Optimization of Fermentation and Transcriptomic Analysis: The Impact of Aspartic Acid on the Antioxidant Activity of Termitomyces
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Method
2.2.1. Strain Culture and Liquid Inoculation
2.2.2. Antioxidant Activity Assay
DPPH Scavenging Activity
ABTS Scavenging Activity
Hydroxyl Radical (•OH) Scavenging Activity
Total Antioxidant Capacity Assay
2.2.3. Comprehensive Evaluation of Antioxidant Activity
Membership Function Method for Strain Screening
Antioxidant Potency Composite
2.2.4. Optimization of Fermentation Conditions
Single-Factor Experiment on Medium Components
Response Surface Methodology (RSM) for Optimization
2.2.5. Transcriptome Analysis
2.2.6. Data Analysis
3. Results
3.1. Screening of High Antioxidant Activity Strains
3.2. Optimization of Fermentation Medium Components for XNQL025
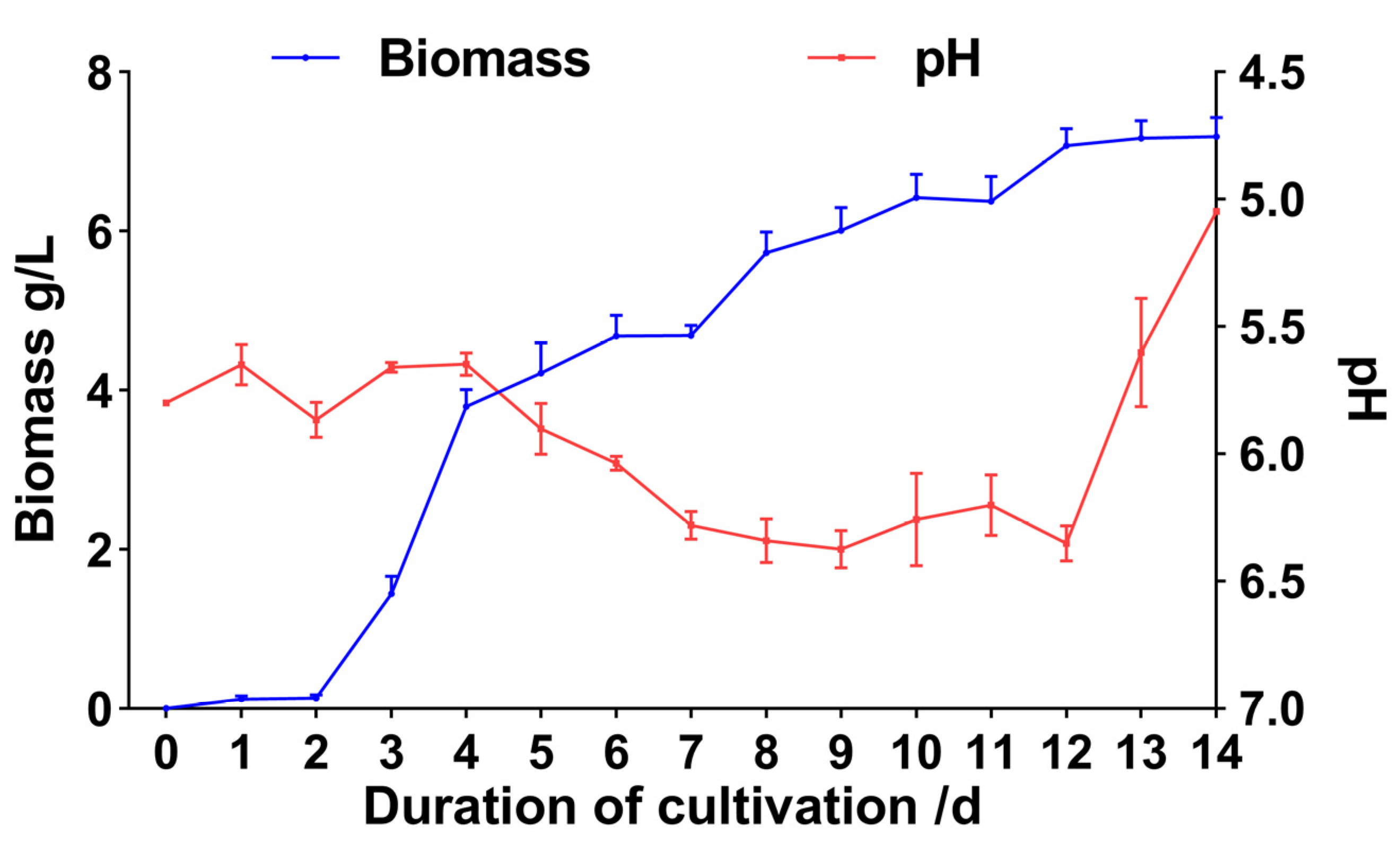
3.2.1. Judgment of Fermentation Endpoint of XNQL025
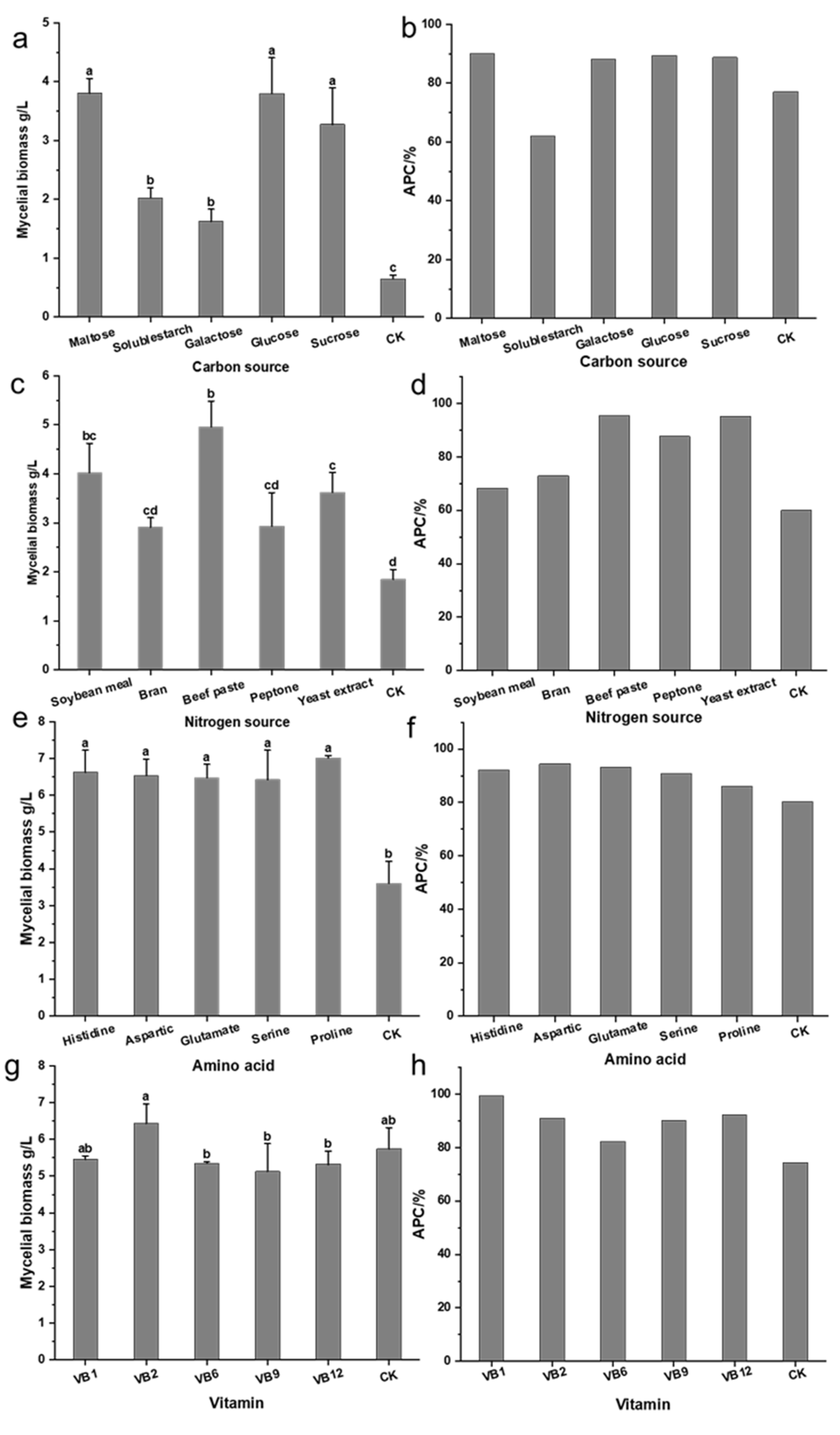
3.2.2. Impact of Different Medium Components on XNQL025 Growth and Antioxidant Activity
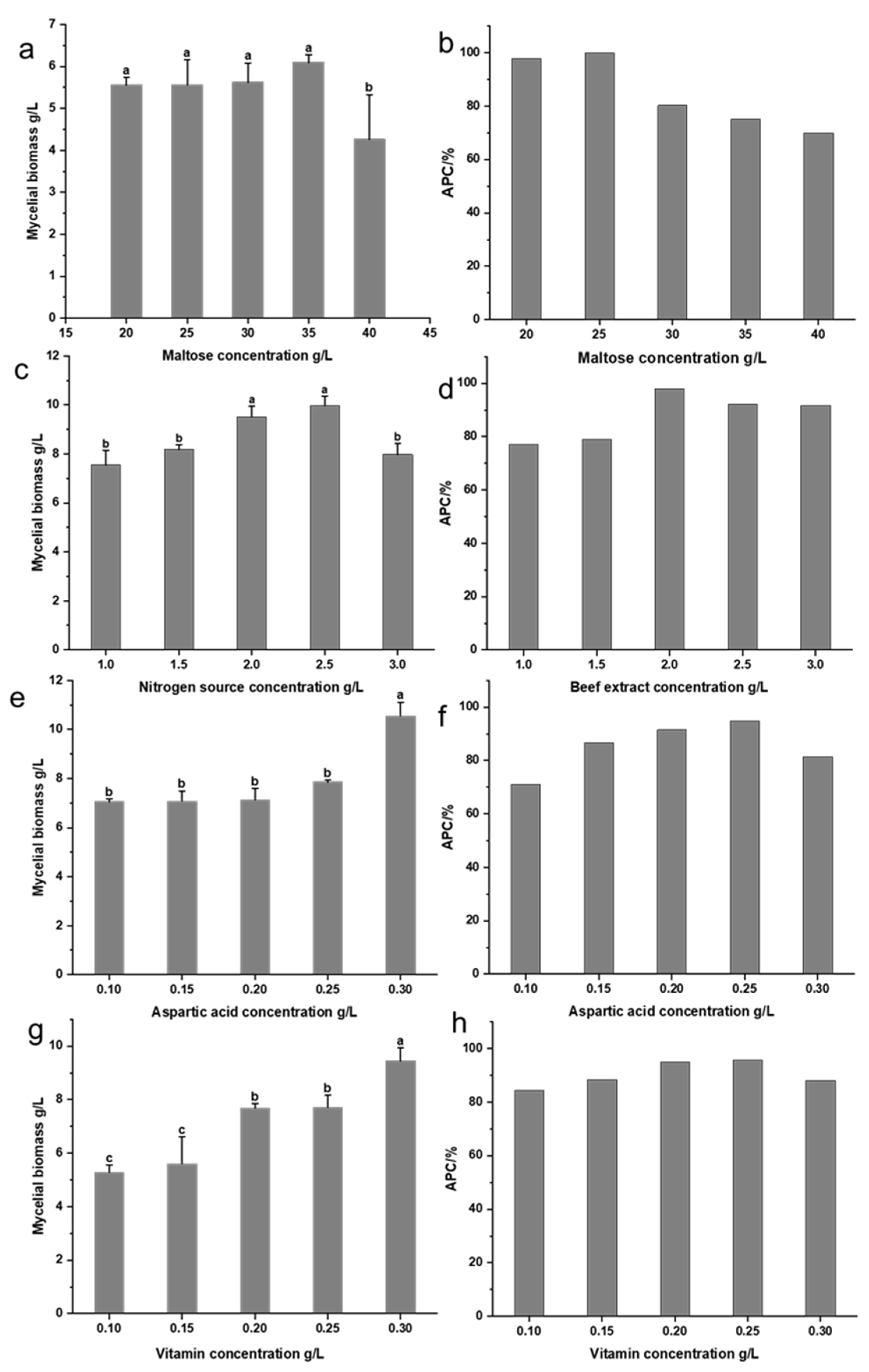
3.2.3. Optimization of Medium Component Concentrations for XNQL025
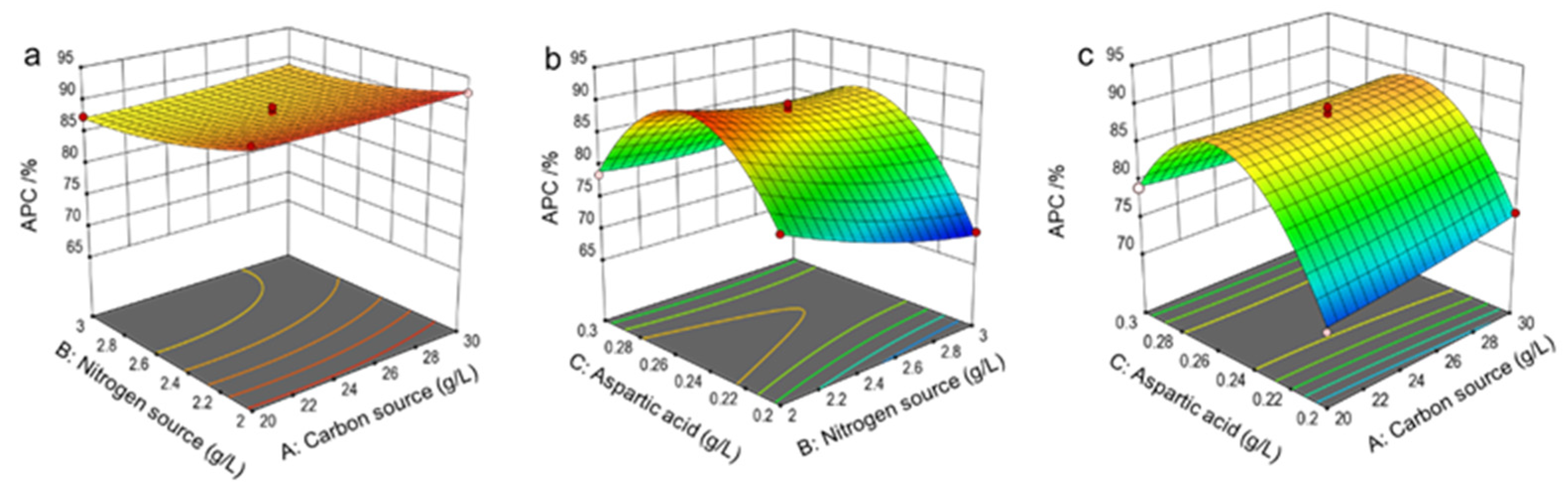
3.2.4. Response Surface Analysis and Model Validation
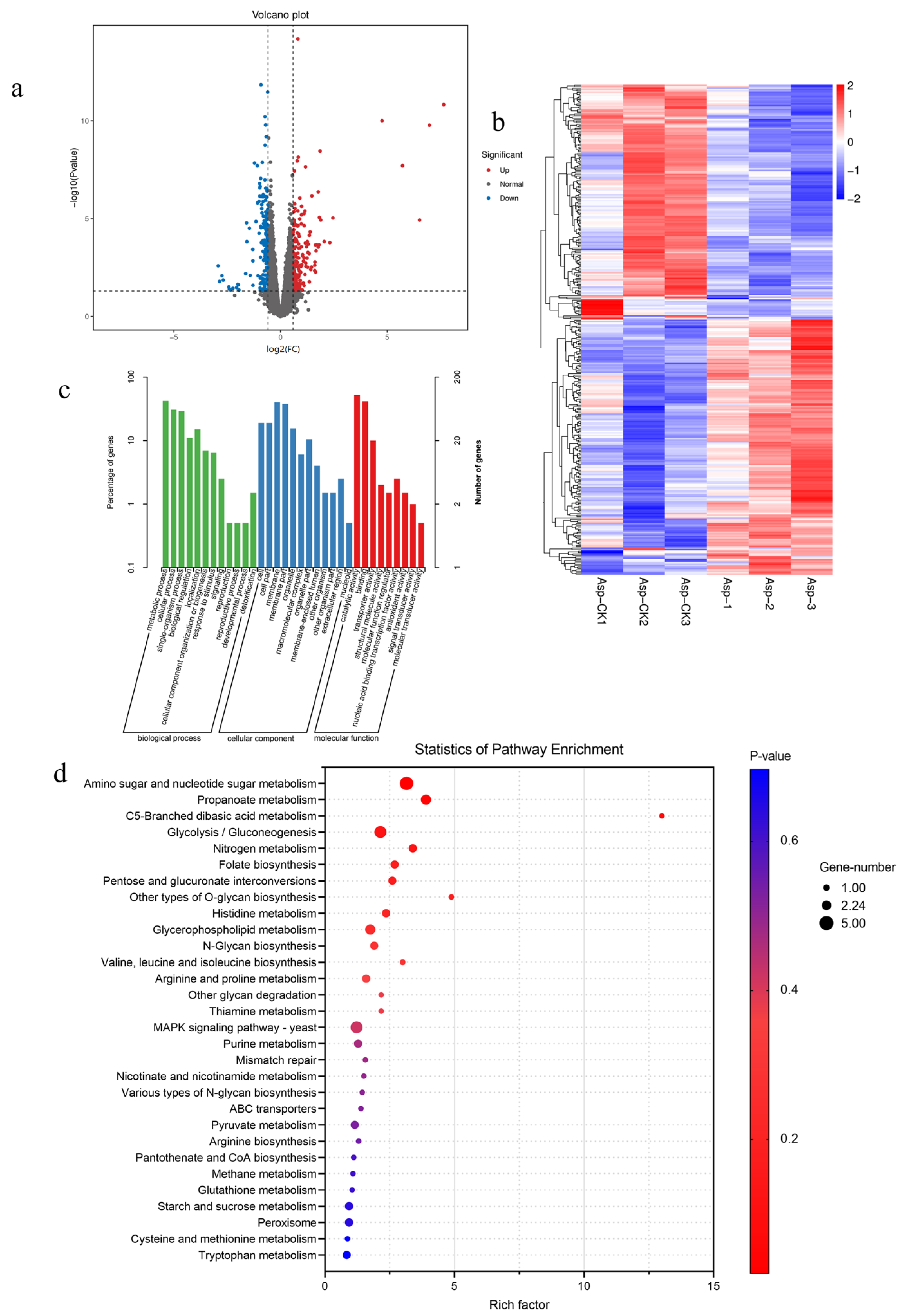
3.3. Transcriptomic Analysis of Aspartic Acid Effects on Antioxidant Activity
3.3.1. Quality Assessment of Transcriptome Data
3.3.2. Functional Annotation and GO Analysis of DEGs
3.3.3. KEGG Pathway Enrichment Analysis of DEGs
4. Discussion
4.1. Impact of Medium Components on Termitomyces Antioxidant Activity
4.2. Asp Regulates Termitomyces Metabolism and Signaling Pathways to Synergistically Enhance Antioxidant Capacity
4.3. Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, M.-Z.; Li, J.-Y. Physiological Regulation of Reactive Oxygen Species in Organisms Based on Their Physicochemical Properties. Acta Physiol. 2020, 228, e13351. [Google Scholar] [CrossRef]
