Recent Biotechnological Applications of Whey: Review and Perspectives
Abstract
1. Introduction
2. Materials and Methods
- Reference framework: PerSPECTiF
- Population (P): biotechnological processes for the valorization of whey.
- Setting (S): biotechnology and energy industry.
- Perspective (P): researchers, biotechnologists, and industrialists in the energy and bioproducts sector.
- Exposure (E): use of whey in producing biofuels and bioproducts (e.g., organic acids, biopolymers, metabolites, etc.) and recombinant proteins.
- Comparison (C): comparison with other residual substrates or conventional carbon sources.
- Time (T): last 5 years (to capture the most recent trends).
- Findings (F): technological, economic, and environmental feasibility of using whey in these processes.
- Documents that report innovations in the use of whey for food applications in the last 5 years;
- Documents that report applications in the use of whey for biotechnological applications in the production of metabolites at an industrial level in the last 5 years;
- Documents that report the use of whey as an alternative substrate for expressing recombinant proteins within the last 5 years;
- Documents that report information on whey in general and for food use, regardless of the publication date;
- Documents that report the use of IPTG and lactose as inducers of recombinant proteins, as well as the interchangeability between the two, regardless of the year of publication.
- Duplicate documents in databases;
- Documents related to whey that do not have food or biotechnological applications;
- Documents that report the use of whey for research related to medical applications, with tissues or human beings;
- Documents older than or equal to 10 years about biotechnological applications of whey.
3. Biotechnological Applications of Whey
3.1. Production of Lactic Acid, Starter Cultures, and Potential Probiotics
3.2. Production of Bioplastics and Biopolymers
3.3. Biofuel Production
3.4. Biomass Production
3.5. Other Metabolites of Industrial Interest
| Category | Product | Microorganism | Reference |
|---|---|---|---|
| Lactic acid, starter cultures, and potential probiotics | Lactic acid | Lacticaseibacillus casei BL23 | [40] (2021) |
| Lactic acid, probiotics | Lacticaseibacillus paracasei ItalPN16 | [28] (2025) | |
| Starter culture | Lactobacillus delbrueckii subsp. lactis, Lactobacillus delbrueckii subsp. jakobsenii, Lactobacillus leichmannii and Lactobacillus crispatus | [41] (2023) | |
| Starter culture, probiotic | Lactiplantibacillus plantarum | [42] (2021) | |
| Starter culture, probiotic | Lactococcus lactis | [43] (2024) | |
| Bioplastics and Biopolymers | Polyhydroxybutyrate (PHB) | Recombinant E. coli | [26] (2024) |
| Polyhydroxyalcanoates (PHA) | Paracoccus homiensis | [44] (2022) | |
| Polyhydroxyalcanoates (PHA) | Several | [45] (2022) | |
| Biopolymers | Lactic Acid Bacteria | [46] (2023) | |
| Polyhydroxybutyrate (PHB) | Recombinant Escherichia coli LSBJ | [31] (2021) | |
| Biomass and Biofuels | Biomass | Chlorella sp. | [35] (2024) |
| Biomass | Yeasts | [47] (2024) | |
| Biomass | Chlorella sorokiniana UTEX 1230 | [36] (2024) | |
| Biomass | Spirulina platensis, Chlorella homosphaera and Scenedesmus obliquus. | [37] | |
| Butyl acetate, butanol | Clostridium saccharoperbutylacetonicum | [33] (2024) | |
| Bioethanol | Recombinant Saccharomyces cerevisiae | [34] (2021) | |
| Bioethanol | Several | [32] (2022) | |
| Enzymes | [NiFe]-hydrogenases | Cupriavidus necator, E. coli | [48] (2024) |
| Endoglucanases | Recombinant Trichoderma reesei, recombinant Aspergillus carneus | [49] (2024) | |
| α-amylase | Recombinant Anoxybacillus karvacharensis | [50] (2024) | |
| β-galactosidase | Several | [51] (2022) | |
| α-Galactosidases | Several | [52] (2020) | |
| [NiFe]-hydrogenases | Escherichia coli, Ralstonia eutropha | [53] (2023) | |
| Other metabolites of industrial interest | L-threonine | Escherichia coli ATCC® 21277 | [39] (2023) |
| 5-Hydroxytryptophan (5-HTP) | E. coli strain C1T7-S337A/F318Y | [54] (2024) | |
| Lactulose | Enzymatic Bioreactor | [38] (2023) | |
| Recombinant hen ovalbumin and bovine β-lactoglobulin | Trichoderma reesei | [55] (2023) | |
| Galactooligosaccharides | Pantoea anthophila | [56] (2021) | |
| β-Farnesene | Recombinant Escherichia coli | [57] (2021) | |
| D-tagatosa | Paracoccus denitrificans | [58] (2023) |
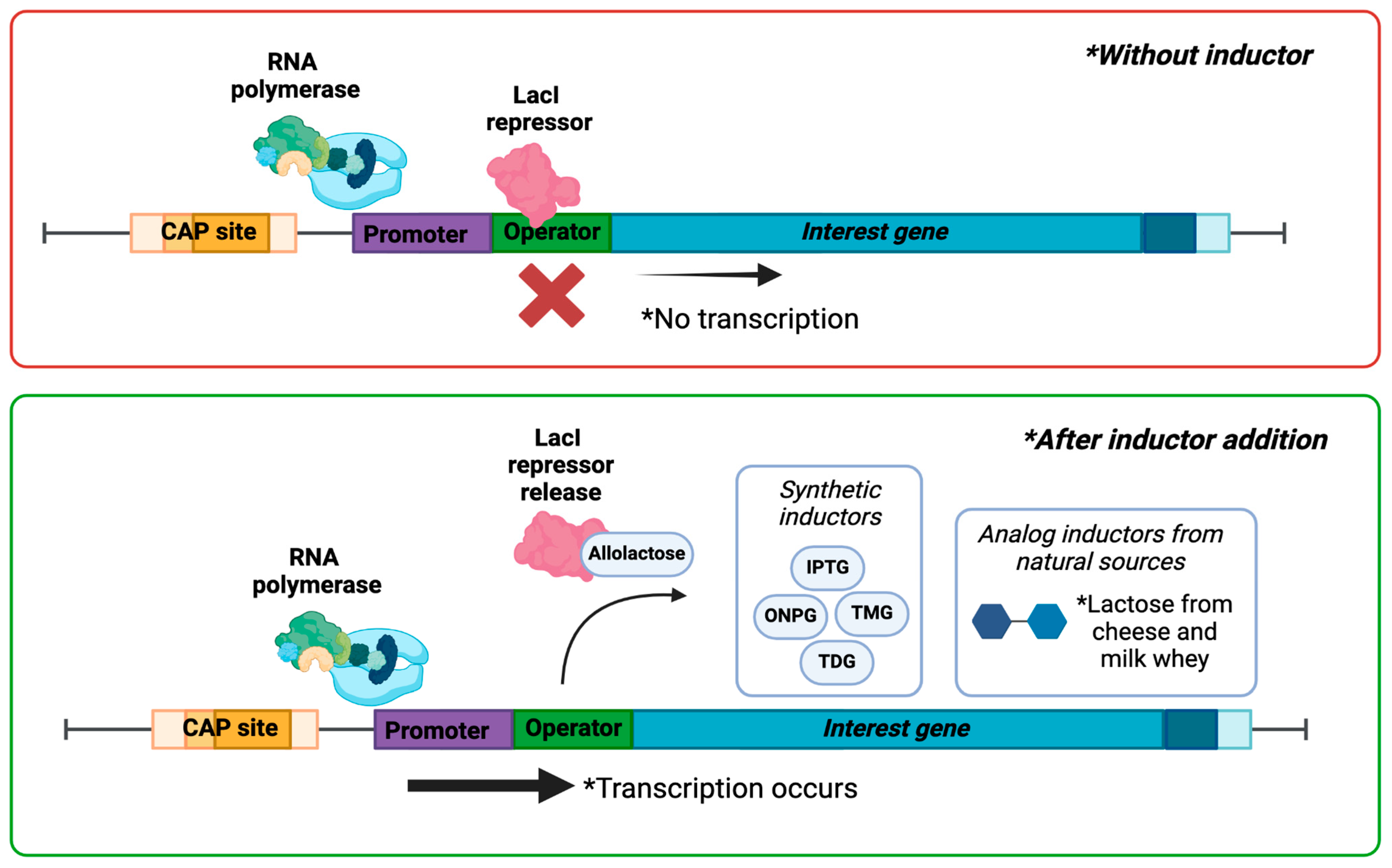
4. The IPTG Inducer
5. Whey as Low-Cost Media for Recombinant Protein Expression
Formulation of Industrial Culture Media Using Whey for Recombinant Protein Expression Induction
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Manzoor, A.; Qazi, J.I.; ul Haq, I.; Mukhtar, H.; Rasool, A. Significantly Enhanced Biomass Production of a Novel Bio-Therapeutic Strain Lactobacillus plantarum (AS-14) by Developing Low Cost Media Cultivation Strategy. J. Biol. Eng. 2017, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Osorio-González, C.S.; Gómez-Falcon, N.; Brar, S.K.; Ramírez, A.A. Cheese Whey as a Potential Feedstock for Producing Renewable Biofuels: A Review. Energies 2022, 15, 6828. [Google Scholar] [CrossRef]
