, 2014, 932486. [Google Scholar] [CrossRef]
- Robinson, B.E. Epidemiology of chronic kidney disease and anemia. J. Am. Med. Dir. Assoc. 2006, 7, S3–S6; quiz S17–S21. [Google Scholar] [CrossRef]
- Al-Sharefi, A.; Mohammed, A.; Abdalaziz, A.; Jayasena, C.N. Androgens and Anemia: Current Trends and Future Prospects. Front. Endocrinol. 2019, 10, 754. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, J.M.; Vermeulen, A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr. Rev. 2005, 26, 833–876. [Google Scholar] [CrossRef] [PubMed]
- Price, E.A.; Mehra, R.; Holmes, T.H.; Schrier, S.L. Anemia in older persons: Etiology and evaluation. Blood Cells Mol. Dis. 2011, 46, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Shavelle, R.M.; MacKenzie, R.; Paculdo, D.R. Anemia and mortality in older persons: Does the type of anemia affect survival? Int. J. Hematol. 2012, 95, 248–256. [Google Scholar] [CrossRef]
- Akman, T.; Arıca, D.; Hatipoğlu, B.; Arslanoğlu, E.; Koca, E.; Karakuş, S.; Akı, Ş.Z. A Cross Sectional Analysis of Etiology of Anemia Among Elderly Patients. Osman. Tıp Derg. 2023, 45, 844–852. [Google Scholar] [CrossRef]
- Girelli, D.; Marchi, G.; Camaschella, C. Anemia in the Elderly. Hemasphere 2018, 2, e40. [Google Scholar] [CrossRef]
- Bach, V.; Schruckmayer, G.; Sam, I.; Kemmler, G.; Stauder, R. Prevalence and possible causes of anemia in the elderly: A cross-sectional analysis of a large European university hospital cohort. Clin. Interv. Aging 2014, 9, 1187–1196. [Google Scholar] [CrossRef]
- Barnett, K.; Mercer, S.W.; Norbury, M.; Watt, G.; Wyke, S.; Guthrie, B. Epidemiology of multimorbidity and implications for health care, research, and medical education: A cross-sectional study. Lancet 2012, 380, 37–43. [Google Scholar] [CrossRef]
- Halawi, R.; Moukhadder, H.; Taher, A. Anemia in the elderly: A consequence of aging? Expert Rev. Hematol. 2017, 10, 327–335. [Google Scholar] [CrossRef]
- Lopez, A.; Cacoub, P.; Macdougall, I.C.; Peyrin-Biroulet, L. Iron deficiency anaemia. Lancet 2016, 387, 907–916. [Google Scholar] [CrossRef]
- Andrès, E.; Federici, L.; Serraj, K.; Kaltenbach, G. Update of nutrient-deficiency anemia in elderly patients. Eur. J. Intern. Med. 2008, 19, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Loikas, S.; Koskinen, P.; Irjala, K.; Löppönen, M.; Isoaho, R.; Kivelä, S.L.; Pelliniemi, T.T. Vitamin B12 deficiency in the aged: A population-based study. Age Ageing 2007, 36, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Goodnough, L.T. Iron deficiency syndromes and iron-restricted erythropoiesis (CME). Transfusion 2012, 52, 1584–1592. [Google Scholar] [CrossRef] [PubMed]
- Goodnough, L.T.; Schrier, S.L. Evaluation and management of anemia in the elderly. Am. J. Hematol. 2014, 89, 88–96. [Google Scholar] [CrossRef]
- Gowanlock, Z.; Sriram, S.; Martin, A.; Xenocostas, A.; Lazo-Langner, A. Erythropoietin Levels in Elderly Patients with Anemia of Unknown Etiology. PLoS ONE 2016, 11, e0157279. [Google Scholar] [CrossRef]
