Enhancing Biohydrogen Production: The Role of Iron-Based Nanoparticles in Continuous Lactate-Driven Dark Fermentation of Powdered Cheese Whey
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrate and Inoculum
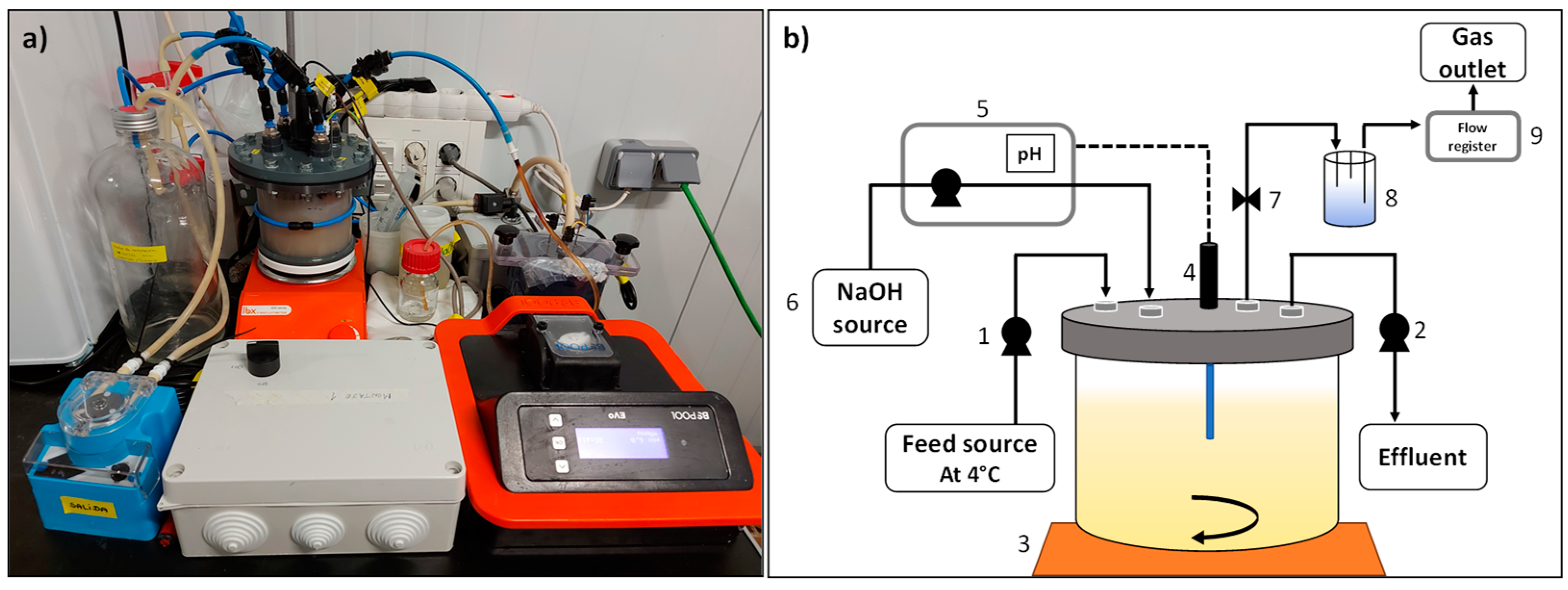
2.2. Experimental Setup and Operational Conditions
2.2.1. Batch Configuration
2.2.2. Continuous Operation
2.3. Analytical Methods
2.4. Data Analysis
2.5. Microbial Community Composition
3. Results and Discussion
3.1. Batch Configuration
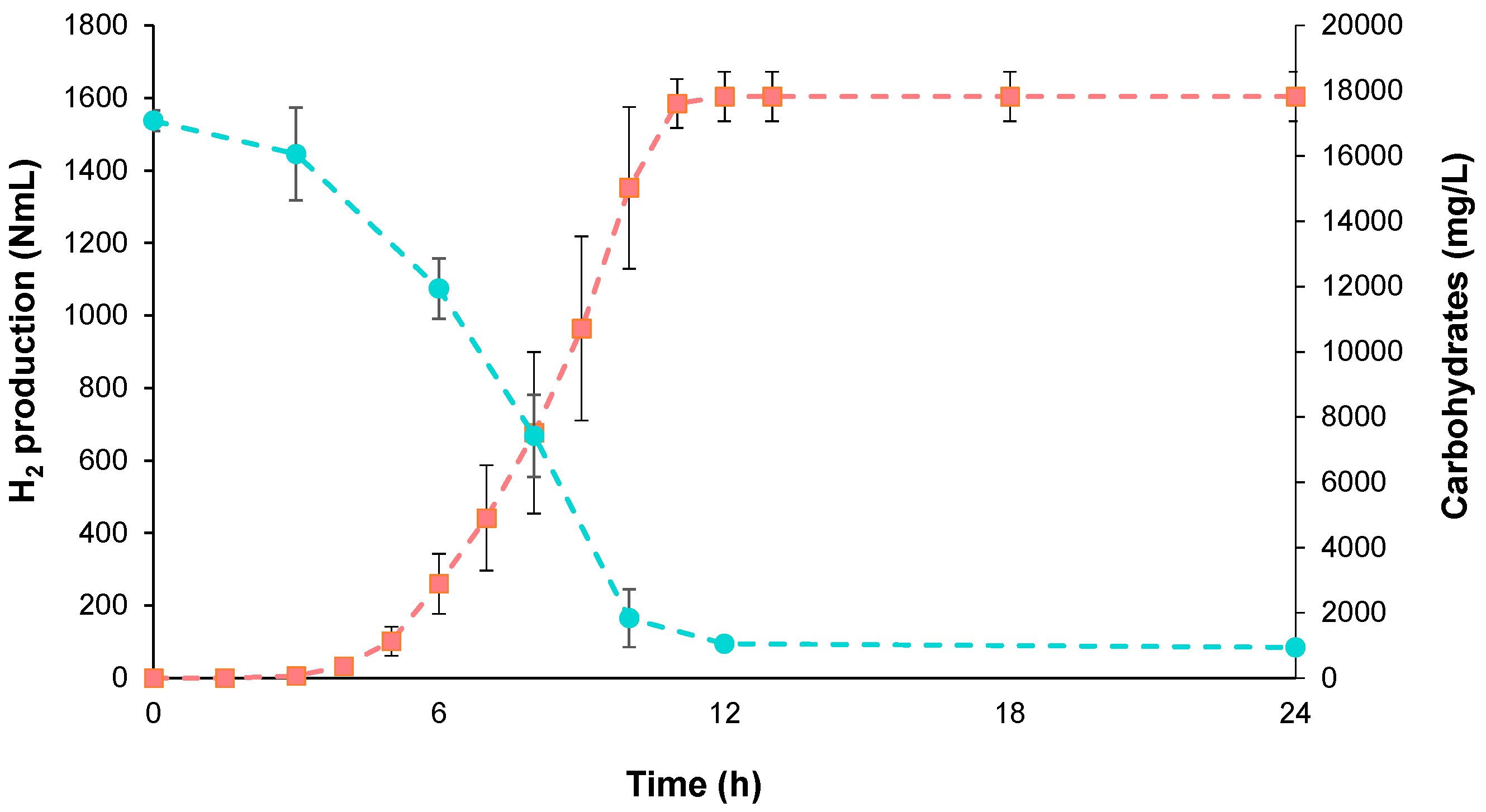
3.1.1. BioH2 Production and Substrate Consumption Efficiency
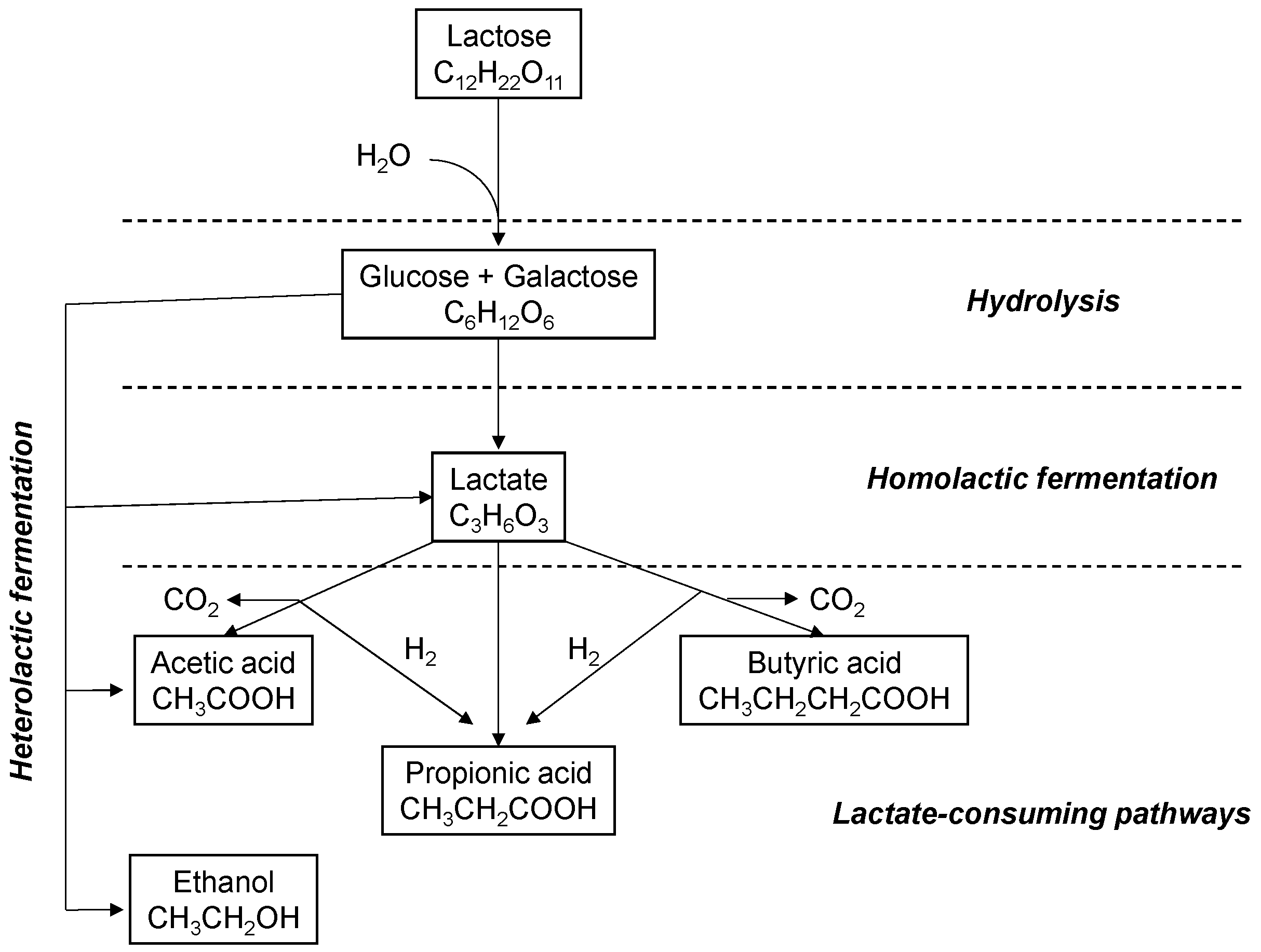
3.1.2. Production of Metabolites
3.2. Continuous Operation
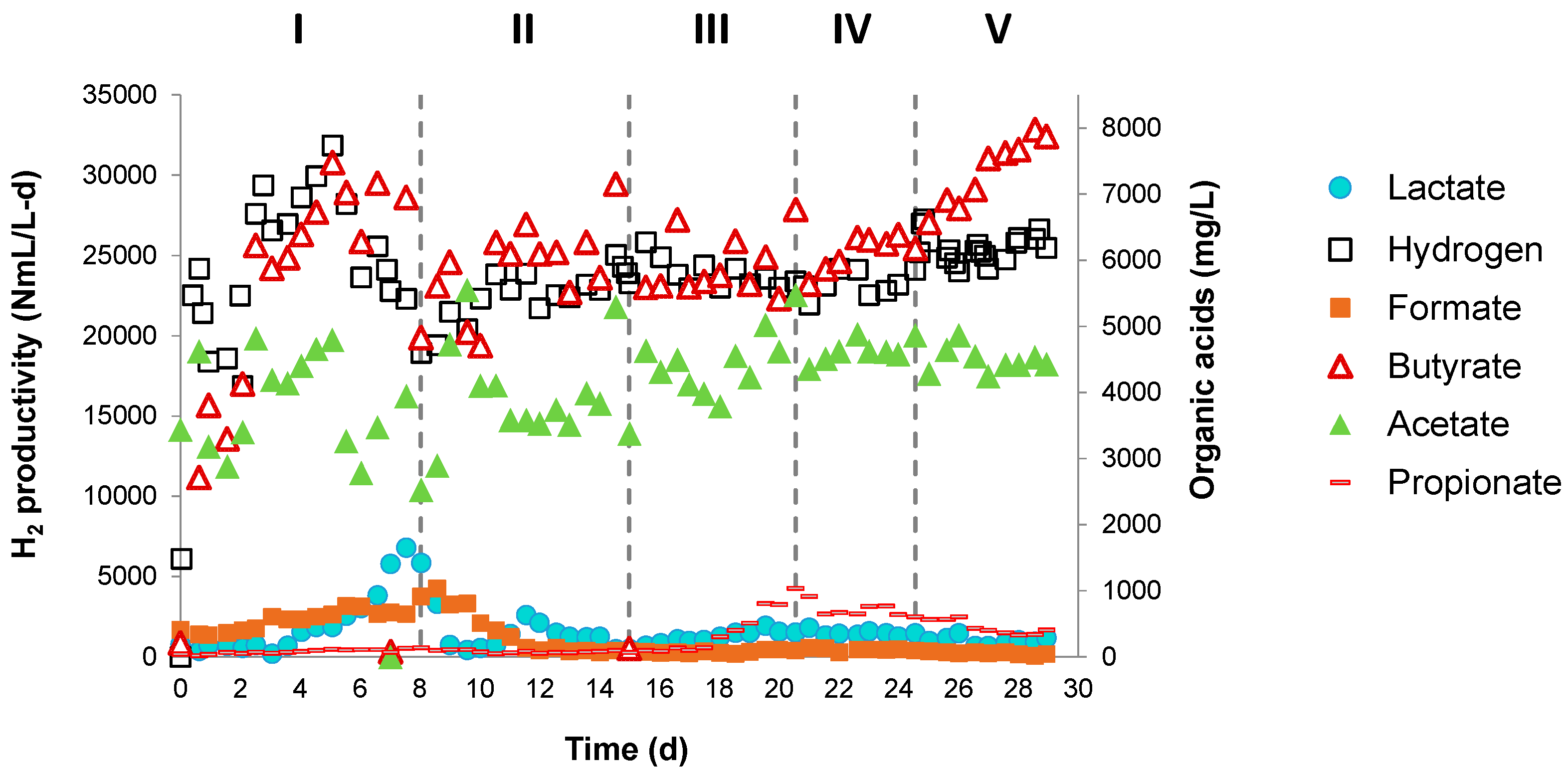
3.2.1. H2 Production and System Stability
3.2.2. Substrate Removal and Biomass Growth
3.2.3. Evolution of Intermediate Metabolites
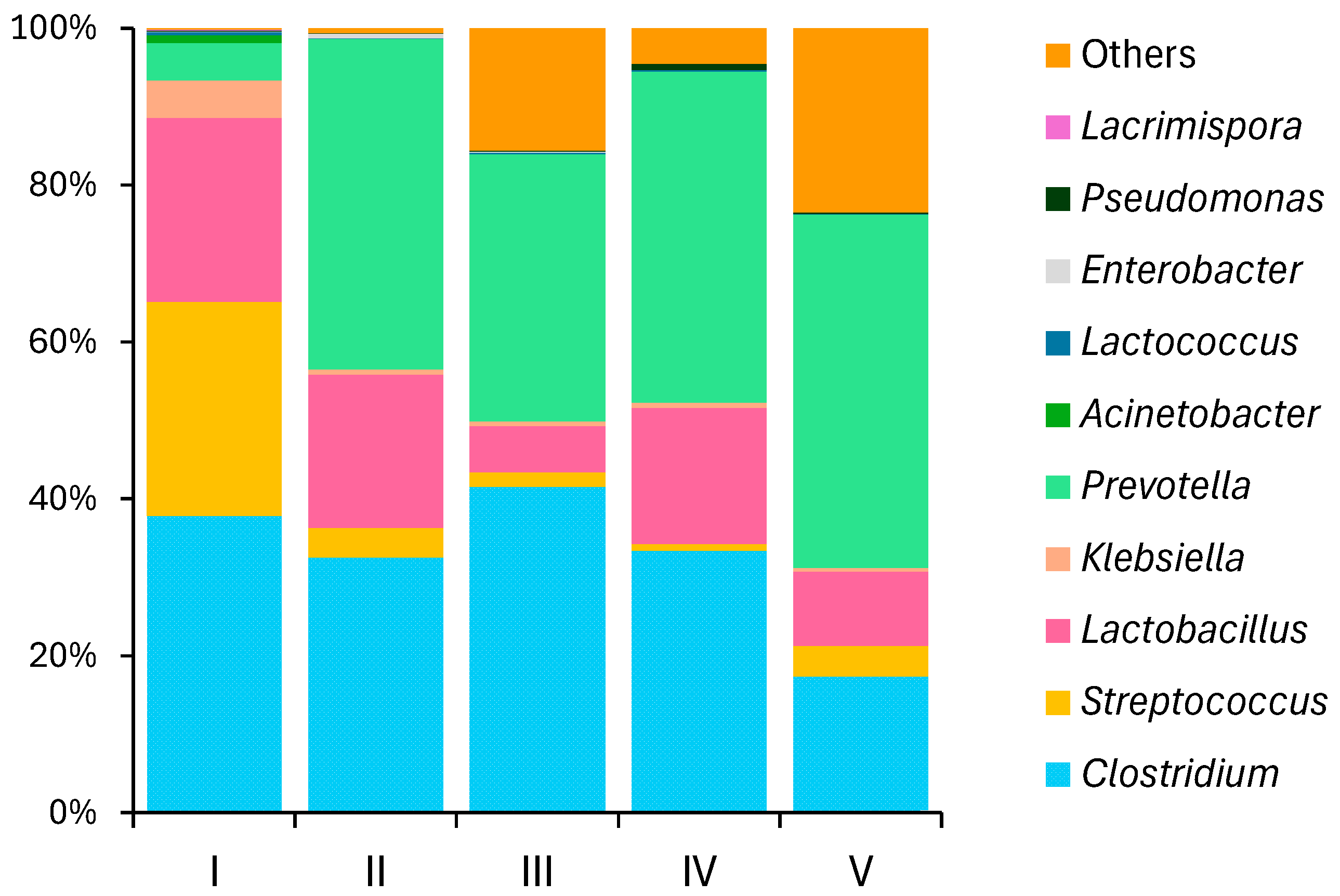
3.2.4. Microbial Community Structure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Litti, Y.V.; Potekhina, M.A.; Zhuravleva, E.A.; Vishnyakova, A.V.; Gruzdev, D.S.; Kovalev, A.A.; Kovalev, D.A.; Katraeva, I.V.; Parshina, S.N. Dark fermentative hydrogen production from simple sugars and various wastewaters by a newly isolated Thermoanaerobacterium thermosaccharolyticum SP-H2. Int. J. Hydrogen Energy 2022, 47, 24310–24327. [Google Scholar] [CrossRef]
- Ahmad Sobri, M.Z.; Redhwan, A.; Ameen, F.; Lim, J.W.; Liew, C.S.; Mong, G.R.; Daud, H.; Sokkalingam, R.; Ho, C.-D.; Usman, A.; et al. A review unveiling various machine learning algorithms adopted for biohydrogen productions from microalgae. Fermentation 2023, 9, 243. [Google Scholar] [CrossRef]
- Cheng, D.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Deng, L.; Chen, Z.; Ye, Y.; Bui, X.T.; Hoang, N.B. Advanced strategies for enhancing dark fermentative biohydrogen production from Biowaste towards sustainable environment. Bioresour. Technol. 2022, 351, 127045. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yin, Y. Clostridium species for fermentative hydrogen production: An overview. Int. J. Hydrogen Energy 2021, 46, 34599–34625. [Google Scholar] [CrossRef]
