Low pH Stress Enhances Gluconic Acid Accumulation with Enzymatic Hydrolysate as Feedstock Using Gluconobacter oxydans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Culture Conditions
2.2. Enzymatic Hydrolysis of Pretreated Corncobs
2.3. Determination of the Enzymatic Activity
2.4. Analytical Methods
3. Results and Discussion
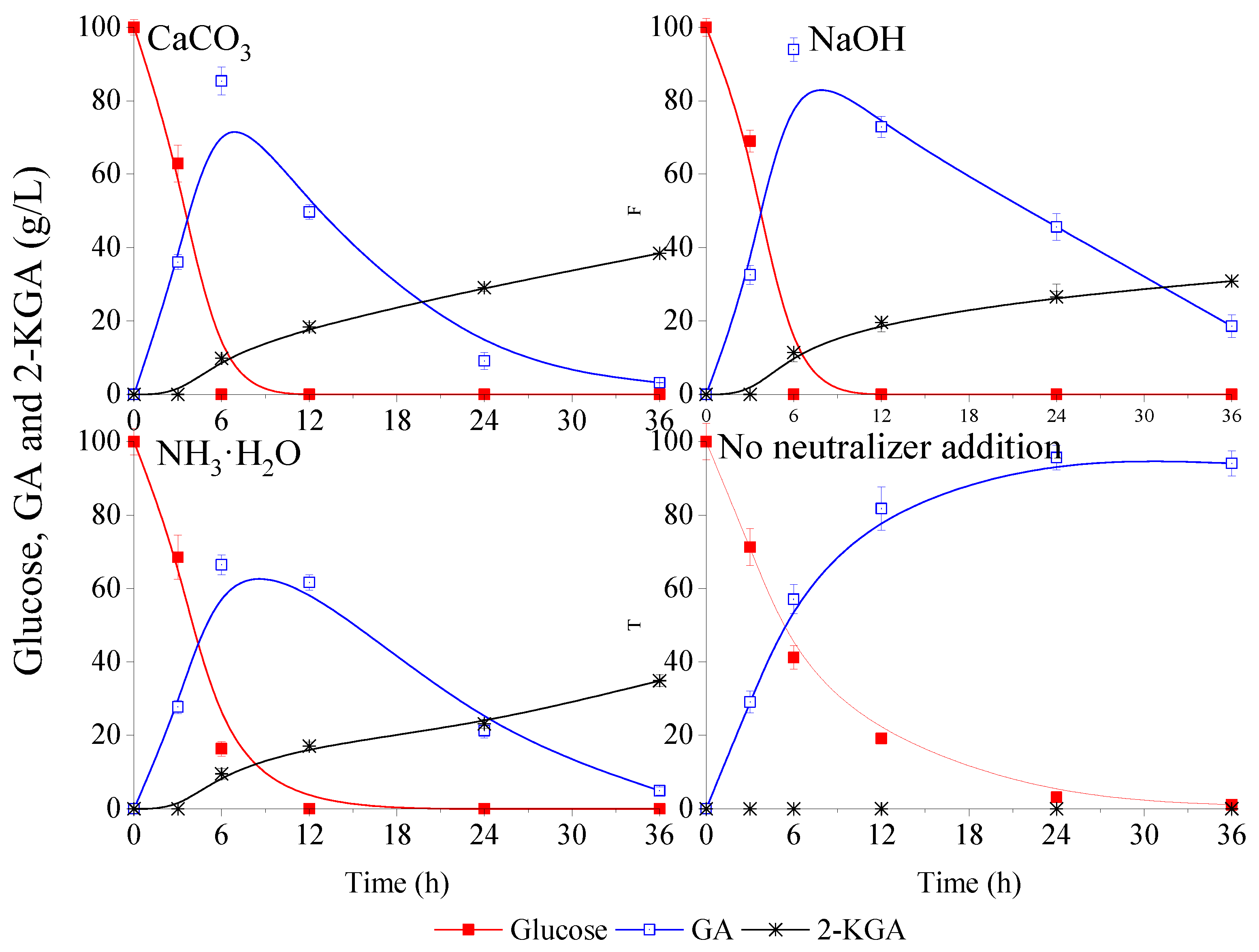
3.1. Effects of Different Neutralizers on Glucose Metabolism of G. oxydans
3.2. The Effects of Different pH Environments on Glucose Metabolism of G. oxydans
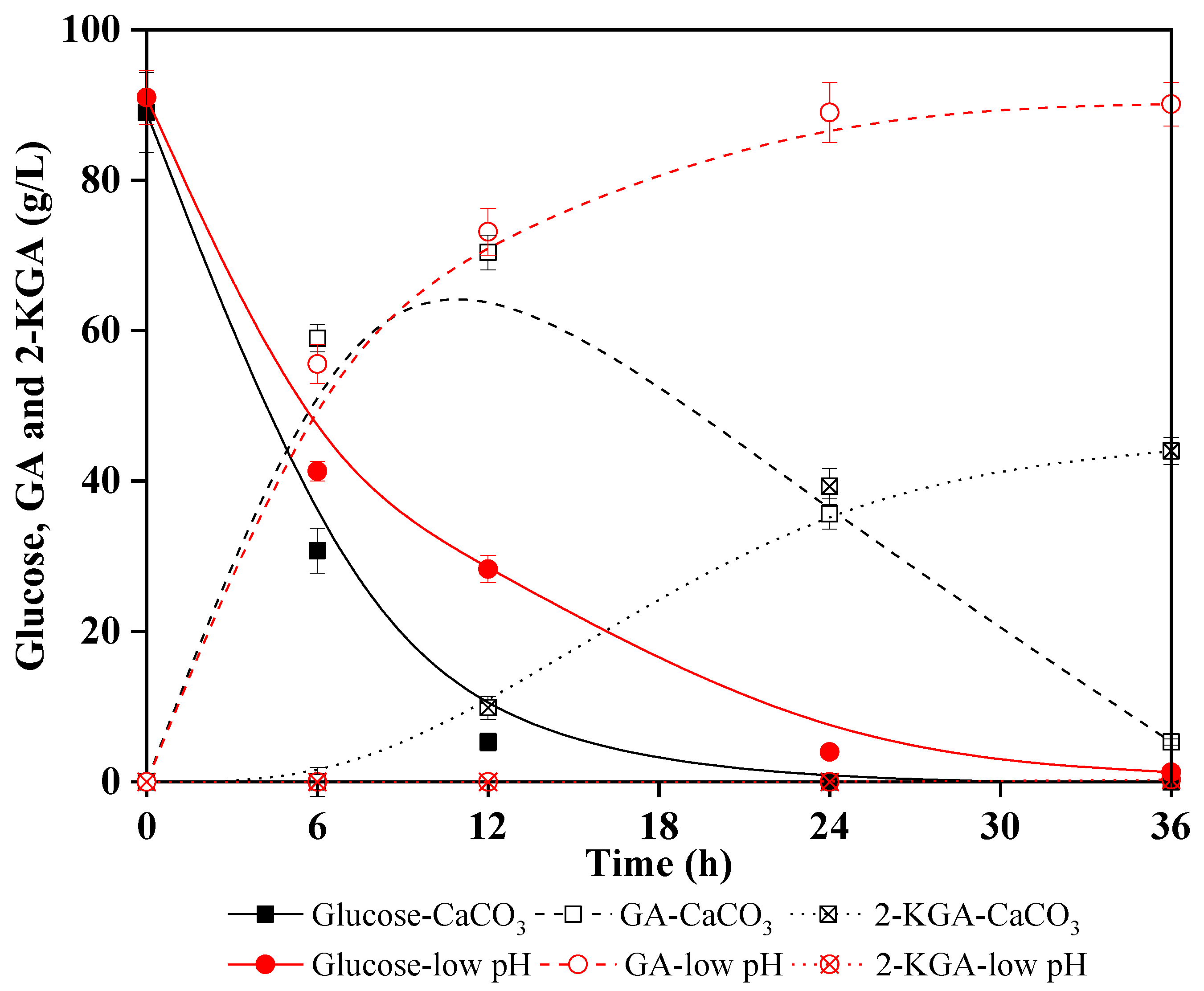
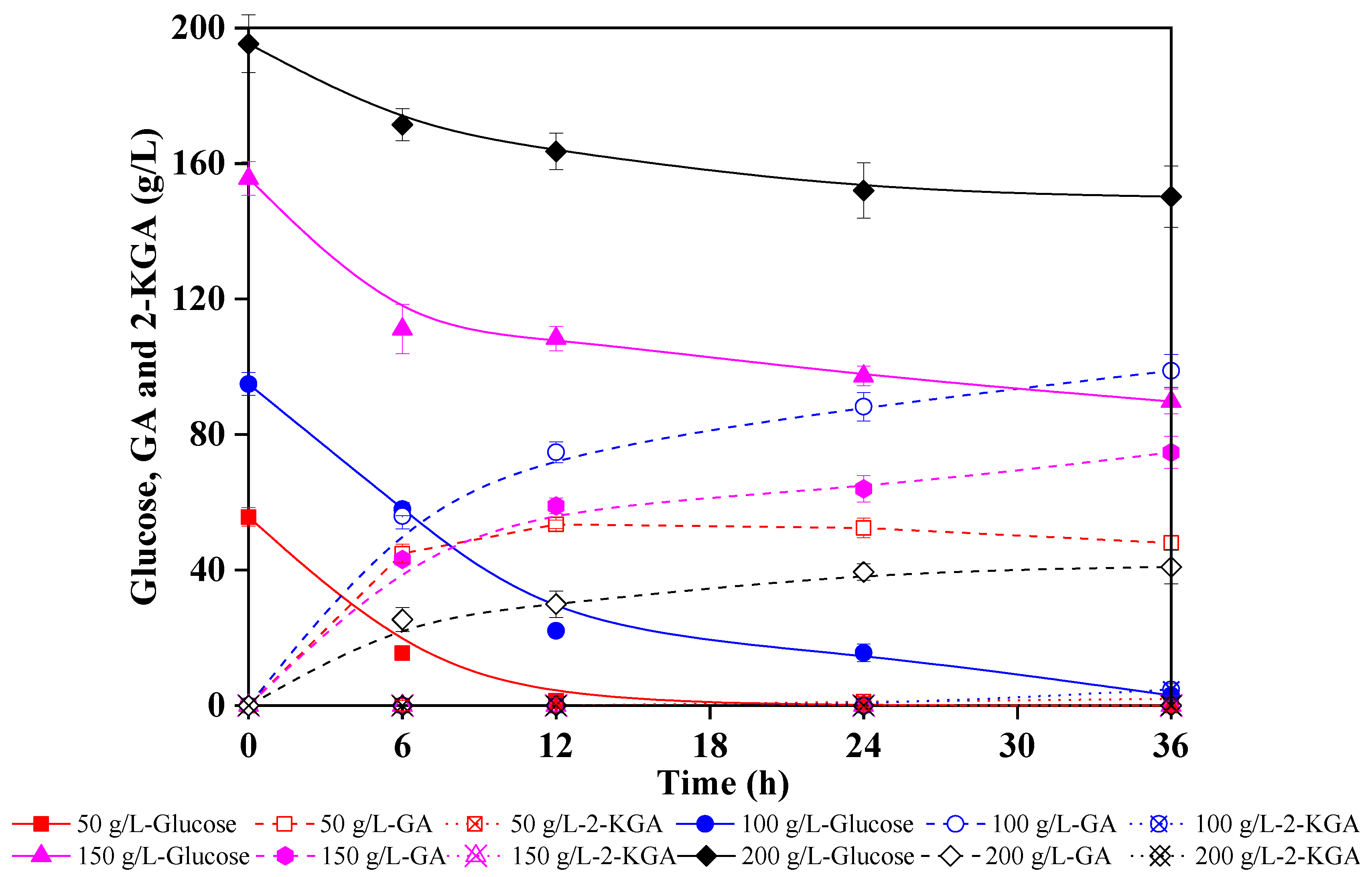
3.3. Direct Production of GA from Lignocellulosic Hydrolysate in Low pH Environment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, J.; Huang, K.; Zhang, S.; Xu, Y. Optimization of selective acidolysis pretreatment for the valorization of wheat straw by a combined chemical and enzymatic process. J. Chem. Technol. Biotechnol. 2019, 95, 694–701. [Google Scholar] [CrossRef]
- Liu, Z.-H.; Hao, N.; Wang, Y.-Y.; Dou, C.; Lin, F.; Shen, R.; Bura, R.; Hodge, D.B.; Dale, B.E.; Ragauskas, A.J.; et al. Transforming biorefinery designs with ‘plug-in processes of lignin’ to enable economic waste valorization. Nat. Commun. 2021, 12, 3912. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Kumar, R.; Pal, P. Fermentative production of gluconic acid: A membrane-integrated green process. J. Taiwan Inst. Chem. Eng. 2018, 84, 76–84. [Google Scholar] [CrossRef]
- Zou, F.; Tan, H.; Guo, Y.; Ma, B.; He, X.; Zhou, Y. Effect of sodium gluconate on dispersion of polycarboxylate superplasticizer with different grafting density in side chain. J. Ind. Eng. Chem. 2017, 55, 91–100. [Google Scholar] [CrossRef]
