In Situ Processing to Achieve High-Performance Epoxy Nanocomposites with Low Graphene Oxide Loading
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
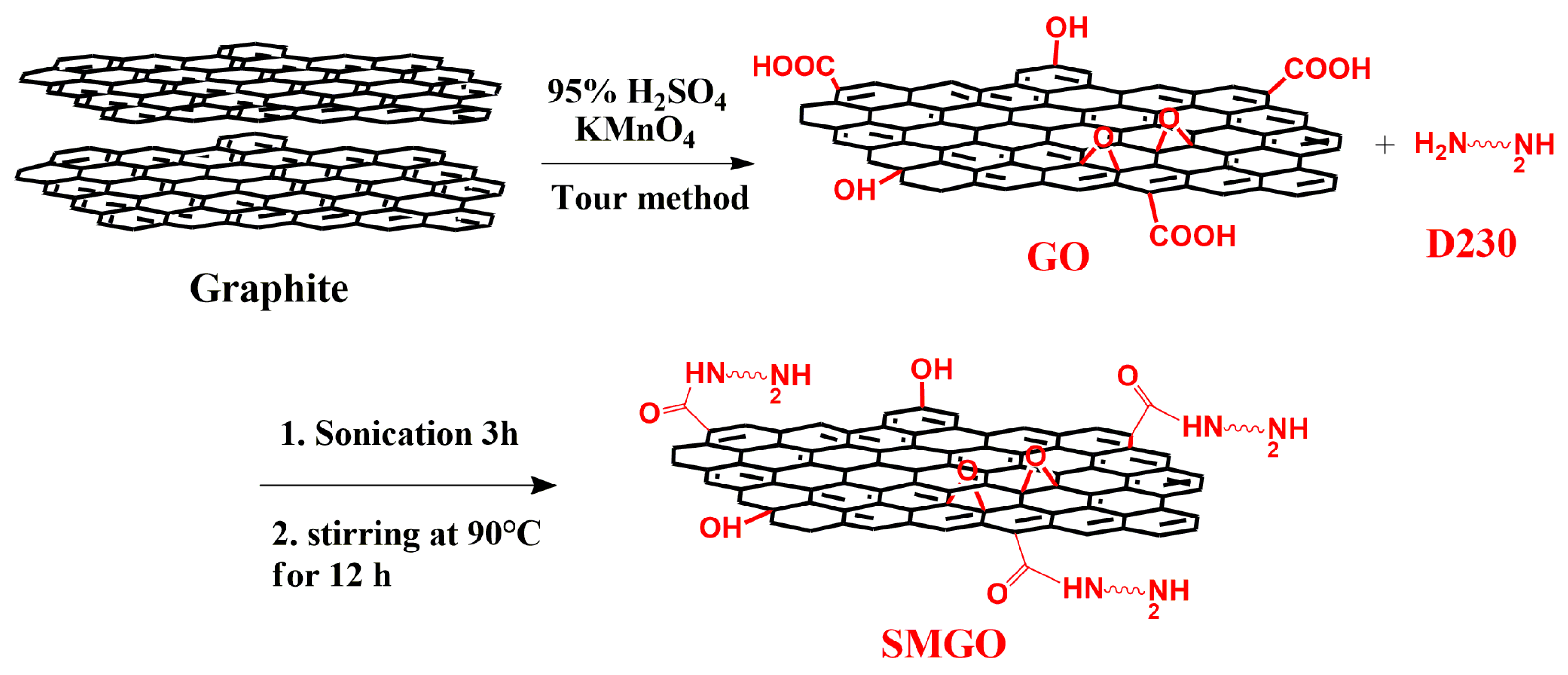
2.2. Synthesis of GO Sheet
2.3. Freeze-Dried Graphene Oxide
2.4. Dispersion of GO Nanofillers
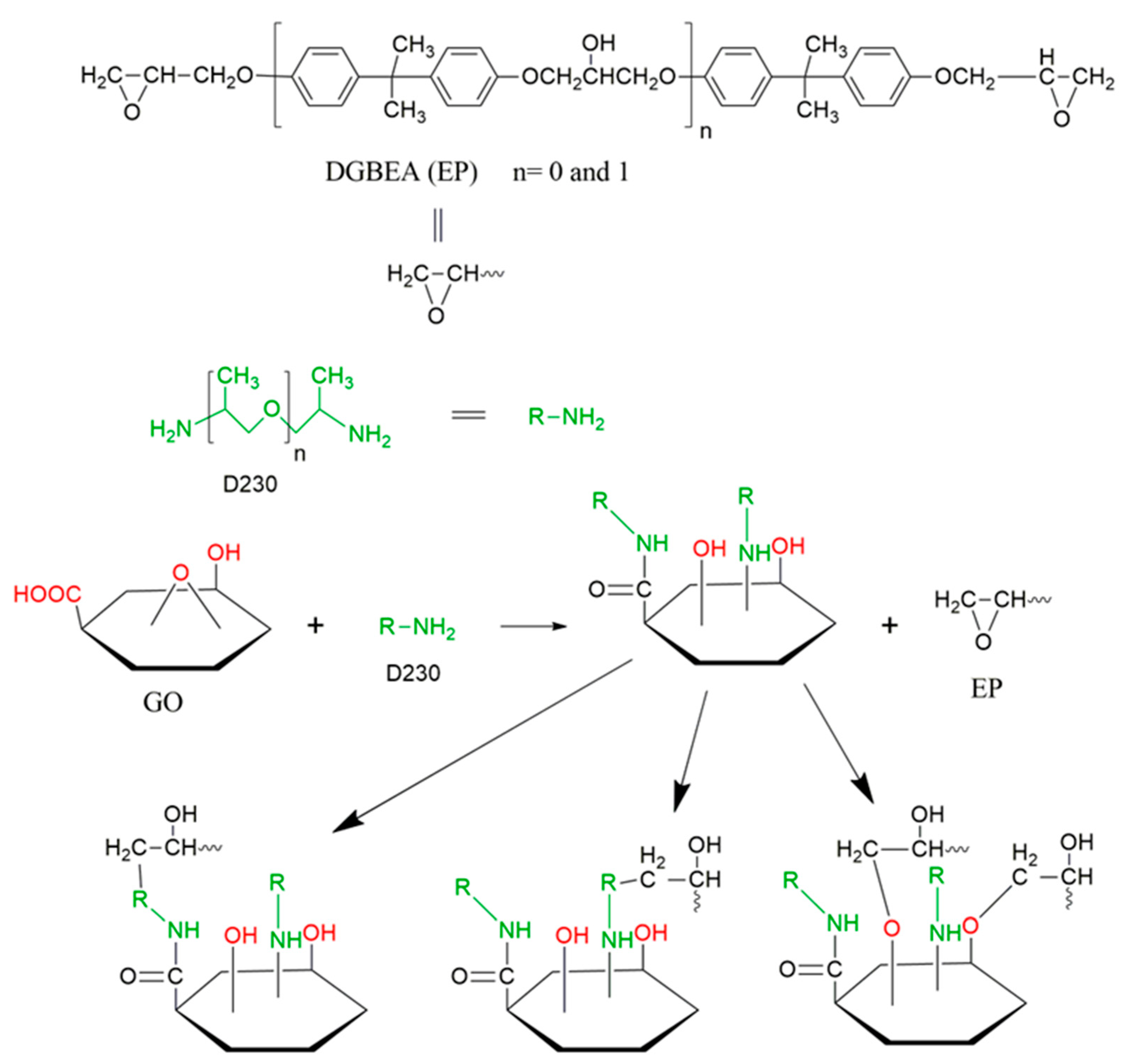
2.5. Fabrication of Epoxy Nanocomposites
2.6. Characterization
3. Results and Discussion
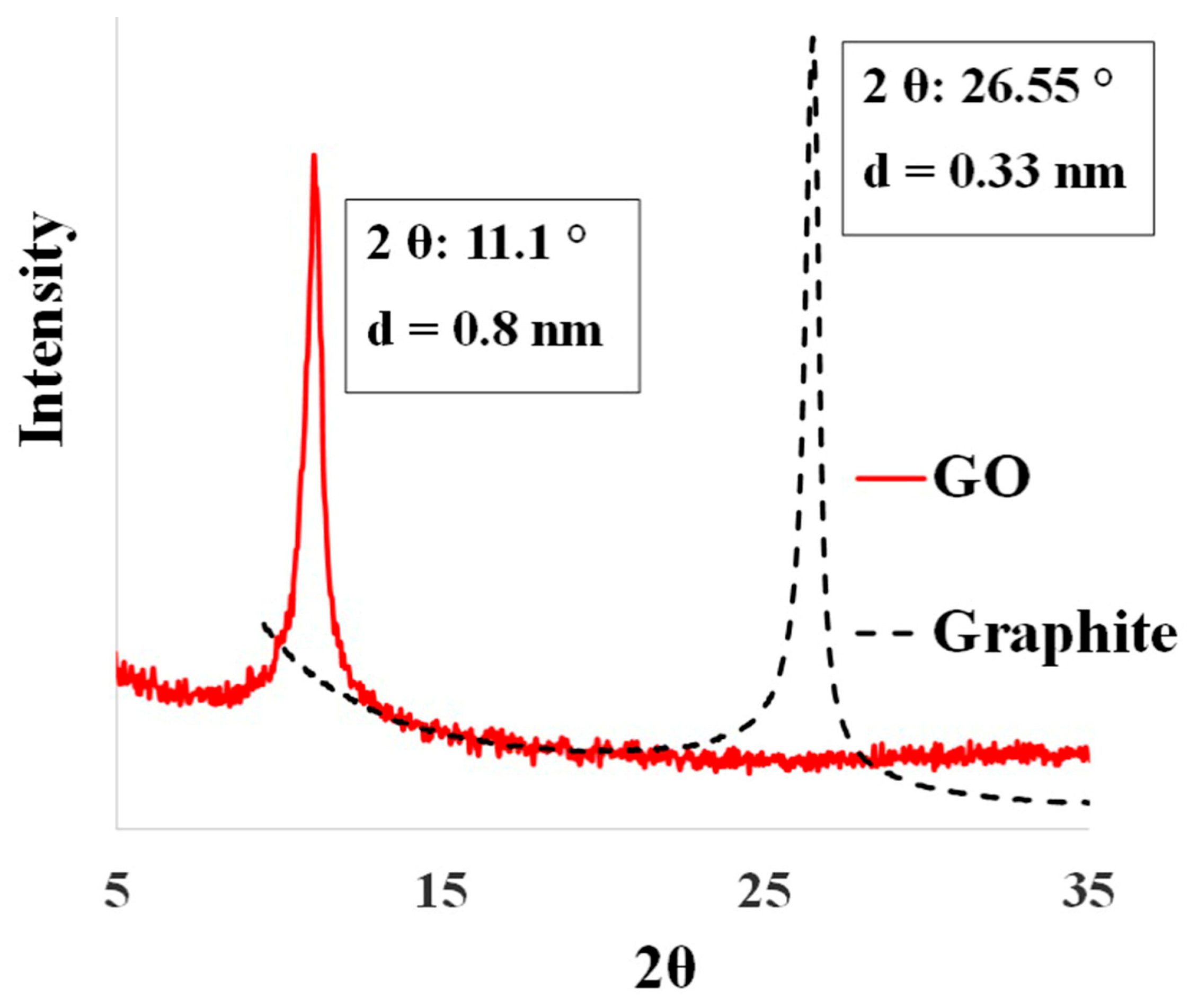
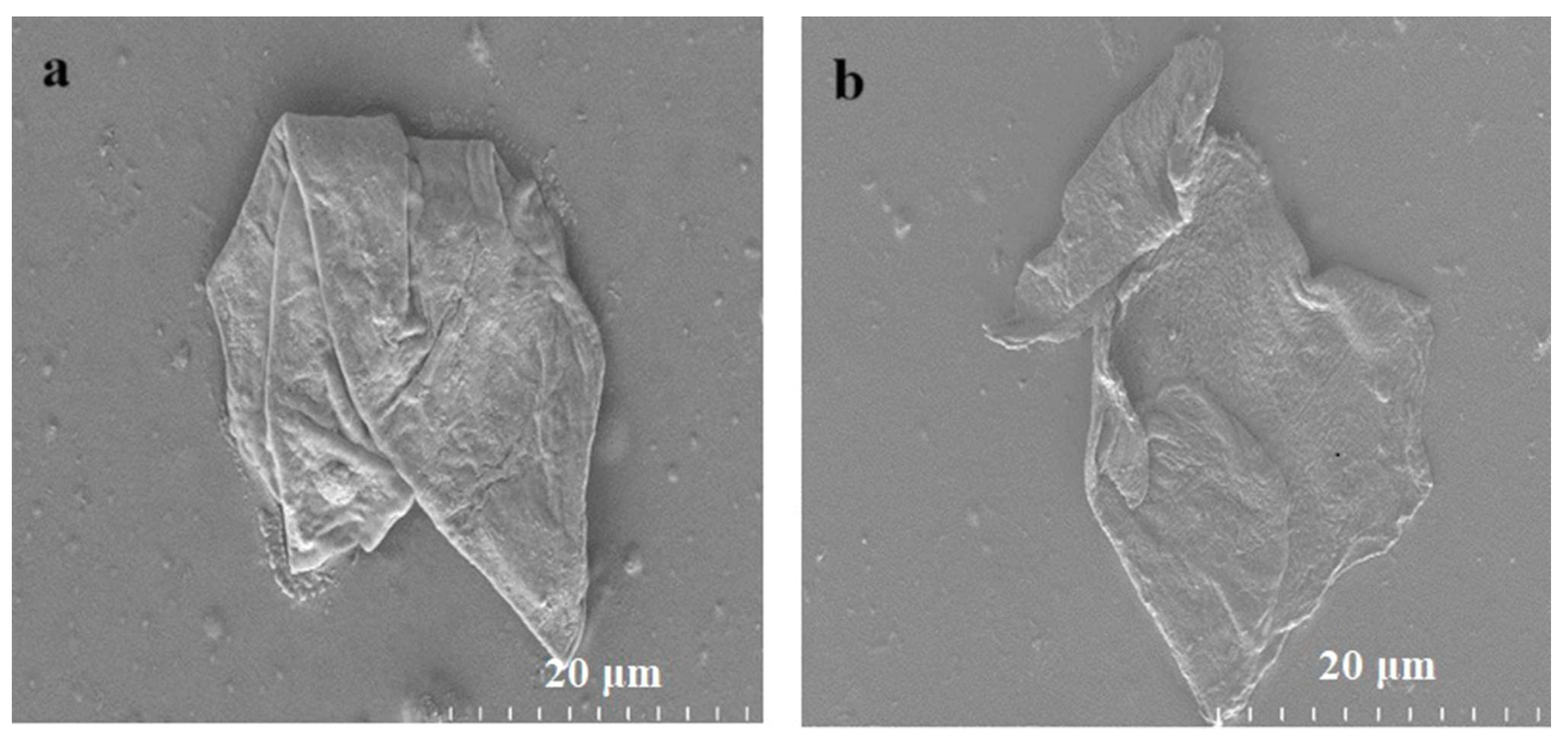
3.1. Characterization of As-Synthesized GO
3.2. Microstructure of Nanocomposites
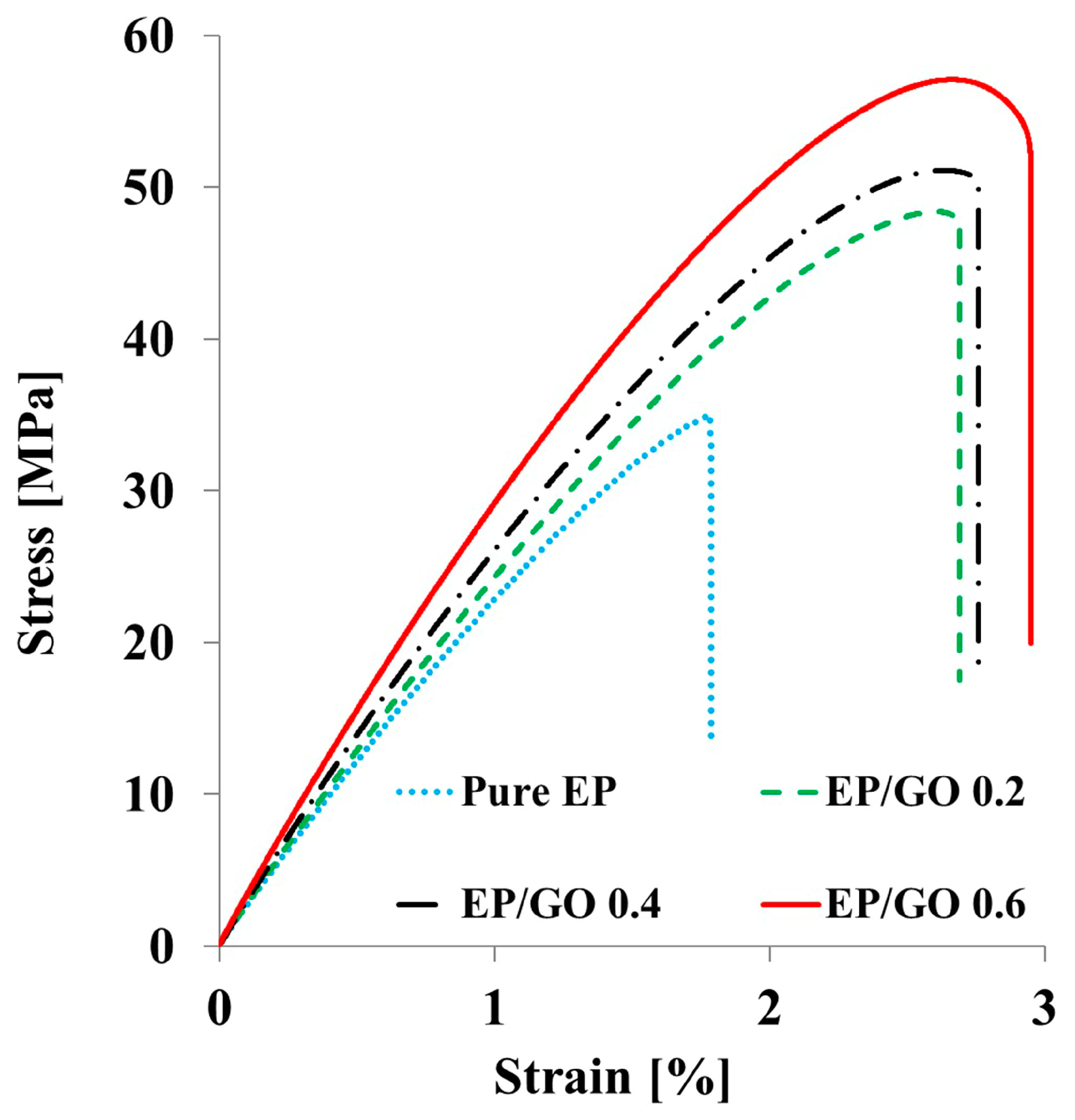
3.3. Tensile Properties
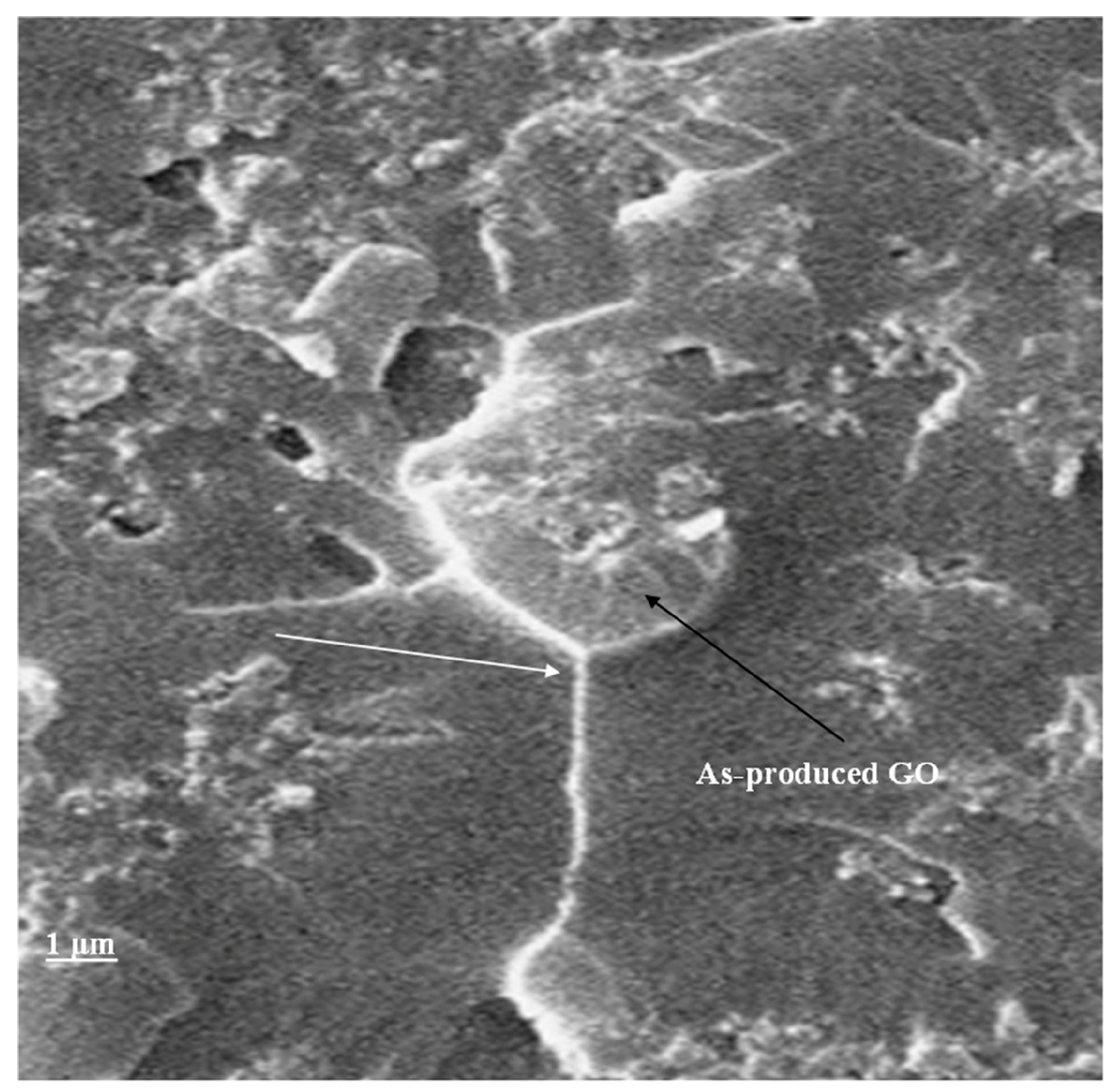
3.4. Fractography and Microstructure Analysis
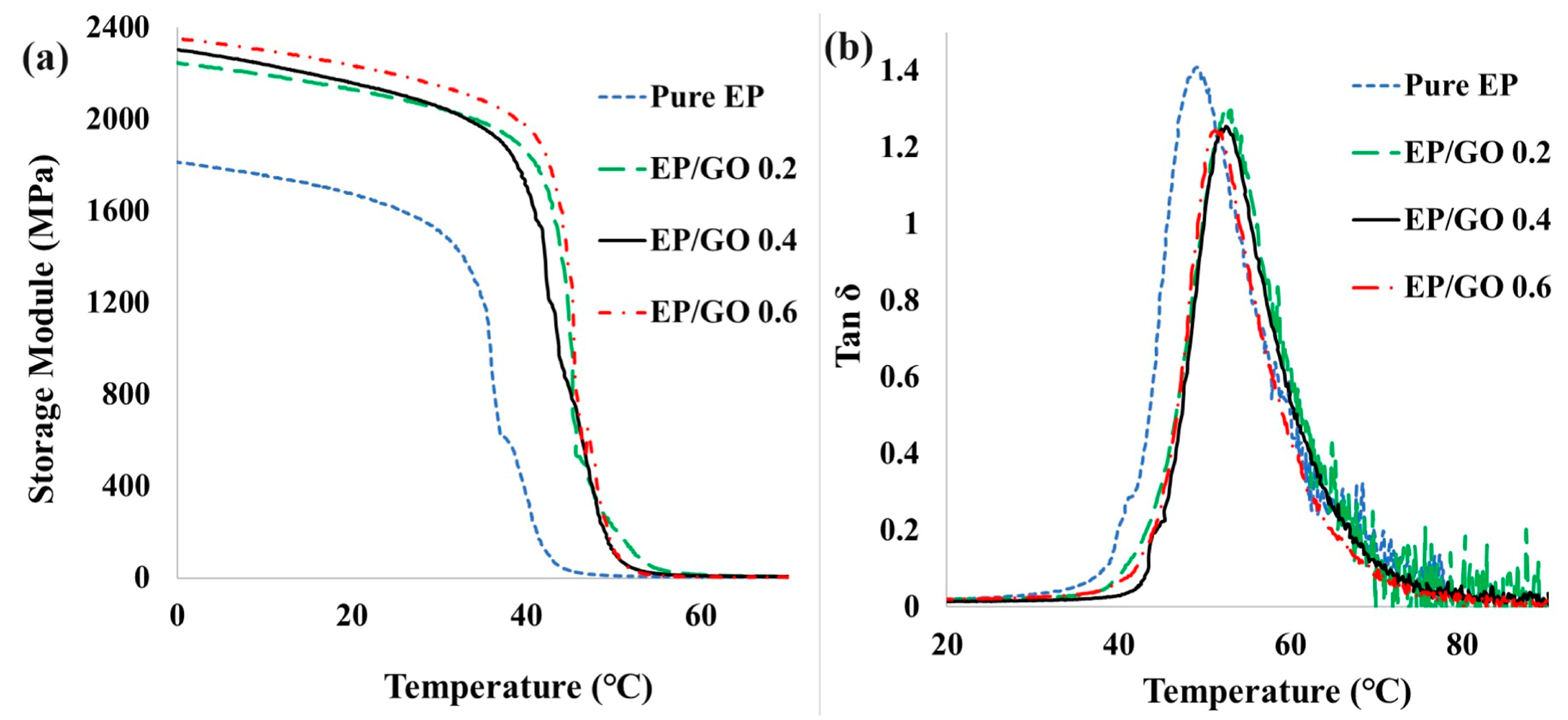
3.5. Thermomechanical Properties
3.6. FTIR Spectroscopy
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, X.; Ding, S.; Qiu, L.; Ashour, A.; Wang, Y.; Han, B.; Ou, J. Improving Bond of Fiber-Reinforced Polymer Bars with Concrete through Incorporating Nanomaterials. Compos. Part B Eng. 2022, 239, 109960. [Google Scholar] [CrossRef]
- Khandelwal, S.; Rhee, K.Y. Recent Advances in Basalt-Fiber-Reinforced Composites: Tailoring the Fiber-Matrix Interface. Compos. Part B Eng. 2020, 192, 108011. [Google Scholar] [CrossRef]
- Li, Z.; Wang, R.; Young, R.; Deng, L.; Yang, F.; Hao, L. Control of the Functionality of Graphene Oxide for Its Application in Epoxy Nanocomposites. Polymer 2013, 54, 6437–6446. [Google Scholar] [CrossRef]