- Ribeiro, J.C.; Mota, V.T.; de Oliveira, V.M.; Zaiat, M. Hydrogen and organic acid production from dark fermentation of cheese whey without buffers under mesophilic condition. J. Environ. Manag. 2022, 304, 114253. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Mohan, S.V. Simultaneous production of green hydrogen and bioethanol from segregated sugarcane bagasse hydrolysate streams with circular biorefinery design. J. Chem. Eng. 2021, 425, 130386. [Google Scholar] [CrossRef]
- Hiloidhari, M.; Bhuyan, N.; Gogoi, N.; Seth, D.; Garg, A.; Singh, A.; Prasad, S.; Kataki, R. Agroindustry wastes: Biofuels and biomaterials feedstocks for Sustainable Rural Development. In Refining Biomass Residues for Sustainable Energy and Bioproducts; Academic Press: Cambridge, MA, USA, 2020; pp. 357–388. [Google Scholar]
- Pattanaik, L.; Pattnaik, F.; Saxena, D.K.; Naik, S.N. Biofuels from agricultural wastes. In Second and Third Generation of Feedstocks; Elsevier: Amsterdam, The Netherlands, 2019; pp. 103–142. [Google Scholar]
- Aranda-Jaramillo, B.; León-Becerril, E.; Aguilar-Juárez, O.; Castro-Muñoz, R.; García-Depraect, O. Feasibility study of biohydrogen production from acid cheese whey via lactate-driven dark fermentation. Fermentation 2023, 9, 644. [Google Scholar] [CrossRef]
- Lopes, A.C.A.; Eda, S.H.; Andrade, R.P.; Amorim, J.C.; Duarte, W.F. New alcoholic fermented beverages—Potentials and challenges. Fermented Beverages 2019, 5, 577–603. [Google Scholar]
- Prazeres, A.R.; Carvalho, F.; Rivas, J. Cheese whey management: A review. J. Environ. Manag. 2012, 110, 48–68. [Google Scholar] [CrossRef]
- Fernández-Gutiérrez, D.; Veillette, M.; Giroir-Fendler, A.; Ramirez, A.A.; Faucheux, N.; Heitz, M. Biovalorization of saccharides derived from industrial wastes such as whey: A review. Rev. Environ. Sci. Biotechnol. 2017, 16, 147–174. [Google Scholar] [CrossRef]
