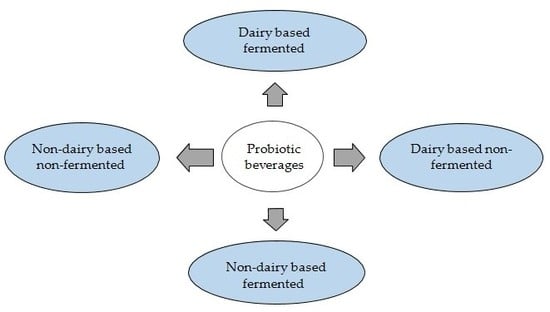
Probiotic Delivery through Fermentation: Dairy vs. Non-Dairy Beverages
Abstract
:1. Probiotics: A Brief Overview
2. Dairy vs. Non-Dairy Food Matrices
3. Fermented vs. Non-Fermented Beverages
4. Conclusions
Author Contributions
Conflicts of Interest
References
- FAO/WHO. Joint FAO/WHO Working Group Report on Drafting Guidelines for the Evaluation of Probiotics in Food; G. P. Putnams’s Sons: London, ON, Canada, 2002. [Google Scholar]
- Ranadheera, R.D.C.S.; Baines, S.K.; Adams, M.C. Importance of food in probiotic efficacy. Food Res. Int. 2010, 43, 1–7. [Google Scholar] [CrossRef]
- Metchnikoff, E. The Prolongation of Life; G. P. Putnams’s Sons: New York, NY, USA, 1907. [Google Scholar]
- Salminen, S.; Wrightb, A.; Morellic, L.; Marteaud, P.; Brassarte, B.; Vosf, W.M.; Fondeng, R.; Saxelinh, M.; Collinsi, K.; Mogensenj, G.; et al. Demonstration of safety of probiotics—A review. Int. J. Food Microbiol. 1998, 44, 93–106. [Google Scholar] [CrossRef]
- Penner, R.; Fedorak, R.N.; Madsen, K.L. Probiotics and nutraceuticals: non-medicinal treatments of gastrointestinal diseases. Curr. Opin. Pharmacol. 2005, 5, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Senok, A.C.; Ismaeel, A.Y.; Botta, G.A. Probiotics: Facts and myths. Clin. Microbiol. Infect. 2005, 11, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.P. Functional cultures and health benifits. Int. Dairy J. 2007, 17, 1262–1277. [Google Scholar] [CrossRef]
- Sari, F.N.; Dizdar, E.A.; Oguz, S.; Erdeve, O.; Uras, N.; Dilmen, U. Oral probiotics: Lactobacillus sporogenes for prevention of necrotizing enterocolitis in very low-birth weight infants: A randomized, controlled trial. Eur. J. Clin. Nutr. 2011, 65, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Caplan, M.; Frost, B. Myth: Necrotizing enterocolitis: Probiotics will end the disease, and surgical intervention improves the outcome. Semin. Fetal Neonatal Med. 2011, 16, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Ranadheera, C.S.; Prasanna, P.H.P.; Vidanarachchi, J.K. Fruit juice as probiotic carriers. In Fruit Juices: Types, Nutritional Composition and Health Benefits; Elder, K.E., Ed.; Nova Science Publishers: New York, NY, USA, 2014; pp. 253–268. [Google Scholar]
- Marques, T.M.; Cryan, J.F.; Shanahan, F.; Fitzgerald, G.F.; Ross, R.P.; Dinan, T.G.; Stanton, C. Gut microbiota modulation and implications for host health: Dietary strategies to influence the gut–brain axis. Innov. Food Sci. Emerg. Technol. 2014, 22, 239–247. [Google Scholar]
- Rasic, J.L. Microflora of the intestine: Probiotics. In Encyclopedia of Food Sciences and Nutrition; Caballero, B., Trugo, L., Finglas, P., Eds.; Academic Press: Oxford, UK, 2003; pp. 3911–3916. [Google Scholar]
- Kim, H.J.; Roque, M.I.V.; Camilleri, M.; Stephens, D.; Burton, D.D.; Baxter, K.; Thomforde, G.; Zinsmeister, A.R. A randomized controlled trial of a probiotic combination VSL# 3 and placebo in irritable bowel syndrome with bloating. Neurogastroenterol. Motil. 2005, 17, 687–696. [Google Scholar] [PubMed]
- Hickson, M.; Souza, A.L.D.; Muthu, N.; Rogers, T.R.; Want, S.; Rajkumar, C.; Bulpitt, C.J. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: Randomised double blind placebo controlled trial. BMJ 2007, 335, 80. [Google Scholar] [CrossRef] [PubMed]
- Cabré, E.; Gassull, M.A. Probiotics for preventing relapse or recurrence in Crohn’s disease involving the ileum: Are there reasons for failure? J. Crohns Colitis 2007, 1, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Quigley, E.M.M. Gut microbiota and the role of probiotics in therapy. Curr. Opin. Pharmacol. 2011, 11, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Luo, T.M.; Jobin, C.; Young, H.A. Gut microbiota and probiotics in colon tumorigenesis. Cancer Lett. 2011, 309, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.F.; Pan, T.M. In Vitro Effects of Lactic acid bacteria on cancer cell viability and antioxidant activity. J. Food Drug Anal. 2010, 18, 77–86. [Google Scholar]
- Liu, C.T.; Chu, F.J.; Chou, C.C.; Yu, R.C. Antiproliferative and anticytotoxic effects of cell fractions and exopolysaccharides from Lactobacillus casei 01. Mutat. Res. 2011, 721, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Lollo, P.C.B.; Moura, C.S.; Morato, P.N.; Cruz, A.G.; Castro, W.F.; Betim, C.B.; Nisishima, L.; Faria, J.A.F.; Junior, M.M.; et al. Probiotic yogurt offers higher immune-protection than probiotic whey beverage. Food Res. Int. 2013, 54, 118–124. [Google Scholar] [CrossRef]
- Prasanna, P.H.P.; Grandison, A.S.; Charalampopoulos, D. Bifidobacteria in milk products: An overview of physiological and biochemical properties, exopolysaccharide production, selection criteria of milk products and health benefits. Food Res. Int. 2014, 55, 247–262. [Google Scholar] [CrossRef]
