Abstract
This review critically examines the multifaceted role of acetic acid bacteria (AAB) in the intricate production process of port wine vinegar, particularly in its transformative process from port wine. With the emergence of port wine vinegar as a distinctive agricultural product in 2018, producers have been faced with a diverse array of challenges, ranging from reducing the high alcohol content to preserving the inherent sweetness. Through an exhaustive exploration of acetic fermentation processes and the indispensable role of AAB, this review meticulously elucidates the complex biochemistry underlying vinegar formation, delving into the nuanced interactions between microbial activity and chemical composition. Furthermore, this review underscores the importance of sensory characteristics and consumer perception derived from vinegar production, providing invaluable insights into these fermented products’ sensory profiles and marketability. In summary, this study offers valuable insights into the evolution of port wine into vinegar, highlighting its significance in agricultural and culinary contexts.
1. Introduction
Fermentation is a pivotal biochemical process within the food industry, notably in the intricate realm of wine production. Governed by microorganisms such as Saccharomyces cerevisiae yeast, fermentation orchestrates the conversion of grape sugars into a complex and revered beverage, globally cherished for its intricacy. Commencing post-harvest, grapes undergo crushing to liberate their inherent sugars, setting the stage for microbial transformation. Saccharomyces cerevisiae yeast, among other microflora, metabolizes these sugars, effectuating the conversion into ethyl alcohol and carbon dioxide, thereby engendering the transformation from grape juice to wine [1,2,3].
In the viticultural landscape of Portugal, port wine reigns as an exemplar of oenological prowess, distinguished by its fortified nature and rich heritage. Rooted in the vineyards of the Douro Demarcated Region, a bastion of UNESCO heritage, port wine embodies centuries of winemaking tradition and craftsmanship. Statistical data from the Instituto dos Vinhos do Douro e do Porto, I.P. (IVDP, IP) attest to the global reach of this esteemed libation, with exports spanning approximately 35 countries, prominently featuring France and the United Kingdom as leading markets [3,4,5,6].
Biological products are inherently dynamic, subject to variations and the influence of diverse microorganisms. In ancient times, the spontaneous acetification of wine gave rise to wine vinegar, a distinctive nutraceutical product synonymous with regions like Modena (Italy) and Jerez de la Frontera (Spain). This culinary evolution culminated in the recognition of “Port Wine Vinegar” in 2018, derived from the acetic oxidation of port wine and compliant with prevailing regulations [7,8]. This evolution enhances local culinary traditions and contributes to the refinement of European gastronomy, introducing gourmet vinegar with distinctive organoleptic qualities.
The production of port wine vinegar presents a significant challenge in reducing the high alcohol content inherent in the original wine, which typically ranges from 18% to 22%. The sweetness of the resulting vinegar is intricately linked to the specific type of port wine utilized in its production [8,9,10].
Acetic acid bacteria (AAB) play a central role in producing port wine vinegar. These microorganisms, including Acetobacter and Gluconacetobacter, thrive in aerobic environments. They catalyze the oxidation of ethanol to acetic acid, a process known as acetification. AAB is pivotal in food preservation, condiment production, and flavor enhancement [11,12,13,14].
Moreover, AABs exhibit remarkable adaptability, allowing them to thrive in diverse fermentation environments and substrates beyond traditional vinegars. Their metabolic versatility extends to other fermented foods and beverages, where they contribute to flavor development, preservation, and probiotic properties. One notable example is Kombucha, a probiotic beverage popular across Asia. Traditionally crafted from sweetened tea and fermented by a symbiotic colony of bacteria and yeasts (SCOBY), Kombucha offers a refreshing fusion of flavors and health benefits [14,15,16]. Additionally, the unique cocoa flavor emerges from the fermentation of cocoa beans. In the initial stages, yeast and lactic acid bacteria (LAB) operate in a low pH, high sugar content environment, with AAB assuming dominance as temperatures rise and alcohol levels accumulate [13,14,17]. The versatile role of AAB in fermentation underscores their significance in shaping the diverse and dynamic landscape of fermented foods and beverages. Beyond its traditional association with vinegar production, AAB inspires culinary innovation and contributes to the depth and complexity of fermented creations enjoyed worldwide.
This review aims to provide a comprehensive analysis of the pivotal role played by acetic acid bacteria (AAB) in the intricate process of producing port wine vinegar. Through meticulous examination, it seeks to elucidate the biochemical mechanisms underlying the transformation of port wine into vinegar, shedding light on the complex processes involved. Furthermore, the review emphasizes various methods of vinegar production and the optimization of AAB acetification during this process. This study offers significant insights into the importance of acetic acid bacteria and their indispensable role in vinegar production.
2. Biochemistry and Physiology of Acetic Acid Bacteria (AAB)
Acetic acid bacteria (AAB) are a diverse group of microorganisms crucial for acetic fermentation. Their role includes their general description, classification, identification, biochemical mechanisms, resistance to acidic environments, and strategies for maintaining optimal bacterial health.
2.1. General Overview, Classification, and Identification
Acetic acid bacteria (AAB) represent a taxonomically diverse group with distinctive features that underscore their ecological preferences and metabolic prowess, Figure 1. As obligate aerobes, their intricate reliance on oxygen intricately shapes their adaptive strategies within aerobic environments, where oxygen becomes a cornerstone for vital metabolic processes. Notably, the Gram-negative or Gram-variable cell wall structure, a taxonomic hallmark, extends beyond mere classification, actively influencing their dynamic interactions with external stimuli [11,17,18,19,20,21,22].
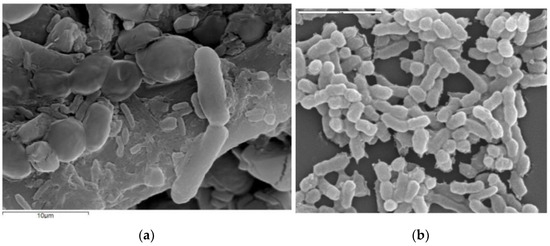
Figure 1.
The figure shows SEM (scanning electron microscope) images of the morphology of acetic acid bacteria in different magnification ranges. (a) Immobilized A. aceti during vinegar fermentation (adapted from Plioni et al. [23]); (b) acetic acid bacteria during vinegar production (adapted from Román-Camacho et al. [21]).
In the inaugural chapter of Matsushita et al.’s (2016) book [24], crafted by Yuzo Yamada, a comprehensive exploration unfolds, encompassing the various genera and species of acetic acid bacteria. The authors adeptly describe the phenotypic characteristics essential for distinguishing these microorganisms within this context. This includes nuanced processes like the oxidation of lactate and acetate, the transformation of ethanol into acetic acid, and the assimilation of ammoniac nitrogen, among other discerning traits.
Regarding morphology, AAB displays a remarkable absence of spore formation, presenting ellipsoidal to rod-shaped cells that can manifest singly, in pairs, or in concise chains. The dimensions of these cells span a range from 0.4 to 1.0 µm in width and 0.8 to 4.5 µm in length, encapsulating a spectrum of forms reflective of their adaptability [14,18,19,22,24].
Pivotal genera governing fermented foods include Acetobacter, Gluconobacter, Gluconacetobacter, and Komagataeibacter, shaping the taxonomic landscape within the Acetobacteraceae family. This diverse landscape, first initiated by Acetobacter and Gluconobacter, now spans over 47 genera and 207 species [21,25,26]. The taxonomic complexity arises from influences such as membrane-bound dehydrogenases, ethanol oxidation capabilities, and the nuances of respiratory coenzyme chain types [13,18,22,27]—the evolving classification landscape benefits from molecular techniques. DNA sequencing, particularly in conserved genes like 16S rRNA, contributes to a more refined understanding of the genetic relationships among different strains and species [13,17,18,21].
Engaging in oxidative fermentation, AAB undergoes an intricate process wherein the direct oxidation of carbohydrates and sugar alcohols yields copious amounts of corresponding oxidation products, thereby extracting vital metabolic energy [21]. Their mesophilic nature manifests with an optimum growth temperature hovering around 30 °C, though certain strains display a commendable thermotolerance, thriving even at elevated temperatures up to 42 °C [14,18,28].
AAB’s habitat diversity encompasses fruit juice, wine, cider, beer, and vinegar, where their metabolic prowess is pronounced [14,17,29]. Acetobacter and Komagataeibacter are pivotal in converting ethanol to acetic acid, while Gluconobacter excels in converting glucose into gluconic acid. In the industrial realm, AAB, particularly exemplified by Acetobacter and Komagataeibacter, orchestrate a pivotal role in vinegar production [17,30,31,32,33]. Their influence extends beyond fermentation, shaping the distinct aroma of vinegar. The quality spectrum of vinegar, influenced by factors like raw materials, technological processes, and aging intricacies, showcases the multifaceted impact of AAB [32,33].
Isolating acetic acid bacteria (AAB) poses significant challenges, primarily due to their demanding nutritional requirements and the phenomenon of the viable but nonculturable (VBNC) state. The selection of growth media is pivotal and tailored to the specific origin of strains, whether derived from carbohydrate-rich or ethanol-acetic acid-rich environments [13,29,34].
Traditional identification methods rely on phenotypic traits and biochemical tests, often centering around the hallmark metabolic activity of AAB—oxidation of ethanol to acetic acid [13,33]. However, molecular techniques have brought about a transformative shift in identification methodologies. Polymerase chain reaction (PCR) and DNA sequencing, offering unprecedented precision, now facilitate discrimination between closely related AAB strains and species. Importantly, genomic approaches, including whole-genome sequencing, provide a comprehensive view of the genetic makeup of AAB, significantly enhancing our ability to discern subtle variations [19,27,29,35,36].
In a study by Gomes et al. (2018) [29] on acetic bacteria in the food industry, the authors explored various culture media for acetic bacteria strains’ growth, recovery, culture, and gene differentiation. Despite presenting a table with 18 different culture media, not all of them can support the development of these bacteria due to the existence of VBNC or the selectivity of the medium. Table 1 summarizes studies conducted in the food industry over the last five years, specifically on wine-derived products like vinegar. Among the media mentioned in Table 1, those most commonly found in recent studies are Carr medium, composed of yeast extract, ethanol, bromocresol green, and agar; GYP medium (glucose–yeast extract–peptone), comprised of glucose, yeast extract, peptone, and agar; and YPE medium (yeast extract–peptone–ethanol), composed of yeast extract, peptone, ethanol, and agar [29].

Table 1.
The table presents an overview of acetic acid bacteria studies in the food industry, including type strains identified or used, culture media, and molecular identification process.
Understanding the biochemical intricacies of acetic acid bacteria is crucial, given their metabolic prowess, which is marked by oxidative fermentation and the direct oxidation of carbohydrates and sugar alcohols. This defines their distinctive biochemistry and underscores their industrial significance, especially in vinegar production.
2.2. Metabolic Pathways and Respiratory Chains in AAB
AAB showcases a distinctive proficiency in oxidative fermentation. It excels in partially oxidizing substrates like ethanol, carbohydrates, and sugar alcohols, unveiling their intricate metabolic pathways and potential applications.
2.2.1. Respiratory Machinery and Energy Yield
In the initial steps of aerobic respiration, the process commences with the thorough oxidation of pyruvate to carbon dioxide (CO2) within the citric acid cycle. Following this, the respiratory chain in the cytoplasmic membrane receives the reduced electron acceptors produced in the citric acid cycle. Within this respiratory chain, components facilitate the oxidation of reduced electron carriers through oxidative phosphorylation. This oxidation leads to the creation of water, concurrently excluding protons from the cytoplasm and establishing a proton gradient [18,54,55,56]. The normalization of this proton-motive force involves transferring protons back into the cell through a transmembrane ATPase (F1F0-type adenosine triphosphate [ATP] synthase), ultimately culminating in the synthesis of energy in the form of ATP [18,54].
Integral to the metabolic processes of acetic acid bacteria (AAB) is the utilization of respiratory chains, predominantly employed for aerobic respiration and energy production. The intricate AAB respiratory machinery comprises two periplasmic dehydrogenases, a membrane-bound proton-pumping transhydrogenase, a nonproton-translocating NADH: ubiquinone oxidoreductase (nicotinamide adenine dinucleotide: ubiquinone oxidoreductase), and two terminal oxidases of the ubiquinol oxidase-type. As an electron shuttle between these components, ubiquinone (UQ) facilitates the regeneration of NADP+ and NAD+ [18,54,57].
Despite the comparatively lower energy and biomass yields in AAB when contrasted with some microorganisms, these bacteria have evolved distinctive adaptations. Notably, the absence of crucial respiratory chain components, such as cytochrome c oxidase and the proton-translocating NADH: ubiquinone oxidoreductase, in G. oxydans 621H, imposes limitations on the proton-motive force and energy transduction in AAB. However, AAB overcomes this limitation through the presence of membrane-bound dehydrogenases, enabling the rapid oxidation of substrates through “oxidative fermentation” at the periplasmic level [18,54,57,58].
2.2.2. Acetic Acid Production: Oxidative Fermentation
Acetic acid bacteria demonstrate their expertise in oxidative fermentation through the established ethanol oxidation process, producing acetic acid. The chemical equation illustrates the complete conversion of ethanol to acetic acid [17,21]:
C2H5OH + O2 → CH3COOH + H2O ΔH° = −520 kJ/mol
As chemoorganotrophic microorganisms, AAB heavily depends on ethanol from an alcoholic medium as their primary carbon reservoir. While the Acetobacter and Komagataeibacter genera consistently display a marked preference for ethanol, specific subsets within the broader AAB category may exhibit proclivities for alternative carbon sources [21,57,59,60,61]. In this intricate two-step process, pivotal membrane-bound enzymes on the outer surface of the cytoplasmic membrane transform ethanol into acetic acid (Figure 2) [17,18,21,59,62,63,64,65,66,67].
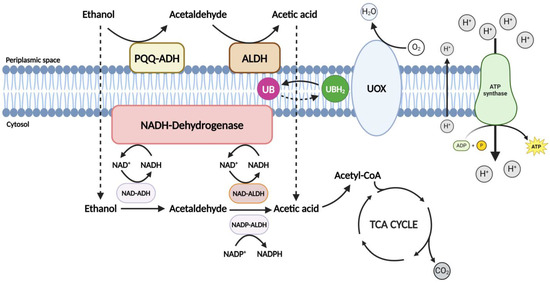
Figure 2.