- García-Depraect, O.; Muñoz, R.; Rodríguez, E.; Rene, E.R.; Leon-Becerril, E. Microbial ecology of a lactate-driven dark fermentation process producing hydrogen under carbohydrate-limiting conditions. Int. J. Hydrogen Energy 2021, 46, 11284–11296. [Google Scholar] [CrossRef]
- Cabrol, L.; Marone, A.; Tapia-Venegas, E.; Steyer, J.P.; Ruiz-Filippi, G.; Trably, E. Microbial ecology of fermentative hydrogen producing bioprocesses: Useful insights for driving the ecosystem function. FEMS Microbiol. Rev. 2017, 41, 158–181. [Google Scholar] [CrossRef] [PubMed]
- Sikora, A.; Błaszczyk, M.; Jurkowski, M.; Zielenkiewicz, U. Lactic acid bacteria in hydrogen-producing consortia: On purpose or by coincidence? In Lactic Acid Bacteria—R & D for Food, Health and Livestock Purposes; InTechOpen: London, UK, 2013; pp. 488–514. [Google Scholar]
- García-Depraect, O.; Valdez-Vázquez, I.; Rene, E.R.; Gómez-Romero, J.; López-López, A.; León-Becerril, E. Lactate-and acetate-based biohydrogen production through dark co-fermentation of tequila vinasse and nixtamalization wastewater: Metabolic and microbial community dynamics. Bioresour. Technol. 2019, 282, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Arimi, M.M.; Knodel, J.; Kiprop, A.; Namango, S.S.; Zhang, Y.; Geißen, S.U. Strategies for improvement of biohydrogen production from organic-rich wastewater: A review. Biomass Bioenergy 2015, 75, 101–118. [Google Scholar] [CrossRef]
- Ji, J.; Cai, Z.; Shen, L. Comparison analysis on biohydrogen production of mesophilic and thermophilic dark fermentation using nickel doped lanthanum-iron oxide nanoparticles as supplementation. Int. J. Hydrogen Energy 2024, 49, 90–106. [Google Scholar] [CrossRef]
- Yildirim, O.; Ozkaya, B. Effect of nanoparticles synthesized from green extracts on dark fermentative biohydrogen production. Biomass Bioenergy 2023, 170, 106707. [Google Scholar] [CrossRef]
