Functional Analysis of a Salicylate Hydroxylase in Sclerotinia sclerotiorum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Strains, Plant Materials, and Culture Conditions
2.2. Bioinformatic Analysis
2.3. Expression Vector Construction and Salicylate Hydroxylase Activity Assay
2.4. RNA Extraction and RT-PCR Analysis
2.5. Mutant Generation and Complementation
2.6. Hypha Growth, Cell Wall Integrity, Oxalic Acid Production, and Exogenous SA Sensitivity Assay
2.7. Pathogenicity Assay
2.8. Transgenic Arabidopsis Construction and Quantification of SA in A. thaliana Leaves
2.9. Statistical Analysis
3. Results
3.1. Identification of Genes Encoding Salicylate Hydroxylase in S. sclerotiorum
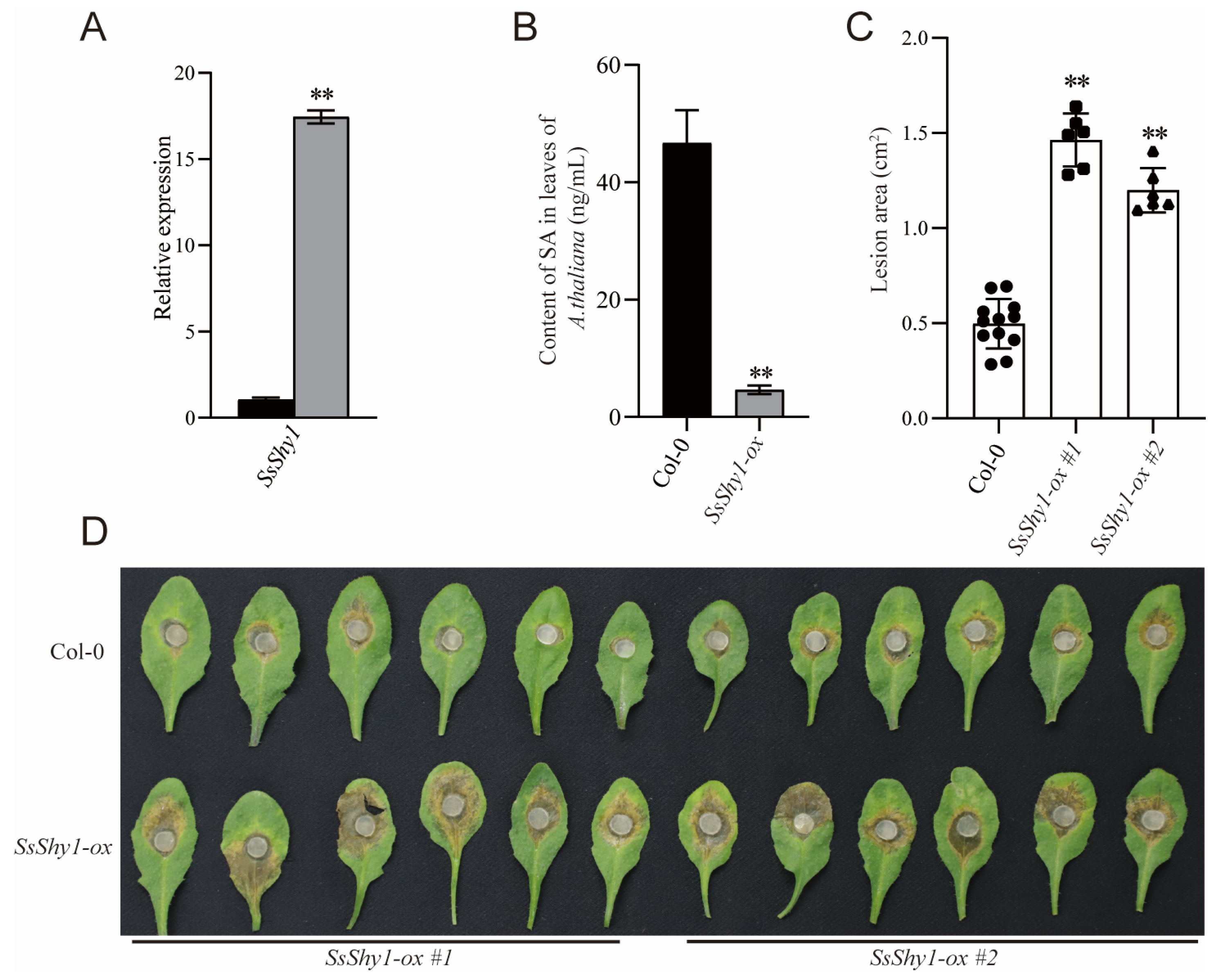
3.2. Overexpression of SsShy1 in Plant Reduced the Content of Plant SA and Increased Susceptibility to S. sclerotiorum
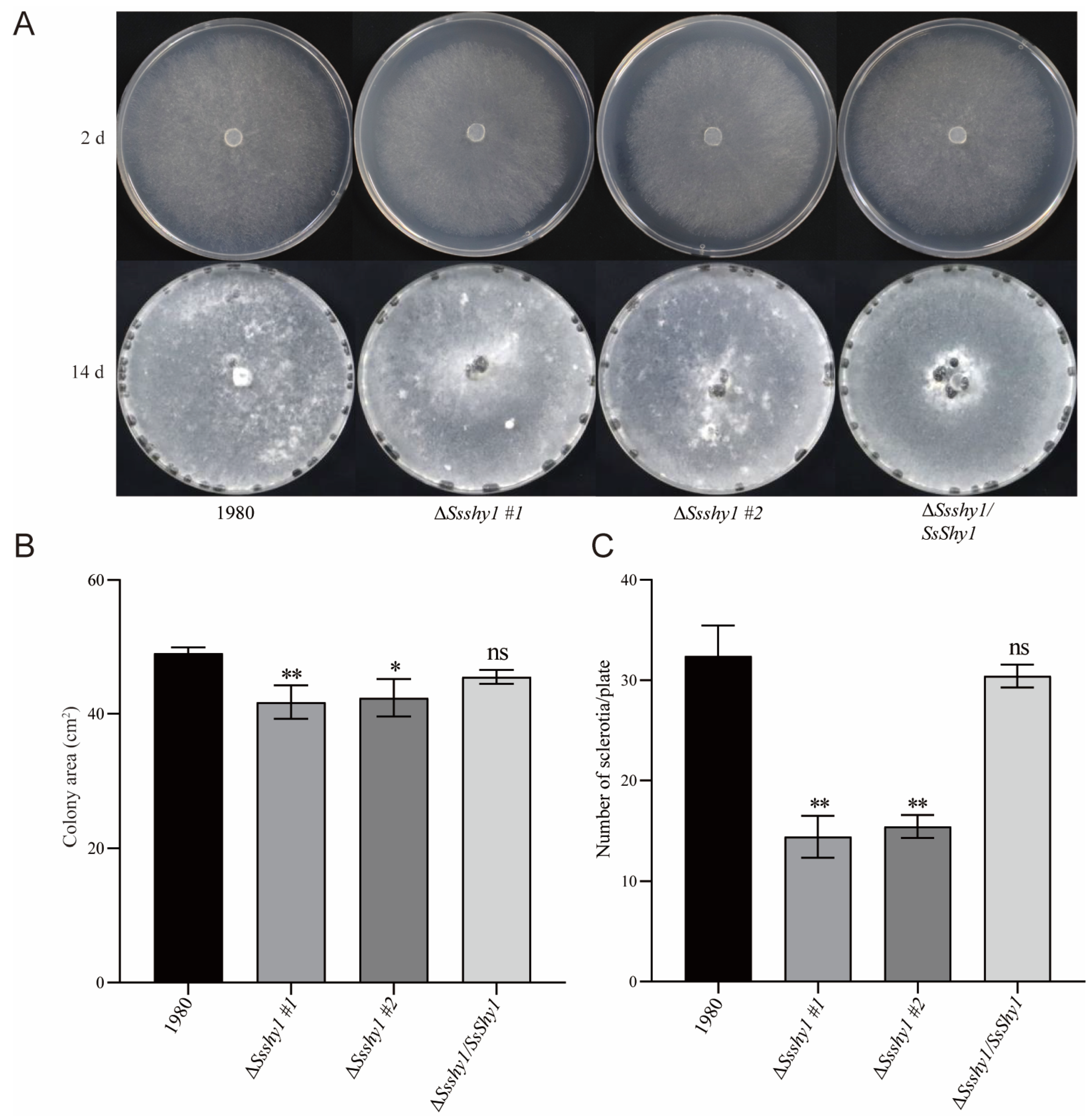
3.3. Deletion of SsShy1 Affected the Hyphae Growth and Sclerotia Production
3.4. SsShy1 Was Related to Cell Wall Integrity but Not OA Secretion
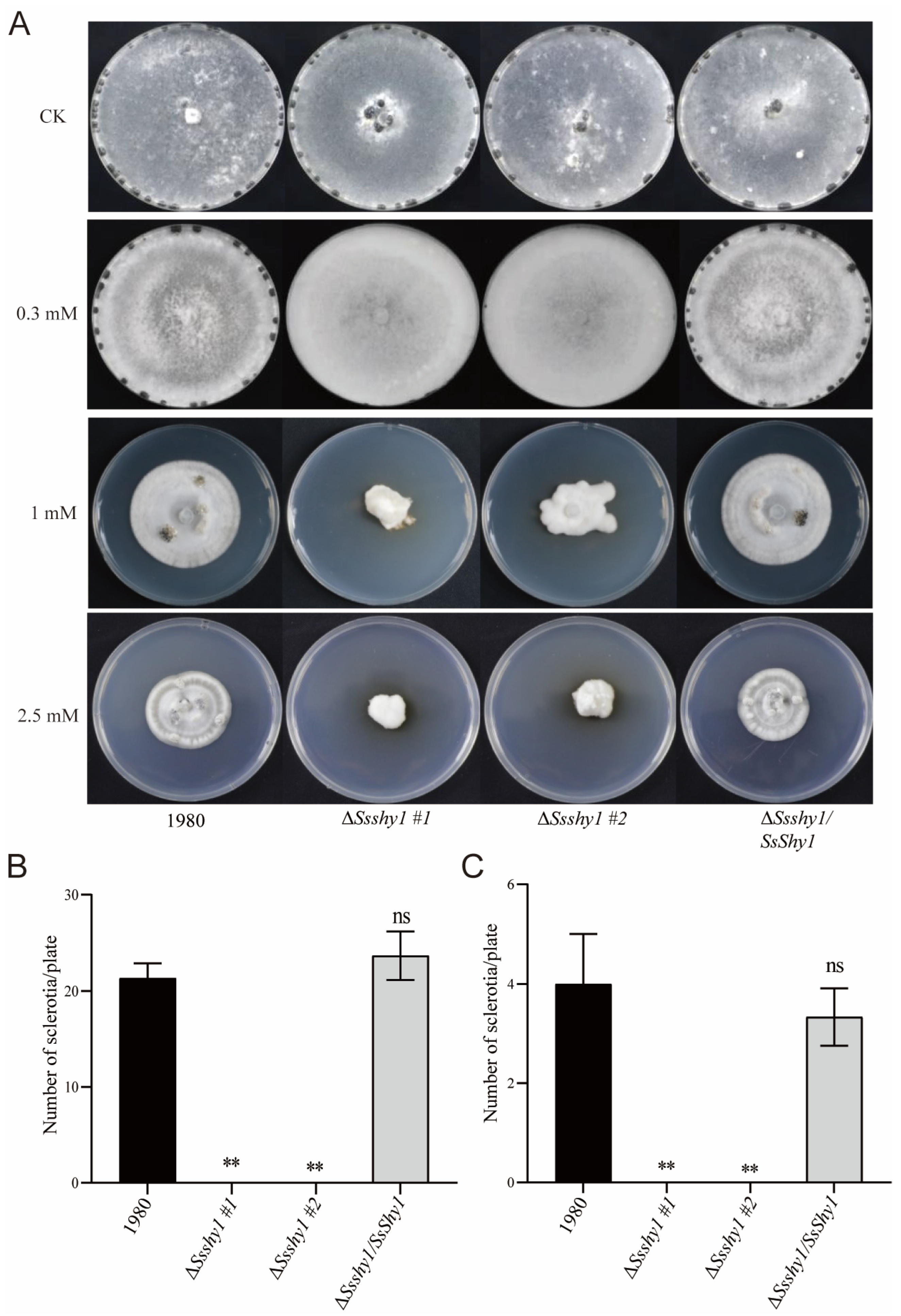
3.5. Deletion of SsShy1 Increased Sensitivity to Exogenous SA
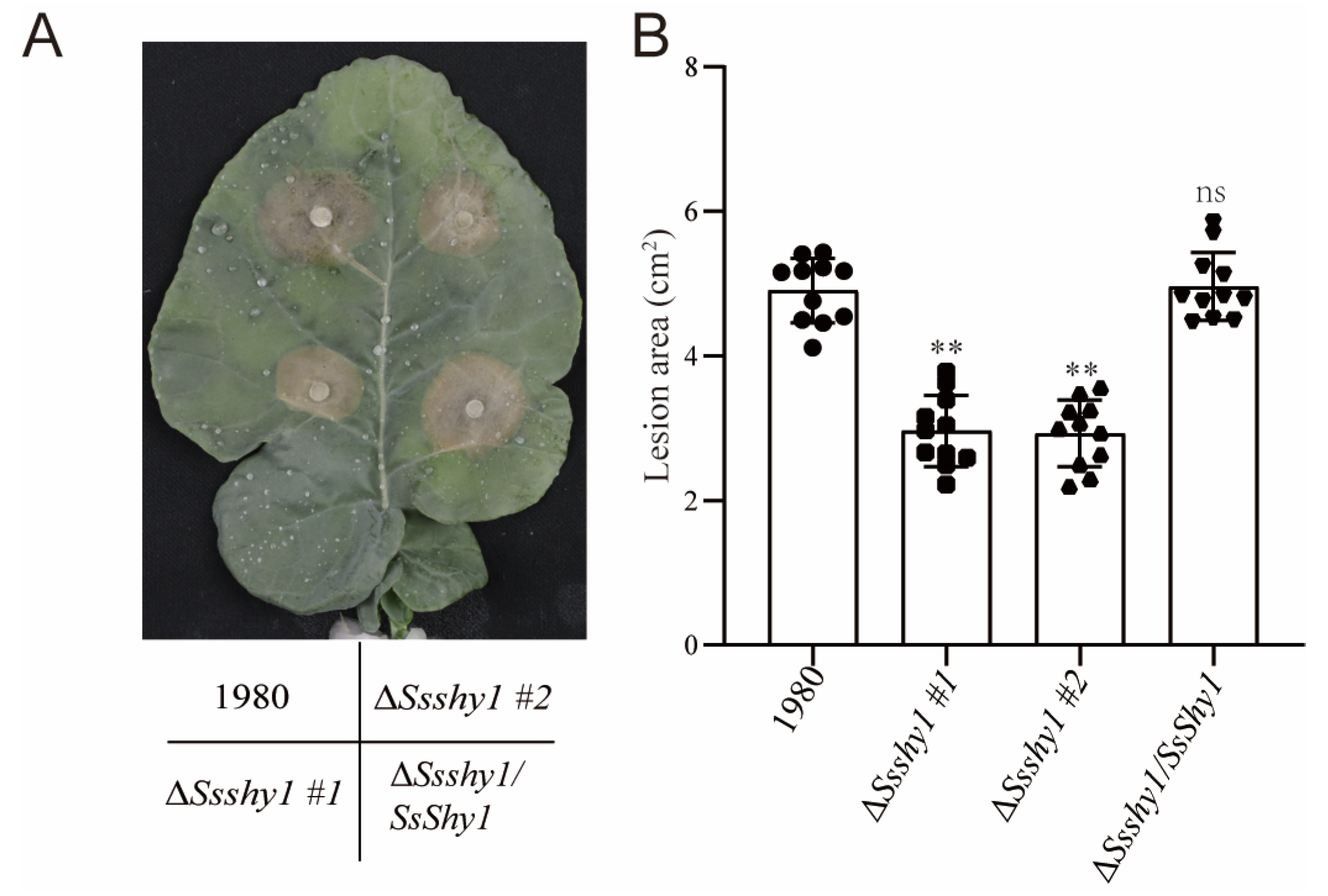
3.6. SsShy Was Required for the Virulence of S. sclerotiorum
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jones, J.D.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.K.; Baek, K.H. Salicylic acid biosynthesis and metabolism: A divergent pathway for plants and bacteria. Biomolecules 2021, 11, 705. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, K.; Sato, M.; Stoddard, T.; Glazebrook, J.; Katagiri, F. Network properties of robust immunity in plants. PLoS Genet. 2009, 5, e1000772. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, X. Salicylic acid: Biosynthesis, perception, and contributions to plant immunity. Curr. Opin. Plant Biol. 2019, 50, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Durrant, W.E.; Dong, X. Systemic acquired resistance. Annu. Rev. Phytopathol. 2004, 42, 185–209. [Google Scholar] [CrossRef] [PubMed]
- Klessig, D.F.; Choi, H.W.; Dempsey, D.A. Systemic acquired resistance and salicylic acid: Past, present, and future. Mol. Plant-Microbe Interact. MPMI 2018, 31, 871–888. [Google Scholar] [CrossRef] [PubMed]
- Ding, P.; Ding, Y. Stories of salicylic acid: A plant defense hormone. Trends Plant Sci. 2020, 25, 549–565. [Google Scholar] [CrossRef]
- Gaffney, T.; Friedrich, L.; Vernooij, B.; Negrotto, D.; Nye, G.; Uknes, S.; Ward, E.; Kessmann, H.; Ryals, J. Requirement of salicylic acid for the induction of systemic acquired resistance. Science 1993, 261, 754–756. [Google Scholar] [CrossRef]
- Delaney, T.P.; Uknes, S.; Vernooij, B.; Friedrich, L.; Weymann, K.; Negrotto, D.; Gaffney, T.; Gut-Rella, M.; Kessmann, H.; Ward, E.; et al. A central role of salicylic acid in plant disease resistance. Science 1994, 266, 1247–1250. [Google Scholar] [CrossRef]
- Wildermuth, M.C.; Dewdney, J.; Wu, G.; Ausubel, F.M. Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 2001, 414, 562–565. [Google Scholar] [CrossRef]