- Besediuk, V.; Yatskov, M.; Korchyk, N.; Kucherova, A.; Maletskyi, Z. Whey-from Waste to a Valuable Resource. J. Agric. Food Res. 2024, 18, 101280. [Google Scholar] [CrossRef]
- Schmidt, R.H.; Packard, V.S.; Morris, H.A. Effect of Processing on Whey Protein Functionality. J. Dairy. Sci. 1984, 67, 2723–2733. [Google Scholar] [CrossRef]
- Shelke, P.A.; Sabikhi, L.; Khetra, Y.; Ganguly, S.; Baig, D. Effect of Skim Milk Addition and Heat Treatment on Characteristics of Cow Milk Ricotta Cheese Manufactured from Cheddar Cheese Whey. LWT 2022, 162, 113405. [Google Scholar] [CrossRef]
- Yazici, F.; Dervisoglu, M.; Akgun, A.; Aydemir, O. Effect of Whey PH at Drainage on Physicochemical, Biochemical, Microbiological, and Sensory Properties of Mozzarella Cheese Made from Buffalo Milk during Refrigerated Storage. J. Dairy. Sci. 2010, 93, 5010–5019. [Google Scholar] [CrossRef]
- Luisa, B.G. Handbook of Milk Composition; Elsevier: Amsterdam, The Netherlands, 1995. [Google Scholar]
- Lin, T.; Meletharayil, G.; Kapoor, R.; Abbaspourrad, A. Bioactives in Bovine Milk: Chemistry, Technology, and Applications. Nutr. Rev. 2021, 79, 48–69. [Google Scholar] [CrossRef]
- Vashisht, P.; Sharma, A.; Awasti, N.; Wason, S.; Singh, L.; Sharma, S.; Charles, A.P.R.; Sharma, S.; Gill, A.; Khattra, A.K. Comparative Review of Nutri-Functional and Sensorial Properties, Health Benefits and Environmental Impact of Dairy (Bovine Milk) and Plant-Based Milk (Soy, Almond, and Oat Milk). Food Humanit. 2024, 2, 100301. [Google Scholar] [CrossRef]
- Haug, A.; Høstmark, A.T.; Harstad, O.M. Bovine Milk in Human Nutrition—A Review. Lipids Health Dis. 2007, 6, 25. [Google Scholar] [CrossRef]
- Madureira, A.R.; Pereira, C.I.; Gomes, A.M.P.; Pintado, M.E.; Malcata, F.X. Bovine Whey Proteins–Overview on Their Main Biological Properties. Food Res. Int. 2007, 40, 1197–1211. [Google Scholar] [CrossRef]
- Stocco, G.; Summer, A.; Cipolat-Gotet, C.; Malacarne, M.; Cecchinato, A.; Amalfitano, N.; Bittante, G. The Mineral Profile Affects the Coagulation Pattern and Cheese-Making Efficiency of Bovine Milk. J. Dairy. Sci. 2021, 104, 8439–8453. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, S.; Barać, M.; Maćej, O. Whey Proteins-Properties and Possibility of Application. Mljekarstvo 2005, 55, 215–233. [Google Scholar]
- Solak, B.B.; Akin, N. Health Benefits of Whey Protein: A Review. J. Food Sci. Eng. 2012, 2, 129. [Google Scholar] [CrossRef]
- Gržinić, G.; Piotrowicz-Cieślak, A.; Klimkowicz-Pawlas, A.; Górny, R.L.; Ławniczek-Wałczyk, A.; Piechowicz, L.; Olkowska, E.; Potrykus, M.; Tankiewicz, M.; Krupka, M.; et al. Intensive Poultry Farming: A Review of the Impact on the Environment and Human Health. Sci. Total Environ. 2023, 858, 160014. [Google Scholar] [CrossRef]
- Marwaha, S.S.; Kennedy, J.F. Whey—Pollution Problem and Potential Utilization. Int. J. Food Sci. Technol. 1988, 23, 323–336. [Google Scholar] [CrossRef]
- Pires, A.F.; Marnotes, N.G.; Rubio, O.D.; Garcia, A.C.; Pereira, C.D. Dairy By-Products: A Review on the Valorization of Whey and Second Cheese Whey. Foods 2021, 10, 1067. [Google Scholar] [CrossRef]
- de Almeida, M.P.; Mockaitis, G.; Weissbrodt, D.G. Got Whey? Sustainability Endpoints for the Dairy Industry through Resource Biorecovery. Fermentation 2023, 9, 897. [Google Scholar] [CrossRef]
- Kosseva, M.R.; Panesar, P.S.; Kaur, G.; Kennedy, J.F. Use of Immobilised Biocatalysts in the Processing of Cheese Whey. Int. J. Biol. Macromol. 2009, 45, 437–447. [Google Scholar] [CrossRef]
- Caltzontzin-Rabell, V.; Feregrino-Pérez, A.A.; Gutiérrez-Antonio, C. Bio-Upcycling of Cheese Whey: Transforming Waste into Raw Materials for Biofuels and Animal Feed. Heliyon 2024, 10, e32700. [Google Scholar] [CrossRef]
- Westers, L.; Westers, H.; Quax, W.J. Bacillus subtilis as Cell Factory for Pharmaceutical Proteins: A Biotechnological Approach to Optimize the Host Organism. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2004, 1694, 299–310. [Google Scholar] [CrossRef]
- Viitanen, M.I.; Vasala, A.; Neubauer, P.; Alatossava, T. Cheese Whey-Induced High-Cell-Density Production of Recombinant Proteins in Escherichia coli. Microb. Cell Fact. 2003, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Molins, F.; Serrano, M.A. Bases Neurales de La Aversión a Las Pérdidas En Contextos Económicos: Revisión Sistemática Según Las Directrices PRISMA. Rev. Neurol. 2019, 68, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Boumaiza, M.; Colarusso, A.; Parrilli, E.; Garcia-Fruitós, E.; Casillo, A.; Arís, A.; Corsaro, M.M.; Picone, D.; Leone, S.; Tutino, M.L. Getting Value from the Waste: Recombinant Production of a Sweet Protein by Lactococcus lactis Grown on Cheese Whey. Microb. Cell Fact. 2018, 17, 126. [Google Scholar] [CrossRef] [PubMed]
- Goyal, C.; Dhyani, P.; Rai, D.C.; Tyagi, S.; Dhull, S.B.; Sadh, P.K.; Duhan, J.S.; Saharan, B.S. Emerging Trends and Advancements in the Processing of Dairy Whey for Sustainable Biorefining. J. Food Process. Preserv. 2023, 2023, 6626513. [Google Scholar] [CrossRef]