- Sun, H.; Shen, J.; Hu, M.; Zhang, J.; Cai, Z.; Zang, L.; Zhang, F.; Ji, D. Manganese ferrite nanoparticles enhanced biohydrogen production from mesophilic and thermophilic dark fermentation. Energy Rep. 2021, 7, 6234–6245. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, W.; Yang, J.; Li, Z.; Zhang, J.; Zang, L. Comparison of mesophilic and thermophilic dark fermentation with nickel ferrite nanoparticles supplementation for biohydrogen production. Bioresour. Technol. 2021, 329, 124853. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Mathimani, T.; Rene, E.R.; Pugazhendhi, A. Application of nanotechnology in dark fermentation for enhanced biohydrogen production using inorganic nanoparticles. Int. J. Hydrogen Energy 2019, 44, 13106–13113. [Google Scholar] [CrossRef]
- Mullai, P.; Yogeswari, M.K.; Sridevi, K.J.B.T. Optimisation and enhancement of biohydrogen production using nickel nanoparticles—A novel approach. Bioresour. Technol. 2013, 141, 212–219. [Google Scholar] [CrossRef]
- Rafrafi, Y.; Trably, E.; Hamelin, J.; Latrille, E.; Meynial-Salles, I.; Benomar, S.; Giudici-Orticoni, M.-T.; Steyer, J.-P. Sub-dominant bacteria as Keystone Species in microbial communities producing bio-hydrogen. Int. J. Hydrogen Energy 2023, 38, 4975–4985. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association (APHA): Washington, DC, USA, 2005; Volume 21. [Google Scholar]
- García-Depraect, O.; León-Becerril, E. Fermentative biohydrogen production from tequila vinasse via the lactate-acetate pathway: Operational performance, kinetic analysis and microbial ecology. Fuel 2018, 234, 151–160. [Google Scholar] [CrossRef]
- García-Depraect, O.; Martínez-Mendoza, L.J.; Diaz, I.; Muñoz, R. Two-stage anaerobic digestion of food waste: Enhanced bioenergy production rate by steering lactate-type fermentation during hydrolysis-acidogenesis. Bioresour. Technol. 2022, 358, 127358. [Google Scholar] [CrossRef]
