The Nutritional Quality of the Culture Medium Influences the Survival of Non-Saccharomyces Yeasts Co-Cultured with Saccharomyces cerevisiae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Yeasts Strains
2.2. Yeast Growth and Viability Monitoring
2.3. Culture Medium and Fermentation Kinetics
2.4. Glucose, Fructose, and Ethanol Quantification
2.5. Identification and Semi-Quantification of Minor Volatile Compounds
2.6. Statistical Analysis
3. Results and Discussion
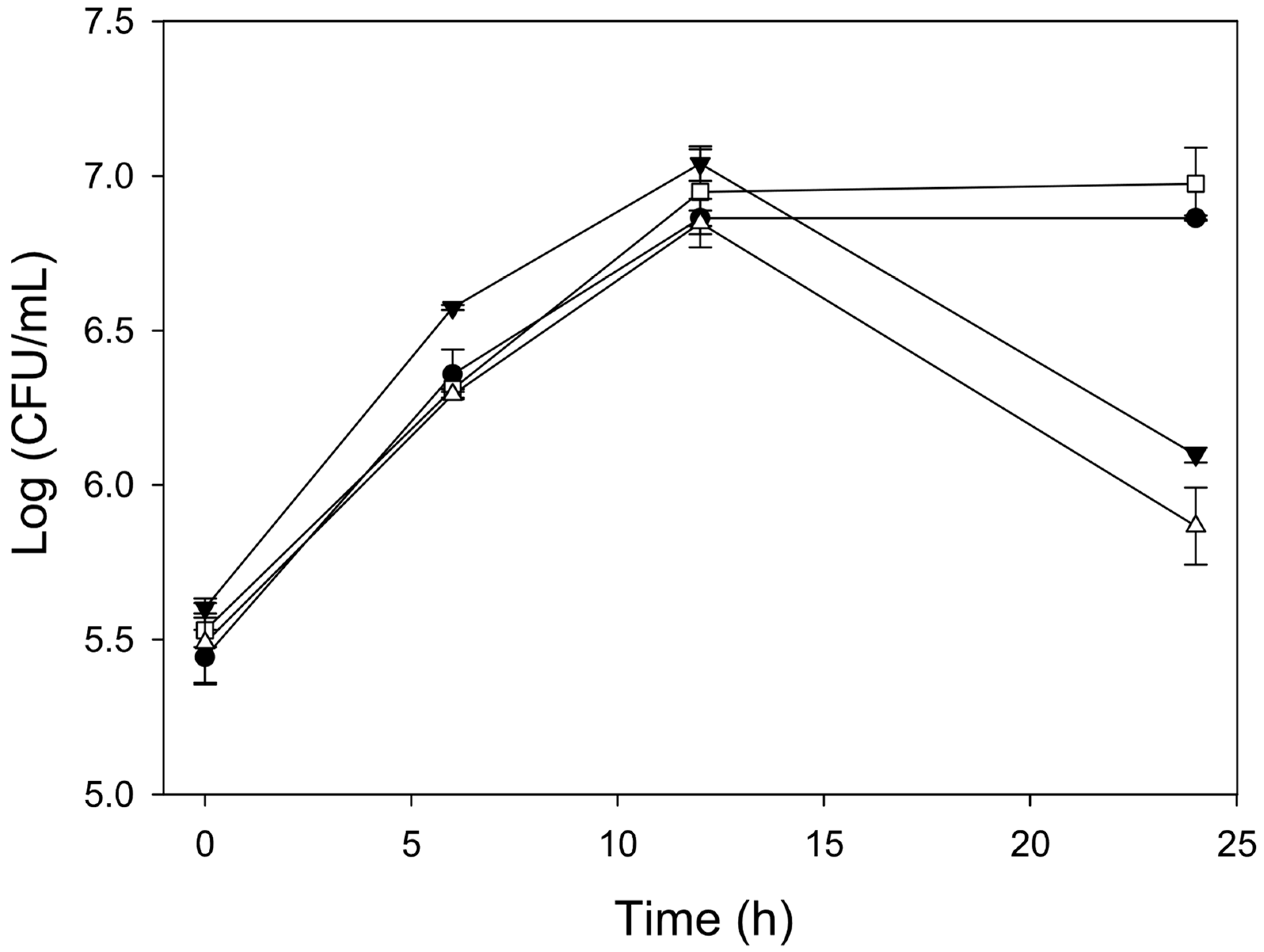
3.1. Monitoring Culture Medium Selection
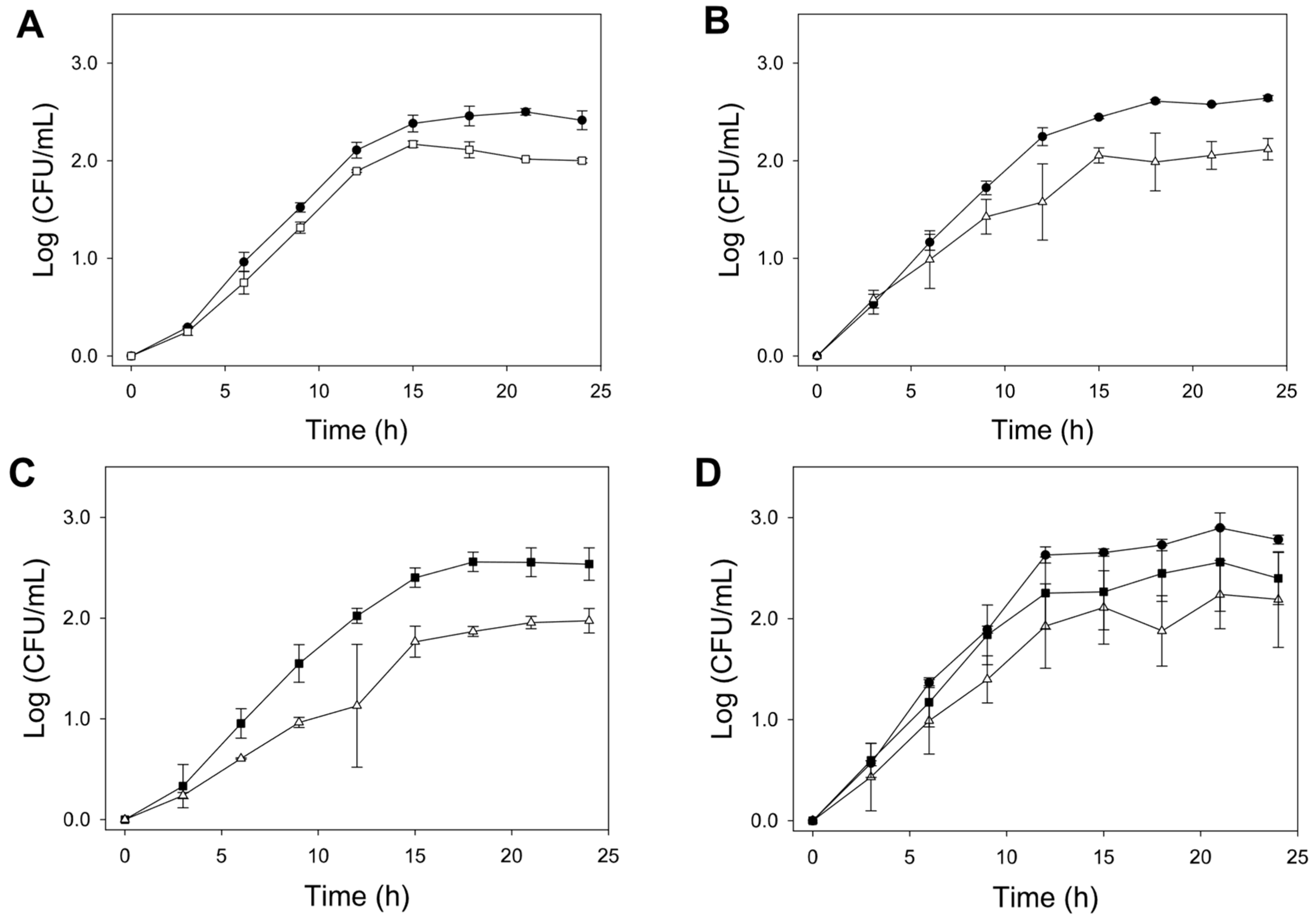
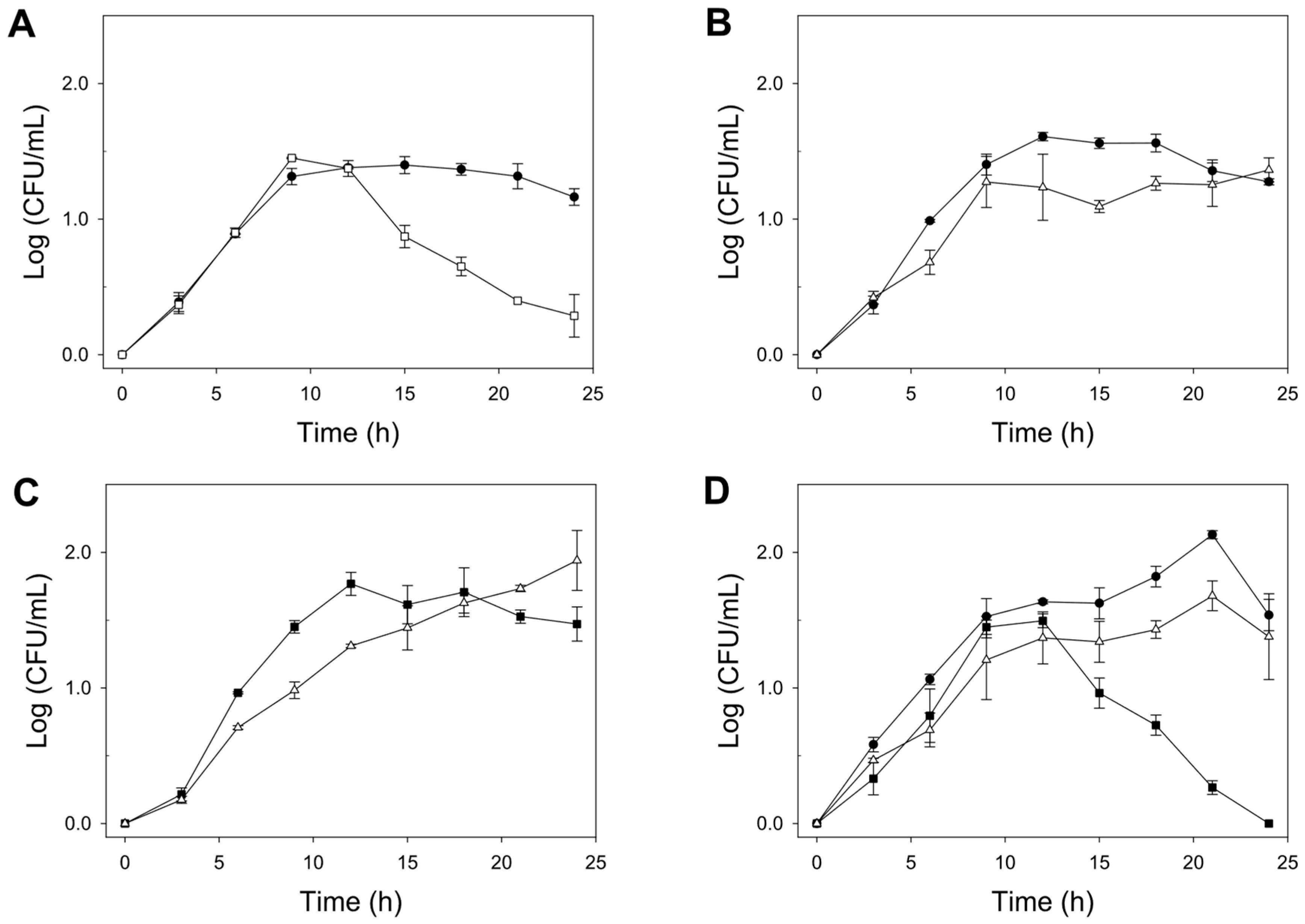
3.2. Fermentation Kinetics
3.3. Production of Minor Volatile Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arellano-Plaza, M.; Paez-Lerma, J.B.; Soto-Cruz, N.O.; Kirchmayr, M.R.; Gschaedler Mathis, A. Mezcal Production in Mexico: Between Tradition and Commercial Exploitation. Front. Sustain. Food Syst. 2022, 6, 1–16. [Google Scholar] [CrossRef]
- Gallegos-Casillas, P.; García-Ortega, L.F.; Espinosa-Cantú, A.; Avelar-Rivas, J.A.; Torres-Lagunes, C.G.; Cano-Ricardez, A.; García-Acero, A.M.; Ruiz-Castro, S.; Flores-Barraza, M.; Castillo, A.; et al. Yeast diversity in open agave fermentations across Mexico. Yeast 2024, 41, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Nolasco-Cancino, H.; Santiago-Urbina, J.A.; Wacher, C.; Ruíz-Terán, F. Predominant yeasts during artisanal mezcal fermentation and their capacity to ferment maguey juice. Front. Microbiol. 2018, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Páez-Lerma, J.B.; Arias-García, A.; Rutiaga-Quiñones, O.M.; Barrio, E.; Soto-Cruz, N.O. Yeasts Isolated from the Alcoholic Fermentation of Agave duranguensis During Mezcal Production. Food Biotechnol. 2013, 27, 342–356. [Google Scholar] [CrossRef]
- Verdugo-Valdez, A.; Segura-Garcia, L.; Kirchmayr, M.; Ramírez-Rodríguez, P.; González-Esquinca, A.; Coria, R.; Gschaedler-Mathis, A. Yeast communities associated with artisanal mezcal fermentations from Agave salmiana. Antonie Van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2011, 100, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Benito, S. The impact of Torulaspora delbrueckii yeast in winemaking. Appl. Microbiol. Biotechnol. 2018, 102, 3081–3094. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Liu, H.; Xue, J.; Tang, C.; Duan, C.; Yan, G. Use of Torulaspora delbrueckii and Hanseniaspora vineae co-fermentation with Saccharomyces cerevisiae to improve aroma profiles and safety quality of Petit Manseng wines. LWT 2022, 161, 113360. [Google Scholar] [CrossRef]