- Lennicke, C.; Cochemé, H.M. Redox Metabolism: ROS as Specific Molecular Regulators of Cell Signaling and Function. Mol. Cell 2021, 81, 3691–3707. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, E.; Sorrentino, C. Oxidative Stress and Age-Related Tumors. Antioxidants 2024, 13, 1109. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.N.; Gupta, G.; Sharma, P. A Comprehensive Review of Free Radicals, Antioxidants, and Their Relationship with Human Ailments. Crit. Rev. Eukaryot. Gene Expr. 2018, 28, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Tang, P.; Yan, S.; Yang, Q.; Zhang, Z.; Gong, W.; Zhu, Z.; Zhou, Y.; Yan, L.; Hu, Z.; et al. Comparative Study on Bioactivities from Lingzhi or Reishi Medicinal Mushroom, Ganoderma lucidum (Agaricomycetes), Gives an Insight into the Fermentation Broth Showing Greater Antioxidative Activities. Int. J. Med. Mushrooms 2020, 22, 627–639. [Google Scholar] [CrossRef]
- Abdelkader, M.-A.E.; Mediatrice, H.; Lin, D.; Lin, Z.; Aggag, S.A. Mitigating Oxidative Stress and Promoting Cellular Longevity with Mushroom Extracts. Foods 2024, 13, 4028. [Google Scholar] [CrossRef]
- Kozarski, M.; Klaus, A.; Jakovljevic, D.; Todorovic, N.; Vunduk, J.; Petrović, P.; Niksic, M.; Vrvic, M.M.; Van Griensven, L. Antioxidants of Edible Mushrooms. Molecules 2015, 20, 19489–19525. [Google Scholar] [CrossRef]
- Hu, Q.; Yuan, B.; Xiao, H.; Zhao, L.; Wu, X.; Rakariyatham, K.; Zhong, L.; Han, Y.; Kimatu, B.M.; Yang, W. Polyphenols-Rich Extract from Pleurotus eryngii with Growth Inhibitory of HCT116 Colon Cancer Cells and Anti-Inflammatory Function in RAW264.7 Cells. Food Funct. 2018, 9, 1601–1611. [Google Scholar] [CrossRef]
- Neergheen, V.S.; Hip Kam, A.; Pem, Y.; Ramsaha, S.; Bahorun, T. Regulation of Cancer Cell Signaling Pathways as Key Events for Therapeutic Relevance of Edible and Medicinal Mushrooms. Semin. Cancer Biol. 2022, 80, 145–156. [Google Scholar] [CrossRef]
- Wisselink, M.; Aanen, D.K.; van ’t Padje, A. The Longevity of Colonies of Fungus-Growing Termites and the Stability of the Symbiosis. Insects 2020, 11, 527. [Google Scholar] [CrossRef] [PubMed]
- Mondal, A.; Banerjee, D.; Majumder, R.; Maity, T.K.; Khowala, S. Evaluation of In Vitro Antioxidant, Anticancer and In Vivo Antitumour Activity of Termitomyces clypeatus MTCC 5091. Pharm. Biol. 2016, 54, 2536–2546. [Google Scholar] [CrossRef] [PubMed]
