Current Applications and Future Trends of Dehydrated Lactic Acid Bacteria for Incorporation in Animal Feed Products
Abstract
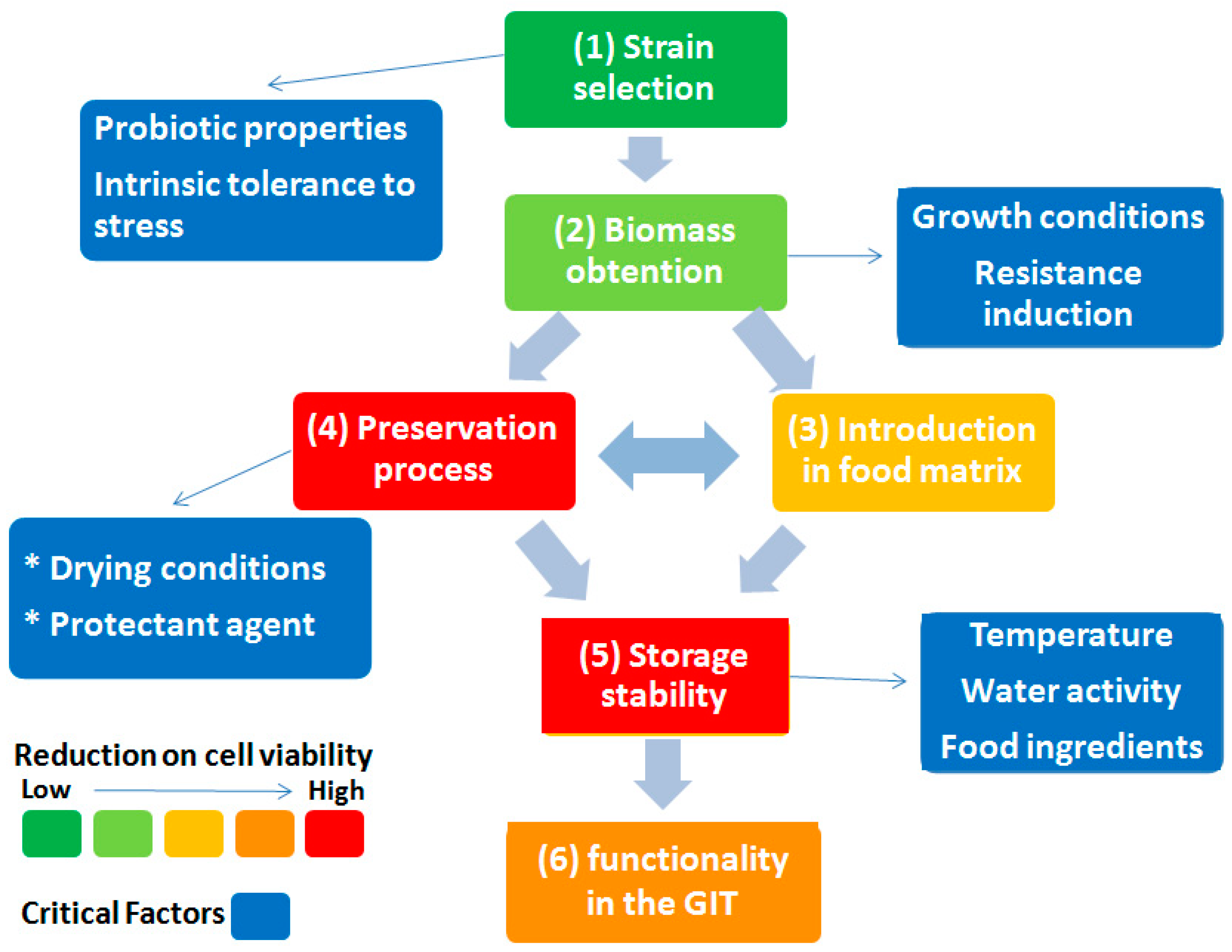
1. Introduction
2. Lactic Acid Bacteria
| Used Group | LAB Species | Main Effects | Addition Method | Ref. |
|---|---|---|---|---|
| Poultry | ||||
| Broiler chicks | Lpb. plantarum | Improved growth performance, intestinal morphology and immune response in broiler chickens under heat stress. | Sprayed on the feed (postbiotic) | [54] |
| 1-day-old chickens | Lgb. salivarius | Improved growth performance (weight and longer shank length), increased relative weights of the immune organs and decreased concentrations of odor-causing compounds. | In diet (107, 108, and 109 CFU/kg of feed) | [55] |
| Broiler chicks | P. acidilactici, Lmb. reuteri, Enterococcus faecium and Lb. acidophilus | Modulates the activation of the innate immune response and inhibits the activation of standard C. perfringens immune responses. | Water (postbiotic) | [56] |
| Broiler chicks | Lgb. salivarius | Improved body weight of broiler under low ambient temperature and a trend in reducing the mortality rate. | Mixed in feed | [57] |
| Broiler | Lb. acidophilus, B. subtilis, S. cerevisiae, A. oryzae | Improved overall weight gain and CP retention. | Mix of probiotics added in basal diet (0–30%) | [58] |
| Swine | ||||
| Weaned piglets | Lpb. plantarum | Increases diversity and richness in the microbial community, promoting intestinal development. | Liquid probiotic via feed (1.25 × 109 CFU/kg of diet). | [59] |
| Weaned piglets | Lpb. plantarum and P. acidilactici | Reduced impact of enterotoxigenic E. coli, being associated with decreased E. coli detection; modulation of the cytokine response, reduction in intestinal damage and clinical signs, and improved growth performance. | Microencapsulated probiotics suspended in sterile peptone water, given orally via sterile syringe (109 CFU/mL) | [60] |
| Weaned piglets | Lb. Johnsonii Lb. mucosae | Higher (p < 0.05) body weight gain, feed intake, and gain/feed ratio than weaned piglets fed basal diet. Probiotic feeding also increased the numbers of lactobacilli and decreased the numbers of E. coli in the feces of weaned piglets. | Probiotic freeze-dried and mixed into the basal diet | [61] |
| Pig farm | Lpb. plantarum | Improved meat quality and physicochemical characteristics. | Drinking water (2.5 × 107 CFU/mL) | [62] |