- Makipour, S.; Kanapuru, B.; Ershler, W.B. Unexplained anemia in the elderly. Semin. Hematol. 2008, 45, 250–254. [Google Scholar] [CrossRef]
- Sriram, S.; Xenocostas, A.; Lazo-Langner, A. Erythropoietin in anemia of unknown etiology: A systematic review and meta-analysis. Hematology 2016, 21, 234–240. [Google Scholar] [CrossRef]
- Lousa, I.; Reis, F.; Beirão, I.; Alves, R.; Belo, L.; Santos-Silva, A. New Potential Biomarkers for Chronic Kidney Disease Management-A Review of the Literature. Int. J. Mol. Sci. 2020, 22, 43. [Google Scholar] [CrossRef]
- Belo, L.; Rocha, S.; Valente, M.J.; Coimbra, S.; Catarino, C.; Bronze-da-Rocha, E.; Rocha-Pereira, P.; do Sameiro-Faria, M.; Oliveira, J.G.; Madureira, J.; et al. Hepcidin and diabetes are independently related with soluble transferrin receptor levels in chronic dialysis patients. Ren. Fail. 2019, 41, 662–672. [Google Scholar] [CrossRef]
- Karimi, Z.; Raeisi Shahraki, H.; Mohammadian-Hafshejani, A. Erythropoiesis-stimulating agents and cardiovascular mortality: A systematic review and meta-analysis of 17 studies and 372,156 hemodialysis patients. Int. J. Cardiol. Cardiovasc. Risk Prev. 2023, 19, 200220. [Google Scholar] [CrossRef]
- Ruperto, M.; Rodríguez-Mendiola, N.; Díaz-Domínguez, M.; Giménez-Moyano, S.; García-Bermejo, M.L.; Fernández-Lucas, M. Effect of oral administration of docohexanoic acid on anemia and inflammation in hemodialysis patients: A randomized controlled clinical trial. Clin. Nutr. ESPEN 2021, 41, 129–135. [Google Scholar] [CrossRef] [PubMed]

|
| Non-Modifiable Risk Factors | Common Modifiable Risk Factors | Less Common Modifiable Risk Factors |
|---|---|---|
|
|
|
| CKD-EPI Creatinine Equation (2021) | eGFRcr = 142 × min(Scr/k,1)α × max(Scr/k,1)−1.200 × 0.9938age × 1.012[if female] |
| CKD-EPI Creatinine-Cystatin C Equation (2021) | eGFRcr−cys = 135 × min(Scr/k,1)α × max(Scr/k,1)−0.544 × min(Scys/0.8,1)−0.323 × max(Scys/0.8,1)−0.778 × 0.9961age × 0.963[if female] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, S.; Lousa, I.; Carvalho, M.; Sameiro-Faria, M.; Santos-Silva, A.; Belo, L. Anemia in Elderly Patients: Contribution of Renal Aging and Chronic Kidney Disease. Geriatrics 2025, 10, 43. https://doi.org/10.3390/geriatrics10020043
Santos S, Lousa I, Carvalho M, Sameiro-Faria M, Santos-Silva A, Belo L. Anemia in Elderly Patients: Contribution of Renal Aging and Chronic Kidney Disease. Geriatrics. 2025; 10(2):43. https://doi.org/10.3390/geriatrics10020043
Chicago/Turabian StyleSantos, Simone, Irina Lousa, Márcia Carvalho, Maria Sameiro-Faria, Alice Santos-Silva, and Luís Belo. 2025. "Anemia in Elderly Patients: Contribution of Renal Aging and Chronic Kidney Disease" Geriatrics 10, no. 2: 43. https://doi.org/10.3390/geriatrics10020043
APA StyleSantos, S., Lousa, I., Carvalho, M., Sameiro-Faria, M., Santos-Silva, A., & Belo, L. (2025). Anemia in Elderly Patients: Contribution of Renal Aging and Chronic Kidney Disease. Geriatrics, 10(2), 43. https://doi.org/10.3390/geriatrics10020043










