Creating Value from Acidogenic Biohydrogen Fermentation Effluents: An Innovative Approach for a Circular Bioeconomy That Is Acquired via a Microbial Biorefinery-Based Framework
Abstract
:1. Introduction
Contribution of this Work to the Scientific Knowledge
2. What Are Acidogenic-Derived VFAs?
3. Types of Feedstocks Used for VFA Production during Acidogenesis
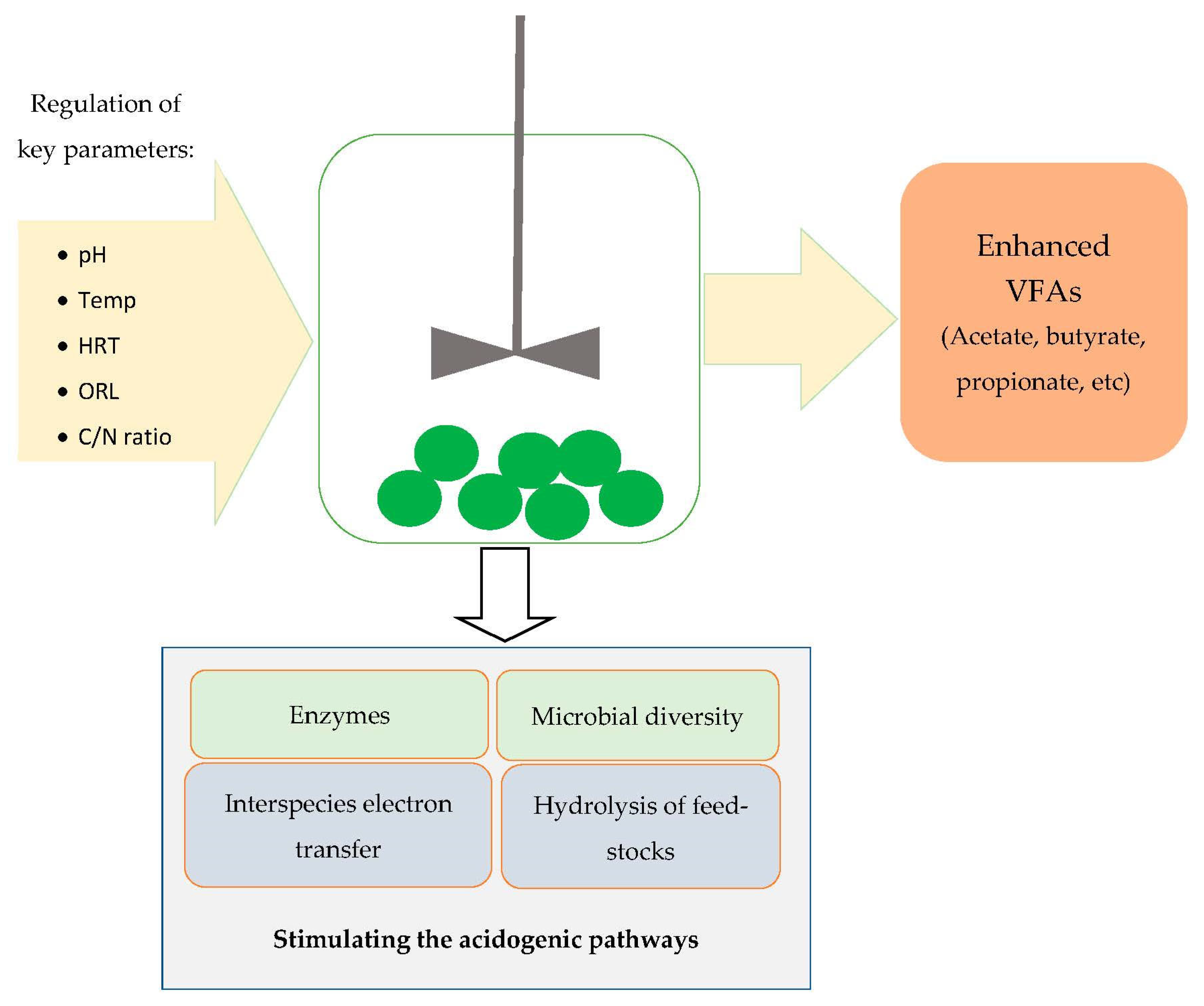
4. The Key Parameters Governing the Recovery of VFAs during Acidogenesis
5. Microbial Biorefinery-Based Processes Involving the Use of Acidogenic VFAs
5.1. Biobased Polymers
5.2. Alternative Fuels
- Electricity—microbial fuel cells (MFCs) are bioelectrochemical reactors that use a wide variety of biofilm-forming microorganisms to harness chemical energy stored in organic wastes for electricity generation [77,78]. These reactor systems consist of an anodic compartment and a cathodic compartment, which are divided by a proton exchange membrane [79]. The anodic compartment is anaerobic so that biofilm-forming communities can effectively convert organic wastes into electrons, protons, and carbon dioxide [80]. These electrons and protons are then transferred into the aerobic cathodic compartment through the means of an electric circuit and proton exchange membrane. In the cathodic chamber, the protons and oxygen combine to generate water. Figure 2 demonstrates the working principles of MFCs with VFA as a model substrate [81]. Equations (1) and (2) show the reactions that occur in the anodic and cathodic chambers, with acetate as a carbon source [82].
- Different MFC designs are used for electricity generation, and these include single-chambered MFCs and two-chambered MFCs, the upflow MFC, and the stacked MFC. These have been detailed by Udama et al. [83] and Ramya and Kumar [81]. The use of acidogenic VFAs could provide many breakthroughs in MFC technology as these compounds can be directly used for bio-electricity generation without the need for pretreatments as opposed to other bioprocesses. Several authors have successfully demonstrated the production of electricity using waste-derived VFAs. For example, Mohanakrishna et al. [84] produced a power density of 111.76 mW/m2 using VFAs obtained from acidogenic fermentation. Asefi et al. [85] achieved an optimum power density of 422 mW/m2 using VFAs from food waste. Microbial characterization studies of the anodic chamber showed the prevalence of Geobacter species which are the predominant bioelectricity-producing microorganisms [85].
- The composition of VFAs in acidogenic effluents has been shown to have a considerable effect on the performance of MFCs during electricity generation. It was reported that the electrical current produced in an acetate fed-MFC was almost two times higher than the electrical current obtained in MFCs fed with other VFA types, resulting in high coulombic efficiency of 93% [86]. Similarly, the acetate-fed-MFC outperformed the butyrate- and propionate-fed MFCs [87]. The 16S ribosomal DNA sequencing results of the anodic biofilms showed the prevalence of Geobacter sp. [87]. It has been shown that acetate is an ideal substrate for electroactive microorganisms due to its biodegradability and stimulatory effects compared to other VFA compounds [88]. Research is ongoing to acquire a deeper understanding of the prevailing bio-electricity anodic biofilms, electron transfer mechanisms, MFC designs, and electrode types to advance this technology.

- Biogas—biogas has received increasing attention over the past two decades due to its high methane content (30–80%), cost-competitiveness, and contribution to the bio-based economy [90]. Biogas occurs in the final step of the anaerobic process, where various bacterial and archaeal species use metabolites (VFAs and CO2) from the acidogenic and acetogenic stages as precursors for biogas formation [91]. Although various microorganisms have been reported in the biosynthesis of biogas, methanogenic species are the predominant species [90]. Biogas can be produced using a single-stage anaerobic process or a two-stage anaerobic process. Single-stage fermentations are not suitable because they generate low biogas content due to variations in the growth requirements of fermentative microorganisms [92]. Two-stage processes produce optimal biogas content because they cater to the growth conditions of different microbial communities. Acidogens are fast-growing species and require acidic pH alongside short HRTs, whereas methanogens are slow-growing species and proliferate under neutral pH and long HRTs [93]. The acidogenic step is used to convert the substrates into VFA-rich effluents, and these are later used by methane-producers in the second step [93]. The co-digestion of food waste and activated sludge was investigated in single-stage and two-stage anaerobic processes under mesophilic conditions. The two-stage process had a remarkable effect on methane content: increased from 61.2% to 70.1% [93]. Two-stage processes are appealing to scientific researchers because they eliminate the pretreatment steps, which are energy-intensive and expensive [94]. More studies are now focusing on biogas-upgrading technologies because these systems can produce high-purity methane (CH4 ≥90% v/v) that is applicable in natural gas pipelines [95].
