Hydrolyzed Agricultural Residues—Low-Cost Nutrient Sources for l-Lactic Acid Production
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Reference Cultivation and Variation of the Complex Nutrient Sources in MRS Medium
3.2. Nutrient Requirements
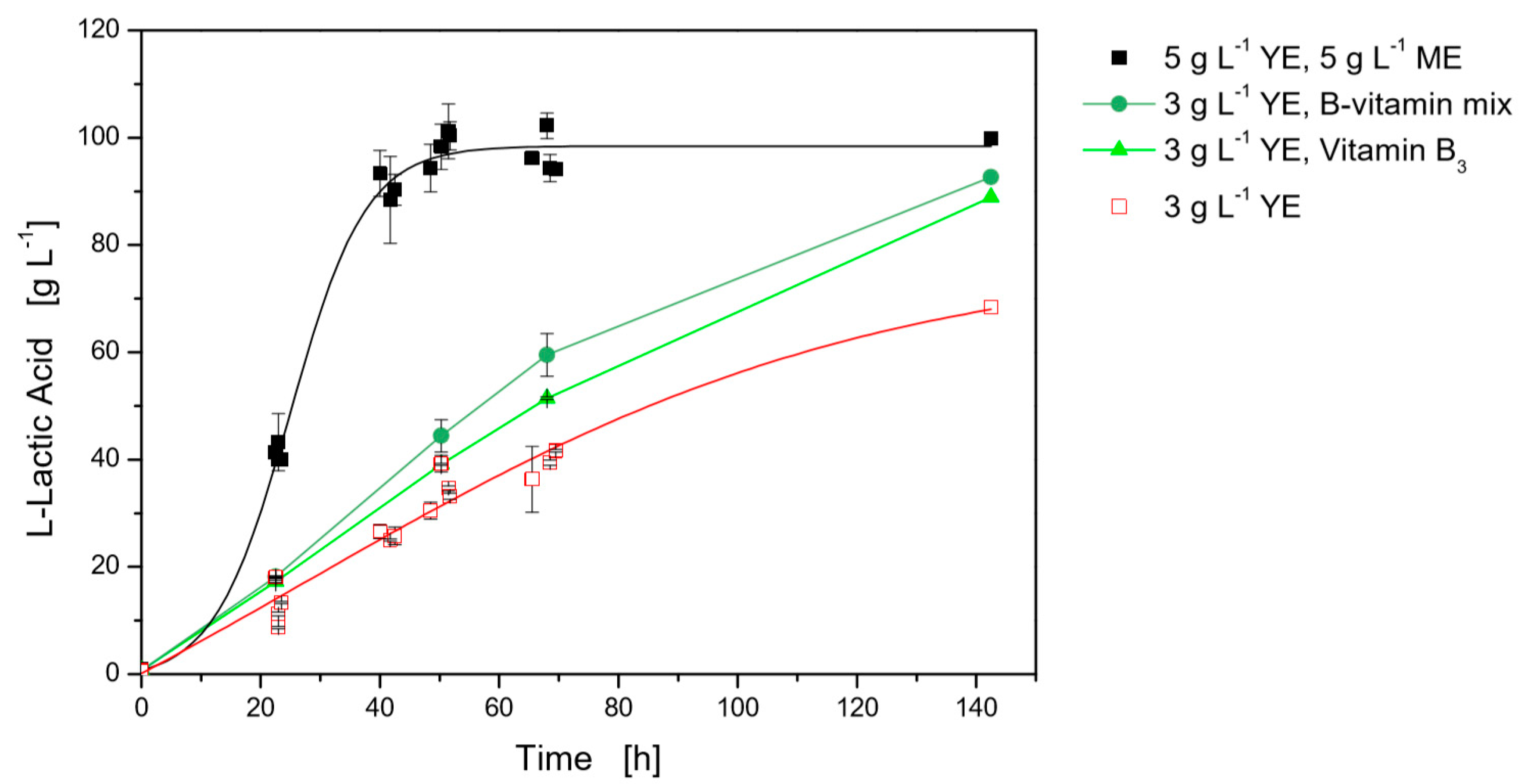
3.2.1. B-Vitamin Requirements
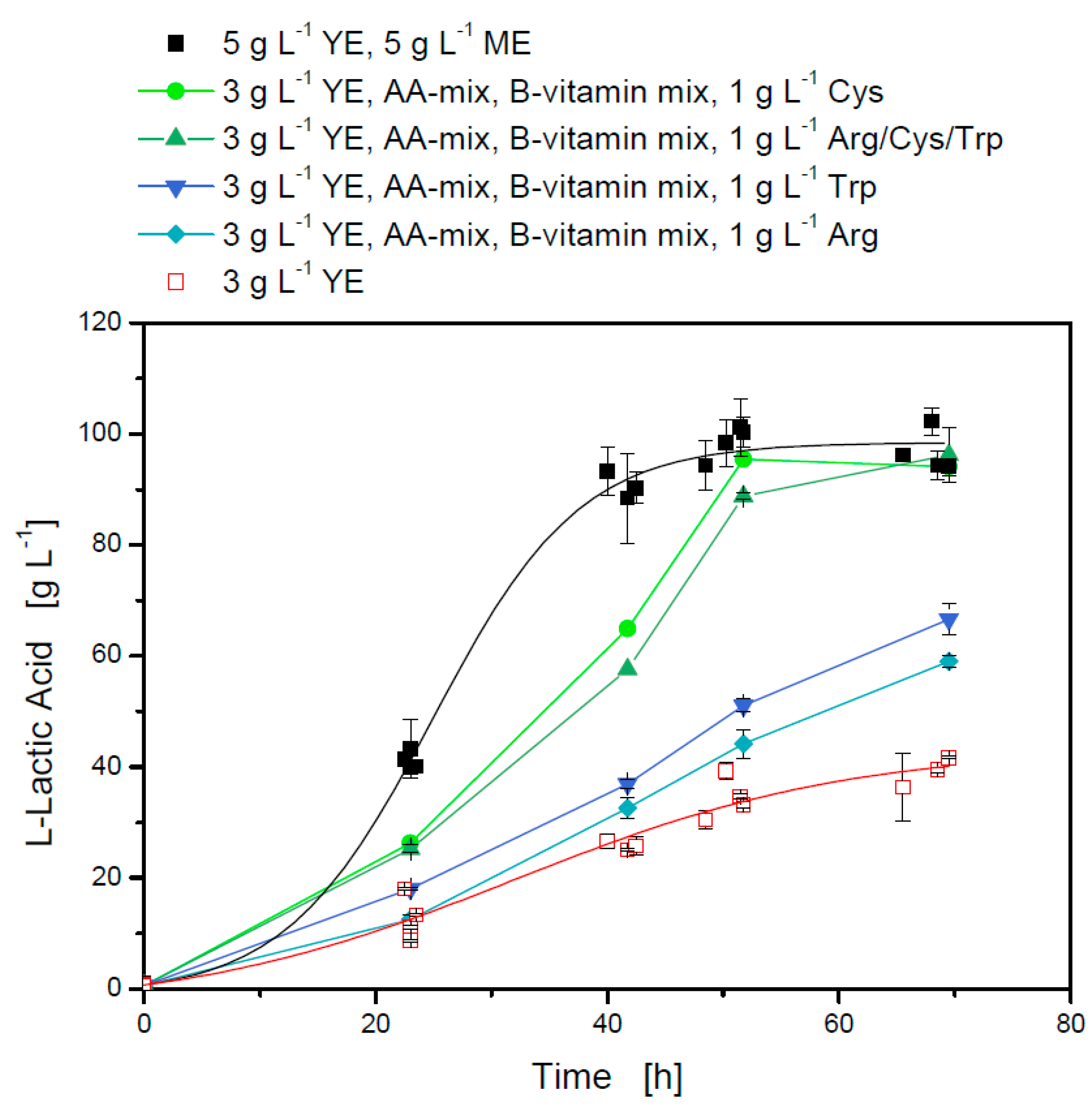
3.2.2. Amino Acid Requirements and the Influence of Different Medium Components
3.3. Agriculture Residue Hydrolysates as Nutrition Source
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Maddipati, P.; Atiyeh, H.K.; Bellmer, D.D.; Huhnke, R.L. Ethanol production from syngas by Clostridium strain P11 using corn steep liquor as a nutrient replacement to yeast extract. Bioresour. Technol. 2011, 102, 6494–6501. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, M.A.; Tashiro, Y.; Sonomoto, K. Recent advances in lactic acid production by microbial fermentation processes. Biotechnol. Adv. 2013, 31, 877–902. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tashiro, Y.; Sonomoto, K. Fermentative production of lactic acid from renewable materials: Recent achievements, prospects, and limits. J. Biosci. Bioeng. 2015, 119, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Chopin, A. Organization and regulation of genes for amino acid biosynthesis in lactic acid bacteria. FEMS Microbiol. Rev. 1993, 12, 21–37. [Google Scholar] [CrossRef]
- Deguchi, Y.; Morishita, T. Nutritional requirements in multiple auxotrophic lactic acid bacteria: Genetic lesions affecting amino acid biosynthetic pathways in Lactococcus lactis, Enterococcus faecium, and Pediococcus acidilactici. Biosci. Biotechnol. Biochem. 1992, 56, 913–918. [Google Scholar] [CrossRef]
- Klotz, S.; Kaufmann, N.; Kuenz, A.; Prüße, U. Biotechnological production of enantiomerically pure d-lactic acid. Appl. Microbiol. Biotechnol. 2016, 100, 9423–9437. [Google Scholar] [CrossRef]
- Liu, S.-Q.; Holland, R.; Crow, V. The potential of dairy lactic acid bacteria to metabolise amino acids via non-transaminating reactions and endogenous transamination. Int. J. Food Microbiol. 2003, 86, 257–269. [Google Scholar] [CrossRef]