- Jia, L.; Kaur, G.; Juneja, A.; Majumder, E.L.-W.; Ramarao, B.V.; Kumar, D. Polyhydroxybutyrate Production from Non-Recyclable Fiber Rejects and Acid Whey as Mixed Substrate by Recombinant Escherichia coli. Biotechnol. Sustain. Mater. 2024, 1, 12. [Google Scholar] [CrossRef]
- Gordillo-Andia, C.; Almirón, J.; Barreda-Del-Carpio, J.E.; Roudet, F.; Tupayachy-Quispe, D.; Vargas, M. Influence of Kluyveromyces lactis and Enterococcus Faecalis on Obtaining Lactic Acid by Cheese Whey Fermentation. Appl. Sci. 2024, 14, 4649. [Google Scholar] [CrossRef]
- Pinilla, C.M.B.; Galland, F.; Pacheco, M.T.B.; Bócoli, P.J.; Borges, D.F.; Alvim, I.D.; Spadoti, L.M.; e Alves, A.T.S. Unraveling the Real Potential of Liquid Whey as Media Culture and Microencapsulation Material for Lactic Acid Bacteria. Innov. Food Sci. Emerg. Technol. 2025, 100, 103885. [Google Scholar] [CrossRef]
- Ascencio-Galván, M.L.; López-Agudelo, V.A.; Gómez-Ríos, D.; Ramirez-Malule, H. A Bibliometric Landscape of Polyhydroxyalkanoates Production from Low-Cost Substrates by Cupriavidus necator and Its Perspectives for the Latin American Bioeconomy. J. Appl. Biol. Biotechnol. 2024, X, 1–14. [Google Scholar] [CrossRef]
- Hasan, S.F.; Elsoud, M.M.A.; Sidkey, N.M.; Elhateir, M.M. Production and Characterization of Polyhydroxybutyrate Bioplastic Precursor from Parageobacillus toebii Using Low-Cost Substrates and Its Potential Antiviral Activity. Int. J. Biol. Macromol. 2024, 262, 129915. [Google Scholar] [CrossRef]
- Hou, L.; Jia, L.; Morrison, H.M.; Majumder, E.L.-W.; Kumar, D. Enhanced Polyhydroxybutyrate Production from Acid Whey through Determination of Process and Metabolic Limiting Factors. Bioresour. Technol. 2021, 342, 125973. [Google Scholar] [CrossRef]
- Zou, J.; Chang, X. Past, Present, and Future Perspectives on Whey as a Promising Feedstock for Bioethanol Production by Yeast. J. Fungi 2022, 8, 395. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Guo, N.; Wang, S.; Wang, Y.; Jiang, Z.; Guo, L.; Luo, W.; Wang, Y. Metabolically Engineer Clostridium saccharoperbutylacetonicum for Comprehensive Conversion of Acid Whey into Valuable Biofuels and Biochemicals. Bioresour. Technol. 2024, 400, 130640. [Google Scholar] [CrossRef] [PubMed]
- Cunha, J.T.; Gomes, D.G.; Romaní, A.; Inokuma, K.; Hasunuma, T.; Kondo, A.; Domingues, L. Cell Surface Engineering of Saccharomyces cerevisiae for Simultaneous Valorization of Corn Cob and Cheese Whey via Ethanol Production. Energy Convers. Manag. 2021, 243, 114359. [Google Scholar] [CrossRef]
- Stratigakis, N.C.; Nazos, T.T.; Chatzopoulou, M.; Mparka, N.; Spantidaki, M.; Lagouvardou-Spantidaki, A.; Ghanotakis, D.F. Cultivation of a Naturally Resilient Chlorella sp.: A Bioenergetic Strategy for Valorization of Cheese Whey for High Nutritional Biomass Production. Algal Res. 2024, 82, 103616. [Google Scholar] [CrossRef]
- Chen, S.; Zhu, H.; Radican, E.; Wang, X.; D’Amico, D.J.; Xiao, Z.; Luo, Y. Perspectives on Optimizing Microalgae Cultivation: Harnessing Dissolved CO2 and Lactose for Sustainable and Cost-Efficient Protein Production. J. Agric. Food Res. 2024, 18, 101387. [Google Scholar] [CrossRef]
- Braun, J.C.A.; Balbinot, L.; Beuter, M.A.; Rempel, A.; Colla, L.M. Mixotrophic Cultivation of Microalgae Using Agro-Industrial Waste: Tolerance Level, Scale up, Perspectives and Future Use of Biomass. Algal Res. 2024, 80, 103554. [Google Scholar] [CrossRef]
- Sitanggang, A.B. Production of Lactulose from Cheese Whey. In Enzymes Beyond Traditional Applications in Dairy Science and Technology; Elsevier: Amsterdam, The Netherlands, 2023; pp. 403–423. [Google Scholar]
- Blandón, J.F.V.; Henao, C.P.S.; Montoya, J.E.Z.; Ochoa, S. L-Threonine Production from Whey and Fish Hydrolysate by E. coli ATCC® 21277TM. Heliyon 2023, 9, e18744. [Google Scholar] [CrossRef]
- Catone, M.V.; Palomino, M.M.; Legisa, D.M.; Fina Martin, J.; Monedero Garcia, V.; Ruzal, S.M.; Allievi, M.C. Lactic Acid Production Using Cheese Whey Based Medium in a Stirred Tank Reactor by a CcpA Mutant of Lacticaseibacillus casei. World J. Microbiol. Biotechnol. 2021, 37, 61. [Google Scholar] [CrossRef]
- Naziri, E.; Papadaki, E.; Savvidis, I.; Botsaris, G.; Gkatzionis, K.; Mugampoza, E.; Mantzouridou, F.T. Exploring the Potential of Halloumi Second Cheese Whey for the Production of Lactic Acid Cultures for the Dairy Industry. Sustainability 2023, 15, 9082. [Google Scholar] [CrossRef]
- Brizuela, N.S.; Arnez-Arancibia, M.; Semorile, L.; Bravo-Ferrada, B.M.; Tymczyszyn, E.E. Whey Permeate as a Substrate for the Production of Freeze-Dried Lactiplantibacillus plantarum to Be Used as a Malolactic Starter Culture. World J. Microbiol. Biotechnol. 2021, 37, 115. [Google Scholar] [CrossRef]
- De Chiara, I.; Marasco, R.; Della Gala, M.; Fusco, A.; Donnarumma, G.; Muscariello, L. Probiotic Properties of Lactococcus lactis Strains Isolated from Natural Whey Starter Cultures. Foods 2024, 13, 957. [Google Scholar] [CrossRef] [PubMed]