- Woldegiorgis, A.Z.; Abate, D.; Haki, G.D.; Ziegler, G.R. Antioxidant Property of Edible Mushrooms Collected from Ethiopia. Food Chem. 2014, 157, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wang, X.; Liu, X.; Zhang, J.; Wan, L.; Jia, L. Antioxidant and Hypolipidemic Activities of Acid-Depolymerised Exopolysaccharides by Termitomyces albuminosus. Oxidative Med. Cell. Longev. 2019, 2019, 1–13. [Google Scholar] [CrossRef]
- Paloi, S.; Kumla, J.; Paloi, B.P.; Srinuanpan, S.; Hoijang, S.; Karunarathna, S.C.; Acharya, K.; Suwannarach, N.; Lumyong, S. Termite Mushrooms (Termitomyces), a Potential Source of Nutrients and Bioactive Compounds Exhibiting Human Health Benefits: A Review. J. Fungi 2023, 9, 112. [Google Scholar] [CrossRef]
- Kumar, K.; Mehra, R.; Guiné, R.P.F.; Lima, M.J.; Kumar, N.; Kaushik, R.; Ahmed, N.; Yadav, A.N.; Kumar, H. Edible Mushrooms: A Comprehensive Review on Bioactive Compounds with Health Benefits and Processing Aspects. Foods 2021, 10, 2996. [Google Scholar] [CrossRef]
- Gebreyohannes, G.; Sbhatu, D.B.; Nyerere, A.; Bii, C.; Gebrehiwot, A.G. Wild Mushrooms: Potential Natural Sources of Antioxidant and Anti-Quorum Sensing Bioactive Compounds for Medical Applications. Evid.-Based Complement. Altern. Med. 2023, 2023, 1–14. [Google Scholar] [CrossRef]
- Yang, G.; Ahmad, F.; Zhou, Q.; Guo, M.; Liang, S.; Gaal, H.A.; Mo, J. Investigation of Physicochemical Indices and Microbial Communities in Termite Fungus-Combs. Front. Microbiol. 2021, 11, 581219. [Google Scholar] [CrossRef]
- Rajagopal, K. Statistical Optimization of Medium Components by Plackett Burman Design and Response Surface Methodology for Enhanced Antioxidant Activity by Xylaria feejeensis HMJAU22039. Int. J. Pharm. Pharm. Sci. 2016, 8, 159–164. [Google Scholar] [CrossRef]
- Jiamworanunkul, S. Effective Antioxidant Production through Submerged Fermentation of Edible Mushrooms. Thai J. Pharm. Sci. 2019, 43, 213–218. [Google Scholar] [CrossRef]
- Gu, D.; Tang, S.; Liu, C.; He, D.; Tian, J.; Yang, Y. Optimization of Liquid Fermentation Conditions for Coprinus comatus to Enhance Antioxidant Activity. Prep. Biochem. Biotechnol. 2024, 54, 830–837. [Google Scholar] [CrossRef]