- Wang, X.; Xing, W.; Zhang, P.; Song, L.; Yang, H.; Hu, Y. Covalent Functionalization of Graphene with Organosilane and Its Use as a Reinforcement in Epoxy Composites. Compos. Sci. Technol. 2012, 72, 737–743. [Google Scholar] [CrossRef]
- Mirzapour, A.; Asadollahi, M.; Baghshaei, S.; Akbari, M. Effect of Nanosilica on the Microstructure, Thermal Properties and Bending Strength of Nanosilica Modified Carbon Fiber/Phenolic Nanocomposite. Compos. Part Appl. Sci. Manuf. 2014, 63, 159–167. [Google Scholar] [CrossRef]
- Wetzel, B.; Rosso, P.; Haupert, F.; Friedrich, K. Epoxy Nanocomposites—Fracture and Toughening Mechanisms. Eng. Fract. Mech. 2006, 73, 2375–2398. [Google Scholar] [CrossRef]
- Kim, J.; Cha, J.; Jun, G.; Yoo, S.C.; Ryu, S.; Hong, H. Fabrication of Graphene Nanoplatelet/Epoxy Nanocomposites for Lightweight and High-Strength Structural Applications. Part. Syst. Charact. 2018, 35, 1700412. [Google Scholar] [CrossRef]
- Meng, Q.; Wu, H.; Zhao, Z.; Araby, S.; Lu, S.; Ma, J. Free-Standing, Flexible, Electrically Conductive Epoxy/Graphene Composite Films. Compos. Part Appl. Sci. Manuf. 2017, 92, 42–50. [Google Scholar] [CrossRef]
- Eslami, Z.; Yazdani, F.; Mirzapour, M. Thermal and Mechanical Properties of Phenolic-Based Composites Reinforced by Carbon Fibres and Multiwall Carbon Nanotubes. Compos. Part Appl. Sci. Manuf. 2015, 72, 22–31. [Google Scholar] [CrossRef]
- Liao, K.; Park, Y.T.; Abdala, A.; Macosko, C. Aqueous Reduced Graphene/Thermoplastic Polyurethane Nanocomposites. Polymer 2013, 54, 4555–4559. [Google Scholar] [CrossRef]