- Hydrogen—VFAs from dark fermentation (DF) processes are also applicable in other hydrogen-producing technologies such as photo-fermentation (PF) [96], microalgal cultivation [97], electrohydrolysis [98], and microbial electrolysis cell (MEC) [99]. In photo-fermentation, purple non-sulfur bacteria use acidogenic compounds as substrates for hydrogen production [100]. Over the past few years, the coupling of DF and PF has been shown as an innovative approach that could augment the energetic gains in hydrogen production studies [101]. However, precautionary measures need to be applied because the main photosynthetic hydrogen producers, such as Rhodobacter sphaeroides, are fastidious and sensitive to certain growth conditions. For example, the acidogenic effluents consisting of VFAs must undergo vigorous pretreatments such as the removal of colloids, pH adjustment, dilution, and nutrient addition [102]. The types of VFAs found in the DF effluents also impact the growth of these photosynthetic species, as it has been reported that acetate- and propionate-rich effluents have a positive effect on hydrogen production yields compared to other VFA compounds [103]. Two-stage sequential batch processes of DK and PF can produce an overall H2 yield of 12 mols, as shown in Equations (3) and (4) [96]. This hybrid system holds a huge potential in the development of hydrogen-related technologies as some researchers have reported an optimal H2 yield of 10.21 mol H2/mol glucose and a VFA recovery of more than 90% [104,105]. Likewise, the use of microalgae is expanding and gaining recognition in hydrogen process development because these microorganisms are easily accessible and can be cultivated under various conditions [106]. Hybrid processes involving a microalgal consortium are therefore seen as a novel approach to harnessing clean hydrogen. Recent studies are focused on developing genetically engineered strains alongside optimal reactor designs to provide insights that could lead to the scalability of this process [107]. A coupled system of DF and the microalgal process was investigated for biohydrogen using food waste [108]. This integrated process generated a high biohydrogen yield of 133.66 mL/g substrate with almost 100% VFA consumption [108]. The energy conversion efficiency also increased from 10.14–24.06% [108].
- Electrohydrolysis of VFA is another option for producing renewable hydrogen, and this biotechnological route applies direct DC voltage into the VFA-rich effluents, causing the release of electrons from the metal electrodes (e.g., copper electrode), and these combine with protons to form hydrogen [109]. The mechanism of this technology is explained using Equations (5)–(7). This technology is advantageous as it is directly coupled in the anaerobic reactor to enable the endogenous (in-situ) production of hydrogen without the need for the pretreatment of effluents. Hydrogen production by electrohydrolysis of acidogenic VFAs was conducted by Tuna et al. [109]. It was observed that increasing the applied voltage from 1–3 V had a significant effect on H2 production [109]. However, studies that focus on the production of hydrogen by electrohydrolysis of waste-derived VFAs are still scarce in the literature. This implies that there are a lot of knowledge gaps in this area.
- Hydrogen is also produced through the means of a MEC. The MEC is derived from the MFC but slightly differs as it requires an external electricity supply [110]. In the anodic compartment, the electrochemically-active bacteria breakdown the VFAs and release protons and electrons to generate hydrogen in the cathodic compartment [111]. It is only in recent years that the acidogenic VFAs were found to be valuable in MECs. These reactor systems were discovered in the early 2000s by scientists from Wageningen University (The Netherlands) and Penn State University (United States) [112] and have since gained considerable attention among researchers, with some studies reaching pilot-scale [113,114]. A diagram summarizing the use of acidogenic VFAs in other bioprocesses is shown in Figure 3.
| Feedstock | Inoculum | Setpoint Conditions | VFA Production | VFA Composition | Reference |
| Food waste | Sludge | Temp = 35–55 °C, pH 5.0–6.0, FM = batch, process time = 5 days, | NA | Acetate, butyrate | [20] |
| Food waste | Sludge | Temp = 30 °C, pH = 6.0, FM = batch, process time = 15 days, | 0.908 g/g VSremoval | Acetate, butyrate | [115] |
| Food waste | Sewage sludge | Temp = 35 °C, pH = 10.0, FM = batch, process time = 35 days | NA | Acetic acid, propionate, butyrate, and valeric acid | [116] |
| Waste activated sludge | - | Temp = 35 °C, pH = 10.0, FM = batch, process time = 8 days | 4619.6 mg COD/L | - | [117] |
| Waste activated sludge | - | Temp = 35 °C, pH = 6.8, FM = batch, process time = 12 days | 327.8 mg COD/g VS | Acetic acid, butyric acid, valeric acid, and propionic acid | [118] |
| OFMSW | Digestate | Temp = 37 °C, pH = 7.0, FM = batch, process time = 180 days | 24.4 g CODVFA/L | Acetic acid, butyric acid, propionic acid, caproic acid, isobutyric acid, isocaproic acid, and isovaleric acid | [119] |
| Potato waste | Rumen fluid | Temp = 39 °C, pH = 6.95, FM = batch, process time = 2 days | NA | Acetic acid, propionic acid, butyric acid, iso-valeric acid, and valeric acid | [120] |
| Organic waste | Granular sludge | Temp = 55 °C, pH = 7.0, FM = batch, process time = 42 days | NA | Acetic acid, butyric acid | [121] |
| Cheese whey | Sludge | Temp = 30 °C, pH = 5.0–6.0, FM = batch, process time = 0.5 day | NA | Butyric acid, propionic acid, and valeric acid | [18] |
- Microbial lipids for biodiesel—biodiesel is an alternative fuel that has received enormous growth over the past two decades, and this technology has reached a pilot scale [122]. It is currently being used as a fuel blend in diesel engines [123]. The majority of the world’s biodiesel is synthesized using lipids extracted from edible crops such as rapeseed oil, sunflower oil, soybean oil, and palm oil [124]. However, this technological route is still being scrutinized by scientists as it disregards the concept of “circular bioeconomy through waste valorization”. The “food vs. fuel” debate has also reinvigorated scientists to search for other sources of feedstocks. It has been proposed that the waste-derived VFAs could play a crucial role in the advancement of biodiesel technologies as these compounds can be used by oleaginous species to produce lipids that are applicable in the transesterification process [125]. Lipids synthesized from waste-derived VFAs have similar fatty acid composition to other well-known biodiesel-producing feedstocks such as jatropha oil and soybean oil [126], making them an attractive feedstock that could compete with the existing carbon-based materials. It was recently reported that the two-stage batch process is the best strategy to obtain the highest lipid content (37% w/w) in microbial lipid synthesis using waste-derived VFAs [127]. Another study demonstrated the production of microbial lipids using VFAs that were obtained from wastepaper [128]. The biomass content achieved from VFAs derived from waste-office paper and the waste newspaper was 4.3 g/L and 2.9 g/L, respectively; the lipid content was 41.2% and 27.7% dry cell weight, respectively [128]. These scientific reports showcase a sustainable, ecologically-friendly, and economical approach to producing microbial lipids using waste-derived VFAs. These findings could serve as a basis upon which further development studies could be carried out in microbial lipid production using waste-derived VFAs.
5.3. Biobased Chemicals
5.4. Wastewater Treatment
5.5. Soil Amelioration
5.6. Nutritional Compounds
6. Recent Progress, Technical Barriers, and Future Outlook in Microbial Biorefineries
The Case of Acidogenic-Based Microbial Systems
| Company | Country | Feedstock | Product(s) | Capacity (Tons) |
|---|---|---|---|---|
| Borregaard | Norway | Woody plants | Cellulose, biovanillin, ethanol | 250,000 |
| Alco Bio Fuel | Belgium | Corn and wheat | Ethanol, protein-rich feed, electricity, liquid CO2 | NA |
| Tereos | Czech Republic | Molasses | Ethanol | 200,000 to 1,000,000 |
| Novozymes | Denmark | Corn | Ethanol | 25,000 |
| Pannonia Bio | Hungary | Corn | Ethanol, distillers’ grain, corn oil | 85,000 |
| Essentica | Bulgaria | Grain | Ethanol | NA |
| Enocell Mill | Finland | Wood | Pulp | 490,000 |
| Lantmännen | Sweden | Grain | Ethanol | 200,000 |
| Abengoa Bioenergy | France | Corn | Ethanol, biodiesel | NA |
| Abengoa Bioenergy | France | Corn | Ethanol, biodiesel | NA |
| Crop Energies | Germany | Sugarcane | Ethanol | 750,000 |
7. Economic Aspects Relating to Biohydrogen and Its Associated Products
8. Conclusions and Suggestions for Future Research
- It is imperative to acquire deeper insights into the various microbial assemblages when using mixed cultures during the acidogenic biohydrogen fermentations, as this will help in the cultivation of the dominant VFA-producing biocatalysts.
- It is crucial to understand the nutrient-rich substrates alongside the optimal bioprocess conditions (especially the synergistic or antagonistic interactions of these setpoint variables), as this will lead to the optimal VFA recovery.
- Conducting pilot-scale studies using acidogenic VFAs as a sole carbon source will also assist in ascertaining the most suitable process dynamics.