The figure shows the oxidative fermentation process of acetic acid bacteria. This biotransformation involves complex biochemical reactions, converting ethanol to acetic acid primarily at the cell membrane level within the periplasmic space. Adapted from Román-Camacho et al. [21].
The process begins with initiation by pyrroloquinoline quinone (PQQ)-dependent alcohol dehydrogenase (ADH), catalyzing the oxidation of ethanol into acetaldehyde (Figure 2). Subsequently, aldehyde dehydrogenase (ALDH) takes charge, further oxidizing acetaldehyde to yield acetic acid. The efficiency of acetobacters in acetic acid production is significantly influenced by the stability of ADH under acidic conditions. Notably, certain strains of Gluconobacter exhibit an inactive form of ADH, impacting the efficiency of ethanol oxidation. Additionally, AAB possesses an inactive form of ADH, characterized by ubiquinol: ferricyanide oxidoreductase activity, playing a role in redox regulation in the cytoplasmic membrane under specific conditions [17,21,66,67,68].
In this enzymatic process, alcohol dehydrogenase (ADH), initially bound to pyrroloquinoline quinone (PQQ), facilitates the oxidation of ethanol into acetaldehyde. The subsequent oxidation of acetaldehyde to acetic acid is carried out by membrane-bound aldehyde dehydrogenase (ALDH). Both enzymes are strategically located on the periplasmic side of the inner cell membrane [17,27,34,64,66,68], as shown in Figure 2. Further metabolic pathways come into play, where acetyl-CoA synthase can completely oxidize the inner acetic acid. This process introduces acetyl-CoA into the tricarboxylic acid (TCA) cycle, producing CO2 and H2O [21,69]. This step provides energy in the form of ATP and detoxifies the cell. AAB’s strictly aerobic metabolism closely connects the ADH-PQQ, ALDH complexes, and the respiratory chain. The respiratory chain facilitates reducing equivalents from donor substrates to ubiquinone (UB). Subsequently, electrons from the reduced UB (ubiquinol, UBH2) are transferred to the final electron acceptor, oxygen (O2), by terminal ubiquinol oxidases (UOXs), resulting in the production of H2O [21,34,69].
In addition to these variations, the divergent metabolic capabilities between Acetobacter and Gluconobacter strains play a crucial role in their distinct utilization of ethanol and acetic acid. Acetobacter can oxidize acetic acid to CO2 and water through the tricarboxylic acid cycle (TCA), particularly in the absence of ethanol. This metabolic pathway is repressed in the presence of ethanol. Similarly, Gluconobacter strains undergo an irreversible metabolic shift after ethanol depletion, limiting their capacity for ethanol oxidation. This intricacy underscores the significant impact of substrate availability on the metabolic pathways of AAB, with implications for acetic acid production in industrial processes [17,70,71,72].
Upon the exhaustive oxidation and depletion of ethanol, specific AAB genera, including Acetobacter, Gluconacetobacter, and Komagataeibacter, display the capacity to assimilate acetic acid, entering a process known as acetate “overoxidation”. This intricate metabolic transformation involves the further oxidation of acetic acid to CO2 and water. The consequence of this irreversible metabolic shift is the incapacitation of these bacteria to oxidize ethanol, consequently influencing the yields of acetic acid. This phenomenon serves as a testament to the intricate nature of AAB metabolic pathways, highlighting the substantial influence of substrate availability on their nuanced and complex metabolic responses [17,18,31,70,73].
In contrast, the stability of ADH activity in Acetobacter species under acidic conditions surpasses that of Gluconobacter species [54,57,68]. This distinction elucidates the heightened proficiency of acetobacters in acetic acid production compared to gluconobacters and gluconacetobacters. Notably, Gluconobacter and other specific AAB genera exhibit a restrained capacity for acetate overoxidation due to the absence of crucial enzymes and glyoxylate shunts in the citric acid cycle. This difference in oxidative potential designates gluconobacters as “under-oxidizers” and characterizes acetobacters (and gluconacetobacters) as “over-oxidizers.” Overoxidation is mitigated by sustaining a small proportion of ethanol in the medium, demonstrating the intricate balance necessary in AAB metabolic dynamics [11,18].
2.2.3. Oxidation of Carbohydrates, Alcohols, and Organic Acids
The metabolic prowess of AAB extends to their adept utilization of a wide array of sugars, including glucose, arabinose, fructose, galactose, mannose, ribose, sorbose, and xylose [21,34,70,74]. This metabolic versatility is orchestrated through the intricate interplay of pathways, primarily relying on the cytoplasmic hexose monophosphate pathway, also known as the Warburg–Dickens pathway. Interestingly, certain AAB strains, especially those involved in cellulose synthesis, demonstrate heightened activity in the Entner–Doudoroff pathway, showcasing their nuanced adaptation to diverse environmental conditions, as presented in Table 2 [34,74,75,76].

Table 2.
The table presents an overview of the metabolic aspects of acetic acid bacteria, with a particular emphasis on Acetobacter and Gluconobacter, highlighting the differences in their metabolic characteristics and the similarities shared by both genera.
The oxidation of glycerol emerges as a critical aspect in the industrial-scale production of gluconic acid by G. oxydans, unfolding with meticulous precision. The process thrives under elevated glucose concentrations, low pH, and vigorous aeration rates. Notably, there is a strategic suppression of ketogluconate formation under low pH conditions, showcasing the nuanced control mechanisms employed by these bacteria for achieving optimal metabolic outcomes [34,74,78].
AAB’s growth phases on alcohols, carbohydrates, or sugar alcohols reveal a biphasic pattern [18]. The majority of energy is derived from respiratory chains and proton-motive force generation. In the second growth phase, some energy is obtained through the assimilation and catabolism of products oxidized in the initial phase. Gluconobacter exhibits an incomplete citric acid cycle due to deficiencies in succinyl-CoA synthetase and succinate dehydrogenase. The initial generation of oxidized products from carbohydrates and sugar alcohols enables AAB to swiftly deplete carbon sources for competing microorganisms. Simultaneously, the accumulation of acidic products induces a low pH, creating an inhibitory environment for competitors. AAB’s competitive advantage extends to the assimilation and partial use of these products as an energy source [18,34,75,78].
2.2.4. Resistance to Acidic Environments
AAB exhibits a remarkable array of mechanisms in microbial adaptation to acidic environments. Its resilience is particularly vital in industrial processes like vinegar fermentation. These bacteria adeptly manage challenges posed by acetic acid, providing valuable insights into their survival strategies.
Effective as an antimicrobial compound, acetic acid’s potency, even at 0.5%, necessitates robust resistance mechanisms [17]. Notably, various AAB genera manifest distinct tolerances. For instance, Acetobacter species endure 6% to 10% acid, while Komagataeibacter strains, such as K. xylinus and K. hansenii, resist 10% to 15% acetic acid. K. europaeus tolerates 15–20% acetic acid, showcasing significant inter-species variations [18,27]. AAB deploys diverse strategies to combat high acetic acid concentrations. These mechanisms encompass the adaptation and protection of intracellular proteins against acid stress, metabolic pathways facilitating acetic acid overoxidation, active acetic acid efflux from the cell, and preventive measures to impede acetic acid entry [18,79].
Structurally, intracellular proteins play a pivotal role in AAB’s resistance. Acid-tolerant strains, exemplified by A. pasteurianus, exhibit modifications in enzymes, with structural alterations contributing to stability in acidic cytoplasm and enhancing overall thermotolerance [18]. The heat-shock systems, GroESL and DnaJK, emerge as crucial players in acetic acid resistance, acting as general stress proteins that shield other proteins from denaturation and aggregation. The disruption of RpoH, a regulator of heat-shock response proteins, diminishes resistance to ethanol and acetic acid stress in A. pasteurianus [18,54,79].
Metabolically, AAB engages in acetic acid oxidation through the citric acid cycle and glyoxylate shunt. Strains with a fully functional citric acid cycle, like Acetobacter and Komagataeibacter, exhibit heightened acetic acid tolerance, while those lacking this cycle, such as Gluconobacter strains, demonstrate comparatively lower resistance [17,18,79].
Efflux systems play a crucial role in mitigating the impact of acetic acid. AAB employs proton-motive force-driven efflux pumps and ATP-binding cassette (ABC) transporters to export intracellular acetate, actively reducing acid stress. This becomes particularly significant under high acidity conditions in industrial vinegar fermentation [18,27,80,81]. Excluding acetic acid from the bacterial cell is another strategy to resist acid stress involving specific membrane compositions [18]. Certain species, like Komagataeibacter, feature higher phosphatidylcholine (PC) content in their lipid membranes. Hopanoids, especially tetrahydoxybacteriohopane, in Komagataeibacter membranes, contribute to acetic acid and ethanol tolerance. Carbohydrate polymers attached to the outer membrane also protect against acetic acid ingress. Strains capable of producing polysaccharides exhibit lower intracellular acetate levels compared to non-polysaccharide-producing counterparts [18,27,82].
Notably, maintaining low residual ethanol levels during vinegar fermentation prevents the overoxidation of acetic acid and averts productivity losses. Consequently, alternative strategies must be employed to sustain acetic acid tolerance. The export of intracellular acetate emerges as a pivotal strategy for AAB to tolerate acetic acid. Two identified export systems—a proton-motive force-driven efflux pump and an ATP-binding cassette (ABC) transporter—are found in Acetobacter and Komagataeibacter species. This transporter confers resistance to acetic acid and other short-chain organic acids like formic, propionic, and lactic acid [18,27,30,82].
Intriguingly, there is a link between acetic acid resistance and productivity during fermentation surfaces. The natural intrinsic tolerance of Komagataeibacter and Acetobacter species to acetic acid contributes to their higher productivity compared to Gluconobacter, which generally displays lower acetic acid productivity [18,82].
The adaptive strategies of AAB reveal an intricate network of mechanisms, showcasing a fascinating interplay of structural, metabolic, and membrane-level strategies. As industries harness the metabolic prowess of AAB, understanding these adaptive mechanisms becomes imperative for optimizing industrial processes and enhancing productivity in acidic conditions.
2.2.5. Resistance to Alcoholic Environments
Ethanol resistance in acetic acid bacteria is critical to their ability to thrive in vinegar production processes, where ethanol is the primary carbon source. These bacteria exhibit varying degrees of tolerance to ethanol, with some strains capable of withstanding high alcohol concentrations.
In a recent study conducted by Kourouma et al. (2022) [83], the researchers delved into the ethanol resistance of acetic acid bacteria isolated from fermented mango alcohol, shedding light on their capacity to thrive in environments rich in alcohol, particularly within vinegar production processes. The investigation unveiled that all strains exhibited robust growth in the presence of ethanol concentrations of up to 15% (v/v). However, as the ethanol concentration surpassed this threshold, the inhibitory effects of ethanol became increasingly pronounced. Select strains attributed to the Gluconoacetobacter genera, specifically VMA1, VMA5, VMA7, and VMAO, displayed notable resilience, showcasing medium growth even in 20% (v/v) ethanol. Remarkably, these strains exhibited heightened tolerance, demonstrating the ability to proliferate in environments containing up to 25% (v/v) ethanol, thus earning classification as alcohol-tolerant strains.
Understanding the mechanisms underlying AAB’s ethanol resistance shows their ability to adapt to challenging environments. One such mechanism involves the enzymatic pathways responsible for ethanol metabolism. Key enzymes like alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH) play pivotal roles in converting ethanol into acetaldehyde and subsequently into acetic acid, the primary product of vinegar fermentation. Studies have shown that these enzymes’ activity and expression are upregulated in response to ethanol stress, facilitating efficient ethanol utilization, even at high concentrations [84,85].
Furthermore, the genetic diversity among AAB strains plays a significant role in determining their ethanol tolerance levels. While certain strains inherently resist ethanol, others may develop tolerance through adaptation to particular environmental circumstances or fermentation techniques. Moreover, environmental factors, including temperature and pH, influence ethanol resistance in AAB. Elevated temperatures, such as those experienced during incubation at 37 °C instead of the standard 30 °C, have been observed to bolster ethanol tolerance in select strains. Additionally, pH levels play a pivotal role, as AAB strains exhibit differing degrees of ethanol tolerance across various pH ranges [83,86,87].
Optimizing vinegar fermentation processes requires precise control over ethanol concentrations to ensure the growth and activity of AAB. High initial ethanol concentrations can be selected for ethanol-tolerant strains and prevent over-oxidation during fermentation. Screening and selecting strains with enhanced ethanol tolerance are essential for improving fermentation efficiency and vinegar quality. By elucidating the intricacies of ethanol tolerance mechanisms, researchers can advance innovations in vinegar production and microbial biotechnology, ultimately improving the efficiency and quality of vinegar fermentation processes.
2.2.6. Extracellular Polymeric Substances Produced by AAB
Extracellular polymeric substances (EPS) produced by AAB exhibit remarkable diversity in chemical structure and composition [14]. Table 3 serves as a comprehensive overview of the diverse EPS produced by AAB, shedding light on their chemical structures, properties, and pivotal roles in the food industry.

Table 3.
The table shows the extracellular polymeric substances (EPS) produced by acetic acid bacteria and their chemical structure, characteristics, and applications.
G. oxydans appears to produce both intracellular and extracellular forms of dextran dextrinase. The extracellular form is particularly abundant in hydrolyzed starch and maltodextrins. However, the relationship between these two enzyme forms and whether the strain is induced to secrete intracellular dextran dextrinase in specific environmental conditions were previously unclear [101]. As shown in Figure 3, the applications of acetan and dextran in the food industry are diverse, reflecting the versatility inherent in other EPS produced by AAB.
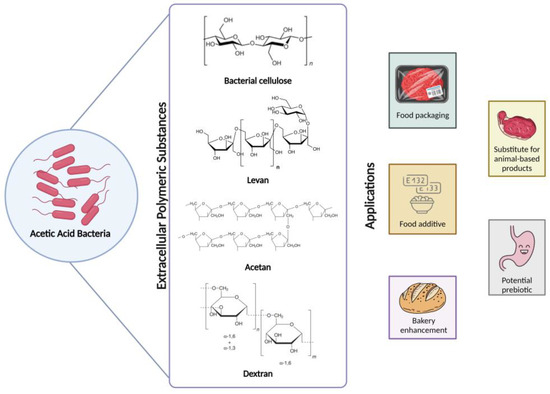
Figure 3.