- Cho, J. Free Vibration Analysis of Functionally Graded Porous Cylindrical Panels Reinforced with Graphene Platelets. Nanomaterials 2023, 13, 1441. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ma, C.; Chen, L.; Jiang, C.; Chen, C.; Xie, X.; Li, A.; Wang, X. Graphene Nanoribbons for Quantum Electronics. Nat. Rev. Phys. 2021, 3, 791–802. [Google Scholar] [CrossRef]
- Zare, Y.; Rhee, K.; Park, S. Progressing of a Power Model for Electrical Conductivity of Graphene-Based Composites. Sci. Rep. 2023, 13, 1596. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Cao, H.; Xue, Y.; Li, B.; Cai, W. Liquid-Phase Exfoliation of Graphene: An Overview on Exfoliation Media, Techniques, and Challenges. Nanomaterials 2018, 8, 942. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Li, J.; Yi, M.; Zhang, X.; Ma, S. Preparation of Graphene by Jet Cavitation. Nanotechnology 2011, 22, 365306. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Gao, C. Supraparamagnetic, Conductive, and Processable Multifunctional Graphene Nanosheets Coated with High-Density Fe3O4 Nanoparticles. ACS Appl. Mater. Interfaces 2010, 2, 3201–3210. [Google Scholar] [CrossRef] [PubMed]
- Pathak, A.; Borah, M.; Gupta, A.; Yokozeki, T.; Dhakate, R. Improved Mechanical Properties of Carbon Fiber/Graphene Oxide-Epoxy Hybrid Composites. Compos. Sci. Technol. 2016, 135, 28–38. [Google Scholar] [CrossRef]
- Fang, M.; Wang, K.; Lu, H.; Yang, Y.; Nutt, S. Covalent Polymer Functionalization of Graphene Nanosheets and Mechanical Properties of Composites. J. Mater. Chem. 2009, 19, 7098–7105. [Google Scholar] [CrossRef]
- Phiri, J.; Gane, P.; Maloney, T. General Overview of Graphene: Production, Properties and Application in Polymer Composites. Mater. Sci. Eng. 2017, 215, 9–28. [Google Scholar] [CrossRef]