- Kołodziej, M.; Szajewska, H. Lactobacillus reuteri DSM 17938 in the prevention of antibiotic-associated diarrhoea in children: Protocol of a randomised controlled trial. BMJ Open 2017, 7, e013928. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, P.J.; Salminen, S.; Saxelin, M.; Hamalainen, P.; Ihanto-Vormisto, A.; Muurasniemi-Isovita, L.; Nikkari, S.; Oksanen, T.; Porsti, I.; Salminen, E.; et al. Prevention of travellers diarrhoea by Lactobacillus GG. Ann. Med. 1990, 22, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Kajander, K.; Hatakka, K.; Poussa, T.; Farkkila, M.; Koppela, R. A probiotic mixture alleviates symptoms in irritable bowel syndrome patients: A controlled 6-month intervention. Aliment. Pharmacol. Ther. 2005, 22, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Guyonnet, D.; Chassany, O.; Ducrotte, P.; Picard, C.; Mouret, M.; Mercier, C.H.; Matuchansky, C. Effect of a fermented milk containing Bifidobacterium animalis DN-173 010 on the health-related quality of life and symptoms in irritable bowel syndrome in adults in primary care: A multicentre, randomized, double-blind, controlled trial. Aliment. Pharmacol. Ther. 2007, 26, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Brigidi, P.; Vitali, B.; Swennen, E.; Bazzocchi, G.; Matteuzzi, D. Effects of probiotic administration upon the composition and enzymatic activity of human fecal microbiota in patients with irritable bowel syndrome or functional diarrhea. Res. Microbiol. 2001, 152, 735–741. [Google Scholar] [CrossRef]
- Nobaek, S.; Johansson, M.L.; Molin, G.; Ahrne, S.; Jeppsson, B. Alteration of intestinal microflora is associated with reduction in abdominal bloating and pain in patients with irritable bowel syndrome. Am. J. Gastroenterol. 2000, 95, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Marteau, P.; Cuillerier, E.; Meance, S.; Gerhardt, M.F.; Myara, A.; Bouvier, M.; Couley, C.; Tondu, F.; Bommelaer, G.; Grimaud, J.C. Bifidobacterium animalis strain DN-173 010 shortens the colonic transit time in healthy women: A double-blind, randomized, controlled study. Aliment. Pharmacol. Ther. 2002, 16, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Campieri, M.; Rizzello, F.; Venturi, A.; Poggioli, G.; Ugolini, F.; Helwing, U.; Amadini, C.; Romboli, E.; Gionchetti, P. Combination of antibiotic and probiotic treatment is efficacious in prophylaxis of post-operative recurrence of Crohn’s disease: A randomized controlled study vs mesalamine. Gastroenterology 2000, 118, A781. [Google Scholar] [CrossRef]
- Kruis, W.; Fric, P.; Pokrotnieks, J.; Lukas, M.; Fixa, B.; Kamm, M.A.; Weismueller, J.; Beglinger, C.; Stolte, M.; et al. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut 2004, 53, 1617–1623. [Google Scholar] [CrossRef] [PubMed]
- Laake, K.O.; Bjorneklett, A.; Aamodt, G.; Aabakken, L.; Jacobsen, M.; Bakka, A.; Vatn, M.H. Outcome of four weeks’ intervention with probiotics on symptoms and endoscopic appearance after surgical reconstruction with a J-configurated ileal-pouch-anal-anastomosis in ulcerative colitis. Scand. J. Gastroenterol. 2005, 40, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Anukam, K.; Osazuwa, E.; Ahonkhai, I.; Ngwu, M.; Osemene, G.; Bruce, A.W.; Reid, G. Augmentation of antimicrobial metronidazole therapy of bacterial vaginosis with oral probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14: Randomized, double-blind, placebo controlled trial. Microbes Infect. 2006, 8, 1450–1454. [Google Scholar] [CrossRef] [PubMed]
- Abrahamsson, T.R.; Jakobsson, T.; Bottcher, M.F.; Fredrikson, M.; Jenmalm, M.C.; Bjorksten, B.; Oldaeus, G. Probiotics in prevention of IgE-associated eczema: A double-blind, randomized, placebo-controlled trial. J. Allergy Clin. Immunol. 2007, 119, 1174–1180. [Google Scholar] [CrossRef] [PubMed]
- Wickens, K.; Black, P.N.; Stanley, T.V.; Mitchell, E.; Fitzharris, P.; Tannock, G.W.; Purdie, G.; Crane, J. A differential effect of 2 probiotics in the prevention of eczema and atopy: A double-blind, randomized, placebo-controlled trial. J. Allergy Clin. Immunol. 2008, 122, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeldt, V.; Benfeldt, E.; Valerius, N.H.; Paerregaard, A.; Michaelsen, K.F. Effect of probiotics on gastrointestinal symptoms and small intestinal permeability in children with atopic dermatitis. J. Pediatr. 2004, 145, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Rø, A.D.B.; Simpson, M.R.; Ro, T.B.; Storro, O.; Johnsen, R.; Videm, V.; Oien, T. Reduced Th22 cell proportion and prevention of atopic dermatitis in infants following maternal probiotic supplementation. Clin. Exp. Allergy 2017, 47, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Ranadheera, C.S. Probiotic Application in the Development of Goat’s Milk Products with Special Reference to Propionibacterium Jensenii 702: Effects on Viability and Functionality. Ph.D. Thesis, The University of Newcastle Australia, Callaghan, Australia, 2012. [Google Scholar]
- Vasiljevic, T.; Shah, N.P. Probiotics—From Metchnikoff to bioactives. Int. Dairy J. 2008, 18, 714–728. [Google Scholar] [CrossRef]
- Ranadheera, C.S.; Evans, C.A.; Adams, M.C.; Baines, S.K. In vitro analysis of gastrointestinal tolerance and intestinal cell adhesion of probiotics in goat’s milk ice cream and yogurt. Food Res. Int. 2012, 49, 619–625. [Google Scholar] [CrossRef]
- McNaught, C.E.; MacFie, J. Probiotics in clinical practice: A critical review of the evidence. Nutr. Res. 2001, 21, 343–353. [Google Scholar] [CrossRef]
- Saarela, M.; Lahteenmaki, L.; Crittenden, R.; Salminen, S.; Mattila-Sandholm, T. Gut bacteria and health foods-the European perspective. Int. J. Food Microbiol. 2002, 78, 99–117. [Google Scholar] [CrossRef]