The figure shows the extracellular polymeric substances produced by acetic acid bacteria, their chemical structure, and their applications in the food industry. Note that the chemical structures for acetan and dextran represent only the repeating unit.
These microbial polysaccharides find widespread use in bakery products, where they contribute to improving rheological properties, texture, and shelf life. Additionally, their properties make them valuable as fat substitutes, stabilizers, and emulsifiers in various food applications, including their incorporation into edible starch films used in food packaging. Beyond their functional roles in food products, the bioactive properties of EPS, such as antitumor, antimicrobial, anti-inflammatory, hypocholesterolemic, antidiabetic, and immunostimulating activities, underscore their potential health benefits when consumed as part of foods [29,89,91,93,94,98,100,103,104,105,106].
Extracellular polymeric substances (EPS) produced by acetic acid bacteria (AAB) offer numerous benefits to these microorganisms. EPS serves as a protective shield, safeguarding AAB from environmental stresses such as pH variations, temperature fluctuations, and changes in osmolarity [107,108,109]. Additionally, EPS facilitates the formation of biofilms, enabling AAB to adhere to surfaces and establish stable microbial communities [108]. This enhances their resilience and persistence in diverse habitats. Moreover, EPS production aids in trapping and retaining nutrients, providing a continuous supply for bacterial growth and metabolism. Furthermore, EPS plays a role in cell-cell communication within microbial communities, allowing for coordinated responses to environmental cues and promoting cooperative behaviors among AAB [107,108]. Overall, the formation of EPS contributes significantly to the survival, persistence, and ecological success of AAB in various environments.
Exploring these EPS continues to drive innovative advancements in various technological fields. They offer a rich spectrum of properties with versatile applications in traditional and modern food production. Additionally, they hold potential benefits in biomedical and cosmetic domains.
3. Overview of Port Wine Production
Acetic acid bacteria play a pivotal role in producing vinegar. Still, the distinctiveness of port wine vinegar is closely linked to the acetic fermentation of port wine—a tradition in Portugal since the 17th century. This unique vinegar, originating from the Demarcated Douro Region (DDR), emerged as a novel addition to the vinegar market in 2018. The Douro and Port Wine Institute (IVDP) officially recognized its significance by endorsing the collective brand.
Port wine, known as Porto in Portugal, has a captivating historical narrative intertwined with the rugged landscape of the Douro Valley. Its origins trace back to around 1670, and significant milestones in its evolution include the standardization of fortification practices in the mid-eighteenth century [3,4,110,111,112]. Maturation and aging occur in lodges in Vila Nova de Gaia, downriver from the Douro Valley, where natural conditions facilitate wine preservation [3,4,112]. Rabelo boats historically transported wines from the Douro Region to Gaia for export, and the panel of tasters at IVDP certifies and approves port wines, ensuring their authenticity and quality [3,111].
The Douro Valley, the hub of port wine production, profoundly influences grape characteristics due to its steep slopes, schist soils, and challenging climate [3,5,111,112]. Red grape cultivars include Touriga Nacional, Touriga Franca, Tinta Roriz, Tinta Barroca, Tinto Cão, and Tinta Amarela, while white cultivars include Malvasia Fina, Viosinho, Donzelinho Branco, and Gouveio [3,6,111,112]. Modern winemaking techniques involve separating white and red grape varieties to control factors like temperature and alcohol content for effective anthocyanin extraction, setting Portuguese ports apart [3,112,113,114].
After arriving at the winery, chemical assessments determine grape juice parameters, and red port production involves destemming, crushing, and intensive maceration for maximal color extraction. Fermentation, typically spontaneous and controlled by natural yeasts, adds grape spirits, known as “aguardente”, to halt fermentation, followed by pressing the pomace to extract the remaining juice [4,112,114]. Halting fermentation midstream retains high acetaldehyde content, promoting color stability through anthocyanin–tannin polymerization [111,112].
Port wines then undergo maturation and aging in various vessels, including wooden or cement tanks and oak casks called pipes. Regular racking, slight fortification, and compensations for volume losses due to evaporation shape the outcome of this process [3,112]. Wines resulting from this process exhibit deep coloration, complex aroma, and a well-balanced mouthfeel characterized by rounded tannins [3,6,112]. Following classification by the IVDP, all port wines mature in oak barrels, determined by their style and category [3,111]. Aroma profiles evolve during aging, transitioning from floral and bergamot-like notes in young wines to maderized, nutty, and spicy flavors in aged ones [6,115,116]. Oxidative reactions during aging increase the content of compounds like acetaldehyde, indicating wine age and creating complex fragrances [3,6,111].
4. Port Wine Vinegar
The evolution of port wine into vinegar reveals grapes’ remarkable versatility and fermentation’s transformative power. From velvety port wine to nuanced vinegar, this journey showcases the grape’s ability to undergo alchemy, guided by fermentation and acetic acid bacteria. It is a tale of evolution in which the vine’s essence transcends its liquid form, offering a symphony of flavors that bridges two extraordinary worlds.
Despite regional differences, food regulations generally consider vinegar the result of double fermentation—alcoholic and acetous—produced from sugars-containing products. Vinegar, a product with a history dating back to 200 BC, has played diverse roles in human culture. Produced through the microbial biotransformation of fermentable carbohydrates, it utilizes various raw materials globally. Europe favors fruit vinegar, while Asia leans towards cereal vinegar, and “white” vinegar, from diluted alcohol, is a global production leader [8,10,31,33,73,117,118,119]. Throughout history, vinegar has served as a culinary staple, as a condiment, a preservative, a disinfectant, and a remedy. During the Middle Ages, vinegar gained recognition for its perceived medical benefits, and in the Great Plague, it was employed in France for protection. In England, it was used to disinfect coins and prevent the spread of disease. Initially considered a byproduct of wine spoilage due to air exposure, vinegar’s microbial aspects were later understood, with Pasteur identifying the role of Mycoderma aceti in wine-to-vinegar conversion. This evolution in understanding has contributed to the diverse applications and global popularity of vinegar across cultures and centuries [8,10,32,119].
Within the European Union, vinegar production is regulated by stringent standards outlined in Regulation (EU) 2016/263 [120]. This comprehensive regulation defines vinegar as a product of agricultural origin obtained through a double fermentation process—alcoholic and acetous. It establishes specific criteria to maintain the quality and authenticity of various vinegar types, including limits on alcohol content (0.5% for most vinegar, 1.5% for wine vinegar) and volatile acidity (above 6%). The regulation recognizes ten distinct types of vinegar, reflecting the diverse range of raw materials and production methods. These types include wine vinegar, fruit vinegar, cider vinegar, alcoholic vinegar, cereal vinegar, malt vinegar, malt distillate vinegar, balsamic vinegar, and “other balsamic vinegar” [121]. Each type of vinegar possesses distinct characteristics and applications, enriching the diverse choices available in the market. These regulations highlight the European Union’s dedication to upholding the quality and authenticity of vinegar crafted within its member states [10,31]. Wine vinegar, notably widespread in Mediterranean regions, is the most prevalent variety [8,117].
Acetic acid bacteria’s production, crucial for vinegar formation, is influenced by temperature, dissolved oxygen availability, ethanol concentration, and acetic acid concentration and their interdependence. Typically, vinegar production by acetic bacteria occurs at around 30 °C [122].
Vinegar exhibits remarkable diversity in global production, stemming from various sources, including byproducts, agricultural surpluses, and premium substrates. This diversity results in unique vinegars like Sherry vinegar from Spain and Aceto Balsamico Tradizionale from Italy. These exceptional kinds of vinegar are characterized by specific substrates such as honey or rice, enriching the range of vinegar options available in the market [8,9,30,117,123].
Vinegar’s role extends beyond culinary applications, encompassing its use as a disinfectant, cleansing agent, and beverage. Additionally, vinegar has potential health benefits, including aiding digestion, stimulating appetite, providing antioxidative properties, aiding fatigue recovery, reducing lipid levels, and regulating blood pressure [9,10,119,122,124,125,126].
4.1. Vinegar Production: From Fermentation to Quality
Vinegar production, facilitated by a particular bacterium called Acetobacter, comprises two key fermentation stages: alcoholic fermentation and acetous fermentation (Figure 4) [9,10,117,127].
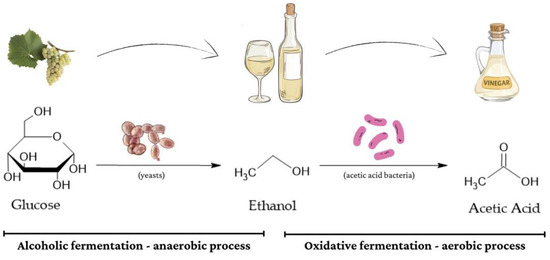
Figure 4.
The figure shows a schematic representation of wine vinegar production bioprocess.
Alcoholic fermentation is instigated by yeasts, commonly strains of Saccharomyces cerevisiae. This stage, usually spanning the initial three weeks, sees the rapid depletion of most sugars as fermentable sugars are converted into ethanol. Notably, this process occurs in anaerobic conditions [8,9,10,127]. Moving on to acetous fermentation, this stage is orchestrated by AAB, where ethanol is further oxidized into acetic acid. It is imperative to highlight that this fermentation unfolds in aerobic conditions, in contrast to the anaerobic setting of alcoholic fermentation [10,117], as shown in Figure 4.
As previously outlined, the acetous fermentation process hinges on the enzymatic action of alcohol dehydrogenase, which converts ethanol into acetaldehyde. This enzyme complex comprises quinoproteins and flavoproteins, each serving distinct functions. Quinoproteins and flavoproteins act as essential cofactors, forming covalent bonds with pyrroloquinolinequinone and flavin adenine dinucleotide. These prosthetic groups are integral to the enzyme’s structure, enabling its catalytic activity in ethanol oxidation [8,9,10,128].
Following alcohol oxidation, acetaldehyde is converted into hydrated acetaldehyde by adding water [8]. Then, alcohol dehydrogenase enzymes transform hydrated acetaldehyde into acetic acid, facilitated by covalent bonds with prosthetic groups [9,10,17].
Controlling dissolved oxygen concentration is crucial for optimizing acetous fermentation. Maintaining specific levels of dissolved oxygen is vital for the efficiency of the process, as oxygen is necessary for the catalytic activity of alcohol dehydrogenase and other associated enzymatic reactions, facilitating the conversion of ethanol to acetic acid. [9,31,117,129,130]. The complex interplay of enzymes and oxygen orchestrates a biochemical symphony that forms the foundation of acetous fermentation, a vital process in vinegar production.
There are distinct methods for vinegar production, each with its characteristics. The Orleans method, harking back to ancient traditions, represents one of the oldest techniques in vinegar production. Characterized by a meticulous and slow acetification process, this method unfolds within the confines of carefully crafted wooden barrels. The choice of wooden vessels contributes a distinct flavor profile and organoleptic complexity to the final product. This traditional approach, however, comes at the cost of time, requiring a patient testing duration of 8 to 14 weeks. During this extended period, the process relies on a static culture of acetic acid bacteria at the liquid-air interface within the barrels. Ethanol gradually transforms into acetic acid, showcasing the artisanal precision inherent in this method. The resulting vinegar is acclaimed for its high quality, a testament to the unhurried maturation process that allows for the development of nuanced flavors [8,9,10,117,130].
While the Orleans Method might be less conducive to large-scale production due to its time-consuming nature, it remains a revered choice for those who value the artistry and authenticity embedded in traditional vinegar craftsmanship. The interaction between the wooden barrels, the static culture of bacteria, and the protracted fermentation period contributes to the unique and cherished characteristics of the Orleans Method-produced vinegar [8,10,117,128].
The Submerged Fermentation Method contrasts the unhurried pace of the Orleans Method, offering a more streamlined and efficient approach to vinegar production. Embracing the advancements of industrialization, this method employs stainless steel fermentation tanks with substantial capacities, typically ranging from 10,000 to 40,000 L. In this method, acetification occurs within these stainless-steel tanks, where Acetobacter, the essential bacteria responsible for converting ethanol to acetic acid, is suspended in the acetifying culture. The larger production scale is facilitated using modern fermentors, allowing for increased aeration, stirring, and control over environmental factors [9,117,128].
One of the hallmark features of the Submerged Fermentation Method is its rapidity. Unlike the prolonged duration associated with the Orleans Method, vinegar production using the submerged approach can be achieved in significantly shorter cycles. This accelerated pace is crucial for meeting the demands of large-scale commercial production [8,30,131].
However, with speed comes a potential trade-off regarding the depth of organoleptic complexity that can be achieved. The efficiency of the Submerged Fermentation Method may prioritize mass production over the intricate flavor development seen in traditional methods. Additionally, while providing advantages in terms of hygiene and scalability, the stainless-steel vessels might lack the nuanced interactions between the vinegar and wooden barrels characteristic of traditional methods [10,30].
The Generator Process, originating in the nineteenth century, showcases the innovative advancements in vinegar-making systems. Also known as the trickle or German rapid acetification method, this approach brings unique principles to vinegar production. The vinegar-making system revolves around a container with two distinct chambers in this method. This innovation allows for an expedited completion of the acetification process, often occurring within 3 to 7 days. Such rapidity makes this method particularly suitable for producing distilled and industrial vinegar [9,10,31,123,128].
The system involves bacteria growing to form a slime coating around a non-compacting material, such as beech wood shavings, charcoal, or coke. This fermentation typically occurs in tanks made of wood or steel, boasting a volume range of 50,000 to 60,000 L. The design includes a packing material, often beech wood shavings, on which the bacteria adhere. The liquid is then sprayed over this material, allowing it to drip through to the bottom of the tank, ensuring the bacteria’s exposure to oxygen [10,17,31,128,132].
Despite its merits, the Generator Process has drawbacks. The risk of clogging looms due to cellulose-producing bacterial growth in the generator. Additional challenges include the accumulation of dead bacteria and the potential for infection with vinegar eels. Moreover, a notable issue of relatively high ethanol loss through evaporation makes it challenging to achieve vinegar with a high acetic acid concentration [10,117].