- Li, F.; Fan, H.; Sun, Q.; Di, Y.; Xia, H. Effects of Medium Additives on the Mycelial Growth and Polysaccharide Biosynthesis in Submerged Culture of Bjerkandera fumosa. Molecules 2024, 29, 422. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-Y.; Park, E.-J.; Ahn, J.-K.; Ka, K.-H. Ergothioneine Contents in Fruiting Bodies and Their Enhancement in Mycelial Cultures by the Addition of Methionine. Mycobiology 2009, 37, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Begum, N.; Khan, Q.U.; Al-Dalali, S.; Lu, D.; Yang, F.; Li, J.; Wu, D.; Li, R.; Wang, J.; Liu, D.; et al. Process Optimization and Identification of Antioxidant Peptides from Enzymatic Hydrolysate of Bovine Bone Extract, a Potential Source in Cultured Meat. Front. Sustain. Food Syst. 2024, 7, 1345833. [Google Scholar] [CrossRef]
- Xie, X.; Zheng, Y.; Liu, X.; Cheng, C.; Zhang, X.; Xia, T.; Yu, S.; Wang, M. Antioxidant Activity of Chinese Shanxi Aged Vinegar and Its Correlation with Polyphenols and Flavonoids During the Brewing Process. J. Food Sci. 2017, 82, 2479–2486. [Google Scholar] [CrossRef]
- Malathi, G.; Murugesan, A.K. Bioconversion, Nutritional Analysis, Radical Scavenging and Characterization of Substrates of Pleurotus flabellatus (Berk. and Br.) Sacc. S. Afr. J. Bot. 2023, 157, 423–437. [Google Scholar] [CrossRef]
- Liu, S.; Zhuang, X.; Zhang, X.; Han, W.; Liu, Y.; Sun, D.; Guo, W. Enzymatic Modification of Rice Bran Polysaccharides by Enzymes from Grifola frondosa: Natural Killer Cell Cytotoxicity and Antioxidant Activity. J. Food Sci. 2018, 83, 1948–1955. [Google Scholar] [CrossRef]
- Li, B.; Zhang, Y.; Kang, Y.; Wang, Y.; Liu, R.; Liu, Q.; Dong, S. Physiological Response to Low-Temperature Stress and Cold Resistance Evaluation of Ziziphus jujuba var. spinosa Clones from Different Provenances. Forests 2024, 15, 1130. [Google Scholar] [CrossRef]
- Toor, B.S.; Kaur, A.; Sahota, P.P.; Kaur, J. Antioxidant Potential, Antinutrients, Mineral Composition and FTIR Spectra of Legumes Fermented with Rhizopus Oligosporus. Food Technol. Biotechnol. 2021, 59, 530–542. [Google Scholar] [CrossRef]
- Luo, D.; Mu, T.; Sun, H. Profiling of Phenolic Acids and Flavonoids in Sweet Potato (Ipomoea Batatas L.) Leaves and Evaluation of Their Anti-Oxidant and Hypoglycemic Activities. Food Biosci. 2021, 39, 100801. [Google Scholar] [CrossRef]
- Fan, X.-Z.; Yao, F.; Yin, C.-M.; Shi, D.-F.; Gao, H. Mycelial Biomass and Intracellular Polysaccharides Production, Characterization, and Activities in Auricularia auricula-judae Cultured with Different Carbon Sources. Int. J. Biol. Macromol. 2023, 244, 125426. [Google Scholar] [CrossRef]