- Liu, C.; Li, M.; Ren, T.; Wang, J.; Niu, C.; Zheng, F.; Li, Q. Effect of Saccharomyces cerevisiae and non-Saccharomyces strains on alcoholic fermentation behavior and aroma profile of yellow-fleshed peach wine. LWT-Food Sci. Technol. 2022, 155, 112993. [Google Scholar] [CrossRef]
- Cioch-Skoneczny, M.; Grabowski, M.; Satora, P.; Skoneczny, S.; Klimczak, K. The use of yeast mixed cultures for deacidification and improvement of the composition of cold climate grape wines. Molecules 2021, 26, 2628. [Google Scholar] [CrossRef]
- Canonico, L.; Agarbati, A.; Comitini, F.; Ciani, M. Assessment of Spontaneous Fermentation and Non-Saccharomyces Sequential Fermentation in Verdicchio Wine at Winery Scale. Beverages 2022, 8, 49. [Google Scholar] [CrossRef]
- Yu, W.; Zhu, Y.; Zhu, R.; Bai, J.; Qiu, J.; Wu, Y.; Zhong, K.; Gao, H. Insight into the characteristics of cider fermented by single and co-culture with Saccharomyces cerevisiae and Schizosaccharomyces pombe based on metabolomic and transcriptomic approaches. Lwt 2022, 163, 113538. [Google Scholar] [CrossRef]
- Nissen, P.; Arneborg, N. Characterization of early deaths of non-Saccharomyces yeasts in mixed cultures with Saccharomyces cerevisiae. Arch. Microbiol. 2003, 180, 257–263. [Google Scholar] [CrossRef]
- Zhu, X.; Torija, M.J.; Mas, A.; Beltran, G.; Navarro, Y. Effect of a multistarter yeast inoculum on ethanol reduction and population dynamics in wine fermentation. Foods 2021, 10, 623. [Google Scholar] [CrossRef] [PubMed]
- Zilelidou, E.A.; Nisiotou, A. Understanding wine through yeast interactions. Microorganisms 2021, 9, 1620. [Google Scholar] [CrossRef] [PubMed]
- Branco, P.; Kemsawasd, V.; Santos, L.; Diniz, M.; Caldeira, J.; Almeida, M.G.; Arneborg, N.; Albergaria, H. Saccharomyces cerevisiae accumulates GAPDH-derived peptides on its cell surface that induce death of non-Saccharomyces yeasts by cell-to-cell contact. FEMS Microbiol. Ecol. 2017, 93, fix055. [Google Scholar] [CrossRef]
- Mannazzu, I.; Domizio, P.; Carboni, G.; Zara, S.; Zara, G.; Comitini, F.; Budroni, M.; Ciani, M. Yeast killer toxins: From ecological significance to application. Crit. Rev. Biotechnol. 2019, 39, 603–617. [Google Scholar] [CrossRef] [PubMed]