- Xue, G.; Xing, J.; Sun, M.; Zhang, X.; Liu, C.; Xue, S.; Yuan, Z.; Zhang, B. In Situ Exfoliation and Surface Functionalization of Graphene Oxide for Epoxy Composites with Improved Thermal and Mechanical Properties. Polym. Compos. 2024, 45, 1826–1838. [Google Scholar] [CrossRef]
- Mirzapour, M.; Robert, M.; Benmokrane, B. Vinyl-Ester Nanocomposites Based on a Binary-Solvent System for Well-Exfoliated Graphene and Enhanced Performance. J. Reinf. Plast. Compos. 2024, accepted. [Google Scholar] [CrossRef]
- Marcano, D.; Kosynkin, D.; Berlin, J.; Sinitskii, A.; Sun, Z.; Slesarev, A. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Tang, L.; Gong, L.; Yan, D.; Li, Y.; Wu, L.; Jiang, J.; Lai, G. Grafting of Epoxy Chains onto Graphene Oxide for Epoxy Composites with Improved Mechanical and Thermal Properties. Carbon 2014, 69, 467–480. [Google Scholar] [CrossRef]
- Kotsyubynsky, V.O.; Boychuk, V.M.; Budzulyak, I.M.; Rachiy, B.I.; Hodlevska, M.A.; Kachmar, A.I.; Hodlevsky, M.A. Graphene Oxide Synthesis Using Modified Tour Method. Adv. Nat. Sci. Nanosci. Nanotechnol. 2021, 12, 035006. [Google Scholar] [CrossRef]
- Chaturvedi, A.; Tiwari, A.; Tiwari, A. Spectroscopic and Morphological Analysis of Graphene Vinylester Nanocomposites. Adv. Mater. Lett. 2013, 4, 656–661. [Google Scholar] [CrossRef]
- Zegeye, E.; Ghamsari, A.; Woldesenbet, E. Mechanical Properties of Graphene Platelets Reinforced Syntactic Foams. Compos. Part B Eng. 2014, 60, 268–273. [Google Scholar] [CrossRef]