With its innovative design and shorter production timeline, the Generator Process addresses the need for efficiency in vinegar production. However, it navigates challenges associated with clogging, bacterial accumulation, and ethanol loss, requiring a delicate balance between speed and product quality.
Vinegar aging is crucial to organoleptic quality despite differences in production methods. Aging involves chemical reactions, evaporation, ester formations, and interactions with wood, which improves the integration of aromas and metabolites [8,10,30,133,134,135,136].
As the vinegar undergoes aging, various chemical reactions unfold within the liquid. These reactions result from residual microorganisms, enzymes, and interactions with the oxygen introduced during fermentation. Over time, these reactions form new compounds, contributing to the richness and complexity of the vinegar’s flavor profile [10,30,133,135]. Evaporation during aging is a noteworthy phenomenon. As the vinegar rests in wooden barrels, some liquid evaporates, concentrating its components. This concentration intensifies the vinegar’s flavors, allowing for a more robust and nuanced taste. Additionally, the porous nature of the wood may permit a controlled exchange of air, contributing to the oxidation of certain compounds and further influencing the final taste [8,30,133].
Ester formations, another essential aspect of aging, involve the creation of esters through the interaction of acids and alcohols. These compounds contribute to the fruity and floral notes found in well-aged vinegars. The longer the aging process, the more time these ester-forming reactions must occur, imparting a distinctive and desirable character to the vinegar. The wooden casks used during aging also play a crucial role. The wood imparts unique flavors, aromas, and even color to the vinegar. Oak barrels, for instance, can contribute vanilla and woody notes, while other types of wood may introduce different elements. The interaction between the vinegar and the wood is a gradual process occurring over an extended aging period [8,9,10,30].
Despite being available commercially since 2018, more scientific articles need to address the production, aging methods, and chemical profile of port wine vinegar. In 2015, Maria Tonello [137] undertook a study on the acetification of Port wine as part of her doctoral research. To encourage the production of this unique vinegar, Tonello developed a gourmet vinegar using a bioreactor with two distinct samples of Port wine. The study’s findings revealed a reduced acetic acid and ethanol reduction during fermentation, while the concentrations of sugars, specifically glucose and fructose, remained constant. Tonello concluded that these measurements did not align with Portuguese legislation standards. Additionally, she proposed that Acetobacter pasteurianus subsp. paradoxus is the predominant AAB responsible for port wine vinegar production. Moreover, the research highlighted that only 9% of the overall bacterial population was cultivable, suggesting the existence of viable but nonculturable (VBNC) bacteria within the microbial community. Figure 5 illustrates the overall process of port wine vinegar production.
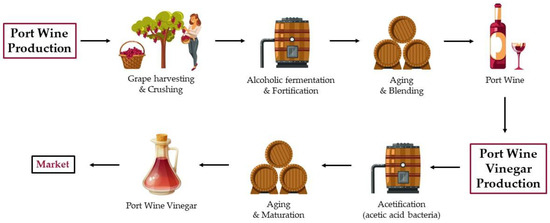
Figure 5.
The figure shows the traditional process of port wine vinegar production.
4.2. Wine Vinegar Characteristics
Wine vinegar, renowned for its unique flavor and aroma, is the result of a meticulous process influenced by various factors, including starter cultures, production methods, and aging [8,137,138].
A fundamental aspect of the quality of wine vinegars lies in their aromatic complexity. The aroma is a complex fraction comprising over 100 different chemical compounds. This complexity evolves during the aging process, mainly when the vinegar is in contact with wood [30,139,140,141].
For instance, Callejón et al. (2008) [141] observed that specific volatile compounds mark Sherry vinegar’s aroma. Diacetyl, recognized for its buttery aroma, enriches the scent with a creamy texture. Isoamyl acetate lends a fruity essence reminiscent of bananas, introducing a tropical sweetness. Isovaleric acid adds an intense note, contributing sharpness to the complexity. Ethyl acetate offers a fruity undertone with its sweet and solvent-like aroma. Sotolon, responsible for Sherry vinegar’s distinctive scent, adds a complex and captivating aromatic dimension. These volatile compounds interact during aging, particularly in the presence of wood, creating a harmonious blend of aromas unique to Sherry vinegar. The interplay of diacetyl’s creaminess, isoamyl acetate’s fruity sweetness, isovaleric acid’s pungency, ethyl acetate’s fruity undertones, and the distinctive character of sotolon forms a sensory tapestry that sets Sherry vinegar apart. This aromatic complexity, shaped by aging, highlights the meticulous craftsmanship of producing top-tier wine vinegar.
Polyphenolic compounds, widely distributed in plant products, play a crucial role as significant quality determinants in vinegar [30]. Beyond their recognized antioxidant activity, these compounds contribute to the vinegar’s color and astringency [142]. The inherently aerobic acetification process depends on oxygen, which is indispensable for bacterial growth, particularly acetic acid bacteria [143]. The interaction between phenolic compounds and oxygen holds considerable importance, and this connection is extensively analyzed for its implications in the color changes observed in wines [111]. During the acetification process, the phenolic composition undergoes alterations influenced by the rate of acetification. This dynamic relationship provides valuable insights into determining the vinegar production method employed. Moreover, the aging method further shapes the phenolic profile of vinegar. As vinegar ages, compounds undergo an ongoing reaction and transformation involving the polymerization and release of substances from the wood used in the aging process. The specific type of wood, such as oak or acacia, its roasting, the ratio of the contact surface to liquid volume, and the duration of aging all contribute to the phenolic composition. Consequently, the amount and nature of phenols present in vinegar become intricately linked to the production and aging methods employed [30,140,143].
For instance, submerged fermentation systems, which use excess oxygen to accelerate the acetification process, may exhibit variations in phenolic composition compared to surface culture systems, where oxygen availability is limited. The rate of acetification, the solubility of oxygen in the medium, and the phenolic composition collectively contribute to the vinegar’s overall quality and characteristics. Furthermore, distinct phenolic compounds released from specific wood types during aging indicate the vinegar’s maturation period and the type of wood utilized in its production [9,30,140,144].
Polyphenolic compounds, encompassing flavonoids, anthocyanins, and other phenolic derivatives, are essential components in vinegar composition. Found abundantly in plant products, they significantly influence the final vinegar product’s sensory attributes, color, and potential health advantages. Flavonoids, a diverse category of polyphenols, are recognized for their antioxidant properties and contribute substantially to vinegar’s color and taste. Anthocyanins, a subset of flavonoids responsible for vivid hues in fruits and vegetables, enhance the visual allure of certain vinegar types. Meanwhile, phenolic derivatives like vanillin, eugenol, and benzaldehyde enhance vinegar’s overall aromatic complexity and flavor spectrum [30,145,146,147].
Consumer perception plays a crucial role in assessing the quality of wine vinegars. Elevated acetic acid levels elicit intense sensations contributing to the vinegar’s pungency, requiring familiarity for accurate tasting. Trained sensory analysis panels utilize solutions containing typical compounds present in wine vinegar to evaluate attributes such as color, aromatic intensity, woody and herbaceous scents, fruity notes, wine aromas, and overall sensory impact [30,117,146].
4.3. Improving Port Wine Production and Its Effect on Vinegar
Producing port wine vinegar encounters a specific challenge: managing the high alcohol levels commonly present in port wine, often surpassing 19% (v/v) [8,137]. Elevated ethanol levels can impede acetic acid bacterial growth, potentially limiting the productivity of the acetification fermentation process [8,32]. Addressing this intricate balance is crucial for achieving optimal acetification. Furthermore, the higher sugar content in port wine can challenge vinegar production [127]. The existence of residual sugars has the potential to perpetuate fermentation, leading to the continuous formation of acetic acid. However, this prolonged fermentation process, coupled with the overoxidation of acetic acid by acetic acid bacteria (AAB), may result in the degradation of the vinegar sample.
The meticulous process of crafting port wine vinegar commences with a discerning grape selection. This initial step necessitates thoroughly evaluating grape varieties, considering their biochemical composition and suitability for vinegar production. Oxygen exposure is carefully managed throughout this process, with the implementation of oxygen-permeable membranes and precise control over the wine’s exposure to oxygen. These measures are crucial in maintaining optimal oxygen levels during the acetification process, ensuring the necessary aeration for ethanol-to-acetic acid conversion while preventing undesirable oxidation. Sulfur dioxide usage, a standard winemaking preservative, becomes a strategic consideration. Its judicious application prevents unwanted microbial growth while preserving the delicate balance of flavors and aromas [8,10,30,117,122].
As fermentation progresses, introducing acetic acid bacteria becomes a transformative moment. Acclimatizing these bacteria to the environment and gradually incorporating them into the fermentation process allows for a seamless transition from alcoholic fermentation to acetic fermentation. This step is a delicate dance, requiring precision to avoid overwhelming the process with acetic acid too soon.
Wine aging, a hallmark of the vinegar crafting process, takes center stage. The choice of barrels, wood type, and aging duration are deliberate decisions that shape the vinegar’s final character. The interaction between the wine and the wood, coupled with controlled exposure to oxygen and using permeable membranes, lends the vinegar a nuanced complexity, distinguishing it as a product of art and science [8,122,143,146].
Conversely, the second perspective in producing port wine vinegar involves enhancing acetification with different types of port wine. Each variant, whether Ruby, Tawny, or Vintage, possesses unique characteristics that merit consideration. This approach entails a nuanced selection process, where various treatments, including pH adjustments, controlled bacterial introduction, and judicious oxidation, come into play. The overarching objective is to expedite the acetification process while harmoniously retaining the distinct qualities of each port wine type, resulting in vinegars that echo the richness and complexity of their wine origins.
Lowering the alcohol concentration in wine, especially in creating exceptional vinegars like port wine vinegar, presents unique hurdles. The conventional dilution method with distilled water can alter the flavor profile, reducing the alcohol content to approximately 6–7% or the desired ethanol concentration. This prompts winemakers to seek alternative techniques to reduce alcohol while maintaining the wine’s nuanced traits [8].
Winemakers employ Reverse Osmosis (RO) to tackle this challenge. In this process, the wine passes through a semi-permeable membrane, allowing water and some volatile compounds to pass while retaining alcohol and larger molecules. This technique requires specialized equipment and expertise but provides a controlled approach to reducing alcohol content without significant flavor loss. Winemakers appreciate RO’s precision in preserving the wine’s aromatic components [148,149,150,151,152,153].
Another technique is the Spinning Cone Column, which utilizes centrifugal force to separate and remove alcohol and volatile compounds from the wine. This approach is gentler than traditional distillation, ensuring a more delicate treatment of the wine’s sensory characteristics. The spinning cone column offers winemakers an efficient means of alcohol reduction while minimizing the impact on the overall flavor profile [152,154].
Vacuum distillation is an alternative approach that operates at lower temperatures than conventional distillation methods. This technique reduces the risk of flavor loss while effectively lowering alcohol content. Winemakers appreciate vacuum distillation for its ability to modify the wine’s composition without compromising its sensory attributes [155,156].
Enzymatic treatment offers a more complex alternative. By introducing enzymes, alcohol can be broken down into its components. However, this approach may not be as practical as others and could impact the wine’s flavor. Precise control over enzymatic treatments is crucial to prevent unintended changes in wine composition. Winemakers must balance reducing alcohol content and maintaining the wine’s distinctive qualities [157,158,159].
The ultimate aim of reducing alcohol concentration in wine is to optimize the acid fermentation process, achieving an ethanol concentration favorable to the development of acetic bacteria. This strategic approach facilitates vinegar production, ensuring a smooth process without hindrance due to the raw material. As winemakers delve into the complexities of producing exceptional vinegar, each decision, whether refining aging techniques, choosing appropriate wood for barrels, or exploring innovative fermentation methods, is carefully considered.
Critically, this deliberate approach raises questions about the impact on the overall flavor profile of the resulting vinegar. Reducing alcohol content while aiding acetic fermentation may introduce a trade-off by diminishing wines’ nuanced and pleasant flavor components. Producers must meticulously assess the advantages of improved acetification against the risk of sensory changes, aiming to find a nuanced equilibrium that enhances the acetic fermentation process while preserving the richness and complexity of the resulting vinegar. Achieving this delicate balance between traditional practices and innovative techniques is crucial. It ensures the creation of exceptional vinegars that showcase the transformative power of acetic fermentation and stand as a testament to the reverence for intrinsic qualities of revered port wines. This nuanced perspective illuminates the artistry and expertise required in producing exceptional vinegar, highlighting the journey from raw material to the final desired product.
5. Exploring Taste: Port Wine and Vinegar Sensory Analysis
Sensory analysis has a rich history dating back to the early 20th century when it emerged as a method for evaluating food products through the perceptions of trained panelists. Initially considered a complement to technological and microbiological assessments, sensory analysis has become a cornerstone of innovation and product development. Traditional techniques such as discrimination tests, descriptive analysis, and consumer tests laid the foundation for more advanced methodologies, including check-all-that-apply (CATA), napping (N), and flash profile (FP), each offering unique insights into product characteristics [160,161,162].
Recent advancements in biometric techniques have revolutionized the field, providing novel ways to measure human responses to stimuli. Facial expression analysis, heart rate monitoring, skin conductance assessment, and eye-tracking technologies offer valuable data on emotional and physiological reactions to food and beverage products. These innovative tools shed light on consumer preferences, helping researchers and industry professionals tailor products to meet evolving tastes and preferences [161,163].
The integration of smart sensors, such as the electronic tongue (E-tongue) and electronic nose (E-nose), represents a significant advancement in sensory science [160]. This cutting-edge technology mimics human taste and smell perception, providing rapid and objective assessments of product taste and aromatic profiles. The E-nose specializes in detecting and analyzing odors, which is crucial for identifying and quantifying volatile compounds responsible for a product’s aroma. Meanwhile, the E-tongue quantifies basic tastes like sweet, sour, bitter, salty, and umami, generating comprehensive fingerprints of food and beverage products. These capabilities facilitate quality control measures and consumer satisfaction initiatives [160,161,163].
Sensory analysis techniques are indispensable for understanding the intricacies of port wine’s sensory attributes [162]. Methods like descriptive analysis and aroma profiling allow wine experts and enthusiasts to explore its nuances. Descriptive analysis, conducted by trained panels systematically evaluating appearance, aroma, flavor, and mouthfeel, provides a comprehensive understanding of port wine’s sensory characteristics [160]. Aroma profiling, on the other hand, identifies and quantifies volatile compounds responsible for their distinctive aromas, offering insights into composition and intensity [162,164].