- Gobert, A.; Tourdot-Maréchal, R.; Morge, C.; Sparrow, C.; Liu, Y.; Quintanilla-Casas, B.; Vichi, S.; Alexandre, H. Non-Saccharomyces Yeasts nitrogen source preferences: Impact on sequential fermentation and wine volatile compounds profile. Front. Microbiol. 2017, 8, 1–13. [Google Scholar] [CrossRef]
- Lleixà, J.; Manzano, M.; Mas, A.; del Portillo, M.C. Saccharomyces and non-Saccharomyces competition during microvinification under different sugar and nitrogen conditions. Front. Microbiol. 2016, 7, 1–10. [Google Scholar] [CrossRef]
- Rollero, S.; Bloem, A.; Ortiz-Julien, A.; Camarasa, C.; Divol, B. Altered Fermentation Performances, Growth, and Metabolic Footprints Reveal Competition for Nutrients between Yeast Species Inoculated in Synthetic Grape Juice-Like Medium. Front. Microbiol. 2018, 9, 1–12. [Google Scholar] [CrossRef]
- Rollero, S.; Bloem, A.; Brand, J.; Ortiz-Julien, A.; Camarasa, C.; Divol, B. Nitrogen metabolism in three non-conventional wine yeast species: A tool to modulate wine aroma profiles. Food Microbiol. 2021, 94, 103650. [Google Scholar] [CrossRef]
- Su, Y.; Seguinot, P.; Sanchez, I.; Ortiz-Julien, A.; Heras, J.M.; Querol, A.; Camarasa, C.; Guillamón, J.M. Nitrogen sources preferences of non-Saccharomyces yeasts to sustain growth and fermentation under winemaking conditions. Food Microbiol. 2020, 85, 103287. [Google Scholar] [CrossRef]
- Lage, P.; Barbosa, C.; Mateus, B.; Vasconcelos, I.; Mendes-faia, A.; Mendes-ferreira, A.H. guilliermondii impacts growth kinetics and metabolic activity of S. cerevisiae: The role of initial nitrogen concentration. Int. J. Food Microbiol. 2014, 172, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Roca-Mesa, H.; Delgado-Yuste, E.; Mas, A.; Torija, M.-J.; Beltran, G. Importance of micronutrients and organic nitrogen in fermentations with Torulaspora delbrueckii and Saccharomyces cerevisiae. Int. J. Food Microbiol. 2022, 381, 109915. [Google Scholar] [CrossRef] [PubMed]
- Guzzon, R.; Widmann, G.; Settanni, L.; Malacarne, M.; Francesca, N.; Larcher, R. Evolution of Yeast Populations during Different Biodynamic Winemaking Processes. S. Afr. J. Enol. Vitic. 2011, 32, 242–250. [Google Scholar] [CrossRef]