- Pinto, A.; Cabral, J.; Tanaka, D.; Mendes, A.M.; Magalhães, F.D. Effect of Incorporation of Graphene Oxide and Graphene Nanoplatelets on Mechanical and Gas Permeability Properties of Poly(Lactic Acid) Films. Polym. Int. 2013, 62, 33–40. [Google Scholar] [CrossRef]
- Guimont, A.; Beyou, E.; Martin, G.; Sonntag, P.; Cassagnau, P. Viscoelasticity of Graphite Oxide-Based Suspensions in PDMS. Macromolecules 2011, 44, 3893–3900. [Google Scholar] [CrossRef]
- Bortz, D.R.; Heras, E.; Martin, G.I. Impressive Fatigue Life and Fracture Toughness Improvements in Graphene Oxide/Epoxy Composites. Macromolecules 2012, 45, 238–245. [Google Scholar] [CrossRef]
- Galpaya, D.; Wang, M.; George, G.; Motta, N.; Waclawik, E.; Yan, C. Preparation of Graphene Oxide/Epoxy Nanocomposites with Significantly Improved Mechanical Properties. J. Appl. Phys. 2014, 116, 053518. [Google Scholar] [CrossRef]
- Park, J.; Kim, D.S. Effects of an Aminosilane and a Tetra-Functional Epoxy on the Physical Properties of Di-Functional Epoxy/Graphene Nanoplatelets Nanocomposites. Polym. Eng. Sci. 2014, 54, 969–976. [Google Scholar] [CrossRef]
- Teng, C.; Ma, C.; Lu, C.H.; Yang, S.Y.; Lee, S.H.; Hsiao, M.C. Thermal Conductivity and Structure of Non-Covalent Functionalized Graphene/Epoxy Composites. Carbon 2011, 49, 5107–5116. [Google Scholar] [CrossRef]
- Jiang, T.; Kuila, T.; Kim, N.H.; Ku, B.C.; Lee, J.H. Enhanced Mechanical Properties of Silanized Silica Nanoparticle Attached Graphene Oxide/Epoxy Composites. Compos. Sci. Technol. 2013, 79, 115–125. [Google Scholar] [CrossRef]