- Pal, P.; Kumar, R.; Banerjee, S. Manufacture of gluconic acid: A review towards process intensification for green production. Chem. Eng. Process. Process Intensif. 2016, 104, 160–171. [Google Scholar] [CrossRef]
- Mu, Q.; Cui, Y.; Tian, Y.; Hu, M.; Tao, Y.; Wu, B. Thermostability improvement of the glucose oxidase from aspergillus niger for efficient gluconic acid production via computational design. Int. J. Biol. Macromol. 2019, 136, 1060–1068. [Google Scholar] [CrossRef]
- Zhong, P.; Zheng, L.; Yang, Y.; Zhou, Y.; Liu, X.; Yang, Q.; Ren, J. Piezoelectric sensing of glucose oxidase activity of aspergillus niger spores pretreated by different methods. Food Chem. 2022, 370, 130901. [Google Scholar] [CrossRef]
- Sankpal, N.V.; Kulkarni, B.D. Optimization of fermentation conditions for gluconic acid production using aspergillus niger immobilized on cellulose microfibrils. Process Biochem. 2002, 37, 1343–1350. [Google Scholar] [CrossRef]
- Ikeda, Y.; Park, E.Y.; Okuda, N. Bioconversion of waste office paper to gluconic acid in a turbine blade reactor by the filamentous fungus aspergillus niger. Bioresour. Technol. 2006, 97, 1030–1035. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.; Chatterjee, S.; Chatterjee, B.P.; Banerjee, P.C.; Guha, A.K. Production of gluconic acid from whey by free and immobilized aspergillus niger. Int. Dairy J. 2005, 15, 299–303. [Google Scholar] [CrossRef]