- Medina, K.; Boido, E.; Dellacassa, E.; Carrau, F. Growth of non-Saccharomyces yeasts affects nutrient availability for Saccharomyces cerevisiae during wine fermentation. Int. J. Food Microbiol. 2012, 157, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Larralde-Corona, C.P.; De la Torre-González, F.J.; Vázquez-Landaverde, P.A.; Hahn, D.; Narváez-Zapata, J.A. Rational Selection of Mixed Yeasts Starters for Agave Must Fermentation. Front. Sustain. Food Syst. 2021, 5, 1–13. [Google Scholar] [CrossRef]
- Olivia Hernandez, A.A.; Taillandier, P.; Reséndez Pérez, D.; Narváez Zapata, J.A.; Larralde-Corona, C.P. The effect of hexose ratios on metabolite production in Saccharomyces cerevisiae strains obtained from the spontaneous fermentation of mezcal. Antonie Van Leeuwenhoek 2013, 103, 833–843. [Google Scholar] [CrossRef]
- Kokoti, K.; Kosma, I.S.; Tataridis, P.; Badeka, A.V.; Kontominas, M.G. Volatile aroma compounds of distilled “ tsipouro ” spirits: Effect of distillation technique. Eur. Food Res. Technol. 2023, 249, 1173–1185. [Google Scholar] [CrossRef]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef]
- Bordet, F.; Joran, A.; Klein, G.; Roullier-Gall, C.; Alexandre, H. Yeast-yeast interactions: Mechanisms, methodologies and impact on composition. Microorganisms 2020, 8, 600. [Google Scholar] [CrossRef]
- Harlé, O.; Legrand, J.; Tesnière, C.; Pradal, M.; Mouret, J.R.; Nidelet, T. Investigations of the mechanisms of interactions between four non-conventional species with Saccharomyces cerevisiae in oenological conditions. PLoS ONE 2020, 15, e0233285. [Google Scholar] [CrossRef] [PubMed]
- Longin, C.; Petitgonnet, C.; Guilloux-Benatier, M.; Rousseaux, S.; Alexandre, H. Application of flow cytometry to wine microorganisms. Food Microbiol. 2017, 62, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Navarro, Y.; Torija, M.J.; Mas, A.; Beltran, G. Viability-PCR allows monitoring yeast population dynamics in mixed fermentations including viable but non-culturable yeasts. Foods 2020, 9, 1373. [Google Scholar] [CrossRef] [PubMed]
- Adams, E. The antibacterial action of crystal violet. J. Pharm. Pharmacol. 1967, 1, 821–826. [Google Scholar] [CrossRef]
- Mani, S.; Bharagava, R.N. Exposure to Crystal Violet, Its Toxic, Genotoxic and Carcinogenic Effects on Environment and Its Degradation and Detoxification for Environmental Safety. In Reviews of Environmental Contamination and Toxicology; de Voogt, P., Ed.; Springer: Cham, Switzerland, 2016; Volume 237. [Google Scholar] [CrossRef]