- Morelli, L. In vitro assessment of probiotic bacteria: From survival to functionality. Int. Dairy J. 2007, 17, 1278–1283. [Google Scholar] [CrossRef]
- Aureli, P.; Capurso, L.; Castellazzi, A.M.; Clerici, M.; Giovannini, M.; Morelli, L.; Poli, A.; Pregliasco, F.; Salvini, F.; Zuccotti, G.V. Probiotics and health: An evidence-based review. Pharmacol. Res. 2011, 63, 366–376. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, J.G.; Chain, F.; Martin, R.; Bermudez-Humaran, L.G.; Courau, S.; Langella, P. Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microb. Cell Factories 2017, 16, 79. [Google Scholar] [CrossRef] [PubMed]
- Cholakov, R.; Tumbarski, Y.; Yanakieva, V.; Dobrev, I.; Salim, Y.; Denkova, Z. Antimicrobial activity of Leuconostoc lactis strain BT1&, isolated from a spontaneously fermented cereal beverage (Boza). J. Microbiol. Biotechnol. Food Sci. 2017, 7, 47–49. [Google Scholar]
- Vidanarachchi, J.K. Regulation of Intestinal Microflora and Productivity of Broiler Chickens by Prebiotic and Bioactive Plant Extracts. Ph.D. Thesis, The University of New England, Armidale, Australia, 2007. [Google Scholar]
- Prado, F.C.; Parada, J.L.; Pandey, A.; Soccol, C.R. Trends in non-dairy probiotic beverages. Food Res. Int. 2008, 41, 111–123. [Google Scholar] [CrossRef]
- Marco, M.L.; Tachon, S. Environmental factors influencing the efficacy of probiotic bacteria. Curr. Opin. Biotechnol. 2013, 24, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Ranadheera, C.S.; Evans, C.A.; Adams, M.A.; Baines, S.K. Effect of dairy probiotic combinations on in vitro gastrointestinal tolerance, intestinal epithelial cell adhesion and cytokine secretion. J. Funct. Foods 2014, 8, 18–25. [Google Scholar] [CrossRef]
- Ranadheera, C.S.; Evans, C.A.; Adams, M.A.; Baines, S.K. Production of probiotic ice cream from goat’s milk and effect of packaging materials on product quality. Small Rumin. Res. 2013, 112, 174–180. [Google Scholar] [CrossRef]
- Lavermicocca, P. Highlights on new food research. Dig. Liver Dis. 2006, 38 (Suppl. S2), S295–S299. [Google Scholar] [CrossRef]
- Rouhi, M.; Sohrabvandi, S.; Mortazavian, A.M. Probiotic Fermented Sausage: Viability of Probiotic Microorganisms and Sensory Characteristics. Crit. Rev. Food Sci. Nutr. 2013, 53, 331–348. [Google Scholar] [CrossRef] [PubMed]
- Klingberg, T.D.; Budde, B.B. The survival and persistence in the human gastrointestinal tract of five potential probiotic lactobacilli consumed as freeze-dried cultures or as probiotic sausage. Int. J. Food Microbiol. 2006, 109, 157–159. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.N.; Sodini, I.; Remeuf, F.; Corrieu, G. Effect of milk supplementation and culture composition on acidification, textural properties and microbiological stability of fermented milks containing probiotic bacteria. Int. Dairy J. 2001, 11, 935–942. [Google Scholar] [CrossRef]
- Martin-Diana, A.B.; Janer, C.; Pelaez, C.; Requena, T. Development of a fermented goat’s milk containing probiotic bacteria. Int. Dairy J. 2003, 13, 827–833. [Google Scholar] [CrossRef]
- 56. Ranadheera, C.S.; Evans, C.A.; Adams, M.A.; Baines, S.K. Co-culturing of probiotics influences the microbial and physico-chemical properties but not sensory quality of fermented dairy drink made from goats’ milk. Small Rumin. Res. 2016, 136, 104–108. [Google Scholar] [CrossRef]
- Maganha, L.C.; Rosim, R.E.; Corassin, C.H.; Cruz, A.G.; Faria, J.A.F. Viability of probiotic bacteria in fermented skim milk produced with different levels of milk powder and sugar. Int. J. Dairy Technol. 2014, 67, 89–94. [Google Scholar] [CrossRef]
- Shah, N.P.; Lankaputhra, W.E.V. Improving viability of Lactobacillus acidophilus and Bifidobacterium spp. in yogurt. Int. Dairy J. 1997, 7, 349–356. [Google Scholar] [CrossRef]
- Ekinci, F.Y.; Gurel, M. Effect of using propionic acid bacteria as an adjunct culture in yogurt production. J. Dairy Sci. 2008, 91, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Kailasapathy, K.; Harmstorf, I.; Phillips, M. Survival of Lactobacillus acidophilus and Bifidobacterium animalis ssp. lactis in stirred fruit yogurts. LWT Food Sci. Technol. 2008, 41, 1317–1322. [Google Scholar] [CrossRef]
- Kumari, A.G.I.P.; Ranadheera, C.S.; Prasanna, P.H.P.; Senevirathne, N.D.; Vidanarachchi, J.K. Development of a rice incorporated synbiotic yogurt with low retrogradation properties. Int. Food Res. J. 2015, 22, 2032–2040. [Google Scholar]
- Prasanna, P.H.P.; Grandison, A.S.; Charalampopoulos, D. Microbiological, chemical and rheological properties of low fat set yoghurt produced with exopolysaccharide (EPS) producing Bifidobacterium strains. Food Res. Int. 2014, 51, 15–22. [Google Scholar] [CrossRef]
- Guler-Akin, M.B.; Akin, M.S. Effects of cysteine and different incubation temperatures on the microflora, chemical composition and sensory characteristics of bio-yogurt made from goat’s milk. Food Chem. 2007, 100, 788–793. [Google Scholar] [CrossRef]
- Ranadheera, C.S.; Evans, C.A.; Adams, M.A.; Baines, S.K. Probiotic viability and physico-chemical and sensory properties of plain and stirred fruit yogurts made from goat’s milk. Food Chem. 2012, 135, 1411–1418. [Google Scholar] [CrossRef] [PubMed]
- Guler-Akin, M.B. The effects of different incubation temperatures on the acetaldehyde content and viable bacteria counts of bio yogurt made from ewe’s milk. Int. J. Dairy Technol. 2005, 58, 174–179. [Google Scholar] [CrossRef]