- Mozejko-Ciesielska, J.; Marciniak, P.; Moraczewski, K.; Rytlewski, P.; Czaplicki, S.; Zadernowska, A. Cheese Whey Mother Liquor as Dairy Waste with Potential Value for Polyhydroxyalkanoate Production by Extremophilic Paracoccus homiensis. Sustain. Mater. Technol. 2022, 33, e00449. [Google Scholar] [CrossRef]
- Kannah, R.Y.; Kumar, M.D.; Kavitha, S.; Banu, J.R.; Tyagi, V.K.; Rajaguru, P.; Kumar, G. Production and Recovery of Polyhydroxyalkanoates (PHA) from Waste Streams—A Review. Bioresour. Technol. 2022, 366, 128203. [Google Scholar] [CrossRef]
- Nicolescu, C.M.; Bumbac, M.; Buruleanu, C.L.; Popescu, E.C.; Stanescu, S.G.; Georgescu, A.A.; Toma, S.M. Biopolymers Produced by Lactic Acid Bacteria: Characterization and Food Application. Polymers 2023, 15, 1539. [Google Scholar] [CrossRef]
- Martin, G.J.O.; Chan, S. Future Production of Yeast Biomass for Sustainable Proteins: A Critical Review. Sustain. Food Technol. 2024, 2, 1592–1609. [Google Scholar] [CrossRef]
- Iskandaryan, M.; Baghdasaryan, L.; Minasyan, E.; Trchounian, K.; Antranikian, G.; Poladyan, A. A Novel, Cost-Effective Approach for the Production of Hydrogenase Enzymes and Molecular Hydrogen from Recycled Whey-Based by-Products. Int. J. Hydrogen Energy, 2024; in press. [Google Scholar] [CrossRef]
- Crament, T.C.; Arendsen, K.; Rose, S.H.; Jansen, T. Cultivation of Recombinant Aspergillus Niger Strains on Dairy Whey as a Carbohydrate Source. J. Ind. Microbiol. Biotechnol. 2024, 51, kuae007. [Google Scholar] [CrossRef]
- Ghevondyan, D.; Soghomonyan, T.; Hovhannisyan, P.; Margaryan, A.; Paloyan, A.; Birkeland, N.-K.; Antranikian, G.; Panosyan, H. Detergent-Resistant α-Amylase Derived from Anoxybacillus karvacharensis K1 and Its Production Based on Whey. Sci. Rep. 2024, 14, 12682. [Google Scholar] [CrossRef]
- Movahedpour, A.; Ahmadi, N.; Ghalamfarsa, F.; Ghesmati, Z.; Khalifeh, M.; Maleksabet, A.; Shabaninejad, Z.; Taheri-Anganeh, M.; Savardashtaki, A. β-Galactosidase: From Its Source and Applications to Its Recombinant Form. Biotechnol. Appl. Biochem. 2022, 69, 612–628. [Google Scholar] [CrossRef]
- Álvarez-Cao, M.-E.; Becerra, M.; González-Siso, M.-I. Biovalorization of Cheese Whey and Molasses Wastes to Galactosidases by Recombinant Yeasts. In Biovalorisation of Wastes to Renewable Chemicals and Biofuels; Elsevier: Amsterdam, The Netherlands, 2020; pp. 149–161. [Google Scholar]
- Poladyan, A.; Trchounian, K.; Paloyan, A.; Minasyan, E.; Aghekyan, H.; Iskandaryan, M.; Khoyetsyan, L.; Aghayan, S.; Tsaturyan, A.; Antranikian, G. Valorization of Whey-Based Side Streams for Microbial Biomass, Molecular Hydrogen, and Hydrogenase Production. Appl. Microbiol. Biotechnol. 2023, 107, 4683–4696. [Google Scholar] [CrossRef]
- Shen, B.; Zhang, L.; Zhou, Y.; Song, F.; You, S.; Su, R.; Qi, W. Efficient Synthesis of 5-Hydroxytryptophan in Escherichia coli by Bifunctional Utilization of Whey Powder as a Substrate for Cell Growth and Inducer Production. J. Biotechnol. 2024, 393, 100–108. [Google Scholar] [CrossRef]
- Aro, N.; Ercili-Cura, D.; Andberg, M.; Silventoinen, P.; Lille, M.; Hosia, W.; Nordlund, E.; Landowski, C.P. Production of Bovine Beta-Lactoglobulin and Hen Egg Ovalbumin by Trichoderma reesei Using Precision Fermentation Technology and Testing of Their Techno-Functional Properties. Food Res. Int. 2023, 163, 112131. [Google Scholar] [CrossRef] [PubMed]
- Yañez-Ñeco, C.V.; Cervantes, F.V.; Amaya-Delgado, L.; Ballesteros, A.O.; Plou, F.J.; Arrizon, J. Synthesis of β (1→ 3) and β (1→ 6) Galactooligosaccharides from Lactose and Whey Using a Recombinant β-Galactosidase from Pantoea anthophila. Electron. J. Biotechnol. 2021, 49, 14–21. [Google Scholar] [CrossRef]
- Ding, J.; You, S.; Ba, W.; Zhang, H.; Chang, H.; Qi, W.; Su, R.; He, Z. Bifunctional Utilization of Whey Powder as a Substrate and Inducer for β-Farnesene Production in an Engineered Escherichia coli. Bioresour. Technol. 2021, 341, 125739. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Li, F.; Li, L.; Li, B.; Niu, K.; Liu, Q.; Han, L.; Han, L.; Fang, X. Production of D-tagatose, bioethanol, and microbial protein from the dairy industry by-product whey powder using an integrated bioprocess. Biotechnol. J. 2024, 19, 2300415. [Google Scholar] [CrossRef]
- Müller, J.; Oehler, S.; Müller-Hill, B. Repression OflacPromoter as a Function of Distance, Phase and Quality of an AuxiliarylacOperator. J. Mol. Biol. 1996, 257, 21–29. [Google Scholar] [CrossRef]
- Jacob, F.; Monod, J. Genetic Regulatory Mechanisms in the Synthesis of Proteins. J. Mol. Biol. 1961, 3, 318–356. [Google Scholar] [CrossRef]
- Lewis, M.; Chang, G.; Horton, N.C.; Kercher, M.A.; Pace, H.C.; Schumacher, M.A.; Brennan, R.G.; Lu, P. Crystal Structure of the Lactose Operon Repressor and Its Complexes with DNA and Inducer. Science 1996, 271, 1247–1254. [Google Scholar] [CrossRef]
- Browning, D.F.; Godfrey, R.E.; Richards, K.L.; Robinson, C.; Busby, S.J.W. Exploitation of the Escherichia coli Lac Operon Promoter for Controlled Recombinant Protein Production. Biochem. Soc. Trans. 2019, 47, 755–763. [Google Scholar] [CrossRef]