- Kazemi, M.A.; Bamdad, H.; Papari, S.; Yaghmaei, S. Modeling and control of dissolved oxygen concentration in the fermentation of glucose to gluconic acid. Period. Polytech. Chem. Eng. 2013, 57, 63. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.; Tian, X.; Zhao, W.; Hang, H.; Chu, J. Oxygen-enriched fermentation of sodium gluconate by aspergillus niger and its impact on intracellular metabolic flux distributions. Bioprocess Biosyst. Eng. 2018, 41, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, S.; Fontanille, P.; Pandey, A.; Larroche, C. Gluconic acid: Properties, applications and microbial production. Food Technol. Biotech. 2006, 44, 185–195. [Google Scholar]
- Zhou, P.; Yao, R.; Zhang, H.; Bao, J. Unique glucose oxidation catalysis ofgluconobacter oxydans constitutes an efficient cellulosic gluconic acid fermentation free of inhibitory compounds disturbance. Biotechnol. Bioeng. 2019, 116, 2191–2199. [Google Scholar] [CrossRef]
- Wei, G.; Yang, X.; Zhou, W.; Lin, J.; Wei, D. Adsorptive bioconversion of ethylene glycol to glycolic acid by gluconobacter oxydans dsm 2003. Biochem. Eng. J. 2009, 47, 127–131. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, X.; Zhang, H.; Cao, R.; Xu, Y. Improving the performance of cell biocatalysis and the productivity of acetoin from 2,3-butanediol using a compressed oxygen supply. Process Biochem. 2018, 64, 46–50. [Google Scholar] [CrossRef]
- Hua, X.; Cao, R.; Zhou, X.; Xu, Y. One-step continuous/semi-continuous whole-cell catalysis production of glycolic acid by a combining bioprocess with in-situ cell recycling and electrodialysis. Bioresour. Technol. 2019, 273, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Hua, X.; Zhou, X.; Xu, Y. Process for the successive production of calcium galactonate crystals by gluconobacter oxydans. Bioresour. Technol. 2018, 261, 458–460. [Google Scholar] [CrossRef] [PubMed]