- Fernandez, S.; Almaguer, L.M.; Sierra, J.A. Detection of Mixtures of Lager Yeast Strains by the Use of Selective Inhibitors in Solid Media. J. Am. Soc. Brew. Chem. 1989, 47, 73–76. [Google Scholar] [CrossRef]
- Lin, C.C.S.; Fung, D.Y.C. Effect of Dyes on the Growth of Food Yeasts. J. Food Sci. 1985, 50, 241–244. [Google Scholar] [CrossRef]
- Tronchoni, J.; Curiel, J.A.; Morales, P.; Torres-Pérez, R.; Gonzalez, R. Early transcriptional response to biotic stress in mixed starter fermentations involving Saccharomyces cerevisiae and Torulaspora delbrueckii. Int. J. Food Microbiol. 2017, 241, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Mas, A.; Esteve-Zarzoso, B. The interaction between Saccharomyces cerevisiae and non-Saccharomyces yeast during alcoholic fermentation is species and strain specific. Front. Microbiol. 2016, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sainz-Mellado, D.C.; Méndez-Hernández, J.E.; López-Miranda, J.; Páez-Lerma, J.B.; Aguilar, C.N.; Soto-Cruz, N.O. Gradually supply of isoamyl alcohol increases the isoamyl acetate production in solid-state fermentation. Lett. Appl. Microbiol. 2023, 76, ovac061. [Google Scholar] [CrossRef]
- Pourcelot, E.; Conacher, C.; Marlin, T.; Bauer, F.; Galeote, V.; Nidelet, T. Comparing the hierarchy of inter- and intra-species interactions with population dynamics of wine yeast cocultures. FEMS Yeast Res. 2023, 23, foad039. [Google Scholar] [CrossRef]
- González-Robles, I.W.; Estarrón-Espinosa, M.; Díaz-Montaño, D.M. Fermentative capabilities and volatile compounds produced by Kloeckera/Hanseniaspora and Saccharomyces yeast strains in pure and mixed cultures during Agave tequilana juice fermentation. Antonie Van Leeuwenhoek 2015, 108, 525–536. [Google Scholar] [CrossRef]
- Tao, Z.; Yuan, H.; Liu, M.; Liu, Q.; Zhang, S.; Liu, H.; Jiang, Y.; Huang, D.; Wang, T. Yeast Extract: Characteristics, Production, Applications and Future Perspectives. J. Microbiol. Biotechnol. 2023, 33, 151–166. [Google Scholar] [CrossRef]
- Walker, G.M. Metals in Yeast Fermentation Processes. Adv. Appl. Microbiol. 2004, 54, 197–229. [Google Scholar] [CrossRef]
- Labuschagne PW, J.; Divol, B. Thiamine: A key nutrient for yeasts during wine alcoholic fermentation. Appl. Microbiol. Biotechnol. 2021, 105, 953–973. [Google Scholar] [CrossRef]
- Kartal, B.; Akcay, A.; Palabiyik, B. Oxidative Stress Upregulates the Transcription of Genes Involved in ThiamineMetabolism. Turk. J. Biol. 2018, 42, 447–452. [Google Scholar] [CrossRef]