- García-Depraect, O.; Muñoz, R.; van Lier, J.B.; Rene, E.R.; Diaz-Cruces, V.F.; León-Becerril, E. Three-stage process for tequila vinasse valorization through sequential lactate, biohydrogen and methane production. Bioresour. Technol. 2020, 307, 123160. [Google Scholar] [CrossRef]
- García-Depraect, O.; Mirzazada, I.; Martínez-Mendoza, L.J.; Regueira-Marcos, L.; Munoz, R. Biotic and abiotic insights into the storage of food waste and its effect on biohydrogen and methane production potential. J. Water Process Eng. 2023, 53, 103840. [Google Scholar] [CrossRef]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 4, e1. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s Q2-feature-classifier plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The Silva ribosomal RNA gene database project: Improved Data Processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef]
- Weiss, S.; Xu, Z.Z.; Peddada, S.; Amir, A.; Bittinger, K.; Gonzalez, A.; Lozupone, C.; Zaneveld, J.R.; Vázquez-Baeza, Y.; Birmingham, A.; et al. Normalization and microbial differential abundance strategies depend upon data characteristics. Microbiome 2017, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- De Gioannis, G.; Friargiu, M.; Massi, E.; Muntoni, A.; Polettini, A.; Pomi, R.; Spiga, D. Biohydrogen production from dark fermentation of cheese whey: Influence of pH. Int. J. Hydrogen Energy 2014, 39, 20930–20941. [Google Scholar] [CrossRef]
- Kargi, F.; Eren, N.S.; Ozmihci, S. Bio-hydrogen production from cheese whey powder (CWP) solution: Comparison of thermophilic and mesophilic dark fermentations. Int. J. Hydrogen Energy 2012, 37, 8338–8342. [Google Scholar] [CrossRef]
- Asunis, F.; De Gioannis, G.; Dessì, P.; Isipato, M.; Lens, P.N.L.; Muntoni, A.; Polettini, A.; Pomi, R.; Rossi, A.; Spiga, D. The dairy biorefinery: Integrating treatment processes for cheese whey valorisation. J. Environ. Manag. 2020, 276, 111240. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Hu, X.; Zhu, X.; Jin, H.; Xu, Z.; Tang, Q.; Li, X. Production of butyrate from lactate by a newly isolated Clostridium sp. BPY5. Appl. Biochem. Biotechnol. 