- Acidogenic microbial-based biorefineries could be implemented with newly developed biochemical engineering tools, such as consolidated bioprocessing, in-vitro synthetic biology, and novel enzymes, in order to create biosynthetic pathways that will allow the biomanufacturing of multiple bio-based compounds.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ramchuran, S.O.; O’Brien, F.; Dube, N.; Ramdas, V. An overview of green processes and technologies, biobased chemicals and products for industrial applications. Curr. Opin. Green Sustain. Chem. 2023, 41, 100832. [Google Scholar] [CrossRef]
- Kora, E.; Patrinou, V.; Antonopoulou, G.; Ntaikou, I.; Tekerlekopoulou, A.G.; Lyberatos, G. Dark fermentation of expired fruit juices for biohydrogen production followed by treatment and biotechnological exploitation of effluents towards bioplastics and microbial lipids. Biochem. Eng. J. 2023, 195, 108901. [Google Scholar] [CrossRef]
- Pereira, A.S.; Lopes, M.; Duarte, M.S.; Alves, M.M.; Belo, I. Integrated bioprocess of microbial lipids production in Yarrowia lipolytica using food-waste derived volatile fatty acids. Ren. Energy 2023, 202, 1470–1478. [Google Scholar] [CrossRef]
- Sarkar, O.; Matsakas, L.; Rova, U.; Christakopoulos, P. Ultrasound-controlled acidogenic valorization of wastewater for biohydrogen and volatile fatty acids production: Microbial community profiling. iScience 2023, 26, 106519. [Google Scholar] [CrossRef] [PubMed]
- Perez-Almada, D.; Galán-Martín, Á.; del Mar Contreras, M.; Castro, E. Integrated techno-economic and environmental assessment of biorefineries: Review and future research directions. Sustain. Energy Fuels 2023, in press. [Google Scholar] [CrossRef]
- Lee, H.; Sohn, Y.J.; Jeon, S.; Yang, H.; Son, J.; Kim, Y.J.; Park, S.J. Sugarcane wastes as microbial feedstocks: A review of the biorefinery framework from resource recovery to production of value-added products. Bioresour. Technol. 2023, 376, 128879. [Google Scholar] [CrossRef]
- Quaid, T.; Reza, T. COSMO-RS predictive screening of type 5 hydrophobic deep eutectic solvents for selective platform chemicals absorption. J. Mol. Liq. 2023, 382, 121918. [Google Scholar] [CrossRef]
- Mineo, A.; Cosenza, A.; Mannina, G. Sewage sludge acidogenic fermentation for organic resource recovery towards carbon neutrality: An experimental survey testing the headspace influence. Bioresour. Technol. 2023, 367, 128217. [Google Scholar] [CrossRef]
- Tabibian, S.S.; Sharifzadeh, M. Statistical and analytical investigation of methanol applications, production technologies, value-chain and economy with a special focus on renewable methanol. Ren. Sustain. Energy Rev. 2023, 179, 113281. [Google Scholar] [CrossRef]
- Tomás-Pejó, E.; González-Fernández, C.; Greses, S.; Kennes, C.; Otero-Logilde, N.; Veiga, M.C.; Bolzonella, D.; Müller, B.; Passoth, V. Production of short-chain fatty acids (SCFAs) as chemicals or substrates for microbes to obtain biochemicals. Biotechnol. Biofuels 2023, 16, 96. [Google Scholar] [CrossRef]
- BCC Research, 2019. Oleochemical Fatty Acids: Global Markets to 2023. Available online: https://www.bccresearch.com/market-research/chemicals/oleochemical-fatty-acids-global-markets.html (accessed on 20 April 2022).
- Turon, V.; Trably, E.; Fouilland, E.; Steyer, J.-P. Potentialities of dark fermentation effluents as substrates for microalgae growth: A review. Process Biochem. 2016, 51, 1843–1854. [Google Scholar] [CrossRef]
- Rama, C.; Rani, P.; Kumar, A. Recent developments in biohydrogen production from wastewater: A review. Biocatal. Biotrans. 2023, 1–18, in press. [Google Scholar] [CrossRef]
- Badawi, E.Y.; Elkharsa, R.A.; Abdelfattah, E.A. Value proposition of bio-hydrogen production from different biomass sources. Energy Nexus 2023, 10, 100194. [Google Scholar] [CrossRef]
- Sganzerla, W.G.; Sillero, L.; Forster-Carneiro, T.; Solera, R.; Perez, M. Determination of Anaerobic Co-fermentation of Brewery Wastewater and Brewer’s Spent Grains for Bio-hydrogen Production. Bioenergy Res. 2023, 16, 1073–1083. [Google Scholar] [CrossRef]
- Dahiya, S.; Lingam, Y.; Venkata Mohan, S. Understanding acidogenesis towards green hydrogen and volatile fatty acid production—Critical analysis and circular economy perspective. Chem. Eng. J. 2023, 464, 141550. [Google Scholar] [CrossRef]
- Sanchez-Ledesma, L.M.; Ramírez-Malule, H.; Rodríguez-Victoria, J.A. Volatile Fatty Acids Production by Acidogenic Fermentation of Wastewater: A Bibliometric Analysis. Sustainability 2023, 15, 2370. [Google Scholar] [CrossRef]
- Liu, H.; Wang, F.; Wang, Z.; Wu, D.; Xing, T.; Kong, X.; Sun, Y. Impact of pH, temperature, and hydraulic residence time on the acidogenic fermentation of fruit and vegetable waste and microbial community analysis. J. Chem. Technol. Biotechnol. 2023, 98, 819–828. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, D.; Du, J.; Zhou, B.; Wang, D.; Liu, X.; Yan, C.; Liang, J.; Zhou, L. Enhancing propionic acid production in the acidogenic fermentation of food waste facilitated by a fungal mash under neutral pH. J. Environ. Manag. 2023, 327, 116901. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhang, M.; Mou, H.; An, Z.; Fu, H.; Su, X.; Chen, C.; Chen, J.; Lin, H.; Sun, F. Comparation of mesophilic and thermophilic anaerobic co-digestion of food waste and waste activated sludge driven by biochar derived from kitchen waste. J. Clean. Product. 2023, 408, 137123. [Google Scholar] [CrossRef]
- Jiang, M.; Qiao, W.; Wang, Y.; Zou, T.; Lin, M.; Dong, R. Balancing acidogenesis and methanogenesis metabolism in thermophilic anaerobic digestion of food waste under a high loading rate. Sci. Total Environ. 2022, 824, 153867. [Google Scholar] [CrossRef]
- Xu, X.; Sun, Y.; Sun, Y.; Li, Y. Bioaugmentation improves batch psychrophilic anaerobic co-digestion of cattle manure and corn straw. Bioresour. Technol. 2022, 343, 126118. [Google Scholar] [CrossRef] [PubMed]
- Strazzera, G.; Battista, F.; Garcia, N.H.; Frison, N.; Bolzonella, D. Volatile fatty acids production from food wastes for biorefinery platforms: A review. J. Environ. Manag. 2018, 226, 278–288. [Google Scholar] [CrossRef]
- Sanjaya, A.; Mondylaksita, K.; Millati, R.; Budhijanto, W. Evaluation of volatile fatty acids (VFAs) production in thermophilic and mesophilic anaerobic digestion of oil palm empty fruit bunch (OPEFB). Conf. Ser. Earth Environ. Sci. 2022, 963, 012049. [Google Scholar] [CrossRef]
- Forrest, A.K.; Hernandez, J.; Holtzapple, M.T. Effects of temperature and pretreatment conditions on mixed-acid fermentation of water hyacinths using a mixed culture of thermophilic microorganisms. Bioresour. Technol. 2010, 101, 7510–7515. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Luo, G.; Wang, W.; He, Y.; Zhang, R.; Liu, G. The effects of pH and temperature on the acetate production and microbial community compositions by syngas fermentation. Fuel 2018, 224, 537–544. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, R.; Liu, F.; Yong, X.; Wu, X.; Zheng, T.; Jiang, M.; Jia, H. Biogas production and microbial community shift through neutral pH control during the anaerobic digestion of pig manure. Bioresour. Technol. 2016, 217, 44–49. [Google Scholar] [CrossRef]
- Kumari, S.; Das, D. Improvement of biohydrogen production using acidogenic culture. Int. J. Hydrogen Energy 2017, 42, 4083–4094. [Google Scholar] [CrossRef]