- Wolak, N.; Kowalska, E.; Kozik, A.; Rapala-kozik, M. Thiamine increases the resistance of baker’s yeast Saccharomyces cerevisiae against oxidative, osmotic and thermal stress, through mechanisms partly independent of thiamine diphosphate-bound enzymes. FEMS Yeast Res. 2014, 14, 1249–1262. [Google Scholar] [CrossRef]
- Mencher, A.; Morales, P.; Curiel, J.A.; Gonzalez, R.; Tronchoni, J. Metschnikowia pulcherrima represses aerobic respiration in Saccharomyces cerevisiae suggesting a direct response to co-cultivation. Food Microbiol. 2021, 94, 1–10. [Google Scholar] [CrossRef]
- Curiel, J.A.; Morales, P.; Gonzalez, R.; Tronchoni, J. Different non-Saccharomyces yeast species stimulate nutrient consumption in S. cerevisiae mixed cultures. Front. Microbiol. 2017, 8, 1–9. [Google Scholar] [CrossRef]
- Vera-Guzmán, A.M.; López, M.G.; Chávez-Servia, J. Chemical composition and volatile compounds in the artisanal fermentation of mezcal in Oaxaca, Mexico. Afr. J. Biotechnol. 2012, 11, 14344–14353. [Google Scholar] [CrossRef]
- Kirchmayr, M.R.; Segura-García, L.E.; Lappe-Oliveras, P.; Moreno-Terrazas, R.; de la Rosa, M.; Gschaedler Mathis, A. Impact of environmental conditions and process modifications on microbial diversity, fermentation efficiency and chemical profile during the fermentation of Mezcal in Oaxaca. LWT-Food Sci. Technol. 2017, 79, 160–169. [Google Scholar] [CrossRef]
- Chen, H.; Fink, G.R. Feedback control of morphogenesis in fungi by aromatic alcohols. Genes Dev. 2006, 20, 1150–1161. [Google Scholar] [CrossRef]
- Cappaert, C.; Larroche, L. Behaviour of dehydrated baker’ s yeast during reduction reactions in a biphasic medium. Appl. Microbiol. Biotechnol. 2004, 64, 686–690. [Google Scholar] [CrossRef]
- Xu, Y.; Zhi, Y.; Wu, Q.; Du, R.; Xu, Y. Zygosaccharomyces bailii Is a Potential Producer of Various Flavor Compounds in Chinese Maotai-Flavor Liquor Fermentation. Front. Microbiol. 2017, 8, 2609. [Google Scholar] [CrossRef]
- Saerens, S.M.G.; Delvaux, F.; Verstrepen, K.J.; Van Dijck, P.; Thevelein, J.M.; Delvaux, F.R. Parameters Affecting Ethyl Ester Production by Saccharomyces cerevisiae during Fermentation. Appl. Environ. Microbiol. 2008, 74, 454–461. [Google Scholar] [CrossRef]