2016, 179, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Lovato, G.; Augusto, I.M.; Ferraz Júnior, A.D.; Albanez, R.; Ratusznei, S.M.; Etchebehere, C.; Zaiat, M.; Rodrigues, J.A. Reactor start-up strategy as key for high and stable hydrogen production from cheese whey thermophilic dark fermentation. Int. J. Hydrogen Energy 2021, 46, 27364–27379. [Google Scholar] [CrossRef]
- Turon, V.; Trably, E.; Fouilland, E.; Steyer, J.P. Potentialities of dark fermentation effluents as substrates for microalgae growth: A review. Process Biochem. 2016, 51, 1843–1854. [Google Scholar] [CrossRef]
- Barca, C.; Soric, A.; Ranava, D.; Giudici-Orticoni, M.T.; Ferrasse, J.H. Anaerobic biofilm reactors for dark fermentative hydrogen production from wastewater: A review. Bioresour. Technol. 2015, 185, 386–398. [Google Scholar] [CrossRef]
- Zhang, J.; Fan, C.; Zhang, H.; Wang, Z.; Zhang, J.; Song, M. Ferric oxide/carbon nanoparticles enhanced bio-hydrogen production from glucose. Int. J. Hydrogen Energy 2018, 43, 8729–8738. [Google Scholar] [CrossRef]
- Liu, Z.; Lv, F.; Zheng, H.; Zhang, C.; Wei, F.; Xing, X.H. Enhanced hydrogen production in a UASB reactor by retaining microbial consortium onto carbon nanotubes (CNTs). Int. J. Hydrogen Energy 2012, 37, 10619–10626. [Google Scholar] [CrossRef]
- Martínez-Mendoza, L.J.; Lebrero, R.; Muñoz, R.; García-Depraect, O. Influence of key operational parameters on biohydrogen production from fruit and vegetable waste via lactate-driven dark fermentation. Bioresour. Technol. 2022, 364, 128070. [Google Scholar] [CrossRef] [PubMed]
- Sivagurunathan, P.; Sahoo, P.C.; Kumar, M.; Gupta, R.P.; Bhattacharyya, D.; Ramakumar, S.S.V. Unrevealing the role of metal oxide nanoparticles on biohydrogen production by Lactobacillus delbrueckii. Bioresour. Technol. 2023, 367, 128260. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, J.; Yang, J.; Zhang, J.; Li, Z.; Yang, Y.; Zang, L. Comparison of copper and aluminum doped cobalt ferrate nanoparticles for improving biohydrogen production. Bioresour. Technol. 