- Rekhter, D.; Lüdke, D.; Ding, Y.; Feussner, K.; Zienkiewicz, K.; Lipka, V.; Wiermer, M.; Zhang, Y.; Feussner, I. Isochorismate-derived biosynthesis of the plant stress hormone salicylic acid. Science 2019, 365, 498–502. [Google Scholar] [CrossRef]
- Huang, J.; Gu, M.; Lai, Z.; Fan, B.; Shi, K.; Zhou, Y.H.; Yu, J.Q.; Chen, Z. Functional analysis of the Arabidopsis PAL gene family in plant growth, development, and response to environmental stress. Plant Physiol. 2010, 153, 1526–1538. [Google Scholar] [CrossRef] [PubMed]
- Vlot, A.C.; Dempsey, D.A.; Klessig, D.F. Salicylic acid, a multifaceted hormone to combat disease. Annu. Rev. Phytopathol. 2009, 47, 177–206. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Oh, J.; Min, K.R.; Kim, Y. Nucleotide sequence of salicylate hydroxylase gene and its 5′-flanking region of Pseudomonas putida KF715. Biochem. Biophys. Res. Commun. 1996, 218, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Asao, E.; Nakamura, Y.; Nakamura, M.; Ohnishi, K.; Fukuda, S. Overexpression of salicylate hydroxylase and the crucial role of lys(163) as its NADH binding site. J. Biochem. 2000, 128, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Lowe-Power, T.M.; Jacobs, J.M.; Ailloud, F.; Fochs, B.; Prior, P.; Allen, C. Degradation of the plant defense signal salicylic acid protects Ralstonia solanacearum from toxicity and enhances virulence on tobacco. mBio 2016, 7, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Pang, Z.; Trivedi, P.; Zhou, X.; Ying, X.; Jia, H.; Wang, N. ‘Candidatus Liberibacter