- Hanke, T.; Nöh, K.; Noack, S.; Polen, T.; Bringer, S.; Sahm, H.; Wiechert, W.; Bott, M. Combined fluxomics and transcriptomics analysis of glucose catabolism via a partially cyclic pentose phosphate pathway in gluconobacter oxydans 621h. Appl. Environ. Microb. 2013, 79, 2336–2348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prust, C.; Hoffmeister, M.; Liesegang, H.; Wiezer, A.; Fricke, W.F.; Ehrenreich, A.; Gottschalk, G.; Deppenmeier, U. Complete genome sequence of the acetic acid bacterium gluconobacter oxydans. Nat. Biotechnol. 2005, 23, 195–200. [Google Scholar] [CrossRef] [Green Version]
- Lian, Z.; Dai, L.; Zhang, R.; Liu, Y.; Zhou, X.; Xu, Y. Efficient aerobic fermentation of gluconic acid by high tension oxygen supply strategy with reusable gluconobacter oxydans hg19 cells. Bioproc. Biosyst. Eng. 2022, 45, 1849–1855. [Google Scholar] [CrossRef] [PubMed]
- Sainz, F.; Navarro, D.; Mateo, E.; Torija, M.J.; Mas, A. Comparison of d-gluconic acid production in selected strains of acetic acid bacteria. Int. J. Food Microbiol. 2016, 222, 40–47. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, X.; Liu, G.; Xu, Y.; Balan, V. Integrated production of gluconic acid and xylonic acid using dilute acid pretreated corn stover by two-stage fermentation. Biochem. Eng. J. 2018, 137, 18–22. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, X.; Huang, L.; Cao, R.; Xu, Y. Efficient coproduction of gluconic acid and xylonic acid from lignocellulosic hydrolysate by zn(ii)-selective inhibition on whole-cell catalysis by gluconobacter oxydans. Bioresour. Technol. 2017, 243, 855–859. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, G.; Zhang, J.; Bao, J. Fermentative production of high titer gluconic and xylonic acids from corn stover feedstock by gluconobacter oxydans and techno-economic analysis. Bioresour. Technol. 2016, 219, 123–131. [Google Scholar] [CrossRef]
- Miao, Y.; Shen, Y.; Xu, Y. Effects of inhibitors on the transcriptional profiling of gluconobater oxydans nl71 genes after biooxidation of xylose into xylonate. Front. Microbiol. 2017, 8, 716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, X.; Zhou, X.; Tang, X.; Xu, Y. Process for calcium xylonate production as a concrete admixture derived from in-situ fermentation of wheat straw pre-hydrolysate. Bioresour. Technol. 2018, 261, 288–293. [Google Scholar] [CrossRef]
- Guo, J.; Cao, R.; Huang, K.; Xu, Y. Comparison of selective acidolysis of xylan and enzymatic hydrolysability of cellulose in various lignocellulosic materials by a novel xylonic acid catalysis method. Bioresour. Technol. 2020, 304, 122943. [Google Scholar] [CrossRef]
- Sugisawa, T.; Hoshino, T.; Nomura, S.; Fujiwara, A. Isolation and characterization of Membrane—Bound L—Sorbose dehydrogenase from gluconobacter melanogenus uv10. Agric. Biol. Chem. 1991, 55, 363–370. [Google Scholar] [CrossRef] [Green Version]
- Sugisawa, T.; Hoshino, T. Purification and properties of Membrane—Bound D—Sorbitol dehydrogenase fromgluconobacter suboxydans ifo 3255. Biosci. Biotechnol. Biochem. 2002, 66, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; He, T.; Zhou, X.; Xu, Y.; Gu, X. Influence of oxygen transfer and uptake rates on xylonic acid production from xylose by gluconobacter oxydans. Biochem. Eng. J. 2021, 176, 108192. [Google Scholar] [CrossRef]