- Marra, L.M.; de Oliveira-Longatti, S.M.; Soares, C.R.; de Lima, J.M.; Olivares, F.L.; Moreira, F.M. Initial pH of medium affects organic acids production but do not affect phosphate solubilization. Braz. J. Microbiol. 2015, 46, 367–375. [Google Scholar] [CrossRef] [Green Version]
- Luo, G.; Jing, Y.; Lin, Y.; Zhang, S.; An, D. A novel concept for syngas biomethanation by two-stage process: Focusing on the selective conversion of syngas to acetate. Sci. Total Environ. 2018, 645, 194–1200. [Google Scholar] [CrossRef]
- Yin, D.M.; Mahboubi, A.; Wainaina, S.; Qiao, W.; Taherzadeh, M.J. The effect of mono- and multiple fermentation parameters on volatile fatty acids (VFAs) production from chicken manure via anaerobic digestion. Bioresour. Technol. 2021, 330, 124992. [Google Scholar] [CrossRef]
- Detman, A.; Laubitz, D.; Chojnacka, A.; Kiela, P.R.; Salamon, A.; Barberán, A.; Chen, Y.; Yang, F.; Błaszczyk, M.K.; Sikora, A. Dynamics of dark fermentation microbial communities in the light of lactate and butyrate production. Microbiome 2021, 9, 158. [Google Scholar] [CrossRef]
- Atasoy, M.; Eyice, O.; Schnürer, A.; Cetecioglu, Z. Volatile fatty acids production via mixed culture fermentation: Revealing the link between pH, inoculum type and bacterial composition. Bioresour. Technol. 2019, 292, 121889. [Google Scholar] [CrossRef]
- Hao, T.; Xiao, Y.; Varjani, S. Transiting from the inhibited steady-state to the steady-state through the ammonium bicarbonate mediation in the anaerobic digestion of low-C/N-ratio food wastes. Bioresour. Technol. 2022, 351, 127046. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Liu, H.; Zhang, L.; Yang, M.; Fu, B.; Liu, H. Novel insight into the relationship between organic substrate composition and volatile fatty acids distribution in acidogenic co-fermentation. Biotechnol. Biofuels 2017, 10, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.; Zhang, L.; Loh, K.C. Review and perspectives of enhanced volatile fatty acids production from acidogenic fermentation of lignocellulosic biomass wastes. Bioresour. Bioprocess 2021, 8, 68. [Google Scholar] [CrossRef]
- Liu, X.; Liu, H.; Chen, Y.; Du, G.; Chen, J. Effects of organic matter and initial carbon-nitrogen ratio on the bioconversion of volatile fatty acids from sewage sludge. J Chem. Technol. Biotechnol. 2008, 83, 1049–1055. [Google Scholar] [CrossRef]
- Paranjpe, A.; Saxena, S.; Jain, P. Biogas yield using single and two stage anaerobic digestion: An experimental approach. Energy Sustain. Develop. 2023, 74, 6–19. [Google Scholar] [CrossRef]
- Franceschi, F.F.; Acosta-González, A.; Vega, L.T.; Gomez, M.F. Improving dry anaerobic methane production from OFMSW by co-digestion with grass waste and pretreatment with white rot fungi. Energy Sustain. Dev. 2023, 74, 6–19. [Google Scholar] [CrossRef]
- Zou, X.; Mengjiao, G.; Mohammed, A.; Liu, Y. Responses of various carbon to nitrogen ratios to microbial communities, kinetics, and nitrogen metabolic pathways in aerobic granular sludge reactor. Bioresour. Technol. 2023, 367, 128225. [Google Scholar] [CrossRef]
- De Groof, V.; Coma, M.; Arnot, T.; Leak, D.J.; Lanham, A.B. Selecting fermentation products for food waste valorisation with HRT and OLR as the key operational parameters. Waste Manag. 2021, 127, 80–89. [Google Scholar] [CrossRef]
- Lim, S.J.; Kim, B.J.; Jeong, C.M.; Choi, J.D.; Ahn, Y.H.; Chang, H.N. Anaerobic organic acid production of food waste in once-a-day feeding and drawing-off bioreactor. Bioresour. Technol. 2008, 99, 7866–7874. [Google Scholar] [CrossRef]
- Lee, W.S.; Chua, A.S.M.; Yeoh, H.K.; Ngoh, G.C. A review of the production and applications of waste-derived volatile fatty acids. Chem. Eng. J. 2014, 235, 83–99. [Google Scholar] [CrossRef]
- Farouk, R.Y.; Mostafa, E.; Wang, Y. Evaluation of hydrogen and volatile fatty acids production system from food waste. Biomass Conv. Bioref. 2023, 13, 5253–5259. [Google Scholar] [CrossRef]
- Tsegaye, D.; Khan, M.; Leta, S. Optimization of Operating Parameters for Two-Phase Anaerobic Digestion Treating Slaughterhouse Wastewater for Biogas Production: Focus on Hydrolytic—Acidogenic Phase. Sustainability 2023, 15, 5544. [Google Scholar] [CrossRef]
- Wijekoon, K.C.; Visvanathan, C.; Abeynayaka, A. Effect of organic loading rate on VFA production, organic matter removal and microbial activity of a two-stage thermophilic anaerobic membrane bioreactor. Bioresour. Technol. 2011, 102, 5353–5360. [Google Scholar] [CrossRef]
- Magdalena, J.A.; Greses, S.; González-Fernández, C. Impact of Organic Loading Rate in Volatile Fatty Acids Production and Population Dynamics Using Microalgae Biomass as Substrate. Sci. Rep. 2019, 9, 18374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ünyay, H.; Yılmaz, F.; Başar, I.A.; Perendeci, N.A.; Çoban, I.; Şahinkaya, E. Effects of organic loading rate on methane production from switchgrass in batch and semi-continuous stirred tank reactor system. Biomass Bioenergy 2022, 156, 106306. [Google Scholar] [CrossRef]
- Zou, X.; Wang, Y.; Dai, Y.; Zhou, S.; Wang, B.; Li, Y.; Li, J. Batch and semi-continuous experiments examining the sludge mesophilic anaerobic digestive performance with different varieties of rice straw. Bioresour. Technol. 2022, 346, 126651. [Google Scholar] [CrossRef]
- Yakaboylu, O.; Albrecht, I.; Harinck, J.; Smit, K.G.; Georgios-Archimidis, T.; Marcello, M.; de Jong, W. Supercritical water gasification of biomass in fluidized bed: First results and experiences obtained from TU Delft/Gensos semi-pilot scale setup. Biomass Bioenergy 2018, 111, 330–342. [Google Scholar] [CrossRef]
- Karthikeyan, O.P.; Selvam, A.; Wong, J.W. Hydrolysis-acidogenesis of food waste in solid-liquid-separating continuous stirred tank reactor (SLS-CSTR) for volatile organic acid production. Bioresour. Technol. 2016, 200, 366–373. [Google Scholar] [CrossRef]
- Kannah, R.Y.; Rohini, K.B.; Gunasekaran, M.; Gokulakrishnan, K.; Kumar, G.; Banu, J.R. Prediction of effective substrate concentration and its impact on biogas production using Artificial Neural Networks in Hybrid Upflow anaerobic Sludge Blanket reactor for treating landfill leachate. Fuel 2022, 313, 122697. [Google Scholar] [CrossRef]
- Safari, M.; Tondro, H.; Zilouei, H. Biohydrogen production from diluted-acid hydrolysate of rice straw in a continuous anaerobic packed bed biofilm reactor. Int. J. Hydrogen Energy 2022, 47, 5879–5890. [Google Scholar] [CrossRef]
- Hanvajanawong, K.; Suyamud, B.; Suwannasilp, B.B.; Lohwacharin, J.; Visvanathan, C. Unravelling capability of two-stage thermophilic anaerobic membrane bioreactors for high organic loading wastewater: Effect of support media addition and irreversible fouling. Bioresour. Technol. 2022, 348, 126725. [Google Scholar] [CrossRef] [PubMed]
- Tsigkou, K.; Terpou, A.; Treu, L.; Kougias, P.G.; Kornaros, M. Thermophilic anaerobic digestion of olive mill wastewater in an upflow packed bed reactor: Evaluation of 16S rRNA amplicon sequencing for microbial analysis. J. Environ. Manag. 2022, 301, 113853. [Google Scholar] [CrossRef] [PubMed]
- Rossi, E.; Becarelli, S.; Pecorini, I.; Di Gregorio, S.; Iannelli, R. Anaerobic Digestion of the Organic Fraction of Municipal Solid Waste in Plug-Flow Reactors: Focus on Bacterial Community Metabolic Pathways. Water 2022, 14, 195. [Google Scholar] [CrossRef]
- Mohapatra, D.P.; Gassara, F.; Brar, S.K. Nanoparticles production and role in biotransformation. J. Nanosci. Nanotechnol. 2011, 11, 899–918. [Google Scholar] [CrossRef]