asiaticus’ encodes a functional salicylic acid (sa) hydroxylase that degrades sa to suppress plant defenses. Mol. Plant-Microbe Interact. MPMI 2017, 30, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Rabe, F.; Ajami-Rashidi, Z.; Doehlemann, G.; Kahmann, R.; Djamei, A. Degradation of the plant defence hormone salicylic acid by the biotrophic fungus Ustilago Maydis. Mol. Microbiol. 2013, 89, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Hao, G.; Naumann, T.A.; Vaughan, M.M.; McCormick, S.; Usgaard, T.; Kelly, A.; Ward, T.J. Characterization of a Fusarium graminearum salicylate hydroxylase. Front. Microbiol. 2018, 9, 3219. [Google Scholar] [CrossRef]
- Rocheleau, H.; Al-Harthi, R.; Ouellet, T. Degradation of salicylic acid by Fusarium graminearum. Fungal Biol. 2019, 123, 77–86. [Google Scholar] [CrossRef]
- Qi, P.F.; Zhang, Y.Z.; Liu, C.H.; Chen, Q.; Guo, Z.R.; Wang, Y.; Xu, B.J.; Jiang, Y.F.; Zheng, T.; Gong, X.; et al. Functional analysis of FgNahG clarifies the contribution of salicylic acid to wheat (Triticum aestivum) resistance against Fusarium head blight. Toxins 2019, 11, 59. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhu, T.; Song, Y.; Feng, L.; Kear, P.J.; Riseh, R.S.; Sitohy, M.; Datla, R.; Ren, M. Salicylic acid fights against Fusarium wilt by inhibiting target of rapamycin signaling pathway in Fusarium oxysporum. J. Adv. Res. 2022, 39, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bolton, M.D.; Thomma, B.P.; Nelson, B.D. Sclerotinia sclerotiorum (Lib.) de Bary: Biology and molecular traits of a cosmopolitan pathogen. Mol. Plant Pathol. 