- Han, J.; Hua, X.; Zhou, X.; Xu, B.; Wang, H.; Huang, G.; Xu, Y. A cost-practical cell-recycling process for xylonic acid bioproduction from acidic lignocellulosic hydrolysate with whole-cell catalysis of gluconobacter oxydans. Bioresour. Technol. 2021, 333, 125157. [Google Scholar] [CrossRef]
- Liu, X.; Cao, R.; Nawaz, A.; Haq, I.U.; Zhou, X.; Xu, Y. Smart removal of monosaccharide contaminants in xylo-oligosaccharide slurry using sandwich-integration bioprocess of whole-cell catalysis combined with electrodialysis separation. Renew. Energy 2021, 168, 1149–1156. [Google Scholar] [CrossRef]
- Peters, B.; Mientus, M.; Kostner, D.; Junker, A.; Liebl, W.; Ehrenreich, A. Characterization of membrane-bound dehydrogenases from gluconobacter oxydans 621h via whole-cell activity assays using multideletion strains. Appl. Microbiol. Biotechnol. 2013, 97, 6397–6412. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, J.; Zhang, X.; Xu, Y. An eco-friendly biorefinery strategy for xylooligosaccharides production from sugarcane bagasse using cellulosic derived gluconic acid as efficient catalyst. Bioresour. Technol. 2019, 289, 121755. [Google Scholar] [CrossRef] [PubMed]
- Adachi, T.; Kitazumi, Y.; Shirai, O.; Kano, K. Direct electron transfer-type bioelectrocatalysis by membrane-bound aldehyde dehydrogenase from gluconobacter oxydans and cyanide effects on its bioelectrocatalytic properties. Electrochem. Commun. 2021, 123, 106911. [Google Scholar] [CrossRef]