- Alamprese, C.; Foschino, R.; Rossi, M.; Pompei, C.; Savani, L. Survival of Lactobacillus johnsonii La 1 and influence of its addition in retail-manufactured ice cream produced with different sugar and fat concentrations. Int. Dairy J. 2002, 12, 201–208. [Google Scholar] [CrossRef]
- Akin, M.B.; Akin, M.S.; Kirmaci, Z. Effects of inulin and sugar levels on the viability of yogurt and probiotic bacteria and the physical and sensory characteristics in probiotic ice cream. Food Chem. 2007, 104, 93–99. [Google Scholar] [CrossRef]
- Ferraz, J.L.; Cruz, A.G.; Cadena, R.S.; Freitas, M.Q.; Pinto, U.M.; Carvalho, C.C.; Faria, J.A.F.; Bolini, H.M.A. Sensory acceptance and survival of probiotic bacteria in ice cream produced with different overrun levels. J. Food Sci. 2012, 77, S24–S28. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, G.E.; Bouchier, P.; O’Sullivan, E.; Kelly, J.; Collins, J.K.; Fitzgerald, G.; Ross, R.P.; Stanton, C. A spray-dried culture for probiotic Cheddar cheese manufacture. Int. Dairy J. 2002, 12, 749–756. [Google Scholar] [CrossRef]
- Ulpathakumbura, C.P.; Ranadheera, C.S.; Senavirathne, N.D.; Jayawardene, L.P.I.N.P.; Prasanna, P.H.P.; Vidanarachchi, J.K. Effect of biopreservatives on microbial, physico-chemical and sensory properties of Cheddar cheese. Food Biosci. 2016, 13, 21–25. [Google Scholar] [CrossRef]
- Buriti, F.C.A.; da Rocha, J.S.; Assis, E.G.; Saad, S.M.I. Probiotic potential of Minas fresh cheese prepared with the addition of Lactobacillus paracasei. LWT Food Sci. Technol. 2005, 38, 173–180. [Google Scholar] [CrossRef]
- Kasimoglu, A.; Goncuoglu, M.; Akgun, S. Probiotic white cheese with Lactobacillus acidophilus. Int. Dairy J. 2004, 14, 1067–1073. [Google Scholar] [CrossRef]
- Bergamini, C.V.; Hynes, E.R.; Quiberoni, A.; Suarez, V.B.; Zalazar, C.A. Probiotic bacteria as adjunct starters: Influence of the addition methodology on their survival in a semi-hard Argentinean cheese. Food Res. Int. 2005, 38, 597–604. [Google Scholar] [CrossRef]
- Vinderola, C.G.; Prosello, W.; Ghiberto, D.; Reinheimer, J.A. Viability of probiotic (Bifidobacterium, Lactobacillus acidophilus and Lactobacillus casei) and nonprobiotic microflora in Argentinian Fresco cheese. J. Dairy Sci. 2000, 83, 1905–1911. [Google Scholar] [CrossRef]
- Madureira, A.R.; Pereira, C.I.; Truszkowska, K.; Gomes, A.M.; Pintado, M.E.; Malcata, F.X. Survival of probiotic bacteria in a whey cheese vector submitted to environmental conditions prevailing in the gastrointestinal tract. Int. Dairy J. 2005, 15, 921–927. [Google Scholar] [CrossRef]
- Gomes, A.M.P.; Malcata, F.X. Development of probiotic cheese manufactured from goat milk: Response surface analysis via technological manipulation. J. Dairy Sci. 1998, 81, 1492–1507. [Google Scholar] [CrossRef]
- Gobbetti, M.; Crosetti, A.; Smacchi, E.; Zocchetti, A.; De Angelis, M. Production of crescenza cheese by incorporation of bifidobacteria. J. Dairy Sci. 1998, 81, 37–47. [Google Scholar] [CrossRef]
- Heenan, C.N.; Adams, M.C.; Hosken, R.W.; Fleet, G.H. Survival and sensory acceptability of probiotic microorganisms in a nonfermented frozen vegetarian dessert. LWT Food Sci. Technol. 2004, 37, 461–466. [Google Scholar] [CrossRef]
- Shimakava, Y.; Matsubara, S.; Yuki, N.; Ikeda, M.; Ishikawa, F. Evaluation of Bifidobacterium breve strain Yakult-fermented soymilk as a probiotic food. Int. J. Food Microbiol. 2003, 81, 131–136. [Google Scholar] [CrossRef]
- Ouwehand, A.C.; Kurvinen, T.; Rissanen, P. Use of a probiotic Bifidobacterium in a dry food matrix, an in vivo study. Int. J. Food Microbiol. 2004, 95, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Helland, M.H.; Wicklund, T.; Narvhus, J.A. Growth and metabolism of selected strains of probiotic bacteria in milk- and water-based cereal puddings. Int. Dairy J. 2004, 14, 957–965. [Google Scholar] [CrossRef]
- Molin, G. Probiotics in foods not containing milk or milk constituents, with special reference to Lactobacillus plantarum 299v. Am. J. Clin. Nutr. 2001, 73, 380S–385S. [Google Scholar] [PubMed]
- Luckow, T.; Delahunty, C. Which juice is healthier? A consumer study of probiotic non-dairy juice drinks. Food Qual. Preference 2004, 15, 751–759. [Google Scholar] [CrossRef]
- Betoret, N.; Puente, L.; Diaz, M.J.; Pagan, M.J.; Garcia, M.J.; Gras, M.L.; Martinez-Monzo, J.; Fito, P. Development of probiotic-enriched dried fruits by vacuum impregnation. J. Food Eng. 2003, 56, 273–277. [Google Scholar] [CrossRef]
- Ding, W.K.; Shah, N.P. Survival of free and microencapsulated probiotic bacteria in orange and apple juices. Int. Food Res. J. 2008, 15, 219–232. [Google Scholar]
- Costa, M.G.M.; Fonteles, T.V.; de Jesus, A.L.T.; Rodrigues, S. Sonicated pineapple juice as substrate for L. casei cultivation for probiotic beverage development: Process optimisation and product stability. Food Chem. 2013, 139, 261–266. [Google Scholar] [CrossRef] [PubMed]
- AdebayoTayo, B.; Akpeji, S. Probiotic viability, physicochemical and sensory properties of probiotic pineapple juice. Fermentation 2016, 2, 20. [Google Scholar] [CrossRef]
- Pereira, A.L.F. Spray-Drying of Probiotic Cashew Apple Juice. Food Bioprocess Technol. 2014, 7, 2492–2499. [Google Scholar] [CrossRef]