- Kim, B.C.; Park, S.W.; Lee, D.G. Fracture Toughness of the Nano-Particle Reinforced Epoxy Composite. Compos. Struct. 2008, 86, 69–77. [Google Scholar] [CrossRef]
- Bagheri, R.; Marouf, B.T.; Pearson, R.A. Rubber-Toughened Epoxies: A Critical Review. Polym. Rev. 2009, 49, 201–225. [Google Scholar] [CrossRef]
- Johnsen, B.B.; Kinloch, A.J.; Mohammed, R.D.; Taylor, A.C.; Sprenger, S. Toughening Mechanisms of Nanoparticle-Modified Epoxy Polymers. Polymer 2007, 48, 530–541. [Google Scholar] [CrossRef]
- Zhao, Q.; Hoa, S.V. Toughening Mechanism of Epoxy Resins with Micro/Nano Particles. J. Compos. Mater. 2007, 41, 201–219. [Google Scholar] [CrossRef]
- Wan, Y.J.; Gong, L.X.; Tang, L.C.; Wu, L.B.; Jiang, J.X. Mechanical Properties of Epoxy Composites Filled with Silane-Functionalized Graphene Oxide. Compos. Part Appl. Sci. Manuf. 2014, 64, 79–89. [Google Scholar] [CrossRef]
- Wang, K.; Chen, L.; Kotaki, M.; He, C. Preparation, Microstructure and Thermal Mechanical Properties of Epoxy/Crude Clay Nanocomposites. Compos. Part Appl. Sci. Manuf. 2007, 38, 192–197. [Google Scholar] [CrossRef]
- Park, Y.T.; Qian, Y.; Chan, C.; Suh, T.; Nejhad, M.G.; Macosko, C.W.; Stein, A. Epoxy Toughening with Low Graphene Loading. Adv. Funct. Mater. 2015, 25, 575–585. [Google Scholar] [CrossRef]
- Ramanathan, T.; Abdala, A.A.; Stankovich, S.; Dikin, D.; Herrera-Alonso, M.; Piner, R.D. Functionalized Graphene Sheets for Polymer Nanocomposites. Nat. Nanotechnol. 2008, 3, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.A.; Singh, S.; Srivastava, A.; Singh, A.P.; Kumar, S. Covalent Attachment of 2D Graphene Oxide (GO) Sheets with Poly Allylamine (PAA) for Enhanced Mechanical Performance: Theoretical and Experimental Study. Polymer 2021, 213, 123195. [Google Scholar] [CrossRef]