2006, 7, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Stotz, H.U. Defense against Sclerotinia sclerotiorum in Arabidopsis is dependent on jasmonic acid, salicylic acid, and ethylene signaling. Mol. Plant-Microbe Interact. MPMI 2007, 20, 1384–1395. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Tan, X.; Zhang, Z.; Gu, S.; Li, G.; Shi, H. Defense to Sclerotinia sclerotiorum in oilseed rape is associated with the sequential activations of salicylic acid signaling and jasmonic acid signaling. Plant Sci. Int. J. Exp. Plant Biol. 2012, 184, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Nováková, M.; Sašek, V.; Dobrev, P.I.; Valentová, O.; Burketová, L. Plant hormones in defense response of Brassica napus to Sclerotinia sclerotiorum—Reassessing the role of salicylic acid in the interaction with a necrotroph. Plant Physiol. Biochem. PPB 2014, 80, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Fan, Y.; Li, X.; Chen, X.; Yu, S.; Wei, S.; Li, S.; Chang, W.; Qu, C.; Li, J.; et al. LESION MIMIC MUTANT 1 confers basal resistance to Sclerotinia sclerotiorum in rapeseed via a salicylic acid-dependent pathway. J. Exp. Bot. 2023, 74, 5620–5634. [Google Scholar] [CrossRef]
- Rollins, J.A. The Sclerotinia sclerotiorum pac1 gene is required for sclerotial development and virulence. Mol. Plant-Microbe Interact. MPMI 2003, 16, 785–795. [Google Scholar] [CrossRef]