| Pigs | Lb. acidophilus, B. subtilis, S. cerevisiae, A. Oryzae | Improved overall performance. The overall gain and apparent total tract digestibility of CP were greater in pigs fed substrate fermentation (SF) diets than in pigs fed a liquid diet (LF). | Basal diets supplemented with 0.30% LF and 0.30% SF multi-microbe probiotic products | [63] |
| Ruminants | ||||
| Post-weaning lambs | Lpb. plantarum | Promotes the development of rumen papillae, enhances the immune status and gastrointestinal health. | In diet (0.9% v/w, CFS, Postbiotic) | [64] |
| Neonatal calves | Lpb. plantarum | Improves gut health to increase growth performance. | Drinking water (probiotic powder, 1.20 × 109 CFU/g) | [65] |
| Preruminant calves | Lb. acidophilus | Improved gut health. Lower incidence of diarrhea and higher cell-mediated immunity in probiotic fed groups. | Fermented milk, microencapsulated and FD (108 CFU/calf/d) were added in the milk or calf starter, depending on calf’s age. | [51] |
| Others | ||||
| Rainbow Trout | Ltb. Sakei | Positive effect on growth, immunity, serum enzyme activity, gut microbiome, and resistance to Aeromonas salmonicida | Commercial diet coated in probiotic (1.0 × 107 CFU/g) | [66] |
| Common carp | E. casseliflavus | Improved growth and non-specific immune responses of common carp fingerlings (highest weight gain and specific growth rate at 1012 group, lowest feed conversion ratio at 1012 group) | In diet (1010, 1011, 1012 CFU/kg feed) | [67] |
| Rainbow trout | Lmb. fermentum | The encapsulated L. fermentum plus lactulose improved growth performance and avoided the absorption and accumulation of heavy metals in rainbow trout liver and gills | Encapsulated in diet (107 CFU g−1) | [68] |
| 1 month old puppies | Lcb. rhamnosus and Lpb. plantarum | Significantly increased Lactobacillus and Faecalibacterium detection in fecal matter. Increased short-chain fatty acids (acetate, propionate and butyrate) concentration in feces. Prevented gastrointestinal infection. | In diet (109 CFU/day) | [69] |
| Young, training and elderly dogs | Lactobacillus casei, Lpb. plantarum and B. animalis | Promoted the average daily feed intake of elderly dogs. Improved average daily weight gain in all dogs. Enhanced the level of serum IgG, IFN-α, and fecal secretory IgA (sIgA), reducing the TNF-α. Increased beneficial bacteria and decreased potentially harmful bacteria. | In diet, 2 × 109 CFU/g (2 g for young, 4 g for training, 10 g for elderly dogs) | [70] |