- Yang, A.; Li, J.; Zhang, C.; Zhang, W.; Ma, N. One-Step Amine Modification of Graphene Oxide to Get a Green Trifunctional Metal-Free Catalyst. Appl. Surf. Sci. 2015, 346, 443–450. [Google Scholar] [CrossRef]
- Chen, B.; Li, J.; Liu, T.; Dai, Z.; Zhao, H. Facile Preparation of Epoxy Based Elastomers with Tunable Tgs and Mechanical Properties. RSC Adv. 2018, 8, 13474–13481. [Google Scholar] [CrossRef] [PubMed]
- Ramajo, L.; Parra, R.; Reboredo, M.; Castro, M.M. Preparation of Amine Coated Silver Nanoparticles Using Triethylenetetramine. J. Chem. Sci. 2009, 121, 83–87. [Google Scholar] [CrossRef]
- Koh, E.; Kim, N.K.; Shin, J.; Kim, Y.W. Polyurethane Microcapsules for Self-Healing Paint Coatings. RSC Adv. 2014, 4, 16214–16223. [Google Scholar] [CrossRef]
- Ferreira, F.V.; Brito, F.S.; Franceschi, W.; Simonetti, E.A.; Cividanes, L.S.; Chipara, M. Functionalized Graphene Oxide as Reinforcement in Epoxy Based Nanocomposites. Surf. Interfaces 2018, 10, 100–109. [Google Scholar] [CrossRef]
- Guan, L.Z.; Wan, Y.J.; Gong, L.X.; Yan, D.; Tang, L.C.; Wu, L.B.; Jiang, J.X.; Lai, G.Q. Toward Effective and Tunable Interphases in Graphene Oxide/Epoxy Composites by Grafting Different Chain Lengths of Polyetheramine onto Graphene Oxide. J. Mater. Chem. A 2014, 2, 15058–15069. [Google Scholar] [CrossRef]




| Specimen | Graphene Oxide (wt%) | Epoxy/Hardener (g) |
|---|---|---|
| Pure Epoxy | 0 | 100 |
| EP/GO 0.2 | 0.2 | 100 |