- Alves, N.N. Spouted bed as an efficient processing for probiotic orange juice drying. Food Res. Int. 2017, 101, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, J.; Borges, S.; Amorim, M.; Pereira, M.J.; Oliveira, A.; Pintado, M.E.; Teixeira, P. Comparison of spray drying, freeze drying and convective hot air drying for the production of a probiotic orange powder. J. Funct. Foods 2015, 17, 340–351. [Google Scholar] [CrossRef]
- Luckow, T.; Delahunty, C. Consumer acceptance of orange juice containing functional ingredients. Food Res. Int. 2004, 37, 805–814. [Google Scholar] [CrossRef]
- Lavermicocca, P.; Valerio, F.; Lonigro, S.L.; Angelis, M.D.; Morelli, L.; Callegari, M.L.; Rizzello, C.G.; Visconti, A. Study of adhesion and survival of lactobacilli and bifidobacteria on table olives with the aim of formulating a new probiotic food. Appl. Environ. Microbiol. 2005, 71, 4233–4240. [Google Scholar] [CrossRef] [PubMed]
- Valero-Cases, E.; Frutos, M.J. Effect of Inulin on the Viability of L. plantarum during Storage and In Vitro Digestion and on Composition Parameters of Vegetable Fermented Juices. Plant Foods Hum. Nutr. 2017, 72, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.Y.; Woodams, E.E.; Hang, Y.D. Fermentation of beet juice by beneficial lactic acid bacteria. LWT Food Sci. Technol. 2005, 38, 73–75. [Google Scholar] [CrossRef]
- Yoon, K.Y.; Woodams, E.E.; Hang, Y.D. Production of probiotic cabbage juice by lactic acid bacteria. Bioresour. Technol. 2006, 97, 1427–1430. [Google Scholar] [CrossRef] [PubMed]
- Valerio, F.; De Bellis, P.; Lonigro, S.L.; Morelli, L.; Visconti, A.; Lavermicocca, P. In vitro and in vivo survival and transit tolerence of potentially probiotic strains carried by Artichokes in the gastrointestinal tract. Appl. Environ. Microbiol. 2006, 72, 3042–3045. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y. Production of Fermented Kale Juices with Lactobacillus Strains and Nutritional Composition. Prev. Nutr. Food Sci. 2017, 22, 231–236. [Google Scholar] [PubMed]
- Battistini, C.; Gullon, B.; Ichimura, E.S.; Gomes, A.M.P.; Ribeiro, E.P.; Kunigk, L.; Moreira, J.U.V.; Jurkiewicz, C.J. Development and characterization of an innovative synbiotic fermented beverage based on vegetable soybean. Braz. J. Microbiol. 2017, in press. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Breidt, F.; Price, R.; Perez-Diaz, I. Survival and Growth of Probiotic Lactic Acid Bacteria in Refrigerated Pickle Products. J. Food Sci. 2017, 82, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Erkkilä, S.; Suihko, M.L.; Eerola, S.; Petaja, E.; Mattila-Sandholm, T. Dry sausage fermented by Lactobacillus rhamnosus strains. Int. J. Food Microbiol. 2001, 64, 205–210. [Google Scholar]
- Speranza, B.; Racioppo, A.; Beneduce, L.; Bevilacqua, A.; Sinigaglia, M.; Corbo, M.R. Autochthonous lactic acid bacteria with probiotic aptitudes as starter cultures for fish-based products. Food Microbiol. 2017, 65, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Ranadheera, C.S.; Evans, C.A.; Adams, M.A.; Baines, S.K. Microencapsulation of Lactobacillus acidophilus LA-5, Bifidobacterium animalis subsp. lactis BB-12 and Propionibacterium jensenii 702 by spray drying in goat’s milk. Small Rumin. Res. 2015, 123, 155–159. [Google Scholar] [CrossRef]
- Silva, M.P.; Tulini, F.L.; Marinho, J.F.U.; Mazzocato, M.C.; De Martinis, E.C.P.; Luccas, V.; Favaro-Trindade, C.S. Semisweet chocolate as a vehicle for the probiotics Lactobacillus acidophilus LA3 and Bifidobacterium animalis subsp. lactis BLC1: Evaluation of chocolate stability and probiotic survival under in vitro simulated gastrointestinal conditions. LWT Food Sci. Technol. 2017, 75, 640–647. [Google Scholar] [CrossRef]
- McMaster, L.D.; Kokott, S.A.; Reid, S.J.; Abratt, V.R. Use of traditional African fermented beverages as delivery vehicles for Bifidobacterium lactis DSM 10140. Int. J. Food Microbiol. 2005, 102, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Mudannayake, D.C.; Silva, K.F.S.T.; Wimalasiri, K.M.S.; Ajlouni, S. In Vitro Prebiotic Properties of Partially Purified Asparagus Falcatus and Taraxacum Javanicum Inulins. J. Food Nutr. Disord. 2016, 5, 1–10. [Google Scholar] [CrossRef]
- Grizard, D.; Barthomeuf, C. Non-digestible oligosaccharides used as prebiotic agents: mode of production and beneficial effects on animal and human health. Reprod. Nutr. Dev. 1999, 39, 588. [Google Scholar] [CrossRef]
- Anal, A.K.; Singh, H. Recent advances in microencapsulation of probiotics for industrial applications and targeted delivery. Trends Food Sci. Technol. 2007, 18, 240–251. [Google Scholar] [CrossRef]
- Abesinghe, N.; Vidanarachchi, J.K.; Silva, S. The effect of Arrowroot (Maranta arundinacea) extract on the survival of probiotic bacteria in set yoghurt. Int. J. Sci. Res. Publ. 2012, 2, 1–4. [Google Scholar]
- Huang, Y.; Adams, M.C. In vitro assessment of the upper gastrointestinal tolerance of potential probiotic dairy propionibacteria. Int. J. Food Microbiol. 2004, 91, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Cruz, A.G.; Antunes, A.E.C.; Sousa, A.L.O.P.; Faria, J.A.F.; Saad, S.M.I. Ice-cream as a probiotic food carrier. Food Res. Int. 2009, 42, 1233–1239. [Google Scholar] [CrossRef]