| Kittens | E. hirae | Promoted intestinal colonization and fecal shedding of live E. hirae during administration. Ameliorated the effects of atypical enteropathogenic E. coli experimental infection on intestinal function and water loss | Probiotic powder (2.85–4.28 × 108 CFU/day) mixed with 100 μL of sterile water and inoculated into canned cat food | [52] |
| Healthy adult cats | Lb. acidophilus | Improved fecal quality parameters, increased Lactobacillus count and decreased total coliform bacteria counts | In diet (5 × 109 CFU/kg of food) | [71] |
| Adult cats | Lb. acidophilus, Lcb. casei, Lb. lactis, B. bifidum, E. faecium and S. cerevisiae | Probiotics and synbiotics positively modulated (p < 0.05) the fecal microbiota of cats, increasing the lactic acid bacteria counts | Commercial kibbles coated with probiotics, supplemented with freeze-dried probiotics and fructooligosaccharides | [72] |
3. Drying of Lactic Acid Bacteria
3.1. Drying Techniques
3.2. Alternative Drying Processes
3.3. Protectant Compounds
3.4. Storage Stability
3.5. Intrinsically Resistant Microorganisms
4. Dehydrated Lactic Acid Bacteria in Animal Food
4.1. Dosage of Probiotics
4.2. Incorporation in Low-Moisture Food Matrices
5. Benefits as Postbiotics
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligné, B.; Gänzle, M.; Kort, R.; Pasin, G.; Pihlanto, A.; et al. Health Benefits of Fermented Foods: Microbiota and Beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Stefanovic, E.; Fitzgerald, G.; McAuliffe, O. Advances in the Genomics and Metabolomics of Dairy Lactobacilli: A Review. Food Microbiol. 2017, 61, 33–49. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Huang, J.; Zhou, R. Genomics of Lactic Acid Bacteria: Current Status and Potential Applications. Crit. Rev. Microbiol. 2017, 43, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, N.G.; Brizuela, N.S.; Tymczyszyn, E.E.; Hollmann, A.; Valdés La Hens, D.; Semorile, L.; Bravo-Ferrada, B.M. Complete Genome Sequencing of Lactobacillus plantarum UNQLp 11 Isolated from a Patagonian Pinot Noir Wine. S. Afr. J. Enol. Vitic. 2020, 41, 197–209. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Mattila-Sandholm, T.; Myllärinen, P.; Crittenden, R.; Mogensen, G.; Fondén, R.; Saarela, M. Technological Challenges for Future Probiotic Foods. Int. Dairy J. 2002, 12, 173–182. [Google Scholar] [CrossRef]
- Farnworth, E.R.; Champagne, C. Production of Probiotic Cultures and Their Incorporation into Foods. In Bioactive Foods in Promoting Health; Academic Press: Cambridge, MA, USA, 2010; pp. 3–17. [Google Scholar] [CrossRef]
- Tripathi, M.K.; Giri, S.K. Probiotic Functional Foods: Survival of Probiotics during Processing and Storage. J. Funct. Foods 2014, 9, 225–241. [Google Scholar] [CrossRef]