| EP/GO 0.4 | 0.4 | 100 |
| EP/GO 0.6 | 0.6 | 100 |
| Sample | Pure PE | PE/GO 0.2 | PE/GO 0.4 | PE/GO 0.6 |
|---|---|---|---|---|
| Tensile strength (MPa) | 37 ± 3 | 46 ± 2 | 49 ± 1 | 54 ± 2 |
| Young’s modulus (GPa) | 2.1 ± 0.2 | 2.7 ± 0.1 | 2.8 ± 0.1 | 2.9 ± 0.1 |
| Elongation at break (%) | 1.8 ± 0.2 | 2.73 ± 0.2 | 2.85 ± 0.1 | 3.0 ± 0.1 |
| Toughness a (MJ m−3) | 0.66 ± 0.05 | 1.24 ± 0.04 | 1.37 ± 0.06 | 1.62 ± 0.03 |
| Sample | Pure EP | EP/GO 0.2 | EP/GO 0.4 | EP/GO 0.6 |
|---|---|---|---|---|
| Storage module at 25 °C (MPa) | 1810 | 2230 | 2295 | 2350 |
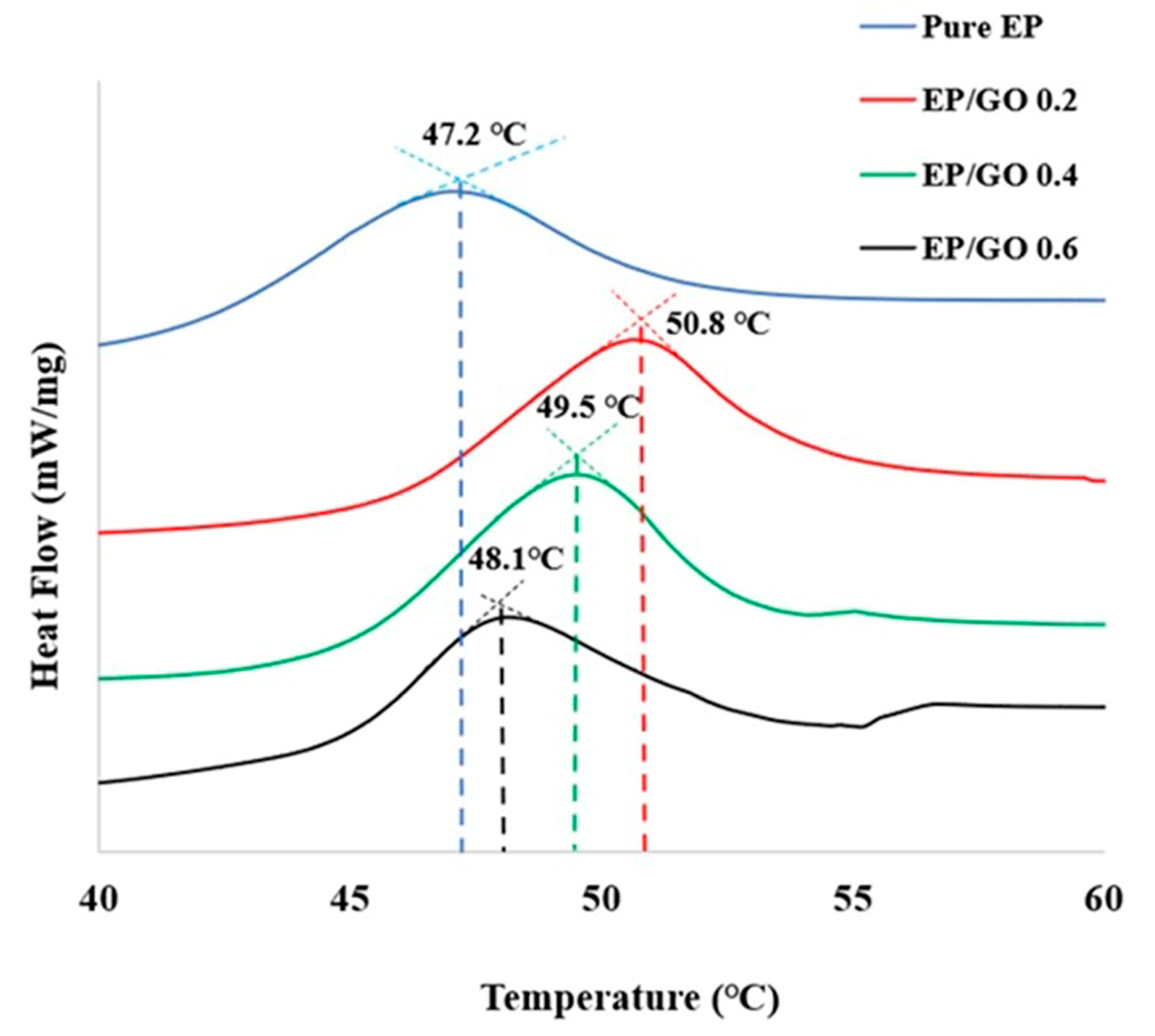
| Tg (°C) (DMA) | 49.3 | 53.1 | 52.7 | 51.5 |
| Tg (°C) DSC | 47.2 | 50.8 | 49.5 | 48.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirzapour, M.; Robert, M.; Benmokrane, B. In Situ Processing to Achieve High-Performance Epoxy Nanocomposites with Low Graphene Oxide Loading. C 2024, 10, 52. https://doi.org/10.3390/c10020052
Mirzapour M, Robert M, Benmokrane B. In Situ Processing to Achieve High-Performance Epoxy Nanocomposites with Low Graphene Oxide Loading. C. 2024; 10(2):52. https://doi.org/10.3390/c10020052
Chicago/Turabian StyleMirzapour, Miraidin, Mathieu Robert, and Brahim Benmokrane. 2024. "In Situ Processing to Achieve High-Performance Epoxy Nanocomposites with Low Graphene Oxide Loading" C 10, no. 2: 52. https://doi.org/10.3390/c10020052
APA StyleMirzapour, M., Robert, M., & Benmokrane, B. (2024). In Situ Processing to Achieve High-Performance Epoxy Nanocomposites with Low Graphene Oxide Loading. C, 10(2), 52. https://doi.org/10.3390/c10020052