Additionally, as previously discussed, the hallmark of port wine production—the aging process—further enhances its sensory allure. Throughout aging, compounds transform, shaping the wine’s distinctive sensory profile. For instance, vanillin, eugenol, and oak lactones extracted from oak barrels introduce vanilla, clove, and coconut flavors, enriching the wine’s aroma and taste. Moreover, oxidative reactions during aging generate compounds like acetaldehyde and furfural, contributing to the wine’s characteristic nutty and caramel-like aromas [165,166,167].
Consumer perception greatly influences the port wine market [168]. Esteemed by enthusiasts and collectors, port wine’s history is often tied to special occasions.
Silva and Rebelo (2019) [168] investigated firms’ and consumers’ perceptions of port wine to address its declining popularity. They found that younger consumers are drawn to its versatility and sophistication, appreciating its sweet, alcoholic nature. Digital platforms and social media have made information about port wine more accessible, sparking interest among younger demographics. Producers can capitalize on this trend by using digital channels to share stories, experiences, and educational content, nurturing a new generation of port wine enthusiasts.
Vilela et al. (2020) [169] innovated in the food sector by creating a perfume infused with the essence of tawny port. Through meticulous sensory analysis of four tawny varieties, they identified benzaldehyde, sotolon, and vanillin as essential compounds to evoke the aroma of tawny port. This study highlights the growing trend of incorporating wine-derived fragrances into enogastronomy, emphasizing perfume development and consumer acceptability.
Port wine vinegar, with its deep ruby hue and intricate flavor profile, offers a sophisticated addition to culinary creations [170]. However, despite its potential, it often needs to be more utilized due to packaging, marketing, and consumer perceptions. Traditional wine vinegar packaging may convey elegance but can also imply exclusivity, deterring some consumers from trying the product [171,172,173]. Moreover, limited marketing and educational initiatives contribute to misconceptions about its value and versatility.
To address these challenges, comprehensive approaches are needed. Educational initiatives, such as cooking demonstrations and tasting events, can help demystify port wine vinegar and showcase its culinary potential. By offering opportunities for consumers to experience its unique qualities firsthand, familiarity and preference for the product can increase over time [174,175,176,177]. Additionally, packaging and marketing strategies emphasizing port wine vinegar’s quality, craftsmanship, and heritage can enhance its perceived value and appeal to discerning consumers.
Furthermore, packaging and marketing strategies emphasizing port wine vinegar’s quality, craftsmanship, and heritage can enhance its perceived value and appeal to discerning consumers. By positioning this type of vinegar as a premium ingredient with distinct sensory attributes and culinary benefits, producers can attract a new generation of consumers who appreciate the depth and complexity of their culinary creations.
Overall, a multifaceted approach that combines education, marketing, and product innovation is essential to elevate the status of port wine vinegar and broaden its adoption in the culinary world.
6. Final Remarks
This review underscores the pivotal role of acetic bacteria in the oxidative fermentation process responsible for producing vinegar, particularly wine vinegar. It introduces a recent culinary innovation: port wine vinegar, introduced in 2018. It highlights the imperative for the wine industry to elevate the appreciation of its byproducts and derivatives. Beyond its conventional role as a beverage, wine harbors considerable potential for developing products endowed with nutraceutical properties.
These insights indicate the wine sector’s readiness to leverage its rich heritage and assets to diversify and innovate its offerings. By leveraging wine’s distinct characteristics, particularly its intricate flavor profiles, and biochemical components, producers can explore opportunities to create value-added products that cater to evolving consumer preferences and health-conscious lifestyles.
Advancing the exploration of port wine vinegar represents a significant stride in tapping into this latent potential. Producers can craft innovative beverages that resonate with diverse palates and address varied consumer preferences by amalgamating this unique vinegar with complementary elements such as fruits, herbs, or botanical extracts. Through strategic collaborations and partnerships with culinary experts and beverage innovators, the wine industry can spearhead the introduction of novel drink concepts that seamlessly blend tradition with innovation, captivating consumers with refreshing sensory experiences and health-promoting attributes.
Author Contributions
Conceptualization, J.M. and A.V.; writing—original draft preparation, J.M.; writing—review and editing, A.V.; supervision, A.V.; funding acquisition, A.V. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Vine & Wine Portugal-Driving Sustainable Growth Through Smart Innovation Project, number 67, AAC: 02/C05-i01/2022, sub-project B1.5.1.—Alcohol a la carte: Reducing alcohol in the wine after fermentation, without any loss of aromas. Financed by the Next Generation EU “Programa de Recuperação e Resiliência (PRR)/Alianças Mobilizadora”. The CQ-VR also funded the study [grant number UIDB/00616/2020 and UIDP/00616/2020—DOI: 10.54499/UIDB/00616/2020], FCT—Portugal, and COMPETE.
Acknowledgments
The authors thank the Chemistry Research Center (CQ-VR) and the project PRR—Vine & Wine Portugal—Driving Sustainable Growth Through Smart Innovation, for their financial support.
Conflicts of Interest
The authors declare no conflicts of interest.
Table of contents
| 1. Introduction……………………………………………………………………………………… | 1 |
| 2. Biochemistry and physiology of Acetic Acid Bacteria (AAB)………………………………. | 2 |
| 2.1. General overview, classification, and identification…………………………………….. | 2 |
| 2.2. Metabolic pathways and respiratory chains in AAB……………………………………. | 6 |
| 2.2.1. Respiratory machinery and energy yield…………………………………………... | 6 |
| 2.2.2. Acetic acid production: Oxidative fermentation…………………………………... | 6 |
| 2.2.3. Oxidation of carbohydrates, alcohols, and organic acids………………………… | 8 |
| 2.2.4. Resistance to acidic environments…………………………………………………... | 9 |
| 2.2.5. Resistance to alcoholic environments………………………………………………. | 10 |
| 2.2.6. Extracellular polymeric substances produced by AAB…………………………… | 11 |
| 3. Overview of Port Wine production……………………………………………………………. | 13 |
| 4. Port Wine Vinegar………………………………………………………………………………. | 13 |
| 4.1. Vinegar production: From fermentation to quality……………………………………… | 14 |
| 4.2. Wine vinegar characteristics……………………………………………………………….. | 18 |
| 4.3. Improving Port Wine production and its effect on vinegar……………………………. | 19 |
| 5. Exploring taste: Port wine and vinegar sensory analysis…………………………………… | 20 |
| 6. Final remarks…………………………………………………………………………………….. | 22 |
| References…………………………………………………………………………………………... | 23 |
References
- Maicas, S. The Role of Yeasts in Fermentation Processes. Microorganisms 2020, 8, 1142. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, D.; Hashimoto, W. Adaptation of Yeast Saccharomyces cerevisiae to Grape-Skin Environment. Sci. Rep. 2023, 13, 9279. [Google Scholar] [CrossRef] [PubMed]
- Moreira, N.; de Pinho, P.G. Port Wine. Adv. Food Nutr. Res. 2011, 63, 119–146. [Google Scholar]
- Martins, J.P. The Pleasure of Port: The Inside Story of a Unique Fortified Wine; LIVROS D’HOJE: Lisbon, Portugal, 2011; ISBN 9789722046619. [Google Scholar]
- Cristovam, E.; Paterson, A. PORT|Composition and Analysis. In Encyclopedia of Food Sciences and Nutrition; Elsevier: Amsterdam, The Netherlands, 2003; pp. 4638–4644. [Google Scholar]
- Perestrelo, R.; Silva, C.; Pereira, J.; Câmara, J.S. Wines: Madeira, Port and Sherry Fortified Wines—The Sui Generis and Notable Peculiarities. Major Differences and Chemical Patterns. In Encyclopedia of Food and Health; Elsevier: Amsterdam, The Netherlands, 2016; pp. 534–555. [Google Scholar]
- Grandes Escolhas Vinagre de Vinho Do Porto Já é Marca Registada. Available online: https://grandesescolhas.com/vinagre-de-vinho-do-porto-ja-e-marca-registada/ (accessed on 31 January 2024).
- Vilela, A. Microbial Dynamics in Sour–Sweet Wine Vinegar: Impacts on Chemical and Sensory Composition. Appl. Sci. 2023, 13, 7366. [Google Scholar] [CrossRef]
- Ho, C.W.; Lazim, A.M.; Fazry, S.; Zaki, U.K.H.H.; Lim, S.J. Varieties, Production, Composition and Health Benefits of Vinegars: A Review. Food Chem. 2017, 221, 1621–1630. [Google Scholar] [CrossRef]
- Bhat, S.V.; Akhtar, R.; Amin, T. An Overview on the Biological Production of Vinegar. Int. J. Fermented Foods 2014, 3, 139. [Google Scholar] [CrossRef]
- Bartowsky, E.J.; Henschke, P.A. Acetic Acid Bacteria Spoilage of Bottled Red Wine—A Review. Int. J. Food Microbiol. 2008, 125, 60–70. [Google Scholar] [CrossRef] [PubMed]
- De Vero, L.; Giudici, P. Genus-Specific Profile of Acetic Acid Bacteria by 16S RDNA PCR-DGGE. Int. J. Food. Microbiol. 2008, 125, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Sengun, I.Y.; Karabiyikli, S. Importance of Acetic Acid Bacteria in Food Industry. Food Control 2011, 22, 647–656. [Google Scholar] [CrossRef]
- Yassunaka Hata, N.N.; Surek, M.; Sartori, D.; Serrato, R.V.; Spinosa, W.A. Role of Acetic Acid Bacteria in Food and Beverages. Food Technol. Biotechnol. 2023, 61, 85–103. [Google Scholar] [CrossRef]
- De Filippis, F.; Troise, A.D.; Vitaglione, P.; Ercolini, D. Different Temperatures Select Distinctive Acetic Acid Bacteria Species and Promotes Organic Acids Production during Kombucha Tea Fermentation. Food Microbiol. 2018, 73, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Laavanya, D.; Shirkole, S.; Balasubramanian, P. Current Challenges, Applications and Future Perspectives of SCOBY Cellulose of Kombucha Fermentation. J. Clean. Prod. 2021, 295, 126454. [Google Scholar] [CrossRef]
- Raspor, P.; Goranovič, D. Biotechnological Applications of Acetic Acid Bacteria. Crit. Rev. Biotechnol. 2008, 28, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Lynch, K.M.; Zannini, E.; Wilkinson, S.; Daenen, L.; Arendt, E.K. Physiology of Acetic Acid Bacteria and Their Role in Vinegar and Fermented Beverages. Compr. Rev. Food Sci. Food Saf. 2019, 18, 587–625. [Google Scholar] [CrossRef] [PubMed]
- Guillamón, J.M.; Mas, A. Chapter 9-Acetic Acid Bacteria. Mol. Wine Microbiol. 2011, 227–255. [Google Scholar] [CrossRef]
- Mizzi, J.; Gaggìa, F.; Bozzi Cionci, N.; Di Gioia, D.; Attard, E. Selection of Acetic Acid Bacterial Strains and Vinegar Production From Local Maltese Food Sources. Front. Microbiol. 2022, 13, 897825. [Google Scholar] [CrossRef] [PubMed]
- Román-Camacho, J.J.; García-García, I.; Santos-Dueñas, I.M.; García-Martínez, T.; Mauricio, J.C. Latest Trends in Industrial Vinegar Production and the Role of Acetic Acid Bacteria: Classification, Metabolism, and Applications—A Comprehensive Review. Foods 2023, 12, 3705. [Google Scholar] [CrossRef] [PubMed]