- Marco, M.L.; Tachon, S. Environmental Factors Influencing the Efficacy of Probiotic Bacteria. Curr. Opin. Biotechnol. 2013, 24, 207–213. [Google Scholar] [CrossRef]
- Dinkçi, N.; Akdeniz, V.; Akalin, A.S. Survival of Probiotics in Functional Foods during Shelf Life. In Food Quality and Shelf Life; Academic Press: Cambridge, MA, USA, 2019; pp. 201–233. [Google Scholar] [CrossRef]
- Mahfuz, S.U.; Nahar, M.; Mo, C.; Gangu, Z.; Zhongjun, L.; Hui, S. Inclusion of Probiotic on Chicken Performance and Inmunity: A Review. Int. J. Poult. Sci. 2017, 16, 328–335. [Google Scholar] [CrossRef]
- Di Gioia, D.; Biavati, B. Probiotics and Prebiotics in Animal Health and Food Safety: Conclusive Remarks and Future Perspectives. In Probiotics and Prebiotics in Animal Health and Food Safety; Springer: Berlin/Heidelberg, Germany, 2018; pp. 269–273. [Google Scholar] [CrossRef]
- Deng, Z.; Hou, K.; Zhao, J.; Wang, H. The Probiotic Properties of Lactic Acid Bacteria and Their Applications in Animal Husbandry. Curr. Microbiol. 2022, 79, 22. [Google Scholar] [CrossRef]
- Blajman, J.; Gaziano, C.; Zbrun, M.V.; Soto, L.; Astesana, D.; Berisvil, A.; Scharpen, A.R.; Signorini, M.; Frizzo, L. In Vitro and in Vivo Screening of Native Lactic Acid Bacteria toward Their Selection as a Probiotic in Broiler Chickens. Res. Vet. Sci. 2015, 101, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Ma, C.; Sun, Z.; Wang, L.; Huang, S.; Su, X.; Xu, J.; Zhang, H. Feed-Additive Probiotics Accelerate yet Antibiotics Delay Intestinal Microbiota Maturation in Broiler Chicken. Microbiome 2017, 5, 91. [Google Scholar] [CrossRef] [PubMed]
- Ramos, O.Y.; Basualdo, M.; Libonatti, C.; Vega, M.F. Current Status and Application of Lactic Acid Bacteria in Animal Production Systems with a Focus on Bacteria from Honey Bee Colonies. J. Appl. Microbiol. 2020, 128, 1248–1260. [Google Scholar] [CrossRef] [PubMed]
- Bahule, C.E.; Silva, T.N.S. Probiotics as a Promising Additive in Broiler Feed: Advances and Limitations. In Advances in Poultry Nutrition Research; IntechOpen: Rijeka, Croatia, 2021. [Google Scholar]
- Grześkowiak, Ł.; Endo, A.; Beasley, S.; Salminen, S. Microbiota and Probiotics in Canine and Feline Welfare. Anaerobe 2015, 34, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.globenewswire.com/news-release/2023/01/31/2598536/0/en/At-CAGR-of-8-8-The-Probiotics-in-Animal-Feed-Market-to-Cross-7-3-billion-by-2026-Globally-Report-by-MarketsandMarkets.html (accessed on 13 July 2023).