- de Oliveira, R.A.; Komesu, A.; Rossell, C.E.V.; Maciel Filho, R. Challenges and opportunities in lactic acid bioprocess design—From economic to production aspects. Biochem. Eng. J. 2018, 133, 219–239. [Google Scholar] [CrossRef]
- Garlotta, D. A literature review of poly (lactic acid). J. Polym. Environ. 2001, 9, 63–84. [Google Scholar] [CrossRef]
- Hofvendahl, K.; Hahn–Hägerdal, B. Factors affecting the fermentative lactic acid production from renewable resources1. Enzym. Microb. Technol. 2000, 26, 87–107. [Google Scholar] [CrossRef]
- González, M.I.; Álvarez, S.; Riera, F.; Álvarez, R. Economic evaluation of an integrated process for lactic acid production from ultrafiltered whey. J. Food Eng. 2007, 80, 553–561. [Google Scholar] [CrossRef]
- Tejayadi, S.; Cheryan, M. Lactic acid from cheese whey permeate. Productivity and economics of a continuous membrane bioreactor. Appl. Microbiol. Biotechnol. 1995, 43, 242–248. [Google Scholar] [CrossRef]
- Sikder, J.; Roy, M.; Dey, P.; Pal, P. Techno-economic analysis of a membrane-integrated bioreactor system for production of lactic acid from sugarcane juice. Biochem. Eng. J. 2012, 63, 81–87. [Google Scholar] [CrossRef]
- Sommer, R. Yeast extracts: Production, properties and components. Food Aust. 1998, 50, 181–183. [Google Scholar]
- Pauli, T.; Fitzpatrick, J.J. Malt combing nuts as a nutrient supplement to whey permeate for producing lactic by fermentation with Lactobacillus casei. Process Biochem. 2002, 38, 1–6. [Google Scholar] [CrossRef]
- Brock, S.; Kuenz, A.; Prüße, U. Impact of hydrolysis methods on the utilization of agricultural residues as nutrient source for D-lactic acid production by Sporolactobacillus inulinus. Fermentation 2019, 5, 12. [Google Scholar] [CrossRef] [Green Version]
- Hujanen, M.; Linko, S.; Linko, Y.-Y.; Leisola, M. Optimisation of media and cultivation conditions for L (+)(S)-lactic acid production by Lactobacillus casei NRRL B-441. Appl. Microbiol. Biotechnol. 2001, 56, 126–130. [Google Scholar] [CrossRef]
- Klotz, S.; Kuenz, A.; Prüße, U. Nutritional requirements and the impact of yeast extract on the d-lactic acid production by Sporolactobacillus inulinus. Green Chem. 2017, 19, 4633–4641. [Google Scholar] [CrossRef]