- Sengun, I.Y.; Kilic, G.; Charoenyingcharoen, P.; Yukphan, P.; Yamada, Y. Investigation of the Microbiota Associated with Traditionally Produced Fruit Vinegars with Focus on Acetic Acid Bacteria and Lactic Acid Bacteria. Food Biosci. 2022, 47, 101636. [Google Scholar] [CrossRef]
- Plioni, I.; Bekatorou, A.; Terpou, A.; Mallouchos, A.; Plessas, S.; Koutinas, A.A.; Katechaki, E. Vinegar Production from Corinthian Currants Finishing Side-Stream: Development and Comparison of Methods Based on Immobilized Acetic Acid Bacteria. Foods 2021, 10, 3133. [Google Scholar] [CrossRef]
- Matsushita, K.; Toyama, H.; Tonouchi, N.; Okamoto-Kainuma, A. Acetic Acid Bacteria; Springer: Tokyo, Japan, 2016; ISBN 978-4-431-55931-3. [Google Scholar]
- Parte, A.C.; Sardà Carbasse, J.; Meier-Kolthoff, J.P.; Reimer, L.C.; Göker, M. List of Prokaryotic Names with Standing in Nomenclature (LPSN) Moves to the DSMZ. Int. J. Syst. Evol. Microbiol. 2020, 70, 5607–5612. [Google Scholar] [CrossRef]
- Hördt, A.; López, M.G.; Meier-Kolthoff, J.P.; Schleuning, M.; Weinhold, L.-M.; Tindall, B.J.; Gronow, S.; Kyrpides, N.C.; Woyke, T.; Göker, M. Analysis of 1000+ Type-Strain Genomes Substantially Improves Taxonomic Classification of Alphaproteobacteria. Front. Microbiol. 2020, 11, 468. [Google Scholar] [CrossRef]
- Qiu, X.; Zhang, Y.; Hong, H. Classification of Acetic Acid Bacteria and Their Acid Resistant Mechanism. AMB Express 2021, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, K.; Azuma, Y.; Kosaka, T.; Yakushi, T.; Hoshida, H.; Akada, R.; Yamada, M. Genomic Analyses of Thermotolerant Microorganisms Used for High-Temperature Fermentations. Biosci. Biotechnol. Biochem. 2016, 80, 655–668. [Google Scholar] [CrossRef] [PubMed]
- Gomes, R.J.; Borges, M.D.F.; Rosa, M.D.F.; Castro-Gómez, R.J.H.; Spinosa, W.A. Acetic Acid Bacteria in the Food Industry: Systematics, Characteristics and Applications. Food Technol. Biotechnol. 2018, 56, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Mas, A.; Torija, M.-J.; del Carmen García-Parrilla, M.C.; Troncoso, A.M. Acetic Acid Bacteria and the Production and Quality of Wine Vinegar. Sci. World J. 2014, 2014, 394671. [Google Scholar] [CrossRef] [PubMed]
- Plessi, M. Vinegar. Encycl. Food Sci. Nutr. 2003, 5996–6004. [Google Scholar] [CrossRef]
- Giudici, P.; Gullo, M.; Solieri, L.; Falcone, P.M. Technological and Microbiological Aspects of Traditional Balsamic Vinegar and Their Influence on Quality and Sensorial Properties. Adv. Food Nutr. Res. 2009, 58, 137–182. [Google Scholar] [CrossRef] [PubMed]
- Gullo, M.; Caggia, C.; De Vero, L.; Giudici, P. Characterization of Acetic Acid. Bacteria in “Traditional Balsamic Vinegar.”. Int. J. Food Microbiol. 2006, 106, 209–212. [Google Scholar] [CrossRef]
- Mamlouk, D.; Gullo, M. Acetic Acid Bacteria: Physiology and Carbon Sources Oxidation. Indian J. Microbiol. 2013, 53, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Cacicedo, M.L.; Castro, M.C.; Servetas, I.; Bosnea, L.; Boura, K.; Tsafrakidou, P.; Dima, A.; Terpou, A.; Koutinas, A.; Castro, G.R. Progress in Bacterial Cellulose Matrices for Biotechnological Applications. Bioresour. Technol. 2016, 213, 172–180. [Google Scholar] [CrossRef]
- González, Á.; Guillamón, J.M.; Mas, A.; Poblet, M. Application of Molecular Methods for Routine Identification of Acetic Acid Bacteria. Int. J. Food Microbiol. 2006, 108, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.C.F.; de Souza, A.C.; Ramos, C.L.; Pereira, A.A.; Schwan, R.F.; Dias, D.R. Sensorial, Antioxidant and Antimicrobial Evaluation of Vinegars from Surpluses of Physalis (Physalis pubescens L.) and Red Pitahaya (Hylocereus monacanthus). J. Sci. Food Agric. 2019, 99, 2267–2274. [Google Scholar] [CrossRef] [PubMed]
- Soumahoro, S.; Ouattara, H.G.; Droux, M.; Nasser, W.; Niamke, S.L.; Reverchon, S. Acetic Acid Bacteria (AAB) Involved in Cocoa Fermentation from Ivory Coast: Species Diversity and Performance in Acetic Acid Production. J. Food Sci. Technol. 2019, 57, 1904–1916. [Google Scholar] [CrossRef] [PubMed]
- Sombolestani, A.S.; Cleenwerck, I.; Cnockaert, M.; Borremans, W.; Wieme, A.D.; De Vuyst, L.; Vandamme, P. Novel acetic acid bacteria from cider fermentations: Acetobacter conturbans sp. nov. and Acetobacter fallax sp. nov. Int. J. Syst. Evol. Microbiol. 2020, 70, 6163–6171. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.H.; Kim, K.H.; Moon, J.Y.; Yeo, S.-H.; Jeon, C.O. Acetobacter Oryzoeni Sp. Nov., Isolated from Korean Rice Wine Vinegar. Int. J. Syst. Evol. Microbiol. 2020, 70, 2026–2033. [Google Scholar] [CrossRef] [PubMed]
- Noman, A.E.; Al-Barha, N.S.; Sharaf, A.-A.M.; Al-Maqtari, Q.A.; Mohedein, A.; Mohammed, H.H.H.; Chen, F. A Novel Strain of Acetic Acid Bacteria Gluconobacter oxydans FBFS97 Involved in Riboflavin Production. Sci. Rep. 2020, 10, 13527. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.; Grandvalet, C.; Verdier, F.; Martin, A.; Alexandre, H.; Tourdot-Maréchal, R. Microbial Dynamics between Yeasts and Acetic Acid Bacteria in Kombucha: Impacts on the Chemical Composition of the Beverage. Foods 2020, 9, 963. [Google Scholar] [CrossRef]
- Angela, C.; Young, J.; Kordayanti, S.; Virgina Partha Devanthi, P.K. Isolation and Screening of Microbial Isolates from Kombucha Culture for Bacterial Cellulose Production in Sugarcane Molasses Medium. KnE Life Sci. 2020. [Google Scholar] [CrossRef]
- Li, H.; Fu, J.; Hu, S.; Li, Z.; Qu, J.; Wu, Z.; Chen, S. Comparison of the Effects of Acetic Acid Bacteria and Lactic Acid Bacteria on the Microbial Diversity of and the Functional Pathways in Dough as Revealed by High-Throughput Metagenomics Sequencing. Int. J. Food Microbiol. 2021, 346, 109168. [Google Scholar] [CrossRef]
- Anguluri, K.; La China, S.; Brugnoli, M.; De Vero, L.; Pulvirenti, A.; Cassanelli, S.; Gullo, M. Candidate Acetic Acid Bacteria Strains for Levan Production. Polymers 2022, 14, 2000. [Google Scholar] [CrossRef]
- Xia, M.; Zhang, X.; Xiao, Y.; Sheng, Q.; Tu, L.; Chen, F.; Yan, Y.; Zheng, Y.; Wang, M. Interaction of Acetic Acid Bacteria and Lactic Acid Bacteria in Multispecies Solid-State Fermentation of Traditional Chinese Cereal Vinegar. Front. Microbiol. 2022, 13, 964855. [Google Scholar] [CrossRef] [PubMed]
- El-Askri, T.; Yatim, M.; Sehli, Y.; Rahou, A.; Belhaj, A.; Castro, R.; Durán-Guerrero, E.; Hafidi, M.; Zouhair, R. Screening and Characterization of New Acetobacter fabarum and Acetobacter pasteurianus Strains with High Ethanol–Thermo Tolerance and the Optimization of Acetic Acid Production. Microorganisms 2022, 10, 1741. [Google Scholar] [CrossRef] [PubMed]
- Brugnoli, M.; La China, S.; Lasagni, F.; Romeo, F.V.; Pulvirenti, A.; Gullo, M. Acetic Acid Bacteria in Agro-Wastes: From Cheese Whey and Olive Mill Wastewater to Cellulose. Appl. Microbiol. Biotechnol. 2023, 107, 3729–3744. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-H.; Kim, S.-H.; Lee, C.-Y.; Jo, H.-W.; Lee, W.-H.; Kim, E.-H.; Choi, B.-K.; Huh, C.-K. Screening of Acetic Acid Bacteria Isolated from Various Sources for Use in Kombucha Production. Fermentation 2023, 10, 18. [Google Scholar] [CrossRef]
- Kim, S.-H.; Jeong, W.-S.; Kim, S.-Y.; Yeo, S.-H. Quality and Functional Characterization of Acetic Acid Bacteria Isolated from Farm-Produced Fruit Vinegars. Fermentation 2023, 9, 447. [Google Scholar] [CrossRef]
- Zgardan, D.; Mitina, I.; Sturza, R.; Mitin, V.; Rubtov, S.; Grajdieru, C.; Behta, E.; Inci, F.; Haciosmanoglu, N. A Survey on Acetic Acid Bacteria Levels and Volatile Acidity in Several Wines of the Republic of Moldova. Biol. Life Sci. Forum 2023, 26, 79. [Google Scholar] [CrossRef]
- Parra, A.; Ovejas, A.; González-Arenzana, L.; Gutiérrez, A.R.; López-Alfaro, I. Development and Validation of a New Method for Detecting Acetic Bacteria in Wine. Foods 2023, 12, 3734. [Google Scholar] [CrossRef]
- Wu, J.; Li, Q.; Hu, K.; Li, J.; Durán-Guerrero, E.; Liu, S.; Guo, M.; Liu, A. Microbial Characterization of Sichuan Baoning Vinegar: Lactic Acid Bacteria, Acetic Acid Bacteria and Yeasts. Arch. Microbiol. 2024, 206, 59. [Google Scholar] [CrossRef]
- Matsushita, K.; Toyama, H.; Adachi, O. Respiratory Chains and Bioenergetics of Acetic Acid Bacteria. Adv. Microb. Physiol. 1994, 36, 247–301. [Google Scholar]
- Ano, Y.; Toyama, H.; Adachi, O.; Matsushita, K. Energy Metabolism of a Unique Acetic Acid Bacterium, Asaia bogorensis, That Lacks Ethanol Oxidation Activity. Biosci. Biotechnol. Biochem. 2008, 72, 989–997. [Google Scholar] [CrossRef]
- Madigan, M.T.; Martinko, J.M.; Bender, K.S.; Buckley, D.H.; Stahl, D.A. Brock Biology of Microorganisms, 14th ed.; Pearson Education: London, UK, 2018; ISBN 9781292235103. [Google Scholar]
- Matsushita, K.; Matsutani, M. Distribution, Evolution, and Physiology of Oxidative Fermentation. In Acetic Acid Bacteria; Springer: Tokyo, Japan, 2016; pp. 159–178. [Google Scholar]
- Hanke, T.; N�h, K.; Noack, S.; Polen, T.; Bringer, S.; Sahm, H.; Wiechert, W.; Bott, M. Combined Fluxomics and Transcriptomics Analysis of Glucose Catabolism via a Partially Cyclic Pentose Phosphate Pathway in Gluconobacter oxydans 621H. Appl. Environ. Microbiol. 2013, 79, 2336–2348. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhang, R.; Yin, H.; Bai, X.; Chang, Y.; Xia, M.; Wang, M. Acetobacter pasteurianus Metabolic Change Induced by Initial Acetic Acid to Adapt to Acetic Acid Fermentation Conditions. Appl. Microbiol. Biotechnol. 2017, 101, 7007–7016. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yan, P.; Lei, Q.; Li, B.; Sun, Y.; Li, S.; Lei, H.; Xie, N. Metabolic Adaptability Shifts of Cell Membrane Fatty Acids of Komagataeibacter Hansenii HDM1-3 Improve Acid Stress Resistance and Survival in Acidic Environments. J. Ind. Microbiol. Biotechnol. 2019, 46, 1491–1503. [Google Scholar] [CrossRef] [PubMed]
- Román-Camacho, J.J.; García-García, I.; Santos-Dueñas, I.M.; Ehrenreich, A.; Liebl, W.; García-Martínez, T.; Mauricio, J.C. Combining Omics Tools for the Characterization of the Microbiota of Diverse Vinegars Obtained by Submerged Culture: 16S RRNA Amplicon Sequencing and MALDI-TOF MS. Front. Microbiol. 2022, 13, 1055010. [Google Scholar] [CrossRef]
- Adachi, O.; Miyagawa, E.; Shinagawa, E.; Matsushita, K.; Ameyama, M. Purification and Properties of Particulate Alcohol Dehydrogenase from Acetobacter Aceti. Agric. Biol. Chem. 1978, 42, 2331–2340. [Google Scholar] [CrossRef]
- Saichana, N.; Matsushita, K.; Adachi, O.; Frébort, I.; Frebortova, J. Acetic Acid Bacteria: A Group of Bacteria with Versatile Biotechnological Applications. Biotechnol. Adv. 2015, 33, 1260–1271. [Google Scholar] [CrossRef]
- Ameyama, M.; Adachi, O. Membrane-Bound. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1982; pp. 450–457. [Google Scholar]
- Adachi, O.; Tayama, K.; Shinagawa, E.; Matsushita, K.; Ameyama, M. Purification and Characterization of Membrane-Bound Aldehyde Dehydrogenase from Gluconobacter suboxydans. Agric. Biol. Chem. 1980, 44, 503–515. [Google Scholar] [CrossRef]
- Saeki, A.; Taniguchi, M.; Matsushita, K.; Toyama, H.; Theeragool, G.; Lotong, N.; Adachi, O. Microbiological Aspects of Acetate Oxidation by Acetic Acid Bacteria, Unfavorable Phenomena in Vinegar Fermentation. Biosci. Biotechnol. Biochem. 1997, 61, 317–323. [Google Scholar] [CrossRef]