- Vinderola, G.; Binetti, A.; Burns, P.; Reinheimer, J. Cell Viability and Functionality of Prebiotic Bacteria in Dairy Products. Front. Microbiol. 2011, 2, 70. [Google Scholar] [CrossRef] [PubMed]
- Gawkowski, D.; Chikindas, M.L. Non-dairy probiotic beverages: The next step into human health. Benef. Microbes 2013, 4, 127–142. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Kenifel, W.; Ouwehand, A.C. Bacteria, Beneficial-Probiotics, Applications in Dairy Products. In Encyclopedia of Dairy Sciences, 2nd ed.; John, W.F., Ed.; Academic Press: San Diego, CA, USA, 2011; pp. 412–419. [Google Scholar]
- Patel, A.R. Probiotic fruit and vegetable juices-recent advances and future perspectives. Int. Food Res. J. 2017, 24, 1850–1857. [Google Scholar]
- Jayawardana, N.W.I.A.; Prasanna, P.H.P.; Ranadheera, C.S.; de Zoysa, H.K.S.; Vidanarachchi, J.K. Probiotics in functional foods. In Fermented Foods: Sources, Consumption and Health Benefits; Nova Science Publishers: New York, NY, USA, 2015; pp. 103–126. [Google Scholar]
- Guarner, F.; Perdigon, G.; Corthier, G.; Salminen, S. Should yoghurt cultures be considered probiotic? Br. J. Nutr. 2005, 93, 783–786. [Google Scholar] [CrossRef] [PubMed]
- Meydani, S.N.; Ha, W.K. Immunologic effects of yogurt. Am. J. Clin. Nutr. 2000, 71, 861–872. [Google Scholar] [PubMed]
- Dave, R.I.; Shah, N.P. Viability of yoghurt and probiotic bacteria in yoghurts made from commercial starter cultures. Int. Dairy J. 1997, 7, 31–41. [Google Scholar] [CrossRef]
- Batista, A.L.D.; Silva, R.; Cappato, L.P.; Almada, C.N.; Garcia, R.K.A.; Silva, M.C.; Raices, R.S.L.; Arellano, D.B.; Sant’Ana, A.S.; Junior, C.A.C.; et al. Quality parameters of probiotic yogurt added to glucose oxidase compared to commercial products through microbiological, physical–chemical and metabolic activity analyses. Food Res. Int. 2015, 77, 627–635. [Google Scholar] [CrossRef]
- Kumar, B.V.; Vijayendra, S.V.N.; Reddy, O.V.S. Trends in dairy and non-dairy probiotic products—A review. J. Food Sci. Technol. 2015, 52, 6112–6124. [Google Scholar] [CrossRef] [PubMed]
- Meybodi, N.M.; Mortazavian, A.M.; da Cruz, A.G.; Mohammadi, R. Probiotic Supplements and Food Products: Comparison for Different Targets. Appl. Food Biotechnol. 2017, 4, 123–132. [Google Scholar]
- Annunziata, A.; Vecchio, R. Consumer perception of functional foods: A conjoint analysis with probiotics. Food Qual. Preference 2013, 28, 348–355. [Google Scholar] [CrossRef]
- Robinson, R.K.; Tamime, A.Y. Types of fermented milks. In Fermented Milks; Blackwell Science Ltd.: Oxford, UK, 2007; pp. 1–10. [Google Scholar]

| Lactobacillus spp. | Bifidobacterium spp. | Other spp. |
|---|---|---|
| L. acidophilus L. casei L. crispatus L. delbrueckii subsp. bulgaricus a L. fermentum L. gasseri L. johnsonii L. paracasei L. plantarum L. reuteri L. rhamnosus L. helveticus L. lactis L. sporogenes | B. bifidum B. breve B. infantis B. longum B. lactis B. animalis B. adolescentis B. essensis B. laterosporus | Escherichia coli Nissle Saccharomyces boulardii Saccharomyces cerevisiae Kluyveromyces lactis Streptococcus thermophilus a S. cremoris S. diacetylactis S. intermedius S. salivarius Enterococcus francium b Propionibacterium freudenreichii P. freudenreichii subsp. shermanii P. jensenii Pediococcus Leuconostoc lactis subsp. cremoris L. lactis subsp. lactis Bacillus cereus Clostridium butyricum |
| Disorder | Probiotic Strain | Mode of Delivery | References |
|---|---|---|---|
| Antibiotic-associated diarrhoea in adults | Mixture of L. casei | Drinking yogurt | [14] |
| L. bulgaricus | |||
| S. thermophilus | |||
| Antibiotic-associated diarrhoea in children | Lactobacillus reuteri | Drops | [22] |
| Traveler’s diarrhoea | Single strain of Lactobacillus GG | Powdered form dissolved in cold water | [23] |
| Irritable bowel syndrome symptoms | Mixture of B. longum, B. infantis, B. breve, L. acidophilus, L. casei, L. delbrueckii, L. plantarum, S. salivarius | Lyophilized powdered form | [13] |
| Mixture of L. rhamnosus, B. breve & P. freudenreichii subsp. shermanii | Capsules | [24] | |
| Mixture of B. animalis, L. bulgaricus & S. thermophilus | Fermented milk | [25] | |
| Mixture of B. longum, B. infantis, B. breve, L. acidophilus, L. casei, L. delbrueckii, L. plantarum, S. salivarius | Lyophilized powdered form | [26] | |
| Single strain of L. plantarum | Rose-hip drink with oat flour | [27] | |
| Single strain of B. animalis | Fermented semi skimmed-milk | [28] | |
| Crohn’s disease Ulcerative colitis Pouchitis | Mixture of B. longum, B. infantis, B. breve, L. acidophilus, L. casei, L. bulgaricus, L. plantarum, S. thermophilus | Lyophilized form | [29] |
| Single strain of E. coli Nissle | Capsules | [30] | |
| Mixture of L. acidophilus La-5, | Fermented milk | [31] | |
| Bifidobacterium Bb 12 | |||
| Bacterial vaginosis | Mixture of L. rhamnosus & L. reuteri | Gelatin capsules | [32] |
| IgE associated eczema Atopic dermatitis Atopic dermatitis in infants | Single strain of L. reuteri | Freeze dried form in coconut or peanut oil droplets | [33] |
| Single strain of L. rhamnosus | Skim milk based freeze-dried form | [34] | |
| Mixture of L. rhamnosus & L. reuteri | Lyophilized powdered form | [35] | |
| Mixture of L. rhamnosus, B. animalis subsp. lactis Bb-12 (Bb-12) & L. acidophilus La-5 | Milk (maternal supplementation) | [36] |