- Rosano, G.L.; Ceccarelli, E.A. Recombinant Protein Expression in Escherichia coli: Advances and Challenges. Front. Microbiol. 2014, 5, 172. [Google Scholar] [CrossRef]
- Tan, J.S.; Ramanan, R.N.; Ling, T.C.; Mustafa, S.; Ariff, A.B. The Role of Lac Operon and Lac Repressor in the Induction Using Lactose for the Expression of Periplasmic Human Interferon-A2b by Escherichia coli. Ann. Microbiol. 2012, 62, 1427–1435. [Google Scholar] [CrossRef]
- Arsov, A.; Armenova, N.; Gergov, E.; Petrov, K.; Petrova, P. Cloning Systems in Bacillus: Bioengineering of Metabolic Pathways for Valuable Recombinant Products. Fermentation 2024, 10, 50. [Google Scholar] [CrossRef]
- Phan, T.T.P.; Tran, L.T.; Schumann, W.; Nguyen, H.D. Development of P Grac 100-Based Expression Vectors Allowing High Protein Production Levels in Bacillus subtilis and Relatively Low Basal Expression in Escherichia coli. Microb. Cell Fact. 2015, 14, 72. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Li, Y.; Bai, Y.; Zhang, T.; Jiang, W.; Shi, T.; Wu, Z.; Zhang, Y.-H.P.J. A Facile and Robust T7-Promoter-Based High-Expression of Heterologous Proteins in Bacillus subtilis. Bioresour. Bioprocess. 2022, 9, 56. [Google Scholar] [CrossRef] [PubMed]
- Krijger, J.-J.; Baumann, J.; Wagner, M.; Schulze, K.; Reinsch, C.; Klose, T.; Onuma, O.F.; Simon, C.; Behrens, S.-E.; Breunig, K.D. A Novel, Lactase-Based Selection and Strain Improvement Strategy for Recombinant Protein Expression in Kluyveromyces lactis. Microb. Cell Fact. 2012, 11, 112. [Google Scholar] [CrossRef]
- Park, J.H.; Bassalo, M.C.; Lin, G.-M.; Chen, Y.; Doosthosseini, H.; Schmitz, J.; Roubos, J.A.; Voigt, C.A. Design of Four Small-Molecule-Inducible Systems in the Yeast Chromosome, Applied to Optimize Terpene Biosynthesis. ACS Synth. Biol. 2023, 12, 1119–1132. [Google Scholar] [CrossRef]
- Swinkels, B.W.; van Ooyen, A.J.J.; Bonekamp, F.J. The Yeast Kluyveromyces lactis as an Efficient Host for Heterologous Gene Expression. Antonie Van Leeuwenhoek 1993, 64, 187–201. [Google Scholar] [CrossRef]
- Myung, S.-H.; Park, J.; Han, J.-H.; Kim, T.-H. Development of the Mammalian Expression Vector System That Can Be Induced by IPTG and/or Lactose. J. Microbiol. Biotechnol. 2020, 30, 1124. [Google Scholar] [CrossRef]
- Faust, G.; Stand, A.; Weuster-Botz, D. IPTG Can Replace Lactose in Auto-Induction Media to Enhance Protein Expression in Batch-Cultured Escherichia coli. Eng. Life Sci. 2015, 15, 824–829. [Google Scholar] [CrossRef]
- Kataoka, K.; Takasu, A. IPTG-Independent Autoinduction of Extracellular Matrix Proteins Using Recombinant E. coli as the Expression Host. Polym. J. 2021, 53, 385–391. [Google Scholar] [CrossRef]
- Xu, J.-M.; Wu, Z.-S.; Zhao, K.-J.; Xi, Z.-J.; Wang, L.-Y.; Cheng, F.; Xue, Y.-P.; Zheng, Y.-G. IPTG-Induced High Protein Expression for Whole-Cell Biosynthesis of L-Phosphinothricin. Biotechnol. J. 2023, 18, 2300027. [Google Scholar] [CrossRef]
- Dvorak, P.; Chrast, L.; Nikel, P.I.; Fedr, R.; Soucek, K.; Sedlackova, M.; Chaloupkova, R.; de Lorenzo, V.; Prokop, Z.; Damborsky, J. Exacerbation of Substrate Toxicity by IPTG in Escherichia coli BL21 (DE3) Carrying a Synthetic Metabolic Pathway. Microb. Cell Fact. 2015, 14, 201. [Google Scholar] [CrossRef]
- Jones, K.L.; Kim, S.-W.; Keasling, J.D. Low-Copy Plasmids Can Perform as Well as or Better than High-Copy Plasmids for Metabolic Engineering of Bacteria. Metab. Eng. 2000, 2, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.A.; Yaverino-Gutierrez, M.A.; Monteiro, M.C.; Souza, B.A.; Bachheti, R.K.; Chandel, A.K. Precision Fermentation in the Realm of Microbial Protein Production: State-of-the-Art and Future Insights. Food Res. Int. 2024, 200, 115527. [Google Scholar] [CrossRef] [PubMed]
- Meshram, P.; Murmu, M.; Barage, S.; Singh, R. Filamentous Fungi as Cell Factories for Heterogeneous Protein Production. In Fundamentals of Recombinant Protein Production, Purification and Characterization; Elsevier: Amsterdam, The Netherlands, 2025; pp. 143–169. [Google Scholar]
- Gombert, A.K.; Kilikian, B.V. Recombinant Gene Expression in Escherichia coli Cultivation Using Lactose as Inducer. J. Biotechnol. 1998, 60, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.U.; Chung, S.I.; Seo, J.H.; Kwon, L.H.; Jung, K.H. Induction of the T7 Promoter Using Lactose for Production of Recombinant Plasminogen Kringle 1-3 in Escherichia coli. J. Microbiol. Biotechnol. 2004, 14, 225–230. [Google Scholar]
- Khani, M.-H.; Bagheri, M. Skimmed Milk as an Alternative for IPTG in Induction of Recombinant Protein Expression. Protein Expr. Purif. 2020, 170, 105593. [Google Scholar] [CrossRef]
- de Divitiis, M.; Ami, D.; Pessina, A.; Palmioli, A.; Sciandrone, B.; Airoldi, C.; Regonesi, M.E.; Brambilla, L.; Lotti, M.; Natalello, A.; et al. Cheese-Whey Permeate Improves the Fitness of Escherichia coli Cells during Recombinant Protein Production. Biotechnol. Biofuels Bioprod. 2023, 16, 30. [Google Scholar] [CrossRef]
- Mobayed, F.H.; Nunes, J.C.; Gennari, A.; de Andrade, B.C.; Ferreira, M.L.V.; Pauli, P.; Renard, G.; Chies, J.M.; Volpato, G.; de Souza, C.F. Effect of By-Products from the Dairy Industry as Alternative Inducers of Recombinant β-Galactosidase Expression. Biotechnol. Lett. 2021, 43, 589–599. [Google Scholar] [CrossRef]