- Adachi, T.; Sowa, K.; Kitazumi, Y.; Shirai, O.; Kano, K. Cyanide sensitivity in direct electron transfer-type bioelectrocatalysis by membrane-bound alcohol dehydrogenase from gluconobacter oxydans. Bioelectrochemistry 2022, 143, 107992. [Google Scholar] [CrossRef]
- Da Silva, G.A.R.; Oliveira, S.S.D.S.; Lima, S.F.; Do Nascimento, R.P.; Baptista, A.R.D.S.; Fiaux, S.B. The industrial versatility of gluconobacter oxydans: Current applications and future perspectives. World J. Microbiol. Biotechnol. 2022, 38, 134. [Google Scholar] [CrossRef] [PubMed]
- Holscher, T.; Gorisch, H. Knockout and overexpression of pyrroloquinoline quinone biosynthetic genes ingluconobacter oxydans 621h. J. Bacteriol. 2006, 188, 7668–7676. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.C.; Zheng, Y.G.; Shen, Y.C. Dissolved-oxygen-stat fed-batch fermentation of 1,3-dihydroxyacetone from glycerol by gluconobacter oxydans zjb09112. Biotechnol. Bioprocess Eng. 2010, 15, 651–656. [Google Scholar] [CrossRef]
- Zhou, X.; Lü, S.; Xu, Y.; Mo, Y.; Yu, S. Improving the performance of cell biocatalysis and the productivity of xylonic acid using a compressed oxygen supply. Biochem. Eng. J. 2015, 93, 196–199. [Google Scholar] [CrossRef]
- Fujiwara, R.; Noda, S.; Tanaka, T.; Kondo, A. Metabolic engineering of escherichia coli for shikimate pathway derivative production from glucose-xylose co-substrate. Nat. Commun. 2020, 11, 279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, B.; Zhang, X.; Lin, Q.; Xin, F.; Sun, R.; Wang, X.; Ren, J. A new approach to recycle oxalic acid during lignocellulose pretreatment for xylose production. Biotechnol. Biofuels 2018, 11, 324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saliu, B.K.; Sani, A. Bioethanol potentials of corn cob hydrolysed using cellulases of aspergillus niger and penicillium decumbens. EXCLI J. 2012, 11, 468–479. [Google Scholar]