- Gao, X.; Kong, J.; Zhu, H.; Mao, B.; Cui, S.; Zhao, J. Lactobacillus, Bifidobacterium and Lactococcus Response to Environmental Stress: Mechanisms and Application of Cross-protection to Improve Resistance against Freeze-drying. J. Appl. Microbiol. 2022, 132, 802–821. [Google Scholar] [CrossRef]
- De Angelis, M.; Gobbetti, M. Environmental Stress Responses in Lactobacillus: A Review. Proteomics 2004, 4, 106–122. [Google Scholar] [CrossRef]
- Santivarangkna, C.; Kulozik, U.; Foerst, P. Inactivation Mechanisms of Lactic Acid Starter Cultures Preserved by Drying Processes. J. Appl. Microbiol. 2008, 105, 1–13. [Google Scholar] [CrossRef]
- Vinderola, G.; Ouwehand, A.; Salminen, S.; Wright, A. Von Lactic Acid Bacteria: Microbiological and Functional Aspects; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Pasolli, E.; De Filippis, F.; Mauriello, I.E.; Cumbo, F.; Walsh, A.M.; Leech, J.; Cotter, P.D.; Segata, N.; Ercolini, D. Large-Scale Genome-Wide Analysis Links Lactic Acid Bacteria from Food with the Gut Microbiome. Nat. Commun. 2020, 11, 2610. [Google Scholar] [CrossRef]
- Vieco-Saiz, N.; Belguesmia, Y.; Raspoet, R.; Auclair, E.; Gancel, F.; Kempf, I.; Drider, D. Benefits and Inputs from Lactic Acid Bacteria and Their Bacteriocins as Alternatives to Antibiotic Growth Promoters during Food-Animal Production. Front. Microbiol. 2019, 10, 422285. [Google Scholar] [CrossRef]
- Sadiq, F.A.; Yan, B.; Tian, F.; Zhao, J.; Zhang, H.; Chen, W. Lactic Acid Bacteria as Antifungal and Anti-Mycotoxigenic Agents: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1403–1436. [Google Scholar] [CrossRef]
- Brashears, M.M.; Amezquita, A.; Jaroni, D. Lactic Acid Bacteria and Their Uses in Animal Feeding to Improve Food Safety. Adv. Food Nutr. Res. 2005, 50, 1–31. [Google Scholar] [CrossRef]
- Yang, S.; Xu, X.; Peng, Q.; Ma, L.; Qiao, Y.; Shi, B. Exopolysaccharides from Lactic Acid Bacteria, as an Alternative to Antibiotics, on Regulation of Intestinal Health and the Immune System. Anim. Nutr. 2023, 13, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Werning, M.L.; Hernández-Alcántara, A.M.; Ruiz, M.J.; Soto, L.P.; Dueñas, M.T.; López, P.; Frizzo, L.S. Biological Functions of Exopolysaccharides from Lactic Acid Bacteria and Their Potential Benefits for Humans and Farmed Animals. Foods 2022, 11, 1284. [Google Scholar] [CrossRef]
- HumayunKober, A.K.M.; Rajoka, M.S.R.; Mehwish, H.M.; Villena, J.; Kitazawa, H. Immunomodulation Potential of Probiotics: A Novel Strategy for Improving Livestock Health, Immunity, and Productivity. Microorganisms 2022, 10, 388. [Google Scholar] [CrossRef]
- Mansilla, F.I.; Ficoseco, C.A.; Miranda, M.H.; Puglisi, E.; Nader-Macías, M.E.F.; Vignolo, G.M.; Fontana, C.A. Administration of Probiotic Lactic Acid Bacteria to Modulate Fecal Microbiome in Feedlot Cattle. Sci. Rep. 2022, 12, 12957. [Google Scholar] [CrossRef] [PubMed]
- Moretti, A.F.; Gamba, R.; De Antoni, G.; Peláez, Á.L.; Golowczyc, M.A. Probiotic Characterization of Indigenous Lactic Acid Bacteria Isolates from Chickens to Be Used as Biocontrol Agents in Poultry Industry. J. Food Process. Preserv. 2022, 46, e17145. [Google Scholar] [CrossRef]