- Montalvo, S.; Vielma, S.; Borja, R.; Huiliñir, C.; Guerrero, L. Increase in biogas production in anaerobic sludge digestion by combining aerobic hydrolysis and addition of metallic wastes. Renew. Energy 2018, 123, 541–548. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Shei, S.-H. Heavy metal effects on fermentative hydrogen production using natural mixed microflora. Int. J. Hydrogen Energy 2008, 33, 587–593. [Google Scholar] [CrossRef]
- Liu, J.; Huang, J.; Li, H.; Shi, B.; Xu, Y.; Liu, J.; Zhang, D.; Tang, J.; Hou, P. Effect of temperature on fermentative VFAs production from waste sludge stimulated by riboflavin and the shifts of microbial community. Water Sci. Technol. 2022, 85, 1191–1201. [Google Scholar] [CrossRef]
- Zhong, L.; Feng, Y.; Wang, G.; Wang, Z.; Bilal, M.; Lv, H.; Jia, S.; Cui, J. Production and use of immobilized lipases in/on nanomaterials: A review from the waste to biodiesel production. Int. J. Biol. Macromol. 2020, 152, 207–222. [Google Scholar] [CrossRef]
- Shahbaz, A.; Hussain, N.; Saleem, M.Z.; Saeed, M.U.; Bilal, M.; Iqbal, H.M.N. Nanoparticles as stimulants for efficient generation of biofuels and renewables. Fuel 2022, 319, 123724. [Google Scholar] [CrossRef]
- Kim, Y.K.; Lee, H. Use of magnetic nanoparticles to enhance bioethanol production in syngas fermentation. Bioresour. Technol. 2016, 204, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, Y.; Bian, Y.; Dong, L.; Zheng, X.; Chen, Y. Long-term effects of copper nanoparticles on volatile fatty acids production from sludge fermentation: Roles of copper species and bacterial community structure. Bioresour. Technol. 2022, 348, 126789. [Google Scholar] [CrossRef]
- Pramanik, N. A tool for biomedical application: Synthesis and modification of polyhydroxyalkanoates. Sustain. Chem. Pharm. 2023, 32, 101041. [Google Scholar] [CrossRef]
- Alves, A.A.; Siqueira, E.C.; Barros, M.P.S.; Silva, P.E.C.; Houllou, L.M. Polyhydroxyalkanoates: A review of microbial production and technology application. Int. J. Environ. Sci. Technol. 2023, 20, 3409–3420. [Google Scholar] [CrossRef]
- Rajvanshi, J.; Sogani, M.; Kumar, A.; Arora, S.; Syed, Z.; Sonu, K.; Gupta, N.S.; Kalra, A. Perceiving biobased plastics as an alternative and innovative solution to combat plastic pollution for a circular economy. Sci. Tot. Environ. 2023, 874, 162441. [Google Scholar] [CrossRef]
- Marreiros, B.C.; Carvalheira, M.; Henriques, C.; Pequito, D.; Nguyen, Y.; Solstad, R.G.; Eksteen, J.J.; Reis, M.A.M. Pilot-scale valorisation of salmon peptone into polyhydroxyalkanoates by mixed microbial cultures under conditions of high ammonia concentration. J. Environ. Chem. Eng. 2023, 11, 110100. [Google Scholar] [CrossRef]
- Zhou, W.; Bergsma, S.; Colpa, D.I.; Euverink, G.-J.W. Polyhydroxyalkanoates (PHAs) synthesis and degradation by microbes and applications towards a circular economy. J. Environ. Manag. 2023, 341, 118033. [Google Scholar] [CrossRef]
- Montiel-Corona, V.; Buitrón, G. Polyhydroxyalkanoates from organic waste streams using purple non-sulfur bacteria. Bioresour. Technol. 2021, 323, 124610. [Google Scholar] [CrossRef]
- Carlozzi, P.; Giovannelli, A.; Traversi, M.L.; Touloupakis, E. Poly(3-hydroxybutyrate) bioproduction in a two-step sequential process using wastewater. J. Water Process Eng. 2021, 39, 101700. [Google Scholar] [CrossRef]
- Cavaliere, C.; Capriotti, A.L.; Cerrato, A.; Lorini, L.; Montone, C.M.; Valentino, F.; Laganà, A.; Majone, M. Identification and Quantification of Polycyclic Aromatic Hydrocarbons in Polyhydroxyalkanoates Produced from Mixed Microbial Cultures and Municipal Organic Wastes at Pilot Scale. Molecules 2021, 26, 539. [Google Scholar] [CrossRef]
- Lee, S.Y.; Choi, J.-I.; Han, K.; Song, J.Y. Removal of endotoxin during purification of poly(3-hydroxybutyrate) from gram-negative bacteria. Appl. Environ. Microbiol. 1999, 65, 2762–2764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, F.; Matos, M.; Pereira, B.; Ralo, C.; Pequito, D.; Marques, N.; Carvalho, G.; Reis, M.A.M. An integrated process for mixed culture production of 3-hydroxyhexanoate-rich polyhydroxyalkanoates from fruit waste. Chem. Eng. J. 2022, 427, 131908. [Google Scholar] [CrossRef]
- Saratale, G.R.; Cho, S.K.; Saratale, D.G.; Kadam, A.A.; Ghodake, G.S.; Kumar, M.; Bharagava, N.R.; Kumar, G.; Kim, S.D.; Mulla, S.I.; et al. A comprehensive overview and recent advances on polyhydroxyalkanoates (PHA) production using various organic waste streams. Bioresour. Technol. 2021, 325, 124685. [Google Scholar] [CrossRef] [PubMed]
- Kalia, V.C.; Singh Patel, S.K.; Shanmugam, R.; Lee, J.K. Polyhydroxyalkanoates: Trends and advances toward biotechnological applications. Bioresour. Technol. 2021, 326, 124737. [Google Scholar] [CrossRef] [PubMed]
- Meylani, V.; Surahman, E.; Fudholi, A.; Almalki, W.H.; Ilyas, N.; Sayyed, R.Z. Biodiversity in microbial fuel cells: Review of a promising technology for wastewater treatment. J. Environ. Chem. Eng. 2023, 11, 109503. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, J.; Zhang, Y.; Guo, Q.; Hu, T.; Xiao, H.; Lu, W.; Jia, J. Progress on anodic modification materials and future development directions in microbial fuel cells. J. Power Sources 2023, 556, 232486. [Google Scholar] [CrossRef]
- Hoang, A.T.; Nižetić, S.; Ng, K.H.; Papadopoulos, A.M.; Le, A.T.; Kumar, S.; Hadiyanto, H.; Pham, V.V. Microbial fuel cells for bioelectricity production from waste as sustainable prospect of future energy sector. Chemosphere 2022, 287, 132285. [Google Scholar] [CrossRef]
- Greenman, J.; Mendis, B.A.; Gajda, I.; Ieropoulos, I.A. Microbial fuel cell compared to a chemostat. Chemosphere 2022, 296, 133967. [Google Scholar] [CrossRef]
- Ramya, M.; Kumar, S.P. A review on recent advancements in bioenergy production using microbial fuel cells. Chemosphere 2022, 288, 132512. [Google Scholar] [CrossRef]
- Mohyudin, S.; Farooq, R.; Jubeen, F.; Rasheed, T.; Fatima, M.; Sher, F. Microbial fuel cells a state-of-the-art technology for wastewater treatment and bioelectricity generation. Environ. Res. 2022, 204, 112387. [Google Scholar] [CrossRef]
- Uduma, R.C.; Oguzie, K.L.; Chijioke, C.F.; Ogbulie, T.E.; Oguzie, E.E. Bioelectrochemical technologies for simultaneous treatment of dye wastewater and electricity generation: A review. Int. J. Environ. Sci. Technol. 2023, in press. [Google Scholar] [CrossRef]
- Mohanakrishna, G.; Mohan, S.V.; Sarma, P.N. Utilizing acid-rich effluents of fermentative hydrogen production process as substrate for harnessing bioelectricity: An integrative approach. Int. J. Hydrogen Energy 2010, 35, 3440–3449. [Google Scholar] [CrossRef]
- Asefi, B.; Li, S.-L.; Moreno, H.A.; Sanchez-Torres, V.; Hu, A.; Li, J.; Yu, C.-P. Characterization of electricity production and microbial community of food waste-fed microbial fuel cells. Process Saf. Environ. Protect. 2019, 125, 83–91. [Google Scholar] [CrossRef]
- Freguia, S.; The, E.H.; Boon, N.; Leung, K.M.; Keller, J.; Rabaey, K. Microbial fuel cells operating on mixed fatty acids. Bioresour. Technol. 2010, 101, 1233–1238. [Google Scholar] [CrossRef]
- Chae, K.J.; Choi, M.J.; Lee, J.W.; Kim, K.Y.; Kim, I.S. Effect of different substrates on the performance, bacterial diversity, and bacterial viability in microbial fuel cells. Bioresour. Technol. 2009, 100, 3518–3525. [Google Scholar] [CrossRef] [PubMed]
- Khater, D.Z.; El-Khatib, K.M.; Hassan, H.M. Microbial diversity structure in acetate single chamber microbial fuel cell for electricity generation. J. Genet. Eng. Biotechnol. 2017, 15, 127–137. [Google Scholar] [CrossRef]