| Criteria | Property/Characteristic | Target and Methods to Be Assessed |
|---|---|---|
| Safety | Origin Pathogenicity and infectivity Virulence factors-toxicity, metabolic activity and intrinsic properties, i.e., antibiotic resistance | Source or origin should be assessed: be isolated from the same species as its intended host is desirable due to higher efficacy in the same species. Probiotics of human origin may be desirable if they are intended for human use. Pre-market clearance and post-market surveillance |
| Technological acceptability | High viability retention during manufacturing and storage of carrier foods Acceptable organoleptic characteristics Ability to produce at large-scale Phage resistance | In vitro studies and food product development Sensory testing of model and final products and consumer studies on product formulations |
| Functionality | Tolerance to gastric acid and juices including acidic conditions and enzymes Bile tolerance Adhesion to mucosal surface and colonization Validated and documented health effects | Model systems for gastric and bile effects (e.g., in vitro, animal and human studies) In vitro adhesion models (e.g., intestinal segments, mucus, cell culture), animal and human studies Health effects confirmed by clinical studies |
| Desirable physiological criteria | Immunomodulation Antagonistic activity towards gastrointestinal pathogens Antimutagenic and anticarcinogenic properties | In vitro/In vivo animal and human studies. Adhesion and competitive exclusion of pathogens in in vitro and in vivo model systems |
| Product Type | Product | Probiotic Strain | Viability at the End of Storage | Total Storage Time | References |
|---|---|---|---|---|---|
| Dairy based | Fermented cow’s milks | L. acidophilus L. rhamnosus | 107 cfu/g | 7 days | [54] |
| Fermented goat’s milk | L. acidophilus Bifidobacterium BB-12 | <106 cfu/g 106–107 cfu/g | 21 days | [55] | |
| Fermented dairy drink from goat’s milk | L. acidophilus B. animalsi ssp. lactis | 107 cfu/mL | 21 days | [56] | |
| Fermented skim milk (cow’s milk) | L. acidophilus B. animalsi ssp. lactis | 106 cfu/mL | 21 days | [57] | |
| Cow’s milk yogurt | L. acidophilus | >106 cfu/g | 42 days | [58] | |
| B. longum | |||||
| B. psedolongum | |||||
| B. infantis | |||||
| B. bifidum | |||||
| P. jensenii | 105 cfu/g | 15 days | [59] | ||
| Cow’s milk fruit yogurt | L. acidophilus B. animalsi ssp. lactis | 106–107 cfu/g | 35 days | [60] | |
| Rice incorporated cow’s milk yogurt | B. animalis subsp. lactis BB-12 | 108 cfu/g | 21 days | [61] | |
| Low fat set yogurts (cow’s milk) | B. infantis, B. longum subsp. infantis | 107 cfu/g | 28 days | [62] | |
| Goat’s milk yogurt | L. acidophilus B. bifidum L. paracasei subsp. casei | 107 cfu/g | 14 days | [63,64] | |
| Ewe’s milk yogurt | L. acidophilus B. bifidum L. casei | 107 cfu/g | 14 days | [65] | |
| Ice cream | L. johnsonii L. acidophilus B. lactis | 107 cfu/g 105–106 cfu/g | 8 months 90 days | [66] [67] | |
| Ice cream (vanilla flavoured) | L. acidophilus | 106 cfu/mL | 60 days | [68] | |
| Goat’s milk ice cream (chocolate flavoured) | L. acidophilus, B. animalis subsp. Lactis, Propionibacterium jensenii | 107–108 cfu/g | 52 weeks | [50] | |
| Cheddar cheese | L. paracasei | 107 cfu/g | 90 days | [69] | |
| Lactococcus lactis subsp. cremoris, L. lactis subsp. lactis, Lactobacillus helvetics, S. thermophiles, Lactobacillus rhamnosus | 108 cfu/g | 4 weeks | [70] | ||
| Fresh Minas cheese | L. paracasei | 108 cfu/g | 21 days | [71] | |
| White Turkish cheese | L. acidophilus | 107 cfu/g | 90 days | [72] | |
| Semi hard Argentinian cheese | L. paracasei L. acidophilus | 108 cfu/g | 60 days | [73] | |
| Argentinian Fresco cheese | B. bifidum B. longum L. acidophilus L. casei | 106 cfu/g | 60 days | [74] | |
| Requeijao-cheese (Portuguese-whey cheese) | L. animalis L. acidophilus L. paracasei L. brevis | 107 cfu/g | 28 days | [75] | |
| Semi hard goat’s cheese | L. acidophilus B. lactis | 106 cfu/g | 70 days | [76] | |
| Crescenza cheese (soft Italian cheese) | B. bifidum B. infantis B. longum | 105 cfu/g | 14 days | [77] | |
| Soya based | Soya frozen dessert | L. acidophilus | 107 cfu/g | 28 weeks | [78] |
| L. paracasei | |||||
| B. lactis | |||||
| L. rhamnosus | |||||
| S. boulardii | ~105 cfu/g | ||||
| Soy milk | B. breve | 109 cfu/mL | 20 days | [79] | |
| Cereal based | Oat bars | B. lactis | 109 cfu/25 g bar | 7–14 days | [80] |
| Milk based maize/rice pudding | B. animalis L. acidophilus L. rhamnosus | 108–109 cfu/g | 21 days | [81] | |
| Oat meal gruel mixed with fruit drinks (i.e., rose hip, strawberry) | L. plantarum | 1010 cfu/mL | 30 days | [82] | |
| Fruit and fruit juice | Blackcurrant | L. plantarum | Not reported | [83] | |
| Dried apple fruits | L. casei | 106 cfu/g | [84] | ||
| Apple juice | L. acidophilus L. rhamnosus L. salivarius L. plantarum L. paracasei B. longum B. lactis type Bi-04 B. lactis type Bi-07 | 106 cfu/mL | 6 weeks | [85] | |
| Pineapple juice | L. casei | 106 cfu/mL | 42 days | [86] | |
| Pediococcus pentosaceus, Lactobacillus rhamnosus, Pediococcus pentosaceus | 109 cfu/mL | 4 weeks | [87] | ||
| Cashew apple juice powder (spray dried) | L. casei NRRL B-442 | ~106 cfu/g | 35 days | [88] | |