- Gennari, A.; Simon, R.; de Andrade, B.C.; Kuhn, D.; Renard, G.; Chies, J.M.; Volpato, G.; de Souza, C.F.V. Recombinant Production in Escherichia coli of a β-Galactosidase Fused to a Cellulose-Binding Domain Using Low-Cost Inducers in Fed-Batch Cultivation. Process Biochem. 2023, 124, 290–298. [Google Scholar] [CrossRef]
- Bianchi, G.; Pessina, A.; Ami, D.; Signorelli, S.; de Divitiis, M.; Natalello, A.; Lotti, M.; Brambilla, L.; Brocca, S.; Mangiagalli, M. Sustainable Production of a Biotechnologically Relevant β-Galactosidase in Escherichia coli Cells Using Crude Glycerol and Cheese Whey Permeate. Bioresour. Technol. 2024, 406, 131063. [Google Scholar] [CrossRef]
- de Souza, T.C.; Oliveira, R.C.; Bezerra, S.G.S.; Manzo, R.M.; Mammarella, E.J.; Hissa, D.C.; Gonçalves, L.R.B. Alternative Heterologous Expression of L-Arabinose Isomerase from Enterococcus Faecium DBFIQ E36 by Residual Whey Lactose Induction. Mol. Biotechnol. 2021, 63, 289–304. [Google Scholar] [CrossRef] [PubMed]
- Alifiya, Q.; Taufik, E.; Soenarno, M.S.; Arifin, M.; Budiman, C. Utilization of Whey-Based Growth Media for Production of Recombinant Papain-like Protease of SARS-CoV-2 under Escherichia coli Host Cells. IOP Conf. Ser. Earth Environ. Sci. 2024, 1359, 12014. [Google Scholar] [CrossRef]
- Bobyr, I.M.; Bondarenko, V.L.; Iungin, O.S. Optimization of Culture Media for Industrial Cultivation of the Recombinant Strain Escherichia coli BL21. Ukr. J. Nat. Sci. 2024, 9, 17–24. [Google Scholar] [CrossRef]
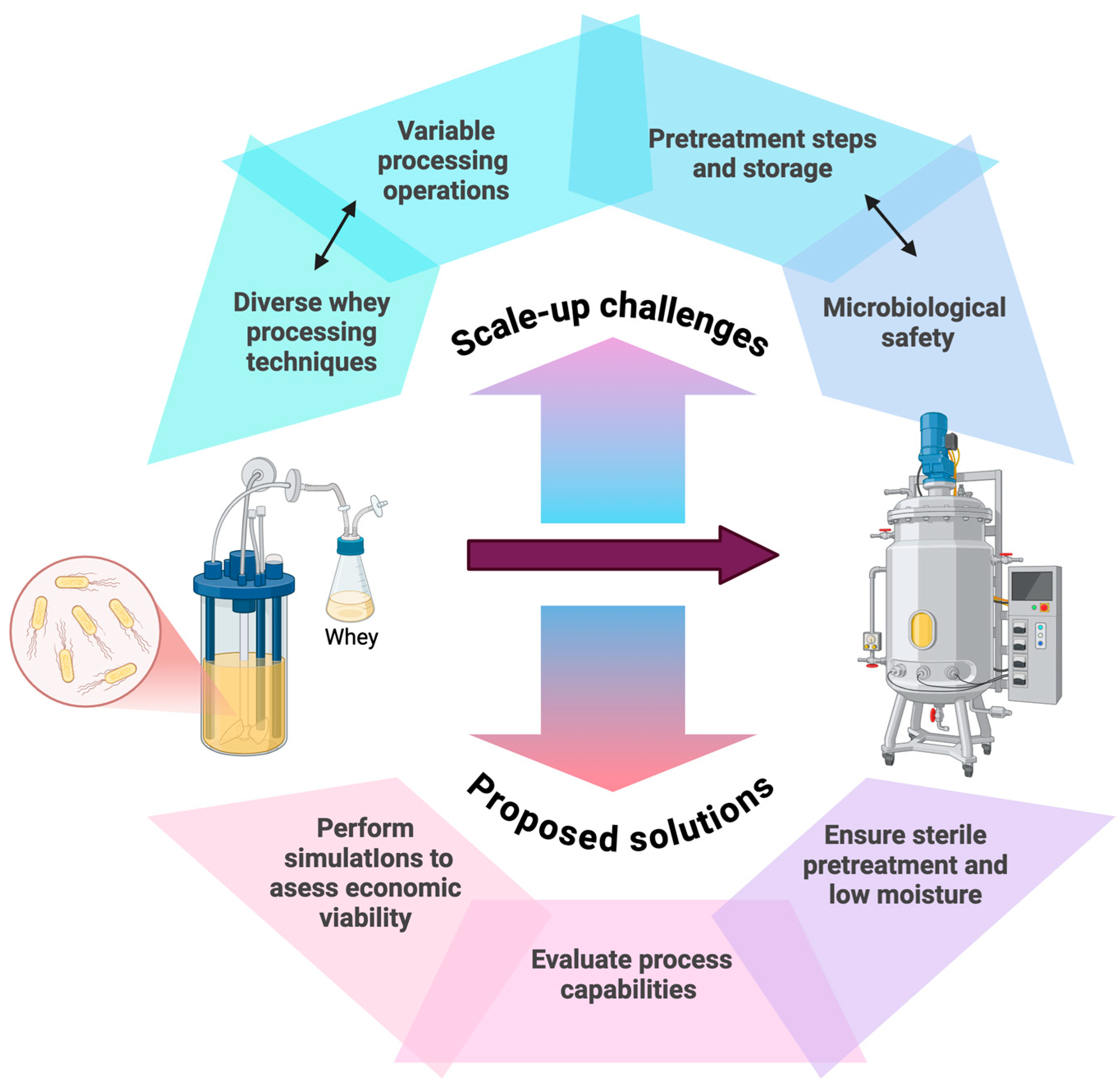
- Buchanan, D.; Martindale, W.; Romeih, E.; Hebishy, E. Recent Advances in Whey Processing and Valorisation: Technological and Environmental Perspectives. Int. J. Dairy. Technol. 2023, 76, 291–312. [Google Scholar] [CrossRef]
- Ozel, B.; McClements, D.J.; Arikan, C.; Kaner, O.; Oztop, M.H. Challenges in Dried Whey Powder Production: Quality Problems. Food Res. Int. 2022, 160, 111682. [Google Scholar] [CrossRef]
- Hebishy, E.; Yerlikaya, O.; Mahony, J.; Akpinar, A.; Saygili, D. Microbiological Aspects and Challenges of Whey Powders–I Thermoduric, Thermophilic and Spore-Forming Bacteria. Int. J. Dairy. Technol. 2023, 76, 779–800. [Google Scholar] [CrossRef]
- Colacicco, M.; De Micco, C.; Macrelli, S.; Agrimi, G.; Janssen, M.; Bettiga, M.; Pisano, I. Process Scale-up Simulation and Techno-Economic Assessment of Ethanol Fermentation from Cheese Whey. Biotechnol. Biofuels Bioprod. 2024, 17, 124. [Google Scholar] [CrossRef]
- Sebastián-Nicolás, J.L.; González-Olivares, L.G.; Vázquez-Rodríguez, G.A.; Lucho-Constatino, C.A.; Castañeda-Ovando, A.; Cruz-Guerrero, A.E. Valorization of Whey Using a Biorefinery. Biofuels Bioprod. Biorefining 2020, 14, 1010–1027. [Google Scholar] [CrossRef]
- Rabell, V.C.; Antonio, C.G. Revalorization of Cheese Whey through a Biorefinery Scheme. Chem. Eng. Trans. 2024, 109, 241–246. [Google Scholar] [CrossRef]
- Ozturk, A.B.; Acar, Z.D.; Yilmaz, F.; Sagdic, O. Whey Conversion Scenarios for Sustainable Lactic Acid, Ethanol and Hydrogen Production: Techno-Economic Aspects of Biofuels and Biochemical Production. Biofuels Bioprod. Biorefining, 2025; early view. [Google Scholar] [CrossRef]
- Mirsalami, S.M.; Mirsalami, M. Advances in Genetically Engineered Microorganisms: Transforming Food Production through Precision Fermentation and Synthetic Biology. Future Foods 2025, 11, 100601. [Google Scholar] [CrossRef]
- Ajayeoba, T.A.; Ijabadeniyi, O.A. Transforming Food for the Future: Precision Fermentation as a Key to Sustainability, Nutrition, and Health. Fermentation, 2025; pre-print version. [Google Scholar] [CrossRef]
- Hupp, B.; Pap, B.; Farkas, A.; Maróti, G. Development of a Microalgae-Based Continuous Starch-to-Hydrogen Conversion Approach. Fermentation 2022, 8, 294. [Google Scholar] [CrossRef]
- da Silva, A.F.; Moreira, A.F.; Miguel, S.P.; Coutinho, P. Recent Advances in Microalgae Encapsulation Techniques for Biomedical Applications. Adv. Colloid. Interface Sci. 2024, 333, 103297. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.E.; Ibrahim, G.E.; Abdella, M.A.A. Enhancement of β-Galactosidase Catalytic Activity and Stability through Covalent Immobilization onto Alginate/Tea Waste Beads and Evaluating Its Impact on the Quality of Some Dairy Products. Int. J. Biol. Macromol. 2024, 278, 134810. [Google Scholar] [CrossRef] [PubMed]
- Abdella, M.A.A.; Hassan, M.E.; Soliman, T.N. Covalently Immobilized β-Galactosidase onto a Novel Alginate/Lemon Peel Carrier: Catalytic, Kinetic, Stability Studies, and Its Application in the Production of Whey High-Protein Beverage. Int. J. Biol. Macromol. 2025, 307, 142222. [Google Scholar] [CrossRef]
- Laksmi, F.A.; Lischer, K.; Nugraha, Y.; Violando, W.A.; Helbert; Nuryana, I.; Khasna, F.N.; Nur, N.; Ramadhan, K.P.; Tobing, D.A.L.; et al. A Robust Strategy for Overexpression of DNA Polymerase from Thermus Aquaticus Using an IPTG-Independent Autoinduction System in a Benchtop Bioreactor. Sci. Rep. 2025, 15, 5891. [Google Scholar] [CrossRef]