2022, 343, 126078. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rangel, M.; Barboza-Corona, J.E.; Navarro-Díaz, M.; Escalante, A.E.; Valdez-Vazquez, I. The duo Clostridium and Lactobacillus linked to hydrogen production from a lignocellulosic substrate. Water Sci. Technol. 2021, 83, 3033–3040. [Google Scholar] [CrossRef] [PubMed]
- García-Depraect, O.; Castro-Muñoz, R.; Muñoz, R.; Rene, E.R.; León-Becerril, E.; Valdez-Vazquez, I.; Kumar, G.; Reyes-Alvarado, L.C.; Martínez-Mendoza, L.J.; Carrillo-Reyes, J.; et al. A review on the factors influencing biohydrogen production from lactate: The key to unlocking enhanced dark fermentative processes. Bioresour. Technol. 2021, 324, 124595. [Google Scholar] [CrossRef] [PubMed]
- Detman, A.; Laubitz, D.; Chojnacka, A.; Wiktorowska-Sowa, E.; Piotrowski, J.; Salamon, A.; Kaźmierczak, W.; Błaszczyk, M.K.; Barberan, A.; Chen, Y.; et al. Dynamics and complexity of dark fermentation microbial communities producing hydrogen from sugar beet molasses in continuously operating packed bed reactors. Front. Microbiol. 2021, 11, 612344. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhang, H.; Zhang, J.; Zhou, C.; Zhang, X.; Yan, X.; Yu, F.; Zhang, J. Comparison of calcium magnesium ferrite nanoparticles for boosting biohydrogen production. Bioresour. Technol. 2024, 395, 130410. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Wang, J. Improving mechanisms of biohydrogen production from grass using zero-valent iron nanoparticles. Bioresour. Technol. 2018, 266, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ma, Y.; Zhang, B.; Sun, H.; Wang, N.; Wang, L.; Zhang, J.; Xue, R. Comparison of magnetite/reduced graphene oxide nanocomposites and magnetite nanoparticles on enhancing hydrogen production in dark fermentation. Int. J. Hydrogen Energy 2022, 47, 22359–22370. [Google Scholar] [CrossRef]