- De Man, J.C.; Rogosa, M.; Sharpe, M.E. A Medium for the Cultivation of Lactobacilli. J. Appl. Bacteriol. 1960, 23, 130–135. [Google Scholar] [CrossRef]
- Olmos-Dichara, A.; Ampe, F.; Uribelarrea, J.L.; Pareilleux, A.; Goma, G. Growth and lactic acid production by Lactobacillus casei ssp. rhamnosus in batch and membrane bioreactor: Influence of yeast extract and Tryptone enrichment. Biotechnol. Lett. 1997, 19, 709–714. [Google Scholar] [CrossRef]
- Amrane, A.; Prigent, Y. Influence of yeast extract concentrationon batch cultures of Lactobacillus helveticus: Growth and production coupling. World J. Microbiol. Biotechnol. 1998, 14, 529–534. [Google Scholar] [CrossRef]
- Nancib, N.; Nancib, A.; Boudjelal, A.; Benslimane, C.; Blanchard, F.; Boudrant, J. The effect of supplementation by different nitrogen sources on the production of lactic acid from date juice by Lactobacillus casei subsp. rhamnosus. Bioresour. Technol. 2001, 78, 149–153. [Google Scholar] [CrossRef]
- Ding, S.; Tan, T. L-lactic acid production by Lactobacillus casei fermentation using different fed-batch feeding strategies. Process Biochem. 2006, 41, 1451–1454. [Google Scholar] [CrossRef]
- Hujanen, M.; Linko, Y.Y. Effect of temperature and various nitrogen sources on L(+)-lactic acid production by Lactobacillus casei. Appl. Microbiol. Biotechnol. 1996, 45, 307–313. [Google Scholar] [CrossRef]
- Kwon, S.; Lee, P.C.; Lee, E.G.; Keun Chang, Y.; Chang, N. Production of lactic acid by Lactobacillus rhamnosus with vitamin-supplemented soybean hydrolysate. Enzym. Microb. Technol. 2000, 26, 209–215. [Google Scholar] [CrossRef]
- Aeschlimann, A.; von Stockar, U. The effect of yeast extract supplementation on the production of lactic acid from whey permeate by Lactobacillus helueticus. Appl. Microbiol. Biotechnol. 1990, 32, 398–402. [Google Scholar] [CrossRef]
- Aspmo, S.I.; Horn, S.J.; Eijsink, V.G.H. Use of hydrolysates from Atlantic cod (Gadus morhua L.) viscera as a complex nitrogen source for lactic acid bacteria. FEMS Microbiol. Lett. 2005, 248, 65–68. [Google Scholar] [CrossRef]