- Wang, Z.; Dai, Y.; Azi, F.; Wang, Z.; Xu, W.; Wang, D.; Dong, M.; Xia, X. Engineering Escherichia coli for Cost-Effective Production of Medium-Chain Fatty Acids from Soy Whey Using an Optimized Galactose-Based Autoinduction System. Bioresour. Technol. 2024, 393, 130145. [Google Scholar] [CrossRef]
- Genç, H.; Friedrich, B.; Alexiou, C.; Pietryga, K.; Cicha, I.; Douglas, T.E.L. Endothelialization of Whey Protein Isolate-Based Scaffolds for Tissue Regeneration. Molecules 2023, 28, 7052. [Google Scholar] [CrossRef]
- Boga, B.; Akbulut, M.; Maytalman, E.; Kozanoglu, I. Effect of Milk and Whey on Proliferation and Differentiation of Placental Stromal Cells. Cytotechnology 2023, 75, 391–401. [Google Scholar] [CrossRef]
- Słota, D.; Głab, M.; Tyliszczak, B.; Douglas, T.E.L.; Rudnicka, K.; Miernik, K.; Urbaniak, M.M.; Rusek-Wala, P.; Sobczak-Kupiec, A. Composites Based on Hydroxyapatite and Whey Protein Isolate for Applications in Bone Regeneration. Materials 2021, 14, 2317. [Google Scholar] [CrossRef]
- Escobar-García, J.D.; Prieto, C.; Pardo-Figuerez, M.; Lagaron, J.M. Room Temperature Nanoencapsulation of Bioactive Eicosapentaenoic Acid Rich Oil within Whey Protein Microparticles. Nanomaterials 2021, 11, 575. [Google Scholar] [CrossRef]
- Al-subhi, F.M.M. Development, Characterization, and Prolonged Shelf Life of Functional Yogurt Enriched with Nanoencapsulated Yellow Capsicum Annuum Extract Using Whey Protein. Appl. Food Res. 2025, 5, 100758. [Google Scholar] [CrossRef]
| Question Number | Science Direct (Advanced Search) | Scopus | Google Scholar |
|---|---|---|---|
| Q1 | 5118 | 231 | 7228 |
| Q2 | 864 | 5 | 528 |
| Q3 | 1565 | 118 | 5344 |
| Total | 7547 | 354 | 13,100 |
| Whey Concentration | Recombinant Microorganism | Productivity/Yield | Scale (Lab/Pilot/Industrial) | Reference |
|---|---|---|---|---|
| Cheese whey and cheese whey permeate (CWP) solution (10 g/L lactose) at bioreactor scale | Recombinant Kluyveromyces sp. β-galactosidase enzyme produced in E. coli pET-30a(+) | Approximately 26 U/mg of recombinant β-galactosidase after 16 h induction at bench bioreactor | Lab: flask and 2 L Biorreactor | [83] (2021) |
| 80 g/L whey powder as a substrate | Engineered E. coli strain F13 with recombinant plasmid with inducible lac UV5 promoter | β-farnesene production for fuel substitution reaching 4.74 g/L | Lab: 7 L batch bioreactor | [57] (2021) |
| 5% w/v of cheese whey powder, supplemented or not with 0.5% yeast extract (Difco) (YE) or 1.5% corn steep liquor (CSL) | ccpA mutant (BL21) of Lacticaseibacillus casei BL23 | Lactic acid production obtained final values of 44.23 g L−1 for BL71 | Lab: bottles (uncontrolled conditions and 5 L Bioreactor (controlled conditions) | [40] (2021) |
| Cheese whey permeate and ricotta whey (5 g/L of lactose) as inducers | E. coli C41(DE3) modified with expression vector pET-35b(+) | β-galactosidase fused to a cellulose-binding domain (CBD) 573.75 and 615.93 U/Lh | Lab: fed-batch cultures in 2 L Stirred Tank Bioreactors | [84] (2023) |
| Sweet and acid whey (SW, AW) were collected, pretreated, and filtrated at a concentration of 36 g/L and 32 g/L dry whey | Hyd mutant E. coli strains | Higher and prolonged H2 production 750–770 mL/g of initial dry whey after 96 h growth | Lab: 250 mL baffled flasks in a batch system | [53] (2023) |
| Crude glycerol (500 g/L), lactose solution (165 g/L) and CWP at a constant rate of 0.2 mL/min | Recombinant β-galactosidase from Marinomonas sp. ef1 (M-βGal) in E. coli | 2000 kU of recombinant M-βGal were successfully produced along with 30 g of galactose accumulated in the culture medium | Lab: shaked flask and 2 L Bioreactor fed-batch, cultures with CWP are comparable with those achieved using lactose | [85] (2024) |
| 10 g/L of whey powder was added as an inducer in 5 L batch fermentations | Recombinant E. coli BL21/CPD/GPS strain (S337A/F318Y) | 5-Hydroxytryptophan (5-HTP) amino acid with a yield of 1.649 g/L | Lab: 5 L fermenter | [54] (2024) |
| Industry | Company Description | URL |
|---|---|---|
| Davisco Foods International | Recognized as one of the leading producers of whey-derived ingredients, such as whey protein concentrates and isolates, used in both functional foods and biotechnological applications. | https://daviscofood.com/ (accessed on 30 March 2025) |
| Hilmar Cheese Company | Specializing in the transformation of whey into high-quality protein products, the company utilizes the by-product of cheesemaking to produce ingredients for the nutrition and food industries. | https://www.hilmar.com/ (accessed on 30 March 2025) |
| Fonterra Co-operative Group | This dairy giant uses whey to produce a wide range of ingredients, including derivatives used in functional formulations and fermentation processes in the biotechnology industry. | https://www.fonterra.com/nz/en.html (accessed on 30 March 2025) |