- Hirst, M.B.; Richter, C.L. Review of aroma formation through metabolic pathways of Saccharomyces cerevisiae in beverage fermentations. Am. J. Enol. Vitic. 2016, 67, 361–370. [Google Scholar] [CrossRef]
- Li, N.; Wang, Q.; Xu, Y.; Li, A.; Tao, Y. Increased glycosidase activities improved the production of wine varietal odorants in mixed fermentation of P. fermentans and high antagonistic S. cerevisiae. Food Chem. 2020, 332, 127426. [Google Scholar] [CrossRef]
- Nuñez-Guerrero, M.E.; Páez-Lerma, J.B.; Rutiaga-Quiñones, O.M.; González-Herrera, S.M.; Soto-Cruz, N.O. Performance of mixtures of Saccharomyces and non-Saccharomyces native yeasts during alcoholic fermentation of Agave duranguensis juice. Food Microbiol. 2016, 54, 91–97. [Google Scholar] [CrossRef]
- Binati, R.L.; Lemos Junior WJ, F.; Luzzini, G.; Slaghenaufi, D.; Ugliano, M.; Torriani, S. Contribution of non-Saccharomyces yeasts to wine volatile and sensory diversity: A study on Lachancea thermotolerans, Metschnikowia spp. and Starmerella bacillaris strains isolated in Italy. Int. J. Food Microbiol. 2020, 318, 108470. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donéche, B.; Lonvaud, A. Handbook of Enology, Volume 1: The Microbiology of Wine and Vinifications; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006; Volume 1. [Google Scholar]
- Pohl, C.H.; Kock JL, F.; Thibane, V.S. Antifungal free fatty acids: A Review. In Science against Microbial Pathogens: Communicating Current Research and Technological Advances; Méndez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2011; Volume 3, pp. 61–71. [Google Scholar]

| µ (h−1) | Residual Fructose (g/L) | Residual Glucose (g/L) | Ethanol Content (g/L) | YP/S (g/g) | ||
|---|---|---|---|---|---|---|
| Monoculture | Sc | 0.59 ± 0.14 a | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 35.70 ± 0.67 b | 0.357 ± 0.007 b |
| Td | 0.34 ± 0.05 a | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 35.77 ± 0.58 b | 0.358 ± 0.006 b | |
| Zb | 0.26 ± 0.09 a | 6.74 ± 0.43 a | 4.19 ± 0.36 a | 25.42 ± 1.56 c | 0.285 ± 0.018 c | |
| Co-culture Sc/Td | Sc | 0.59 ± 0.01 a | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 38.98 ± 2.30 ab | 0.39 ± 0.023 ab |
| Td | 0.57 ± 0.02 a | |||||
| Co-culture Sc/Zb | Sc | 0.39 ± 0.02 a | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 39.97 ± 0.67 ab | 0.40 ± 0.007 ab |
| Zb | 0.41 ± 0.06 a | |||||
| Co-culture Td/Zb | Td | 0.48 ± 0.02 a | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 43.03 ± 3.43 a | 0.43 ± 0.034 a |