- Rogosa, M.; Franklin, J.G.; Perry, K.D. Correlation of the vitamin requirements with cultural and biochemical characters of Lactobacillus spp. J. Gen. Microbiol. 1961, 25, 473–482. [Google Scholar] [CrossRef] [Green Version]
- Koser, S.A. Vitamin requirements of bacteria and yeasts. Arch. Intern. Med. 1968, 122, 192. [Google Scholar] [CrossRef]
- Ledesma, O.V.; De Ruiz Holgado, A.P.; Oliver, G.; De Giori, G.S.; Raibaud, P.; Galpin, J.V. A synthetic medium for comparative nutritional studies of lactobacilli. J. Appl. Bacteriol. 1977, 42, 123–133. [Google Scholar] [CrossRef]
- Yoo, I.-K.; Chang, H.N.; Lee, E.G.; Chang, Y.K.; Moon, S.-H. Effect of B vitamin supplementation on lactic acid production by Lactobacillus casei. J. Ferment Bioeng. 1997, 84, 172–175. [Google Scholar] [CrossRef]
- Dunn, M.S.; Shankman, S.; Camien, M.N.; Block, H. The amino acid requirements of twenty-three lactic acid bacteria. J. Biol. Chem. 1947, 168, 1–22. [Google Scholar] [PubMed]
- Bogicevic, B.; Berthoud, H.; Portmann, R.; Bavan, T.; Meile, L.; Irmler, S. Cysteine biosynthesis in Lactobacillus casei: Identification and characterization of a serine acetyltransferase. FEMS Microbiol. Lett. 2016, 363. [Google Scholar] [CrossRef] [Green Version]
- Irmler, S.; Schafer, H.; Beisert, B.; Rauhut, D.; Berthoud, H. Identification and characterization of a strain-dependent cystathionine beta/gamma-lyase in Lactobacillus casei potentially involved in cysteine biosynthesis. FEMS Microbiol. Lett. 2009, 295, 67–76. [Google Scholar] [CrossRef]
- Fountoulakis, M.; Lahm, H.-W. Hydrolysis and amino acid composition analysis of proteins. J. Chromatogr. A 1998, 826, 109–134. [Google Scholar] [CrossRef]
- Stein, H.H. Distillers dried grains with solubles (DDGS) in diets fed to swine. Swine Focus 2007, 1, 1–8. [Google Scholar]
- Proplanta. Hamburg Großhandelspreise vom 18.08.2020-Getreide, Mühlenprodukte, Futtermittel und Ölsaaten. Available online: https://www.proplanta.de/markt-und-preis/hamburger-getreideboerse/hamburg-grosshandelspreise-vom-18-08-2020-getreide-muehlenprodukte-futtermittel-und-oelsaaten_notierungen1597770118.html (accessed on 23 August 2020).