- Tang, C.-Y.; Wang, J.; Liu, X.; Chen, J.-B.; Liang, J.; Wang, T.; Simpson, W.R.; Li, Y.-L.; Li, X.-Z. Medium Optimization for High Mycelial Soluble Protein Content of Ophiocordyceps Sinensis Using Response Surface Methodology. Front. Microbiol. 2022, 13, 1055055. [Google Scholar] [CrossRef]
- Kirsch, L.D.S.; De Macedo, A.J.P.; Teixeira, M.F.S. Production of Mycelial Biomass by the Amazonian Edible Mushroom Pleurotus albidus. Braz. J. Microbiol. 2016, 47, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Lin, Y.Q.; Yang, C.; Ying, Z.H.; Jiang, X.L. Production of Liquid Spawn of an Edible Mushroom, Sparassis latifolia by Submerged Fermentation and Mycelial Growth on Pine Wood Sawdust. Sci. Hortic. 2016, 209, 22–30. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, Y.-H.; Li, M.; Zhu, M.-L.; Wen, T.-Y.; Wu, X.-Q. Medium Optimization to Analyze the Protein Composition of Bacillus pumilus HR10 Antagonizing Sphaeropsis sapinea. AMB Expr. 2022, 12, 61. [Google Scholar] [CrossRef]
- Liu, F.; Ma, L.; Chen, W.; Wang, S.; Wei, C.; Huang, C.; Jiang, Y.; Wang, S.; Lin, H.; Chen, J.; et al. Preliminary Study on the Anti-CO2 Stress and Growth Ability of Hypsizygus marmoreus Mutant Strain HY68. BMC Microbiol. 2023, 23, 293. [Google Scholar] [CrossRef] [PubMed]
- Tepwong, P.; Giri, A.; Sasaki, F.; Fukui, R.; Ohshima, T. Mycobial Enhancement of Ergothioneine by Submerged Cultivation of Edible Mushroom Mycelia and Its Application as an Antioxidative Compound. Food Chem. 2012, 131, 247–258. [Google Scholar] [CrossRef]
- Fendt, S.-M.; Bell, E.L.; Keibler, M.A.; Olenchock, B.A.; Mayers, J.R.; Wasylenko, T.M.; Vokes, N.I.; Guarente, L.; Heiden, M.G.V.; Stephanopoulos, G. Reductive Glutamine Metabolism Is a Function of the α-Ketoglutarate to Citrate Ratio in Cells. Nat. Commun. 2013, 4, 2236. [Google Scholar] [CrossRef]
- Kandasamy, P.; Gyimesi, G.; Kanai, Y.; Hediger, M.A. Amino Acid Transporters Revisited: New Views in Health and Disease. Trends Biochem. Sci. 2018, 43, 752–789. [Google Scholar] [CrossRef]
- Orasch, T.; Dietl, A.-M.; Shadkchan, Y.; Binder, U.; Bauer, I.; Lass-Flörl, C.; Osherov, N.; Haas, H. The Leucine Biosynthetic Pathway Is Crucial for Adaptation to Iron Starvation and Virulence in Aspergillus fumigatus. Virulence 2019, 10, 925–934. [Google Scholar] [CrossRef]