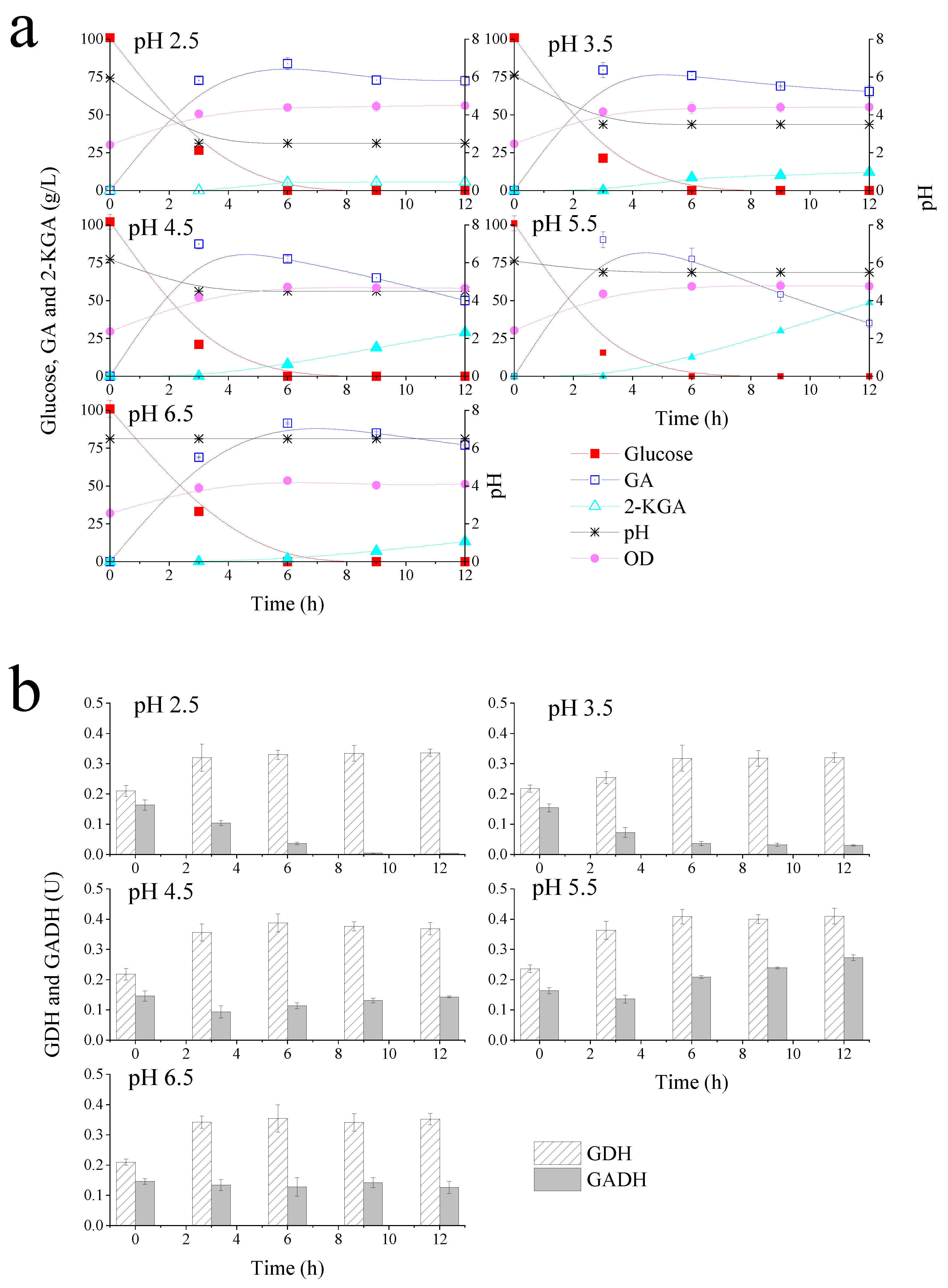
| pH | Production (G/L) | Average Productivity (G/L/h) | ||
|---|---|---|---|---|
| 3 H (GA) | 12 H (2-KGA) | 0–3 H (GA) | 9–12 H (2-KGA) | |
| 2.5 | 72.7 | 5.8 | 24.2 | 0.08 |
| 3.5 | 79.6 | 12.1 | 26.5 | 0.69 |
| 4.5 | 87.3 | 28.9 | 29.1 | 3.33 |
| 5.5 | 90.3 | 48.6 | 30.1 | 6.20 |
| 6.5 | 69.0 | 13.2 | 23.0 | 2.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, L.; Lian, Z.; Fu, Y.; Zhou, X.; Xu, Y.; Zhou, X.; Kuznetsov, B.N.; Jiang, K. Low pH Stress Enhances Gluconic Acid Accumulation with Enzymatic Hydrolysate as Feedstock Using Gluconobacter oxydans. Fermentation 2023, 9, 278. https://doi.org/10.3390/fermentation9030278
Dai L, Lian Z, Fu Y, Zhou X, Xu Y, Zhou X, Kuznetsov BN, Jiang K. Low pH Stress Enhances Gluconic Acid Accumulation with Enzymatic Hydrolysate as Feedstock Using Gluconobacter oxydans. Fermentation. 2023; 9(3):278. https://doi.org/10.3390/fermentation9030278
Chicago/Turabian StyleDai, Lin, Zhina Lian, Yixiu Fu, Xin Zhou, Yong Xu, Xuelian Zhou, Boris N. Kuznetsov, and Kankan Jiang. 2023. "Low pH Stress Enhances Gluconic Acid Accumulation with Enzymatic Hydrolysate as Feedstock Using Gluconobacter oxydans" Fermentation 9, no. 3: 278. https://doi.org/10.3390/fermentation9030278
APA StyleDai, L., Lian, Z., Fu, Y., Zhou, X., Xu, Y., Zhou, X., Kuznetsov, B. N., & Jiang, K. (2023). Low pH Stress Enhances Gluconic Acid Accumulation with Enzymatic Hydrolysate as Feedstock Using Gluconobacter oxydans. Fermentation, 9(3), 278. https://doi.org/10.3390/fermentation9030278