| Orange juice after Spouted bed drying | L. casei | ~106 cfu/g | 5 weeks | [89] | |
| Orange juice powder (spray and freeze dried) | L. plantarum 299v | 106 cfu/g | 180 days | [90] | |
| Orange | Lactobacillus GG | Not reported | [91] | ||
| Vegetable based | Table olives | L. rhamnosus L. paracasei B. bifidum B. longum | 106–108 cfu/g | 90 days | [92] |
| Carrot blended with orange juice | L. plantarum CECT 220 | 108–109 cfu/mL | 30 days | [93] | |
| Tomato juice | L. plantarum L. acidophilus L. casei L. delbrueckii | 104–108 cfu/g | 30 days | [51] | |
| Beet juice | L. plantarum L. acidophilus L. casei L. delbrueckii | 106–108 cfu/mL | 4 weeks | [94] | |
| Cabbage juice | L. plantarum L. delbrueckii | 107 cfu/mL 105 cfu/mL | 4 weeks | [95] | |
| Artichokes | L. plantarum L. paracasei | 107–108 cfu/g | 90 days | [96] | |
| Fermented Kale juice (Brassica oleraceae) | L. plantarum L. casei L. acidophilus L. brevis | 108 cfu/mL | 4 weeks | [97] | |
| Fermented vegetable soybean beverage | L. acidophilus La-5, B. animalis Bb-12 | ~106 cfu/mL 108 cfu/mL | 28 days | [98] | |
| Vegetable pickle products | L. casei LA284 | 104–108cfu/g | 70 days | [99] | |
| Meat and fish products | Fermented sausage | L. plantarum | - | - | [53] |
| Dry sausages-beef + pork | L. rhamnosus | 108 cfu/g | 28 days | [100] | |
| Fermented fish sausage | Lactobacillus spp. | satisfactory | 7 days | [101] | |
| Miscellaneous | Encapsulated and spray dried milk powder | L. acidophilus, B. animalis subsp. Lactis, Propionibacterium jensenii | 105–107 cfu/g | 24 weeks | [102] |
| Chocolate | L. acidophilus LA3, B. animalis subsp. lactis BLC1 | 107–108 cfu/g | 120 days | [103] | |
| African beverages made from maize and milk | B. lactis | 107 cfu/mL | 21 days | [104] | |
| Product Label | Manufacturer | Major Characteristics | Probiotic Strain/s |
|---|---|---|---|
| Golden Circle Healthy Life Probiotic Juice | Golden Circle, Australia | Mixture of apple juice and mango puree or orange, apple, pineapple, passionfruit with banana puree | L. paracasei 8700:2 and L. plantarum HEAL 9 |
| PERKii Probiotic Water | PERKii, Australia | Fruit juice mixtures such as raspberry and pomegranate, lime and coconut, mango and passionfruit and strawberry and watermelon | Lactobacillus casai Lc431 |
| Bravo Friscus | Probi AB, Swdeen | Orange apple and tropical fruit juices | L. plantarum HEAL9 and L. paracasei 8700:2 |
| ProViva | EMEA Probi AB, Sweden | Fruit juice (orange, strawberry or blackcurrant) | L. plantarum 299v |
| Bio-Live Gold & Dark | Bio-Live/Microbz Ltd., UK | Mixtures of fruit juices such as acai berry, cherry, goji, noni, pomegranate, lemon, and various herbs | Mixture of 13 strains including L. acidophilus, L bulgaricus, L. casei, L. fermentum, L. plantarum, Lactococcus lactis, Bacillus subtilis, B. bifidum, B. Infantis B longum; Streptococcus thermophilus, Comobcillus and Saccharomyces cerevisiae |
| Biola | TINE, Norway | Mixture of apple, grapes and passion fruit or orange and tangerine | L. rhamnosus GG |
| Malee Probiotics | Malee Enterprise Compny Ltd., Thailand | Fruit juices such as prune, grape and orange | L. paracasei |
| GoodBelly® Carrot Ginger Flavor Probiotics Juice Drink. | Goodbelly, USA | Carrot Juice, ginger extract and cane sugar contains 2% or less of gluten-free oat flour, | L. Plantarum 299v |
| KEVITA | KEVITA, USA | Various fruit based mixtures such as strawberry and coconut, lime, mint and coconut, mango and coconut, pineapple and coconut | Bacillus coagulans GBI-30 6086, L. rhamnosus, L. plantarum, L. paracasei |
| Tropicana probiotics | Tropicana, USA | Fruit juice mixtures such as strawberry and banana, pineapple and mango and peach passion fruit | B. lactis |
| Probiotic Naked Juice | Naked® Juice, USA | Mixture of apple, orange, pineapple juices and mango and banana puree with fructooligosacccharides | Bifidobacterium |
| Fermentation Type | Type of Microorganism Involved | Beverage Products |
|---|---|---|
| Lactic Fermentation | Mesophilic type | Cultured buttermilk |
| Thermophilic type | Bulgarian buttermilk, Drinking yogurt | |
| Therapeutic | Acidophilus milk, Yakult | |
| Yeast-lactic Fermentation | Yeast and lactic acid bacteria | Kefir, Acidophilus yeast milk |
| Mould-lactic Fermentation | Mould and lactic acid bacteria | Villi |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ranadheera, C.S.; Vidanarachchi, J.K.; Rocha, R.S.; Cruz, A.G.; Ajlouni, S. Probiotic Delivery through Fermentation: Dairy vs. Non-Dairy Beverages. Fermentation 2017, 3, 67. https://doi.org/10.3390/fermentation3040067
Ranadheera CS, Vidanarachchi JK, Rocha RS, Cruz AG, Ajlouni S. Probiotic Delivery through Fermentation: Dairy vs. Non-Dairy Beverages. Fermentation. 2017; 3(4):67. https://doi.org/10.3390/fermentation3040067
Chicago/Turabian StyleRanadheera, Chaminda Senaka, Janak K. Vidanarachchi, Ramon Silva Rocha, Adriano G. Cruz, and Said Ajlouni. 2017. "Probiotic Delivery through Fermentation: Dairy vs. Non-Dairy Beverages" Fermentation 3, no. 4: 67. https://doi.org/10.3390/fermentation3040067
APA StyleRanadheera, C. S., Vidanarachchi, J. K., Rocha, R. S., Cruz, A. G., & Ajlouni, S. (2017). Probiotic Delivery through Fermentation: Dairy vs. Non-Dairy Beverages. Fermentation, 3(4), 67. https://doi.org/10.3390/fermentation3040067