- Steyer, J.T.; Todd, R.B. Branched-Chain Amino Acid Biosynthesis in Fungi. Essays Biochem. 2023, 67, 865–876. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Corona, J.F.; González-Hernández, G.A.; Padilla-Guerrero, I.E.; Olmedo-Monfil, V.; Martínez-Rocha, A.L.; Patiño-Medina, J.A.; Meza-Carmen, V.; Torres-Guzmán, J.C. Fungal Alcohol Dehydrogenases: Physiological Function, Molecular Properties, Regulation of Their Production, and Biotechnological Potential. Cells 2023, 12, 2239. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Zhang, H.; Wu, D.; Zhang, Z.; Zhang, J.; Bao, D.; Yang, Y. Key Metabolism Pathways and Regulatory Mechanisms of High Polysaccharide Yielding in Hericium erinaceus. BMC Genom. 2021, 22, 160. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.; Alrumaihi, F.A.; Sarwar, T.; Babiker, A.; Khan, A.; Prabhu, S.V.; Rahmani, A. Exploring Therapeutic Potential of Catalase: Strategies in Disease Prevention and Management. Biomolecules 2024, 14, 697. [Google Scholar] [CrossRef]
- Wagner, S.; Stuttmann, J.; Rietz, S.; Guerois, R.; Brunstein, E.; Bautor, J.; Niefind, K.; Parker, J.E. Structural Basis for Signaling by Exclusive EDS1 Heteromeric Complexes with SAG101 or PAD4 in Plant Innate Immunity. Cell Host Microbe 2013, 14, 619–630. [Google Scholar] [CrossRef]
- Mo, X.; Yu, X.; Cui, H.; Xiong, K.; Yang, S.; Su, C.; Lu, Y. In Vivo RNA Sequencing Reveals a Crucial Role of Fus3-Kss1 MAPK Pathway in Candida Glabrata Pathogenicity. mSphere 2024, 9, e00715-24. [Google Scholar] [CrossRef]
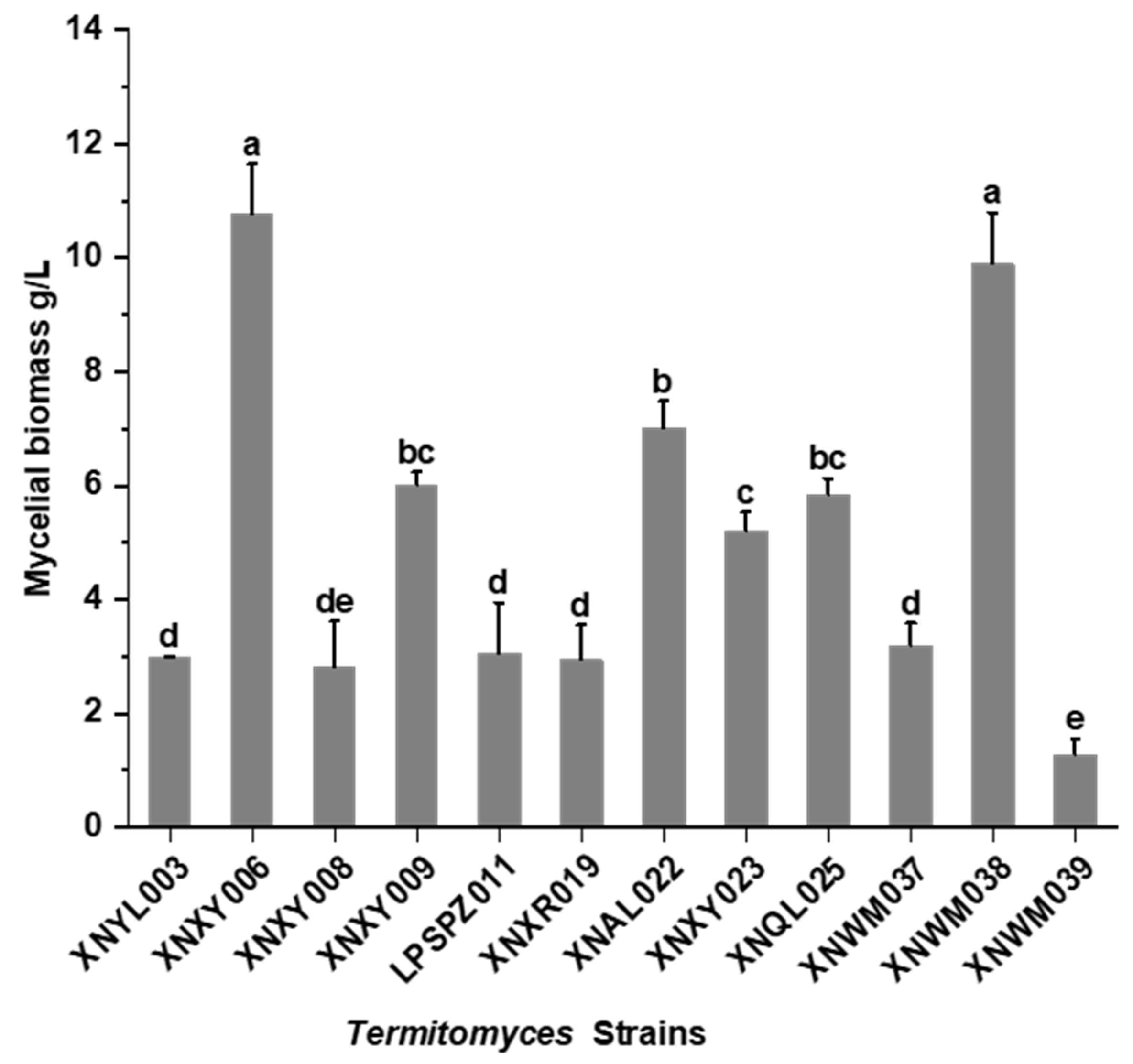


| Termitomyces Strains | Antioxidant Activity Assay | D Value | |||
|---|---|---|---|---|---|
| ABTS/% | •OH/% | FRAP/(mM FeSO4) | DPPH/% | ||
| XNYL003 | 97.23 ± 2 a | 92.23 ± 4 a | 5.58 ± 0.29 a | 63.40 ± 5 d | 0.69 |
| XNXY006 | 81.04 ± 1 bc | 77.95 ± 5 cd | 2.41 ± 0.37 bc | 57.66 ± 3 d | 0.55 |
| XNXY008 | 42.80 ± 23 e | 77.74 ± 8 cd | 5.91 ± 0.24 a | 55.49 ± 1 d | 0.32 |
| XNXY009 | 42.96 ± 19 e | 69.15 ± 10 d | 5.71 ± 0.36 a | 79.42 ± 5 bc | 0.40 |
| LPSPZ011 | 68.65 ± 23 d | 93.38 ± 1 a | 5.88 ± 0.04 a | 71.85 ± 4 c | 0.61 |
| XNXR019 | 82.28 ± 4 bc | 87.19 ± 1 abc | 5.63 ± 0.61 a | 38.93 ± 3 e | 0.48 |