- Siciliano, I.; Amaral Carneiro, G.; Spadaro, D.; Garibaldi, A.; Gullino, M.L. Jasmonic acid, abscisic acid, and salicylic acid are involved in the phytoalexin responses of rice to Fusarium fujikuroi, a high gibberellin producer pathogen. J. Agric. Food Chem. 2015, 63, 8134–8142. [Google Scholar] [CrossRef]
- Cessna, S.G.; Sears, V.E.; Dickman, M.B.; Low, P.S. Oxalic acid, a pathogenicity factor for Sclerotinia sclerotiorum, suppresses the oxidative burst of the host plant. Plant Cell 2000, 12, 2191–2200. [Google Scholar] [CrossRef]
- Ambrose, K.V.; Tian, Z.; Wang, Y.; Smith, J.; Zylstra, G.; Huang, B.; Belanger, F.C. Functional characterization of salicylate hydroxylase from the fungal endophyte Epichloë festucae. Sci. Rep. 2015, 5, 10939. [Google Scholar] [CrossRef] [PubMed]
- Lubbers, R.J.M.; Dilokpimol, A.; Visser, J.; Hildén, K.S.; Mäkelä, M.R.; de Vries, R.P. Discovery and functional analysis of a salicylic acid hydroxylase from Aspergillus niger. Appl. Environ. Microbiol. 2021, 87, e02701-20. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Dutta, T.; Varman, A.M.; Eudes, A.; Manalansan, B.; Loqué, D.; Singh, S. Lignin valorization: Two hybrid biochemical routes for the conversion of polymeric lignin into value-added chemicals. Sci. Rep. 2017, 7, 8420. [Google Scholar] [CrossRef] [PubMed]
- Curran, K.A.; Leavitt, J.M.; Karim, A.S.; Alper, H.S. Metabolic engineering of muconic acid production in Saccharomyces cerevisiae. Metab. Eng. 2013, 15, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Lin, Y.; Huang, Q.; Yuan, Q.; Yan, Y. A novel muconic acid biosynthesis approach by shunting tryptophan biosynthesis via anthranilate. Appl. Environ. Microbiol. 