| Glanbia Nutritionals | Develops and supplies dairy-based ingredients, including whey derivatives, used in both food applications and fermentation and bioprocessing processes. | https://www.glanbianutritionals.com/es-es (accessed on 30 March 2025) |
| DuPont Nutrition & Biosciences (formerly Danisco) | It uses whey derivatives in the design of culture media and in fermentation processes to produce enzymes, cultures, and other biotechnological products. It has also used whey derivatives as an economical and sustainable medium in recombinant expression processes, facilitating the production of enzymes and other biotechnological products. | https://www.iff.com/ (accessed on 30 March 2025) |
| Kerry Group | This company develops ingredient solutions for the food industry, leveraging whey components to produce proteins and other derivatives that provide functionality and nutritional value. Kerry has developed ingredient solutions based on dairy products. Its biotechnology strategies include processes that utilize whey to produce bioactive proteins and optimize the expression of recombinant proteins, leveraging the nutritional benefits of whey. | https://www.kerry.com/ (accessed on 30 March 2025) |
| Agropur Cooperative | In addition to its dairy products, Agropur has implemented processes to valorize whey, transforming it into ingredients used in the food industry and in sustainable biotechnology applications. | https://www.agropur.com/fr (accessed on 30 March 2025) |
| Lactalis Ingredients | As part of the Lactalis Group, this division specializes in the production of dairy-derived ingredients, including those derived from whey, which are utilized in various industrial applications. | https://www.lactalisingredients.com/ (accessed on 30 March 2025) |
| Fermentec | This company specializes in fermentation technologies and has developed processes that utilize dairy by-products, such as whey, for the production of biofuels, thereby contributing to the valorization of industrial waste. | https://fermentec.com.br/ (accessed on 30 March 2025) |
| Technology/Research Area | Description/Potential | References |
|---|---|---|
| Integrated Dairy Biorefinery | Integrate the conversion of whey into multiple high-value products, including bioethanol, bioplastics, bioactives, enzymes, and biomass, in a biorefinery that simultaneously exploits all its fractions. | [93,94,95] |
| Precision Fermentation and Metabolic Engineering | Optimize the expression of recombinant proteins and specific metabolites through strain engineering (using bacteria, yeasts, or fungi) to more efficiently utilize lactose in whey. Research and scale-up the production of biopolymers (PHB, PHA) from whey, using modified microorganisms and optimized fermentation processes to compete with traditional sources. | [96,97] |
| Co-cultures and Hybrid Systems with Microalgae | Develop co-culture systems where whey serves as a nutrient source for microalgae, combining biomass production with bioremediation and the generation of bioactive compounds or biofuels. | [36,37,98,99] |
| Enzymes and Bioactives via Enzymatic Hydrolysis | Develop enzymatic processes, including immobilized systems, to produce bioactive peptides and other functional compounds from whey with applications in the food and pharmaceutical industries. | [100,101] |
| Continuous Fermentation Cells and Autoinduction Systems | Optimize high-performance fermenters using autoinduction systems based on lactose, reducing costs and improving scalability in the expression of recombinant proteins. | [33,102,103] |
| Cell Cultures and Regenerative Medicine | Explore the use of whey components as a substrate in in vitro protein synthesis systems, enabling rapid and controlled protein production without the need for living cells. | [104,105,106] |
| Nanotechnology and Nutrient Encapsulation | Apply micro- and nanocapsule techniques to protect and gradually release whey’s bioactive compounds, enhancing their stability and efficacy in therapeutic and nutraceutical applications. | [107,108] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delgado-Macuil, R.J.; Perez-Armendariz, B.; Cardoso-Ugarte, G.A.; Tolibia, S.E.M.; Benítez-Rojas, A.C. Recent Biotechnological Applications of Whey: Review and Perspectives. Fermentation 2025, 11, 217. https://doi.org/10.3390/fermentation11040217
Delgado-Macuil RJ, Perez-Armendariz B, Cardoso-Ugarte GA, Tolibia SEM, Benítez-Rojas AC. Recent Biotechnological Applications of Whey: Review and Perspectives. Fermentation. 2025; 11(4):217. https://doi.org/10.3390/fermentation11040217
Chicago/Turabian StyleDelgado-Macuil, Raúl J., Beatriz Perez-Armendariz, Gabriel Abraham Cardoso-Ugarte, Shirlley E. Martinez Tolibia, and Alfredo C. Benítez-Rojas. 2025. "Recent Biotechnological Applications of Whey: Review and Perspectives" Fermentation 11, no. 4: 217. https://doi.org/10.3390/fermentation11040217
APA StyleDelgado-Macuil, R. J., Perez-Armendariz, B., Cardoso-Ugarte, G. A., Tolibia, S. E. M., & Benítez-Rojas, A. C. (2025). Recent Biotechnological Applications of Whey: Review and Perspectives. Fermentation, 11(4), 217. https://doi.org/10.3390/fermentation11040217