| XNAL022 | 78.48 ± 10 c | 89.13 ± 2 ab | 2.35 ± 0.31 bc | 58.06 ± 1 d | 0.63 |
| XNXY023 | 84.92 ± 5 bc | 86.14 ± 5 abc | 1.99 ± 0.09 c | 87.90 ± 7 a | 0.75 |
| XNQL025 | 87.53 ± 8 bc | 96.25 ± 1 a | 5.43 ± 0.07 a | 86.43 ± 1 ab | 0.80 |
| XNWM037 | 37.61 ± 36 f | 81.64 ± 2 bc | 5.68 ± 0.15 a | 26.72 ± 8 f | 0.20 |
| XNWM038 | 88.45 ± 9 b | 91.64 ± 6 ab | 2.68 ± 0.24 b | 78.25 ± 1 bc | 0.78 |
| XNWM039 | 39.38 ± 47 f | 91.71 ± 7 ab | 5.96 ± 0.12 a | 15.89 ± 3 g | 0.21 |
| XNYL003 | 97.23 ± 2 a | 92.23 ± 4 a | 5.58 ± 0.29 a | 63.40 ± 5 d | 0.69 |
| XNXY006 | 81.04 ± 1 bc | 77.95 ± 5 cd | 2.41 ± 0.37 bc | 57.66 ± 3 d | 0.55 |
| Run | Maltose (g/L) | Beef Extract (g/L) | Asp (g/L) | APC |
|---|---|---|---|---|
| 1 | 25 | 2.5 | 0.25 | 89.58 |
| 2 | 20 | 3.0 | 0.25 | 87.42 |
| 3 | 30 | 2.0 | 0.25 | 92.61 |
| 4 | 20 | 2.5 | 0.30 | 79.03 |
| 5 | 25 | 2.0 | 0.20 | 79.57 |
| 6 | 30 | 3.0 | 0.25 | 88.17 |
| 7 | 25 | 2.5 | 0.25 | 87.87 |
| 8 | 25 | 2.5 | 0.25 | 87.60 |
| 9 | 20 | 2.5 | 0.20 | 71.42 |
| 10 | 25 | 2.5 | 0.25 | 89.75 |
| 11 | 30 | 2.5 | 0.20 | 75.72 |
| 12 | 20 | 2.0 | 0.25 | 92.77 |
| 13 | 30 | 2.5 | 0.30 | 78.68 |
| 14 | 25 | 2.5 | 0.25 | 88.86 |
| 15 | 25 | 2.0 | 0.30 | 78.57 |
| 16 | 25 | 3.0 | 0.20 | 70.19 |
| 17 | 25 | 3.0 | 0.30 | 79.90 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 0.0820 | 9 | 0.0091 | 106.2500 | <0.0001 |
| A-Maltose (g/L) | 0.0003 | 1 | 0.0003 | 3.0200 | 0.1260 |
| B-Beef Extract (g/L) | 0.0040 | 1 | 0.0040 | 46.4500 | 0.0002 |
| C- Asp (g/L) | 0.0046 | 1 | 0.0046 | 54.1000 | 0.0002 |
| AB | 0.0000 | 1 | 0.0000 | 0.2437 | 0.6366 |
| AC | 0.0005 | 1 | 0.0005 | 6.3100 | 0.0403 |
| BC | 0.0029 | 1 | 0.0029 | 33.4700 | 0.0007 |
| A2 | 0.0000 | 1 | 0.0000 | 0.5402 | 0.4863 |
| B2 | 0.0006 | 1 | 0.0006 | 6.7800 | 0.0352 |
| C2 | 0.0696 | 1 | 0.0696 | 810.9600 | <0.0001 |
| Residual | 0.0006 | 7 | 0.0001 | ||
| Lack of Fit | 0.0002 | 3 | 0.0001 | 0.7787 | 0.5641 |
| Pure Error | 0.0004 | 4 | 0.0001 |
| Group | Biomass/g/L | ABTS /% | DPPH/% | •OH/% |
|---|---|---|---|---|
| Asp | 9.80 ± 0.23 | 82.5 ± 0.82 | 84.08 ± 3.73 | 87.15 ± 2.51 |
| CK | 7.86 ± 0.26 | 74.28 ± 6.68 | 65.82 ± 1.42 | 75.76 ± 2.43 |
| KEGG | Gene ID | Function | log2 (Fold Change Asp/CK) |
|---|---|---|---|
| Glycolysis/Gluconeogenesis | TRINITY_DN1172_c0_g1 | adh | 1.35 |