- Zhang, J.; Li, W.; Yang, J.; Li, Z.; Zhang, J.; Zhao, W.; Zang, L. Cobalt ferrate nanoparticles improved dark fermentation for hydrogen evolution. J. Clean. Prod. 2021, 316, 128275. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, H.; Zhang, J.; Zhou, C.; Pei, Y.; Zang, L. Improved biohydrogen evolution through calcium ferrite nanoparticles assisted dark fermentation. Bioresour. Technol. 2022, 361, 127676. [Google Scholar] [CrossRef] [PubMed]



| Phase | Reads | Richness | Shannon | Simpson |
|---|---|---|---|---|
| I | 59,951 | 15 | 1.46 | 0.72 |
| II | 61,220 | 14 | 1.29 | 0.68 |
| III | 62,705 | 15 | 1.45 | 0.69 |
| IV | 50,542 | 14 | 1.33 | 0.68 |
| V | 56,452 | 13 | 1.56 | 0.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leroy-Freitas, D.; Muñoz, R.; Martínez-Mendoza, L.J.; Martínez-Fraile, C.; García-Depraect, O. Enhancing Biohydrogen Production: The Role of Iron-Based Nanoparticles in Continuous Lactate-Driven Dark Fermentation of Powdered Cheese Whey. Fermentation 2024, 10, 296. https://doi.org/10.3390/fermentation10060296
Leroy-Freitas D, Muñoz R, Martínez-Mendoza LJ, Martínez-Fraile C, García-Depraect O. Enhancing Biohydrogen Production: The Role of Iron-Based Nanoparticles in Continuous Lactate-Driven Dark Fermentation of Powdered Cheese Whey. Fermentation. 2024; 10(6):296. https://doi.org/10.3390/fermentation10060296
Chicago/Turabian StyleLeroy-Freitas, Deborah, Raúl Muñoz, Leonardo J. Martínez-Mendoza, Cristina Martínez-Fraile, and Octavio García-Depraect. 2024. "Enhancing Biohydrogen Production: The Role of Iron-Based Nanoparticles in Continuous Lactate-Driven Dark Fermentation of Powdered Cheese Whey" Fermentation 10, no. 6: 296. https://doi.org/10.3390/fermentation10060296
APA StyleLeroy-Freitas, D., Muñoz, R., Martínez-Mendoza, L. J., Martínez-Fraile, C., & García-Depraect, O. (2024). Enhancing Biohydrogen Production: The Role of Iron-Based Nanoparticles in Continuous Lactate-Driven Dark Fermentation of Powdered Cheese Whey. Fermentation, 10(6), 296. https://doi.org/10.3390/fermentation10060296