| Zb | 0.43 ± 0.32 a | |||||
| Ternary co-culture | Sc | 0.56 ± 0.08 a | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 37.42 ± 3.7 b | 0.374 ± 0.037 b |
| Td | 0.58 ± 0.03 a | |||||
| Zb | 0.44 ± 0.05 a |
| µ (h−1) | Residual Fructose (g/L) | Residual Glucose (g/L) | Ethanol Content (g/L) | YP/S (g/g) | ||
|---|---|---|---|---|---|---|
| Monoculture | Sc | 0.36 ± 0.01 a | 60.52 ± 3.22 b | 1.37 ± 0.28 cd | 12.28 ± 1.14 a | 0.325 ± 0.052 ab |
| Td | 0.39 ± 0.01 a | 70.09 ± 8.31 a | 2.5 ± 0.39 bc | 6.00 ± 0.38 cd | 0.242 ± 0.084 bc | |
| Zb | 0.22 ± 0.01 a | 44.72 ± 1.19 c | 2.43 ± 0.23 ab | 6.73 ± 0.48 c | 0.129 ± 0.007 d | |
| Co-culture Sc/Td | Sc | 0.46 ± 0.08 a | 59.66 ± 2.46 b | 1.31 ± 0.22 cd | 5.36 ± 0.42 d | 0.138 ± 0.015 d |
| Td | 0.42 ± 0.03 a | |||||
| Co-culture Sc/Zb | Sc | 0.52 ± 0.20 a | 46.22 ± 5.28 c | 0.36 ± 0.07 d | 12.56 ± 0.95 a | 0.238 ± 0.039 c |
| Zb | 0.40 ± 0.10 a | |||||
| Co-culture Td/Zb | Td | 0.48 ± 0.02 a | 65.91 ± 3.98 ab | 4.35 ± 0.30 a | 9.69 ± 0.63 b | 0.332 ± 0.058 a |
| Zb | 0.22 ± 0.06 | |||||
| Ternary co-culture | Sc | 0.43 ± 0.04 a | 50.20 ± 3.19 c | 3.12 ± 0.33 ab | 11.53 ± 0.45 a | 0.248 ± 0.021 abc |
| Td | 0.48 ± 0.07 a | |||||
| Zb | 0.35 ± 0.14 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acosta-García, E.D.; Soto-Cruz, N.O.; Valdivia-Hernández, E.A.; Rojas-Contreras, J.A.; Moreno-Jiménez, M.R.; Páez-Lerma, J.B. The Nutritional Quality of the Culture Medium Influences the Survival of Non-Saccharomyces Yeasts Co-Cultured with Saccharomyces cerevisiae. Fermentation 2024, 10, 400. https://doi.org/10.3390/fermentation10080400
Acosta-García ED, Soto-Cruz NO, Valdivia-Hernández EA, Rojas-Contreras JA, Moreno-Jiménez MR, Páez-Lerma JB. The Nutritional Quality of the Culture Medium Influences the Survival of Non-Saccharomyces Yeasts Co-Cultured with Saccharomyces cerevisiae. Fermentation. 2024; 10(8):400. https://doi.org/10.3390/fermentation10080400
Chicago/Turabian StyleAcosta-García, Erick D., Nicolás O. Soto-Cruz, Edwin A. Valdivia-Hernández, Juan A. Rojas-Contreras, Martha R. Moreno-Jiménez, and Jesús B. Páez-Lerma. 2024. "The Nutritional Quality of the Culture Medium Influences the Survival of Non-Saccharomyces Yeasts Co-Cultured with Saccharomyces cerevisiae" Fermentation 10, no. 8: 400. https://doi.org/10.3390/fermentation10080400