- Xu, X.; Luo, D.; Bao, Y.; Liao, X.; Wu, J. Characterization of Diversity and Probiotic Efficiency of the Autochthonous Lactic Acid Bacteria in the Fermentation of Selected Raw Fruit and Vegetable Juices. Front. Microbiol. 2018, 9, 388519. [Google Scholar] [CrossRef]
- Moretti, A.F.; Gamba, R.R.; Puppo, J.; Malo, N.; Gómez-Zavaglia, A.; Peláez, Á.L.; Golowczyc, M.A. Incorporation of Lactobacillus plantarum and Zeolites in Poultry Feed Can Reduce Aflatoxin B1 Levels. J. Food Sci. Technol. 2018, 55, 431–436. [Google Scholar] [CrossRef]
- Victor-Aduloju, A.T.; Anyamene, C.; Ogbu, K.N.; Ishiwu, C.N. Isolation and Identification of Probiotic Lactobacillus Species from Traditional Drink Kunun-Zaki Fortified with Paddy Rice and Sweet Potatoes. Afr. J. Food Sci. 2018, 12, 230–237. [Google Scholar] [CrossRef]
- Bajagai, Y.S.; Klieve, A.V.; Dart, P.J.; Bryden, W.L. Probiotics in Animal Nutrition: Production, Impact and Regulation; FAO: Rome, Italy, 2016. [Google Scholar]
- Sornplang, P.; Piyadeatsoontorn, S. Probiotic Isolates from Unconventional Sources: A Review. J. Anim. Sci. Technol. 2016, 58, 26. [Google Scholar] [CrossRef]
- Lambring, C.B.; Siraj, S.; Patel, K.; Sankpal, U.T.; Mathew, S.; Basha, R. Impact of the Microbiome on the Immune System. Crit. Rev. Immunol. 2019, 39, 313–328. [Google Scholar] [CrossRef] [PubMed]
- Trinh, P.; Zaneveld, J.R.; Safranek, S.; Rabinowitz, P.M. One Health Relationships between Human, Animal, and Environmental Microbiomes: A Mini-Review. Front. Public Health 2018, 6, 385281. [Google Scholar] [CrossRef] [PubMed]
- Wagar, L.E.; Difazio, R.M.; Davis, M.M. Advanced Model Systems and Tools for Basic and Translational Human Immunology. Genome Med. 2018, 10, 73. [Google Scholar] [CrossRef]
- Wang, L.; Liu, C.; Chen, M.; Ya, T.; Huang, W.; Gao, P.; Zhang, H. A Novel Lactobacillus plantarum Strain P-8 Activates Beneficial Immune Response of Broiler Chickens. Int. Immunopharmacol. 2015, 29, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Pringsulaka, O.; Rueangyotchanthana, K.; Suwannasai, N.; Watanapokasin, R.; Amnueysit, P.; Sunthornthummas, S.; Sukkhum, S.; Sarawaneeyaruk, S.; Rangsiruji, A. In Vitro Screening of Lactic Acid Bacteria for Multi-Strain Probiotics. Livest. Sci. 2015, 174, 66–73. [Google Scholar] [CrossRef]
- Chaves, B.D.; Brashears, M.M.; Nightingale, K.K. Applications and Safety Considerations of Lactobacillus salivarius as a Probiotic in Animal and Human Health. J. Appl. Microbiol. 2017, 123, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Xing, S.; He, L.; Li, C.; Wang, X.; Zeng, X.; Dai, Y. Characterization, High-Density Fermentation, and the Production of a Directed Vat Set Starter of Lactobacilli Used in the Food Industry: A Review. Foods 2022, 11, 3063. [Google Scholar] [CrossRef]