- Escapa, A.; Mateos, R.; Martinez, E.J.; Blanes, J. Microbial electrolysis cells: An emerging technology for wastewater treatment and energy recovery. From laboratory to pilot plant and beyond. Ren. Sustain. Energy Rev. 2016, 55, 942–956. [Google Scholar] [CrossRef]
- Gkotsis, P.; Kougias, P.; Mitrakas, M.; Zouboulis, A. Biogas upgrading technologies—Recent advances in membrane-based processes. Int. J. Hydrogen Energy 2023, 48, 3965–3993. [Google Scholar] [CrossRef]
- Kougias, P.G.; Angelidaki, I. Biogas and its opportunities-A review. Front. Environ. Sci. Eng. 2018, 12, 14. [Google Scholar] [CrossRef]
- Dareioti, M.A.; Vavouraki, A.I.; Tsigkou, K.; Kornaros, M. Assessment of Single- vs. Two-Stage Process for the Anaerobic Digestion of Liquid Cow Manure and Cheese Whey. Energies 2021, 14, 5423. [Google Scholar] [CrossRef]
- Baldi, F.; Pecorini, I.; Iannelli, R. Comparison of single-stage and two-stage anaerobic co-digestion of food waste and activated sludge for hydrogen and methane production. Renew. Energy 2019, 143, 1755–1765. [Google Scholar] [CrossRef]
- Sasidhar, K.B.; Kumar, P.S.; Xiao, L. A critical review on the two-stage biohythane production and its viability as a renewable fuel. Fuel 2022, 317, 123449. [Google Scholar] [CrossRef]
- Angelidaki, I.; Treu, L.; Tsapekos, P.; Luo, G.; Campanaro, S.; Wenzel, H.; Kougias, P.G. Biogas upgrading and utilization: Current status and perspectives. Biotechnol. Adv. 2018, 36, 452–466. [Google Scholar] [CrossRef] [Green Version]
- Rao, R.; Basak, N. Process optimization and mathematical modelling of photo-fermentative hydrogen production from dark fermentative cheese whey effluent by Rhodobacter sphaeroides O.U.001 in 2-L cylindrical bioreactor. Biomass Conv. Bioref. 2023, 13, 3929–3952. [Google Scholar] [CrossRef]
- Chang, H.; Feng, H.; Wang, R.; Zhang, X.; Wang, J.; Li, C.; Zhang, Y.; Li, L.; Ho, S.-H. Enhanced energy recovery from landfill leachate by linking light and dark bio-reactions: Underlying synergistic effects of dual microalgal interaction. Water Res. 2023, 231, 119578. [Google Scholar] [CrossRef]
- Moreno, R.; Escapa, A.; Cara, J.; Carracedo, B.; Gómez, X. A two-stage process for hydrogen production from cheese whey: Integration of dark fermentation and biocatalyzed electrolysis. Int. J. Hydrogen Energy 2015, 40, 168–175. [Google Scholar] [CrossRef]
- Phan, T.P.; Ta, Q.T.H.; Nguyen, P.K.T. Maximizing performance of microbial electrolysis cell fed with dark fermentation effluent from water hyacinth. Int. J. Hydrogen Energy 2023, 48, 5447–5462. [Google Scholar] [CrossRef]
- Attia, Y.A.; Samer, M.; Moselhy, M.A.; Arisha, A.H.; Abdelqader, A.A.; Abdelsalam, E.E. Influence of laser photoactivated graphitic carbon nitride nanosheets and nickel nanoparticles on purple non-sulfur bacteria for biohydrogen production from biomass. J. Clean. Prod. 2021, 299, 126898. [Google Scholar] [CrossRef]
- Brar, K.K.; Cortez, A.A.; Pellegrini, V.O.A.; Amulya, K.; Polikarpov, I.; Magdouli, S.; Kumar, M.; Yang, Y.-H.; Bhatia, S.K.; Brar, S.K. An overview on progress, advances, and future outlook for biohydrogen production technology. Int. J. Hydrogen Energy 2022, 47, 37264–37281. [Google Scholar] [CrossRef]
- Mirza, S.S.; Qazi, J.I.; Liang, Y.; Chen, S. Growth characteristics and photofermentative biohydrogen production potential of purple non sulfur bacteria from sugar cane bagasse. Fuel 2019, 255, 115805. [Google Scholar] [CrossRef]
- Uyar, B.; Eroglu, I.; Yücel, M.; Gündüz, U. Photofermentative hydrogen production from volatile fatty acids present in dark fermentation effluents. Int. J. Hydrogen Energy 2009, 34, 4517–4523. [Google Scholar] [CrossRef]
- Su, H.; Cheng, J.; Zhou, J.; Song, W.; Cen, K. Combination of dark- and photo-fermentation to enhance hydrogen production and energy conversion efficiency. Int. J. Hydrogen Energy 2009, 34, 8846–8853. [Google Scholar] [CrossRef]
- Sharma, S.; Basu, S.; Shetti, N.P.; Aminabhavi, T.M. Waste-to-energy nexus for circular economy and environmental protection: Recent trends in hydrogen energy. Sci. Total Environ. 2020, 713, 136633. [Google Scholar] [CrossRef]
- Mutanda, T.; Naidoo, D.; Bwapwa, J.K.; Anandraj, A. Biotechnological Applications of Microalgal Oleaginous Compounds: Current Trends on Microalgal Bioprocessing of Products. Front. Energy Res. 2020, 17, 1–21. [Google Scholar] [CrossRef]
- Khetkorn, W.; Rastogi, R.P.; Incharoensakdi, A.; Lindblad, P.; Madamwar, D.; Pandey, A.; Larroche, C. Microalgal hydrogen production-A review. Bioresour. Technol. 2017, 243, 1194–1206. [Google Scholar] [CrossRef]
- Ren, H.-Y.; Liu, B.-F.; Kong, F.; Zhao, L.; Ma, J.; Ren, N.-Q. Favorable energy conversion efficiency of coupling dark fermentation and microalgae production from food wastes. Energy Conv. Manag. 2018, 165, 156–162. [Google Scholar] [CrossRef]
- Tuna, E.; Kargi, F.; Argun, H. Hydrogen gas production by electrohydrolysis of volatile fatty acid (VFA) containing dark fermentation effluent. Int. J. Hydrogen Energy 2009, 34, 262–269. [Google Scholar] [CrossRef]
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial fuel cells: Methodology and technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef]
- Hua, T.; Li, S.; Li, F.; Zhou, Q.; Ondon, B.S. Microbial electrolysis cell as an emerging versatile technology: A review on its potential application, advance and challenge. J. Chem. Technol. Biotechnol. 2009, 94, 1697–1711. [Google Scholar] [CrossRef]
- Liu, H.; Grot, S.; Logan, B.E. Electrochemically assisted microbial production of hydrogen from acetate. Environ. Sci. Technol. 2005, 39, 4317–4320. [Google Scholar] [CrossRef] [PubMed]
- Heidrich, E.S.; Edwards, S.R.; Dolfing, J.; Cotterill, S.E.; Curtis, T.P. Performance of a pilot scale microbial electrolysis cell fed on domestic wastewater at ambient temperatures for a 12 month period. Bioresour. Technol. 2014, 173, 87–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sim, J.; Reid, R.; Hussain, A.; An, J.; Lee, H.S. Hydrogen peroxide production in a pilot-scale microbial electrolysis cell. Biotechnol. Rep. 2018, 19, e00276. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Wang, K.; Yang, Y.; Shen, D.; Wang, M.; Mo, H. Improving production of volatile fatty acids from food waste fermentation by hydrothermal pretreatment. Bioresour. Technol. 2014, 171, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Wu, M.; Jiang, Y.; Wang, H. Effects of different temperatures and pH values on volatile fatty acids production during codigestion of food waste and thermal-hydrolysed sewage sludge and subsequent volatile fatty acids for polyhydroxyalkanoates production. Bioresour. Technol. 2021, 333, 125149. [Google Scholar] [CrossRef]
- Pang, J.; He, J.; Ma, Y.; Pan, X.; Zheng, Y.; Yu, H.; Yan, Z.; Nan, J. Enhancing volatile fatty acids production from waste activated sludge by a novel cation-exchange resin assistant strategy. J. Clean. Prod. 2021, 278, 123236. [Google Scholar] [CrossRef]
- Xin, X.; She, Y.; Hong, J. Insights into microbial interaction profiles contributing to volatile fatty acids production via acidogenic fermentation of waste activated sludge assisted by calcium oxide pretreatment. Bioresour. Technol. 2021, 320, 124287. [Google Scholar] [CrossRef]
- Valentino, F.; Munarin, G.; Biasiolo, M.; Cavinato, C.; Bolzonella, D.; Pavan, P. Enhancing volatile fatty acids (VFA) production from food waste in a two-phases pilot-scale anaerobic digestion process. J. Environ. Chem. Eng. 2021, 9, 106062. [Google Scholar] [CrossRef]