| TRINITY_DN8757_c0_g1 | adhp | −0.74 | |
| TRINITY_DN1413_c2_g2 | pdc | 1.22 | |
| TRINITY_DN255_c0_g1 | pdc | −1.63 | |
| Propanoate metabolism | TRINITY_DN5447_c0_g1 | prpB | 1.19 |
| TRINITY_DN2667_c0_g1 | GRE2 | 1.47 | |
| TRINITY_DN8350_c0_g1 | GRE2 | −0.90 | |
| amino sugar and nucleoside sugar synthesis | TRINITY_DN3396_c0_g1 | chitinase | 1.19 |
| TRINITY_DN34_c0_g1; | chitinase | 0.80 | |
| TRINITY_DN5903_c0_g1 | chitinase | 0.88 | |
| TRINITY_DN3754_c0_g1 | chitin synthase | −0.92 | |
| TRINITY_DN3754_c0_g1 | N-acetylglucosamine-6-phosphate deacetylase | −0.92 | |
| MAPK signaling pathway | TRINITY_DN959_c0_g1 | Fus3 | 0.60 |
| TRINITY_DN959_c0_g1 | Kss1 | 0.60 | |
| TRINITY_DN2315_c0_g1 | Ctt1 | 0.90 | |
| TRINITY_DN2667_c0_g1 | Gre1 | 1.47 | |
| TRINITY_DN8350_c0_g1 | Gre1 | −0.90 | |
| peroxisome | TRINITY_DN4239_c0_g2 | AMACR | 0.91 |
| TRINITY_DN2315_c0_g1 | catalase | 0.90 | |
| tryptophan metabolism | TRINITY_DN3484_c0_g1 | CYP1A1 | 1.81 |
| TRINITY_DN2315_c0_g1 | catalase | 0.90 | |
| valine, leucine and isoleucine biosynthesis | TRINITY_DN5827_c0_g2 | 3-isopropylmalate dehydrogenase | 0.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Yi, W.; Yang, Y.; Peng, J.; Zhong, W.; Xu, X. Optimization of Fermentation and Transcriptomic Analysis: The Impact of Aspartic Acid on the Antioxidant Activity of Termitomyces. Fermentation 2025, 11, 202. https://doi.org/10.3390/fermentation11040202
Zhou J, Yi W, Yang Y, Peng J, Zhong W, Xu X. Optimization of Fermentation and Transcriptomic Analysis: The Impact of Aspartic Acid on the Antioxidant Activity of Termitomyces. Fermentation. 2025; 11(4):202. https://doi.org/10.3390/fermentation11040202
Chicago/Turabian StyleZhou, Jingfei, Wenhui Yi, Yunfan Yang, Jiahui Peng, Wujie Zhong, and Xuefeng Xu. 2025. "Optimization of Fermentation and Transcriptomic Analysis: The Impact of Aspartic Acid on the Antioxidant Activity of Termitomyces" Fermentation 11, no. 4: 202. https://doi.org/10.3390/fermentation11040202
APA StyleZhou, J., Yi, W., Yang, Y., Peng, J., Zhong, W., & Xu, X. (2025). Optimization of Fermentation and Transcriptomic Analysis: The Impact of Aspartic Acid on the Antioxidant Activity of Termitomyces. Fermentation, 11(4), 202. https://doi.org/10.3390/fermentation11040202