- Saeki, A.; Matsushita, K.; Takeno, S.; Taniguchi, M.; Toyama, H.; Theeragool, G.; Lotong, N.; Adachi, O. Enzymes Responsible for Acetate Oxidation by Acetic Acid Bacteria. Biosci. Biotechnol. Biochem. 1999, 63, 2102–2109. [Google Scholar] [CrossRef][Green Version]
- Yakushi, T.; Matsushita, K. Alcohol Dehydrogenase of Acetic Acid Bacteria: Structure, Mode of Action, and Applications in Biotechnology. Appl. Microbiol. Biotechnol. 2010, 86, 1257–1265. [Google Scholar] [CrossRef]
- He, Y.; Xie, Z.; Zhang, H.; Liebl, W.; Toyama, H.; Chen, F. Oxidative Fermentation of Acetic Acid Bacteria and Its Products. Front. Microbiol. 2022, 13, 879246. [Google Scholar] [CrossRef]
- Rahman, M.S. Handbook of Food Preservation; CRC Press: Boca Raton, FL, USA, 2020; ISBN 9780429091483. [Google Scholar]
- Drysdale, G.S.; Fleet, G.H. The Effect of Acetic Acid Bacteria upon the Growth and Metabolism of Yeasts during the Fermentation of Grape Juice. J. Appl. Bacteriol. 1989, 67, 471–481. [Google Scholar] [CrossRef]
- Adams, M.; Moss, M.O.; McClure, P. Food Microbiology, 4th ed.; The Royal Society of Chemistry: London, UK, 2015; ISBN 978-1-84973-960-3. [Google Scholar]
- Ebner, H.; Sellmer, H.F.S. Vinegar. In Biotechnology Set; Wiley: Hoboken, NJ, USA, 2001; pp. 579–591. [Google Scholar]
- De Ley, J.; Gillis, M.; Swings, J.; Family, V.I. Acetobacteriaceae, Bergey’s Manual of Systematic Bacteriology, 1st ed.; Krieg, N.R., Holt, J.G., Eds.; Williams and Wilkins Co: Baltimore, MD, USA, 1984; Volume 1. [Google Scholar]
- Drysdale, G.S.; Fleet, G.H. Acetic Acid Bacteria in Winemaking: A Review. Am. J. Enol. Vitic. 1988, 39, 143–154. [Google Scholar] [CrossRef]
- White, G.; Wang, C. The Dissimilation of Glucose and Gluconate by Acetobacter xylinum. 1. The Origin and the Fate of Triose Phosphate. Biochem. J. 1964, 90, 408–423. [Google Scholar] [CrossRef]
- Pedraza, R.O. Recent Advances in Nitrogen-Fixing Acetic Acid Bacteria. Int. J. Food Microbiol. 2008, 125, 25–35. [Google Scholar] [CrossRef]
- García-García, I.; Cañete-Rodríguez, A.M.; Santos-Dueñas, I.M.; Jiménez-Hornero, J.E.; Ehrenreich, A.; Liebl, W.; García-Martínez, T.; Mauricio, J.C. Biotechnologically Relevant Features of Gluconic Acid Production by Acetic Acid Bacteria. Acetic Acid. Bact. 2017, 6, 7–10. [Google Scholar] [CrossRef]
- Andrés-Barrao, C.; Barja, F. Acetic Acid Bacteria Strategies Contributing to Acetic Acid Resistance During Oxidative Fermentation. In Acetic Acid Bacteria; CRC Press: Boca Raton, FL, USA, 2017; pp. 92–119. [Google Scholar]
- Nakano, S.; Fukaya, M.; Horinouchi, S. Putative ABC Transporter Responsible for Acetic Acid Resistance in Acetobacter Aceti. Appl. Environ. Microbiol. 2006, 72, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Nakano, S.; Fukaya, M. Analysis of Proteins Responsive to Acetic Acid in Acetobacter: Molecular Mechanisms Conferring Acetic Acid Resistance in Acetic Acid Bacteria. Int. J. Food Microbiol. 2008, 125, 54–59. [Google Scholar] [CrossRef]
- Nakano, S.; Ebisuya, H. Physiology of Acetobacter and Komagataeibacter Spp.: Acetic Acid Resistance Mechanism in Acetic Acid Fermentation. In Acetic Acid Bacteria; Springer: Tokyo, Japan, 2016; pp. 223–234. [Google Scholar]
- Kourouma, M.C.; Mbengue, M.; Sarr, N.C.D.; Sarr, K.; Kane, C.T. Thermoresistant, Ethanol-Resistant and Acid-Resistant Properties of Acetic Acid Bacteria Isolated from Fermented Mango Alcohol. Adv. Microbiol. 2022, 12, 177–191. [Google Scholar] [CrossRef]
- Chen, Y.; Bai, Y.; Li, D.; Wang, C.; Xu, N.; Wu, S.; He, S.; Hu, Y. Correlation between Ethanol Resistance and Characteristics of PQQ-Dependent ADH in Acetic Acid Bacteria. Eur. Food Res. Technol. 2016, 242, 837–847. [Google Scholar] [CrossRef]
- Kanchanarach, W.; Theeragool, G.; Yakushi, T.; Toyama, H.; Adachi, O.; Matsushita, K. Characterization of Thermotolerant Acetobacter pasteurianus Strains and Their Quinoprotein Alcohol Dehydrogenases. Appl. Microbiol. Biotechnol. 2010, 85, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhang, H.; Wang, S.; Zhao, A.; Qu, L.; Xiong, W.; Alam, A.; Ma, W.; Lv, Y.; Xu, J. Screening a Panel of Acid-Producing Strains by Developing a High-Throughput Method. Biotechnol. Bioprocess. Eng. 2022, 27, 810–817. [Google Scholar] [CrossRef]
- Es-sbata, I.; Lakhlifi, T.; Yatim, M.; El-Abid, H.; Belhaj, A.; Hafidi, M.; Zouhair, R. Screening and Molecular Characterization of New Thermo- and Ethanol-tolerant Acetobacter malorum Strains Isolated from Two Biomes Moroccan Cactus Fruits. Biotechnol. Appl. Biochem. 2021, 68, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Tonouchi, N. Cellulose and Other Capsular Polysaccharides of Acetic Acid Bacteria. In Acetic Acid Bacteria; Springer: Tokyo, Japan, 2016; pp. 299–320. [Google Scholar]
- Shi, Z.; Zhang, Y.; Phillips, G.O.; Yang, G. Utilization of Bacterial Cellulose in Food. Food Hydrocoll. 2014, 35, 539–545. [Google Scholar] [CrossRef]
- Paximada, P.; Koutinas, A.A.; Scholten, E.; Mandala, I.G. Effect of Bacterial Cellulose Addition on Physical Properties of WPI Emulsions. Comparison with Common Thickeners. Food Hydrocoll. 2016, 54, 245–254. [Google Scholar] [CrossRef]
- Vigentini, I.; Fabrizio, V.; Dellacà, F.; Rossi, S.; Azario, I.; Mondin, C.; Benaglia, M.; Foschino, R. Set-Up of Bacterial Cellulose Production From the Genus Komagataeibacter and Its Use in a Gluten-Free Bakery Product as a Case Study. Front. Microbiol. 2019, 10, 1953. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, X.; Hao, W.; Xie, Y.; Chen, L.; Li, Z.; Zhu, B.; Feng, X. Nano-Bacterial Cellulose/Soy Protein Isolate Complex Gel as Fat Substitutes in Ice Cream Model. Carbohydr. Polym. 2018, 198, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Okiyama, A.; Motoki, M.; Yamanaka, S. Bacterial Cellulose IV. Application to Processed Foods. Food Hydrocoll. 1993, 6, 503–511. [Google Scholar] [CrossRef]
- Srikanth, R.; Reddy, C.H.S.S.S.; Siddartha, G.; Ramaiah, M.J.; Uppuluri, K.B. Review on Production, Characterization and Applications of Microbial Levan. Carbohydr. Polym. 2015, 120, 102–114. [Google Scholar] [CrossRef]
- Ua-Arak, T.; Jakob, F.; Vogel, R.F. Influence of Levan-Producing Acetic Acid Bacteria on Buckwheat-Sourdough Breads. Food Microbiol. 2017, 65, 95–104. [Google Scholar] [CrossRef]
- Han, Y.W. Microbial Levan. Adv. Appl. Microbiol. 1990, 35, 171–194. [Google Scholar] [PubMed]
- Öner, E.T.; Hernández, L.; Combie, J. Review of Levan Polysaccharide: From a Century of Past Experiences to Future Prospects. Biotechnol. Adv. 2016, 34, 827–844. [Google Scholar] [CrossRef] [PubMed]
- Acetic Acid Bacteria; Sengun, I.Y., Ed.; CRC Press: Boca Raton, FL, USA, 2017; ISBN 9781315153490. [Google Scholar]
- Srikanth, R.; Siddartha, G.; Sundhar Reddy, C.H.S.S.; Harish, B.S.; Janaki Ramaiah, M.; Uppuluri, K.B. Antioxidant and Anti-Inflammatory Levan Produced from Acetobacter xylinum NCIM2526 and Its Statistical Optimization. Carbohydr. Polym. 2015, 123, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Mantovan, J.; Bersaneti, G.T.; Faria-Tischer, P.C.S.; Celligoi, M.A.P.C.; Mali, S. Use of Microbial Levan in Edible Films Based on Cassava Starch. Food Packag. Shelf Life 2018, 18, 31–36. [Google Scholar] [CrossRef]
- Naessens, M.; Cerdobbel, A.; Soetaert, W.; Vandamme, E.J. Dextran Dextrinase and Dextran of Gluconobacter oxydans. J. Ind. Microbiol. Biotechnol. 2005, 32, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Amaretti, A.; Bottari, B.; Morreale, F.; Savo Sardaro, M.L.; Angelino, D.; Raimondi, S.; Rossi, M.; Pellegrini, N. Potential Prebiotic Effect of a Long-Chain Dextran Produced by Weissella cibaria: An in vitro Evaluation. Int. J. Food Sci. Nutr. 2020, 71, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Roca, C.; Alves, V.D.; Freitas, F.; Reis, M.A.M. Exopolysaccharides Enriched in Rare Sugars: Bacterial Sources, Production, and Applications. Front. Microbiol. 2015, 6, 288. [Google Scholar] [CrossRef] [PubMed]
- Czaja, W.K.; Young, D.J.; Kawecki, M.; Brown, R.M. The Future Prospects of Microbial Cellulose in Biomedical Applications. Biomacromolecules 2007, 8, 1–12. [Google Scholar] [CrossRef]
- Islam, S.U.; Ul-Islam, M.; Ahsan, H.; Ahmed, M.B.; Shehzad, A.; Fatima, A.; Sonn, J.K.; Lee, Y.S. Potential Applications of Bacterial Cellulose and Its Composites for Cancer Treatment. Int J Biol Macromol 2021, 168, 301–309. [Google Scholar] [CrossRef]
- Angelin, J.; Kavitha, M. Exopolysaccharides from Probiotic Bacteria and Their Health Potential. Int. J. Biol. Macromol. 2020, 162, 853–865. [Google Scholar] [CrossRef]
- Netrusov, A.I.; Liyaskina, E.V.; Kurgaeva, I.V.; Liyaskina, A.U.; Yang, G.; Revin, V.V. Exopolysaccharides Producing Bacteria: A Review. Microorganisms 2023, 11, 1541. [Google Scholar] [CrossRef] [PubMed]
- Costa, O.Y.A.; Raaijmakers, J.M.; Kuramae, E.E. Microbial Extracellular Polymeric Substances: Ecological Function and Impact on Soil Aggregation. Front. Microbiol. 2018, 9, 1636. [Google Scholar] [CrossRef]
- Kaur, N.; Dey, P. Bacterial Exopolysaccharides as Emerging Bioactive Macromolecules: From Fundamentals to Applications. Res. Microbiol. 2023, 174, 104024. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.M. Porto: Um Vinho Com História. Available online: https://www.ivdp.pt/pt/vinhos/vinhos-do-porto/historia/ (accessed on 1 February 2024).
- Milheiro, J.; Cosme, F.; Filipe-Ribeiro, L.; Nunes, F.M. Port Wine: Production and Ageing. In Chemistry and Biochemistry of Winemaking, Wine Stabilization and Aging; IntechOpen: Rijeka, Croatia, 2021. [Google Scholar]
- Jackson, R.S. Specific and Distinctive Wine Styles. Wine Sci. 2008, 520–576. [Google Scholar] [CrossRef]
- Instituto dos Vinhos do Douro e do Porto Processo de Vinificação Ao Longo Dos Tempos. Available online: https://www.ivdp.pt/pt/vinhos/vinhos-do-porto/enologia/ (accessed on 1 February 2024).
- TAYLOR’S Como é Feito o Vinho Do Porto? Available online: https://www.taylor.pt/pt/o-que-e-o-vinho-do-porto/como-e-feito-o-vinho-do-porto (accessed on 1 February 2024).
- Ferreira, V.; Escudero, A.; Fernández, P.; Cacho, J.F. Changes in the Profile of Volatile Compounds in Wines Stored under Oxygen and Their Relationship with the Browning Process. Z. Für Leb. Und -Forsch. A 1997, 205, 392–396. [Google Scholar] [CrossRef]
- De Pinho, P.G.; Falqué, E.; Castro, M.; E Silva, H.O.; Machado, B.; Ferreira, A.C.S. Further Insights into the Floral Character of Touriga Nacional Wines. J. Food Sci. 2007, 72, S396–S401. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, W.; Morales, M.L.; García-Parrilla, M.C.; Troncoso, A.M. Wine Vinegar: Technology, Authenticity and Quality Evaluation. Trends Food Sci. Technol. 2002, 13, 12–21. [Google Scholar] [CrossRef]
- Sellmer-Wilsberg, S. Wine and Grape Vinegars. In Vinegars of the World; Springer: Milano, Italy, 2009; pp. 145–156. [Google Scholar]
- Solieri, L.; Giudici, P. Vinegars of the World; Springer: Milano, Italy, 2009; ISBN 978-88-470-0865-6. [Google Scholar]
- European Commission Regulation (EU) 2016/263; European Parliament: Brussels, Belgium, 2016.
- Decreto-Lei n.o 174/2007 de 8 de Maio Do Ministério Da Agricultura, Do Desenvolvimento Rural e Das Pescas; Diário da República, 1.a série: Lisboa, Portugal, 2007; pp. 1–3.