- DTN. Progressive Farmer, DDG Weekly Update-DTN Weekly Average DDG Price Unchanged 2/24/2020. Available online: https://www.dtnpf.com/agriculture/web/ag/livestock/article/2020/02/24/dtn-weekly-average-ddg-price (accessed on 23 August 2020).
- DKL Engineering, I. Sulphuric acid on the webᵀᴹ-Market and cost information. Available online: www.sulphuric-acid.com/sulphuric-acid-on-the-web/Market-Info.htm (accessed on 23 August 2020).
- Schnippe, F. Löschkalk als preisgünstige Alternative. Top Agrar 2004, 6, 6–9. [Google Scholar]

| YE (g·L−1) | P (g·L−1) | ME (g·L−1) | TN (g·L−1) | Glucose (g·L−1) | l-Lactic Acid (g·L−1) | Max. Productivity (g·L−1 h−1) |
|---|---|---|---|---|---|---|
| 5 | 10 | 10 | 2.97 | 0 | 106.3 ± 5.5 | 4.63 ± 0.06 |
| 5 | 0 | 5 | 1.11 | 0 | 113.3 ± 3.7 | 4.76 ± 0.08 |
| 5 | 5 | 5 | 1.72 | 0 | 105.1 ± 0.5 | 4.39 ± 0.22 |
| 10 | 0 | 0 | 0.95 | 0 | 113.3 ± 1.1 | 3.02 ± 0.08 |
| 5 | 5 | 0 | 1.09 | 0 | 108.1 ± 2.9 | 2.67 ± 0.26 |
| 0 | 0 | 10 | 1.25 | 0 | 102.1 ± 1.1 | 1.89 ± 0.01 |
| 5 | 0 | 0 | 0.48 | 33.5 ± 0.9 | 87.2 ± 0.5 | 1.69 ± 0.04 |
| 0 | 5 | 5 | 1.24 | 38.7 ± 0.1 | 64.36 ± 0.5 | 1.62 ± 0.02 |
| 0 | 0 | 5 | 0.63 | 54.8 ± 6.1 | 59.0 ± 4.8 | 1.62 ± 0.38 |
| 3 | 0 | 0 | 0.29 | 65.4 ± 0.6 | 57.9 ± 0.1 | 1.02 ± 0.01 |
| 0 | 10 | 0 | 1.24 | 75.7 ± 2.8 | 33.8 ± 1.7 | 0.93 ±0.01 |
| 0 | 5 | 0 | 0.62 | 78.8 ± 4.3 | 18.7 ± 0.8 | 0.54 ± 0.01 |
| 0 | 0 | 0 | 0 | 114 ± 0.1 | 6.1 ± 0.1 | 0.21 ± 0.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krull, S.; Brock, S.; Prüße, U.; Kuenz, A. Hydrolyzed Agricultural Residues—Low-Cost Nutrient Sources for l-Lactic Acid Production. Fermentation 2020, 6, 97. https://doi.org/10.3390/fermentation6040097
Krull S, Brock S, Prüße U, Kuenz A. Hydrolyzed Agricultural Residues—Low-Cost Nutrient Sources for l-Lactic Acid Production. Fermentation. 2020; 6(4):97. https://doi.org/10.3390/fermentation6040097
Chicago/Turabian StyleKrull, Susan, Silvia Brock, Ulf Prüße, and Anja Kuenz. 2020. "Hydrolyzed Agricultural Residues—Low-Cost Nutrient Sources for l-Lactic Acid Production" Fermentation 6, no. 4: 97. https://doi.org/10.3390/fermentation6040097
APA StyleKrull, S., Brock, S., Prüße, U., & Kuenz, A. (2020). Hydrolyzed Agricultural Residues—Low-Cost Nutrient Sources for l-Lactic Acid Production. Fermentation, 6(4), 97. https://doi.org/10.3390/fermentation6040097