- Pourbayramian, R.; Abdi-Benemar, H.; Seifdavati, J.; Greiner, R.; Elghandour, M.M.M.Y.; Salem, A.Z.M. Bioconversion of potato waste by rumen fluid from slaughterhouses to produce a potential feed additive rich in volatile fatty acids for farm animals. J. Clean. Prod. 2021, 280, 124411. [Google Scholar] [CrossRef]
- Weide, T.; Brügging, E.; Wetter, C.; Ierardi, A.; Wichern, M. Use of organic waste for biohydrogen production and volatile fatty acids via dark fermentation and further processing to methane. Int. J. Hydrogen Energy 2019, 44, 24110–24125. [Google Scholar] [CrossRef]
- Abderrahim, B.; Diaz, Y.; Martinez, M.; Aracil, J. Pilot plant studies of biodiesel production using Brassica carinata as raw material. Catal. Today 2005, 106, 193–196. [Google Scholar]
- Soccol, C.R.; Dalmas Neto, C.J.; Soccol, V.T.; Sydney, E.B.; da Costa, E.S.F.; Medeiros, A.B.P.; Vandenberghe, L.P.S. Pilot scale biodiesel production from microbial oil of Rhodosporidium toruloides DEBB 5533 using sugarcane juice: Performance in diesel engine and preliminary economic study. Bioresour. Technol. 2017, 223, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Sharma, D.; Soni, S.L.; Sharma, S.; Sharma, P.K.; Jhalani, A. A review on feedstocks, production processes, and yield for different generations of biodiesel. Fuel 2020, 262, 116553. [Google Scholar] [CrossRef]
- Zhang, L.; Loh, K.C.; Kuroki, A.; Dai, Y.; Tong, Y.W. Microbial biodiesel production from industrial organic wastes by oleaginous microorganisms: Current status and prospects. J. Hazard Mat. 2021, 402, 123543. [Google Scholar] [CrossRef]
- Fei, Q.; Chang, H.N.; Shang, L.; Choi, J.D.; Kim, N.; Kang, J. The effect of volatile fatty acids as a sole carbon source on lipid accumulation by Cryptococcus albidus for biodiesel production. Bioresour. Technol. 2011, 102, 2695–2701. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.S.; Lopes, M.; Miranda, S.M.; Belo, I. Bio-oil production for biodiesel industry by Yarrowia lipolytica from volatile fatty acids in two-stage batch culture. Appl. Microbiol. Biotechnol. 2022, 106, 2869–2881. [Google Scholar] [CrossRef]
- Annamalai, N.; Sivakumar, N.; Fernandez-Castane, A.; Oleskowicz-Popiel, P. Production of microbial lipids utilizing volatile fatty acids derived from wastepaper: A biorefinery approach for biodiesel production. Fuel 2020, 276, 118087. [Google Scholar] [CrossRef]
- Angenent, L.T.; Karim, K.; Al-Dahhan, M.H.; Wrenn, B.A.; Domíguez-Espinosa, R. Production of bioenergy and biochemicals from industrial and agricultural wastewater. Trends Biotechnol. 2004, 22, 477–485. [Google Scholar] [CrossRef]
- Stylianou, E.; Pateraki, C.; Ladakis, D.; Cruz-Fernández, M.; Latorre-Sánchez, M.; Coll, C.; Koutinas, A. Evaluation of organic fractions of municipal solid waste as renewable feedstock for succinic acid production. Biotechnol. Biofuels 2020, 13, 72. [Google Scholar] [CrossRef] [Green Version]
- Yankov, D. Fermentative Lactic Acid Production from Lignocellulosic Feedstocks: From Source to Purified Product. Front. Chem. 2022, 10, 823005. [Google Scholar] [CrossRef]
- Dhillon, S.G.; Brar, K.S.; Verma, M.; Tyagi, R.D. Recent Advances in Citric Acid Bio-Production and Recovery. Food Biop. Technol. 2011, 4, 505–529. [Google Scholar] [CrossRef]
- Behera, B.C.; Mishra, R.; Mohapatra, S. Microbial citric acid: Production, properties, application, and future perspectives. Food Front. 2021, 2, 62–76. [Google Scholar] [CrossRef]
- Zubair, M.; Wang, S.; Zhang, P.; Ye, J.; Liang, J.; Nabi, M.; Zhou, Z.; Tao, X.; Chen, N.; Sun, K.; et al. Biological nutrient removal and recovery from solid and liquid livestock manure: Recent advance and perspective. Bioresour. Technol. 2020, 301, 122823. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Peng, Y.; Chen, Y. Advanced nitrogen and phosphorus removal in A2O-BAF system treating low carbon-to-nitrogen ratio domestic wastewater. Front. Environ. Sci. Eng. China 2011, 5, 474. [Google Scholar] [CrossRef]
- Liu, S.; Daigger, G.T.; Liu, B.; Zhao, W.; Liu, J. Enhanced performance of simultaneous carbon, nitrogen and phosphorus removal from municipal wastewater in an anaerobic-aerobic-anoxic sequencing batch reactor (AOA-SBR) system by alternating the cycle times. Bioresour. Technol. 2020, 301, 122750. [Google Scholar] [CrossRef]
- Zhu, G.; Peng, Y.; Li, B.; Guo, J.; Yang, Q.; Wang, S. Biological removal of nitrogen from wastewater. Rev. Environ. Cont. Toxicol. 2008, 192, 159–195. [Google Scholar]
- Wang, J.; Li, Z.; Wang, Q.; Lei, Z.; Yuan, T.; Shimizu, K.; Zhang, Z.; Adachi, Y.; Lee, D.J.; Chen, R. Achieving stably enhanced biological phosphorus removal from aerobic granular sludge system via phosphorus rich liquid extraction during anaerobic period. Bioresour. Technol. 2022, 346, 126439. [Google Scholar] [CrossRef]
- Lim, S.-J.; Choi, D.W.; Lee, W.G.; Kwon, S.; Chang, H.N. Volatile fatty acids production from food wastes and its application to biological nutrient removal. Bioprocess Eng. 2000, 22, 543–545. [Google Scholar] [CrossRef]
- Sun, J.; Song, J.; Fang, W.; Cao, H. Enhanced nitrogen removal upon the addition of volatile fatty acids from activated sludge by combining calcium peroxide and low-thermal pretreatments. J. Environ. Sci. 2021, 108, 145–151. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Li, Y.; Zhao, H.; Peng, H. Nitrogen removal during anaerobic digestion of wasted activated sludge under supplementing Fe(III) compounds. Chem. Eng. J. 2018, 332, 711–716. [Google Scholar] [CrossRef]
- Stewart, R.D.; Bashar, R.; Amstadt, C.; Uribe-Santos, G.A.; McMahon, K.D.; Seib, M.; Noguera, D.R. Pilot-scale comparison of biological nutrient removal (BNR) using intermittent and continuous ammonia-based low dissolved oxygen aeration control systems. Water Sci. Technol. 2022, 85, 578–590. [Google Scholar] [CrossRef]
- Kim, D.; Kim, K.Y.; Ryu, H.D.; Min, K.K.; Lee, S.I. Long term operation of pilot-scale biological nutrient removal process in treating municipal wastewater. Bioresour. Technol. 2009, 100, 3180–3184. [Google Scholar] [CrossRef]
- Al-Mallahi, J.; Ishii, K. Attempts to alleviate inhibitory factors of anaerobic digestate for enhanced microalgae cultivation and nutrients removal: A review. J. Environ. Manag. 2022, 304, 114266. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Pivato, A. Sustainable Management of Digestate from the Organic Fraction of Municipal Solid Waste and Food Waste Under the Concepts of Back to Earth Alternatives and Circular Economy. Waste Biomass Val. 2019, 10, 465–481. [Google Scholar] [CrossRef]
- Chong, C.C.; Cheng, Y.K.; Ishak, S.; Lam, M.K.; Lim, J.W.; Tan, I.S.; Show, P.L.; Lee, K.T. Anaerobic digestate as a low-cost nutrient source for sustainable microalgae cultivation: A way forward through waste valorization approach. Sci. Total Environ. 2022, 803, 150070. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhou, P.; Chen, Y.C.; Cao, Q.; Liu, X.F.; Li, D. The production of single cell protein from biogas slurry with high ammonia-nitrogen content by screened Nectaromyces rattus. Poult. Sci. 2021, 100, 101334. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhao, D.; Zhu, X.; Su, Y.; Angelidaki, I.; Zhang, Y. Biogas upgrading and valorization to single-cell protein in a bioinorganic electrosynthesis system. Chem. Eng. J. 2021, 426, 131837. [Google Scholar] [CrossRef]
- Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. Lignocellulosic Biorefineries in Europe: Current State and Prospects. Trends Biotechnol. 2019, 37, 231–234. [Google Scholar] [CrossRef] [Green Version]
- Dahiya, S.; Kumar, A.N.; Sravan, S.J.; Chatterjee, S.; Sarkar, O.; Venkata Mohan, V. Food waste biorefinery: Sustainable strategy for circular bioeconomy. Bioresour. Technol. 2018, 248, 2–12. [Google Scholar] [CrossRef]
- Qi, Z.; Wang, Z.; Chen, M.; Xiong, D. Pilot-Scale Anaerobic Treatment of Printing and Dyeing Wastewater and Performance Prediction Based on Support Vector Regression. Fermentation 2022, 8, 99. [Google Scholar] [CrossRef]
- Liu, H.; Han, P.; Liu, H.; Zhou, G.; Fu, B.; Zheng, Z. Full-scale production of VFAs from sewage sludge by anaerobic alkaline fermentation to improve biological nutrients removal in domestic wastewater. Bioresour. Technol. 2018, 260, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Tu, W.; Wu, M.; Zhang, Z.; Wang, H. Pilot-scale fermentation of urban food waste for volatile fatty acids production: The importance of pH. Bioresour. Technol. 2021, 332, 125116. [Google Scholar] [CrossRef] [PubMed]
- Da Ros, C.; Conca, V.; Eusebi, A.L.; Frison, N.; Fatone, F. Sieving of municipal wastewater and recovery of bio-based volatile fatty acids at pilot scale. Water Res. 2020, 174, 115633. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-W.; Chung, J. Bioproduction of hydrogen from food waste by pilot-scale combined hydrogen/methane fermentation. Int. J. Hydrogen Energy 2020, 35, 11746–11755. [Google Scholar] [CrossRef]
- Singhvi, M.S.; Gokhale, D.V. Lignocellulosic biomass: Hurdles and challenges in its valorization. Appl. Microbiol. Biotechnol. 2019, 103, 9305–9320. [Google Scholar] [CrossRef]
- Maleki, F.; Changizian, M.; Zolfaghari, N.; Rajaei, S.; Noghabi, K.A.; Zahiri, H.S. Consolidated bioprocessing for bioethanol production by metabolically engineered Bacillus subtilis strains. Sci. Rep. 2021, 11, 13731. [Google Scholar] [CrossRef]
- Zhang, K.; Lu, X.; Li, Y.; Jiang, X.; Liu, L.; Wang, H. New technologies provide more metabolic engineering strategies for bioethanol production in Zymomonas mobilis. Appl. Microbiol. Biotechnol. 2019, 103, 2087–2099. [Google Scholar] [CrossRef]
- Hughes, S.R.; Jones, M.A. Green Energy to Sustainability: Strategies for Global Industries; Chapter 7; Wiley: Hoboken, NJ, USA, 2020; p. 145. [Google Scholar]
- The Bio-Based Industries Consortium. 2017. Available online: https://biconsortium.eu/news/mapping-european-biorefineries (accessed on 20 April 2022).
- Li, Y.-C.; Liu, Y.-F.; Chang, P.-L.; Hsu, C.-W.; Lin, P.-J.; Wu, S.-Y. Techno-economic evaluation of biohydrogen production from wastewater and agricultural waste. Int. J. Hydrogen Energy 2012, 37, 15704–15710. [Google Scholar] [CrossRef]
- Mutsvene, B.; Chetty, M.; Kumari, S.; Bux, F. Biohydrogen production from brewery wastewater in an Anaerobic baffled reactor. A preliminary techno-economic evaluation. S. Afr. J. Chem. Eng. 2023, 43, 9–23. [Google Scholar] [CrossRef]
- Han, W.; Liu, Z.; Fang, J.; Huang, J.; Zhao, H.; Li, Y. Techno-economic analysis of dark fermentative hydrogen production from molasses in a continuous mixed immobilized sludge reactor. J. Clean. Product. 2016, 127, 567–572. [Google Scholar] [CrossRef]
- Han, W.; Fang, J.; Liu, Z.; Tang, J. Techno-economic evaluation of a combined bioprocess for fermentative hydrogen production from food waste. Bioresour. Technol. 2016, 202, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Ladakis, D.; Stylianou, E.; Ioannidou, S.-M.; Koutinas, A.; Pateraki, C. Biorefinery development, techno-economic evaluation and environmental impact analysis for the conversion of the organic fraction of municipal solid waste into succinic acid and value-added fractions. Bioresour. Technol. 2022, 354, 127172. [Google Scholar] [CrossRef]
- Kwan, T.H.; Hu, Y.; Lin, C.S.K. Techno-economic analysis of a food waste valorisation process for lactic acid, lactide and poly(lactic acid) production. J. Clean. Product. 2018, 181, 72–87. [Google Scholar] [CrossRef]
- Mahmod, S.S.; Jahim, J.; Abdul, P.M.; Luthfi, A.A.I.; Takriff, M.S. Techno-economic analysis of two-stage anaerobic system for biohydrogen and biomethane production from palm oil mill effluent. J. Environ. Chem. Eng. 2021, 9, 105679. [Google Scholar] [CrossRef]
- Ahmad, A.; Rambabu, K.; Hasan, S.W.; Show, P.L.; Banat, F. Biohydrogen production through dark fermentation: Recent trends and advances in transition to a circular bioeconomy. Int. J. Hydrogen Energy 2023, in press. [Google Scholar] [CrossRef]
- Mahata, C.; Dhar, S.; Ray, S.; Das, D. Biohydrogen production from starchy wastewater in upflow anaerobic sludge blanket (UASB) reactor: Possibilities toward circular bioeconomy. Environ. Technol. Innov. 2023, 30, 103044. [Google Scholar] [CrossRef]
- Machineni, L.; Deepanraj, B.; Chew, K.W.; Rao, A.G. Biohydrogen production from lignocellulosic feedstock: Abiotic and biotic methods. Ren. Sustain. Energy. Rev. 2023, 182, 113344. [Google Scholar] [CrossRef]
- Ahmad, S.I.; Rashid, R.; Hashim, Z.; Meng, C.C.; Lun, C.K.; Jumaatuden, D.M.H.; Yasin, N.A.; Jati, A.; Hassim, M. M Economic study on biohydrogen production from liquid pineapple waste. Clean Technol. Environ. Pol. 2023, 25, 703–716. [Google Scholar] [CrossRef]
- Goria, K.; Singh, H.M.; Singh, A.; Kothari, R.; Tyagi, V.V. Insights into biohydrogen production from algal biomass: Challenges, recent advancements and future directions. Int. J. Hydrogen Energy 2023, in press. [Google Scholar] [CrossRef]
- Goveas, L.C.; Nayak, S.; Kumar, P.S.; Vinayagam, R.; Selvaraj, R.; Rangasamy, G. Recent advances in fermentative biohydrogen production. Int. J. Hydrogen Energy 2023, in press. [Google Scholar] [CrossRef]
- Rene, E.R.; Khanongnuch, R.; Race, M.; Pugazhendhi, A. Eco-technologies for waste to energy conversion: Applying the concepts of cleaner production, circular economy, and biorefinery. Clean Technol. Environ. Pol. 2023, 25, 311–312. [Google Scholar] [CrossRef]
- Vinayak, V.; Sirotiya, V.; Khandelwal, P.; Rai, A.; Jadhav, D.A.; Pugazhendhi, A.; Schoefs, B.; Marchand, J.; Chae, K.-J. Recent trends in engineering algae for biohydrogen production: State of art strategies. Fuel 2023, 348, 128636. [Google Scholar] [CrossRef]
- Yang, E.; Chon, K.; Kim, K.Y.; Le, G.T.; Nguyen, H.Y.; Le, T.T.; Nguyen, H.T.; Jae, M.R.; Ahmad, I.; Oh, S.E.; et al. Pretreatments of lignocellulosic and algal biomasses for sustainable biohydrogen production: Recent progress, carbon neutrality, and circular economy. Bioresour. Technol. 2023, 369, 128380. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sekoai, P.T.; Chunilall, V.; Ezeokoli, O. Creating Value from Acidogenic Biohydrogen Fermentation Effluents: An Innovative Approach for a Circular Bioeconomy That Is Acquired via a Microbial Biorefinery-Based Framework. Fermentation 2023, 9, 602. https://doi.org/10.3390/fermentation9070602
Sekoai PT, Chunilall V, Ezeokoli O. Creating Value from Acidogenic Biohydrogen Fermentation Effluents: An Innovative Approach for a Circular Bioeconomy That Is Acquired via a Microbial Biorefinery-Based Framework. Fermentation. 2023; 9(7):602. https://doi.org/10.3390/fermentation9070602
Chicago/Turabian StyleSekoai, Patrick T., Viren Chunilall, and Obinna Ezeokoli. 2023. "Creating Value from Acidogenic Biohydrogen Fermentation Effluents: An Innovative Approach for a Circular Bioeconomy That Is Acquired via a Microbial Biorefinery-Based Framework" Fermentation 9, no. 7: 602. https://doi.org/10.3390/fermentation9070602