- García-García, I.; Santos-Dueñas, I.M.; Jiménez-Ot, C.; Jiménez-Hornero, J.E.; Bonilla-Venceslada, J.L. Vinegar Engineering. In Vinegars of the World; Springer: Milano, Italy, 2009; pp. 97–120. [Google Scholar]
- Budak, N.H.; Aykin, E.; Seydim, A.C.; Greene, A.K.; Guzel-Seydim, Z.B. Functional Properties of Vinegar. J. Food Sci. 2014, 79, R757–R764. [Google Scholar] [CrossRef]
- Chen, H.; Chen, T.; Giudici, P.; Chen, F. Vinegar Functions on Health: Constituents, Sources, and Formation Mechanisms. Compr. Rev. Food Sci. Food Saf. 2016, 15, 1124–1138. [Google Scholar] [CrossRef]
- Qiu, J.; Ren, C.; Fan, J.; Li, Z. Antioxidant Activities of Aged Oat Vinegar in Vitro and in Mouse Serum and Liver. J. Sci. Food Agric. 2010, 90, 1951–1958. [Google Scholar] [CrossRef]
- Nanda, K.; Miyoshi, N.; Nakamura, Y.; Shimoji, Y.; Tamura, Y.; Nishikawa, Y.; Uenakai, K.; Kohno, H.; Tanaka, T. Extract of Vinegar “Kurosu” from Unpolished Rice Inhibits the Proliferation of Human Cancer Cells. J. Exp. Clin. Cancer Res. 2004, 23, 69–75. [Google Scholar]
- María Luzón-Quintana, L.; Castro, R.; Durán-Guerrero, E. Biotechnological processes in fruit vinegar production. Foods 2021, 10, 945. [Google Scholar] [CrossRef]
- Singh, A.K. Overview of Vinegar Production. Palarch’s J. Archaeol. Egypt/Egyptol. 2020, 17, 4027–4037. [Google Scholar]
- Ubeda, C.; Callejón, R.M.; Hidalgo, C.; Torija, M.J.; Mas, A.; Troncoso, A.M.; Morales, M.L. Determination of Major Volatile Compounds during the Production of Fruit Vinegars by Static Headspace Gas Chromatography–Mass Spectrometry Method. Food Res. Int. 2011, 44, 259–268. [Google Scholar] [CrossRef]
- Dabija, A.; Hatnean, C.A. Study Concerning the Quality of Apple Vinegar Obtained through Classical Method. J. Agroaliment. Process. Technol. 2014, 20, 304–310. [Google Scholar]
- Gullo, M.; Verzelloni, E.; Canonico, M. Aerobic Submerged Fermentation by Acetic Acid Bacteria for Vinegar Production: Process and Biotechnological Aspects. Process Biochem. 2014, 49, 1571–1579. [Google Scholar] [CrossRef]
- Vidra, A.; Németh, Á. Bio-Produced Acetic Acid: A Review. Period. Polytech. Chem. Eng. 2017, 62, 245–256. [Google Scholar] [CrossRef]
- Morales, L.; González, G.; Casas, J.; Troncoso, A. Multivariate Analysis of Commercial and Laboratory Produced Sherry Wine Vinegars: Influence of Acetification and Aging. Eur. Food Res. Technol. 2001, 212, 676–682. [Google Scholar] [CrossRef]
- Andreou, V.; Giannoglou, M.; Xanthou, M.Z.; Metafa, M.; Katsaros, G. Aging Acceleration of Balsamic Vinegar Applying Micro-Oxygenation Technique. Food Chem. 2023, 419, 136077. [Google Scholar] [CrossRef]
- Nie, J.; Li, Y.; Xing, J.; Chao, J.; Qin, X.; Li, Z. Comparison of Two Types of Vinegar with Different Aging Times by NMR-based Metabolomic Approach. J. Food Biochem. 2019, 43, e12835. [Google Scholar] [CrossRef]
- Li, H.; Ming, X.; Liu, Z.; Xu, L.; Xu, D.; Hu, L.; Mo, H.; Zhou, X. Accelerating Vinegar Aging by Combination of Ultrasonic and Magnetic Field Assistance. Ultrason. Sonochem. 2021, 78, 105708. [Google Scholar] [CrossRef] [PubMed]
- Tonello, M.J. Acetification of Porto Wine. A Preliminary Step for Vinegar Making. Ph.D. Thesis, University of Porto, Porto, Portugal, 2015. [Google Scholar]
- Durán-Guerrero, E.; Castro, R. Novel Analysis on Aroma Compounds of Wine, Vinegar and Derived Products. Foods 2021, 10, 1245. [Google Scholar] [CrossRef] [PubMed]
- Callejón, R.M.; Torija, M.J.; Mas, A.; Morales, M.L.; Troncoso, A.M. Changes of Volatile Compounds in Wine Vinegars during Their Elaboration in Barrels Made from Different Woods. Food Chem. 2010, 120, 561–571. [Google Scholar] [CrossRef]
- Callejón, R.M.; Tesfaye, W.; Torija, M.J.; Mas, A.; Troncoso, A.M.; Morales, M.L. Volatile Compounds in Red Wine Vinegars Obtained by Submerged and Surface Acetification in Different Woods. Food Chem. 2009, 113, 1252–1259. [Google Scholar] [CrossRef]
- Callejón, R.M.; Morales, M.L.; Ferreira, A.C.S.; Troncoso, A.M. Defining the Typical Aroma of Sherry Vinegar: Sensory and Chemical Approach. J. Agric. Food Chem. 2008, 56, 8086–8095. [Google Scholar] [CrossRef] [PubMed]
- García-Parrilla, M.C.; González, G.A.; Heredia, F.J.; Troncoso, A.M. Differentiation of Wine Vinegars Based on Phenolic Composition. J. Agric. Food Chem. 1997, 45, 3487–3492. [Google Scholar] [CrossRef]
- Andlauer, W.; Stumpf, C.; Fürst, P. Influence of the Acetification Process on Phenolic Compounds. J. Agric. Food Chem. 2000, 48, 3533–3536. [Google Scholar] [CrossRef] [PubMed]
- Budak, H.N.; Guzel-Seydim, Z.B. Antioxidant Activity and Phenolic Content of Wine Vinegars Produced by Two Different Techniques. J. Sci. Food Agric. 2010, 90, 2021–2026. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Tang, G.Y.; Zhao, C.N.; Gan, R.Y.; Li, H. Bin Antioxidant Activities, Phenolic Profiles, and Organic Acid Contents of Fruit Vinegars. Antioxidants 2019, 8, 78. [Google Scholar] [CrossRef]
- Tesfaye, W.; Morales, M.L.; Garcia-Parrilla, M.C.; Troncoso, A.M. Improvement of Wine Vinegar Elaboration and Quality Analysis: Instrumental and Human Sensory Evaluation. Food Rev. Int. 2009, 25, 142–156. [Google Scholar] [CrossRef]
- Kašpar, M.; Bajer, T.; Bajerová, P.; Česla, P. Comparison of Phenolic Profile of Balsamic Vinegars Determined Using Liquid and Gas Chromatography Coupled with Mass Spectrometry. Molecules 2022, 27, 1356. [Google Scholar] [CrossRef] [PubMed]
- Sam, F.E.; Ma, T.; Liang, Y.; Qiang, W.; Atuna, R.A.; Amagloh, F.K.; Morata, A.; Han, S. Comparison between Membrane and Thermal Dealcoholization Methods: Their Impact on the Chemical Parameters, Volatile Composition, and Sensory Characteristics of Wines. Membranes 2021, 11, 957. [Google Scholar] [CrossRef] [PubMed]
- Sam, F.E.; Ma, T.-Z.; Salifu, R.; Wang, J.; Jiang, Y.-M.; Zhang, B.; Han, S.-Y. Techniques for Dealcoholization of Wines: Their Impact on Wine Phenolic Composition, Volatile Composition, and Sensory Characteristics. Foods 2021, 10, 2498. [Google Scholar] [CrossRef] [PubMed]
- Pilipovik, M.V.; Riverol, C. Assessing Dealcoholization Systems Based on Reverse Osmosis. J. Food Eng. 2005, 69, 437–441. [Google Scholar] [CrossRef]
- Massot, A.; Mietton-Peuchot, M.; Peuchot, C.; Milisic, V. Nanofiltration and Reverse Osmosis in Winemaking. Desalination 2008, 231, 283–289. [Google Scholar] [CrossRef]
- Loyola García, R.; Gutiérrez-Gamboa, G.; Medel-Marabolí, M.; Díaz-Gálvez, I. Lowering Wine Alcohol Content by Reverse Osmosis and Spinning Cone Columns: Effects on Sensory Characteristics of the Beverages. IVES Tech. Rev. Vine Wine 2021. [Google Scholar] [CrossRef]
- Gil, M.; Estévez, S.; Kontoudakis, N.; Fort, F.; Canals, J.M.; Zamora, F. Influence of Partial Dealcoholization by Reverse Osmosis on Red Wine Composition and Sensory Characteristics. Eur. Food Res. Technol. 2013, 237, 481–488. [Google Scholar] [CrossRef]
- Belisario-Sánchez, Y.Y.; Taboada-Rodríguez, A.; Marín-Iniesta, F.; Iguaz-Gainza, A.; López-Gómez, A. Aroma Recovery in Wine Dealcoholization by SCC Distillation. Food Bioproc. Tech. 2012, 5, 2529–2539. [Google Scholar] [CrossRef]
- Petrozziello, M.; Panero, L.; Guaita, M.; Prati, R.; Marani, G.; Zinzani, G.; Bosso, A. Effect of the Extent of Ethanol Removal on the Volatile Compounds of a Chardonnay Wine Dealcoholized by Vacuum Distillation. BIO Web Conf. 2019, 12, 02020. [Google Scholar] [CrossRef]
- Motta, S.; Guaita, M.; Petrozziello, M.; Ciambotti, A.; Panero, L.; Solomita, M.; Bosso, A. Comparison of the Physicochemical and Volatile Composition of Wine Fractions Obtained by Two Different Dealcoholization Techniques. Food Chem. 2017, 221, 1–10. [Google Scholar] [CrossRef]
- Röcker, J.; Schmitt, M.; Pasch, L.; Ebert, K.; Grossmann, M. The Use of Glucose Oxidase and Catalase for the Enzymatic Reduction of the Potential Ethanol Content in Wine. Food Chem. 2016, 210, 660–670. [Google Scholar] [CrossRef] [PubMed]
- Scutaraşu, E.C.; Cotea, V.V.; Luchian, C.E.; Colibaba, L.C.; Katalin, N.; Oprean, R.; Niculaua, M. Influence of Enzymatic Treatments on White Wine Composition. BIO Web Conf. 2019, 15, 02032. [Google Scholar] [CrossRef]
- Gonzalez, R.; Guindal, A.M.; Tronchoni, J.; Morales, P. Biotechnological Approaches to Lowering the Ethanol Yield during Wine Fermentation. Biomolecules 2021, 11, 1569. [Google Scholar] [CrossRef] [PubMed]
- Marques, C.; Correia, E.; Dinis, L.T.; Vilela, A. An Overview of Sensory Characterization Techniques: From Classical Descriptive Analysis to the Emergence of Novel Profiling Methods. Foods 2022, 11, 255. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.S.Q.; Dias, L.G.; Teixeira, A. Emerging Methods for the Evaluation of Sensory Quality of Food: Technology at Service. Curr. Food Sci. Technol. Rep. 2024, 2, 77–90. [Google Scholar] [CrossRef]
- Barbe, J.-C.; Garbay, J.; Tempère, S. The Sensory Space of Wines: From Concept to Evaluation and Description. A review. Foods 2021, 10, 1424. [Google Scholar] [CrossRef] [PubMed]
- Torrico, D.D.; Mehta, A.; Borssato, A.B. New Methods to Assess Sensory Responses: A Brief Review of Innovative Techniques in Sensory Evaluation. Curr. Opin. Food Sci. 2023, 49, 100978. [Google Scholar] [CrossRef]
- Ríos-Reina, R.; Azcarate, S.M.; Camiña, J.M.; Callejón, R.M. Sensory and Spectroscopic Characterization of Argentinean Wine and Balsamic Vinegars: A Comparative Study with European Vinegars. Food Chem. 2020, 323, 126791. [Google Scholar] [CrossRef] [PubMed]
- G. Pereira, A.; Fraga, M.; Garcia-Oliveira, P.; Carpena, M.; Jimenez-Lopez, C.; Lourenço-Lopes, C.; Barros, L.; C.F.R. Ferreira, I.; Angel Prieto, M.; Simal-Gandara, J. Management of Wine Aroma Compounds: Principal Basis and Future Perspectives. In Chemistry and Biochemistry of Winemaking, Wine Stabilization and Aging; IntechOpen: Rijeka, Croatia, 2021. [Google Scholar]
- Jeromel, A.; Korenika, A.-M.J.; Tomaz, I. An Influence of Different Yeast Species on Wine Aroma Composition. In Fermented Beverages; Elsevier: Amsterdam, The Netherlands, 2019; pp. 171–285. [Google Scholar]
- Carpena, M.; Fraga-Corral, M.; Otero, P.; Nogueira, R.A.; Garcia-Oliveira, P.; Prieto, M.A.; Simal-Gandara, J. Secondary Aroma: Influence of Wine Microorganisms in Their Aroma Profile. Foods 2020, 10, 51. [Google Scholar] [CrossRef]
- Silva, A.P.; Rebelo, J. Port Wine, an Established Product a New Market: A Comparative Analysis of Perceptions of Firms and Consumers. Wine Econ. Policy 2019, 8, 59–68. [Google Scholar] [CrossRef]
- Vilela, A.; Ferreira, R.; Nunes, F.; Correia, E. Creation and Acceptability of a Fragrance with a Characteristic Tawny Port Wine-Like Aroma. Foods 2020, 9, 1244. [Google Scholar] [CrossRef] [PubMed]
- Gallo Worldwide Vinho de Porto—Vinagre. Available online: https://www.galloportugal.com/vinagres-pt-pt/vinho-do-porto/ (accessed on 7 February 2024).
- Torri, L.; Jeon, S.-Y.; Piochi, M.; Morini, G.; Kim, K.-O. Consumer Perception of Balsamic Vinegar: A Cross-Cultural Study between Korea and Italy. Food Res. Int. 2017, 91, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Ker, J.-K.; Lee, C.-S.; Chen, Y.-C.; Chiang, M.-C. Exploring Taiwanese Consumer Dietary Preferences for Various Vinegar Condiments: Novel Dietary Patterns across Diverse Cultural Contexts. Nutrients 2023, 15, 3845. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, E.; Oliveras, I.; Romero del Castillo, M.R.; Salazar, A. Influence of the Sociocultural Perspective on the Sensory Perception of Wine Consumers in Mexico and Spain. Front. Psychol. 2023, 14, 1171289. [Google Scholar] [CrossRef] [PubMed]
- Fondberg, R.; Lundström, J.N.; Seubert, J. Odor–Taste Interactions in Food Perception: Exposure Protocol Shows No Effects of Associative Learning. Chem. Senses 2021, 46, bjab003. [Google Scholar] [CrossRef]
- Spill, M.K.; Johns, K.; Callahan, E.H.; Shapiro, M.J.; Wong, Y.P.; Benjamin-Neelon, S.E.; Birch, L.; Black, M.M.; Cook, J.T.; Faith, M.S.; et al. Repeated Exposure to Food and Food Acceptability in Infants and Toddlers: A Systematic Review. Am. J. Clin. Nutr. 2019, 109, 978S–989S. [Google Scholar] [CrossRef] [PubMed]
- Appleton, K.M.; Hemingway, A.; Rajska, J.; Hartwell, H. Repeated Exposure and Conditioning Strategies for Increasing Vegetable Liking and Intake: Systematic Review and Meta-Analyses of the Published Literature. Am. J. Clin. Nutr. 2018, 108, 842–856. [Google Scholar] [CrossRef]
- Schicker, D.; Rramani, Q.; Lim, S.X.L.; Saruco, E.; Pleger, B.; Weber, B.; Schultz, J.; Freiherr, J.; Ohla, K. Taste It! 7-Day Exposure to a Protein-Enriched Milk Drink Increases Its Smell, Taste, and Flavor Familiarity and Facilitates Acquisition of Taste Familiarity of a Novel Protein Drink. Food Qual. Prefer. 2023, 106, 104808. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).