2013, 79, 4024–4030. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, L.Y.; Li, X.; Zhao, F.Y.; Sarwar, R.; Cao, J.; Li, Y.L.; Ding, L.N.; Zhu, K.M.; Yang, Y.H.; et al. Genome-wide identification of the NPR1-like gene family in Brassica napus and functional characterization of BnaNPR1 in resistance to Sclerotinia sclerotiorum. Plant Cell Rep. 2020, 39, 709–722. [Google Scholar] [CrossRef] [PubMed]
- van Wees, S.C.; Glazebrook, J. Loss of non-host resistance of Arabidopsis NahG to Pseudomonas syringae pv. phaseolicola is due to degradation products of salicylic acid. Plant J. Cell Mol. Biol. 2003, 33, 733–742. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, S.; Huang, K.; Li, B.; Lu, G.; Wang, A. Functional Analysis of a Salicylate Hydroxylase in Sclerotinia sclerotiorum. J. Fungi 2023, 9, 1169. https://doi.org/10.3390/jof9121169
He S, Huang K, Li B, Lu G, Wang A. Functional Analysis of a Salicylate Hydroxylase in Sclerotinia sclerotiorum. Journal of Fungi. 2023; 9(12):1169. https://doi.org/10.3390/jof9121169
Chicago/Turabian StyleHe, Shengfei, Kun Huang, Baoge Li, Guodong Lu, and Airong Wang. 2023. "Functional Analysis of a Salicylate Hydroxylase in Sclerotinia sclerotiorum" Journal of Fungi 9, no. 12: 1169. https://doi.org/10.3390/jof9121169
APA StyleHe, S., Huang, K., Li, B., Lu, G., & Wang, A. (2023). Functional Analysis of a Salicylate Hydroxylase in Sclerotinia sclerotiorum. Journal of Fungi, 9(12), 1169. https://doi.org/10.3390/jof9121169