- Dong, Z.; Gu, L.; Zhang, J.; Wang, M.; Du, G.; Chen, J.; Li, H. Optimisation for High Cell Density Cultivation of Lactobacillus salivarius BBE 09-18 with Response Surface Methodology. Int. Dairy J. 2014, 34, 230–236. [Google Scholar] [CrossRef]
- Cui, S.; Hu, M.; Sun, Y.; Mao, B.; Zhang, Q.; Zhao, J.; Tang, X.; Zhang, H. Effect of Trehalose and Lactose Treatments on the Freeze-Drying Resistance of Lactic Acid Bacteria in High-Density Culture. Microorganisms 2023, 11, 48. [Google Scholar] [CrossRef]
- Wang, T.; Lu, Y.; Yan, H.; Li, X.; Wang, X.; Shan, Y.; Yi, Y.; Liu, B.; Zhou, Y.; Lü, X. Fermentation Optimization and Kinetic Model for High Cell Density Culture of a Probiotic Microorganism: Lactobacillus rhamnosus LS-8. Bioprocess Biosyst. Eng. 2020, 43, 515–528. [Google Scholar] [CrossRef]
- Golowczyc, M.A.; Silva, J.; Teixeira, P.; De Antoni, G.L.; Abraham, A.G. Cellular Injuries of Spray-Dried Lactobacillus spp. Isolated from Kefir and Their Impact on Probiotic Properties. Int. J. Food Microbiol. 2011, 144, 556–560. [Google Scholar] [CrossRef]
- Paéz, R.; Lavari, L.; Vinderola, G.; Audero, G.; Cuatrin, A.; Zaritzky, N.; Reinheimer, J. Effect of Heat Treatment and Spray Drying on Lactobacilli Viability and Resistance to Simulated Gastrointestinal Digestion. Food Res. Int. 2012, 48, 748–754. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Kim, J.S.; Kwon, H.J.; Kang, C.H. The Effect of a Glutathione (GSH)-Containing Cryo-Protectant on the Viability of Probiotic Cells Using a Freeze-Drying Process. Fermentation 2023, 9, 187. [Google Scholar] [CrossRef]
- Kumar, M.; Kala, A.; Chaudhary, L.C.; Agarwal, N.; Kochewad, S.A. Microencapsulated and Lyophilized Lactobacillus acidophilus Improved Gut Health and Immune Status of Preruminant Calves. Probiotics Antimicrob. Proteins 2022, 14, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Watson, V.E.; Jacob, M.E.; Bruno-Bárcena, J.M.; Amirsultan, S.; Stauffer, S.H.; Píqueras, V.O.; Frias, R.; Gookin, J.L. Influence of the Intestinal Microbiota on Disease Susceptibility in Kittens with Experimentally-Induced Carriage of Atypical Enteropathogenic Escherichia coli. Vet. Microbiol. 2019, 231, 197–206. [Google Scholar] [CrossRef]
- Fernández-Ciganda, S.; Fraga, M.; Zunino, P. Probiotic Lactobacilli Administration Induces Changes in the Fecal Microbiota of Preweaned Dairy Calves. Probiotics Antimicrob. Proteins 2022, 14, 804–815. [Google Scholar] [CrossRef] [PubMed]
- Humam, A.M.; Loh, T.C.; Foo, H.L.; Samsudin, A.A.; Mustapha, N.M.; Zulkifli, I.; Izuddin, W.I. Effects of Feeding Different Postbiotics Produced by Lactobacillus plantarum on Growth Performance, Carcass Yield, Intestinal Morphology, Gut Microbiota Composition, Immune Status, and Growth Gene Expression in Broilers under Heat Stress. Animals 2019, 9, 644. [Google Scholar] [CrossRef]
- Wang, J.; Ishfaq, M.; Guo, Y.; Chen, C.; Li, J. Assessment of Probiotic Properties of Lactobacillus salivarius. Front. Vet. Sci. 2020, 7, 415. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.N.; Kogut, M.H.; Genovese, K.; He, H.; Kazemi, S.; Arsenault, R.J. Administration of a Postbiotic Causes Immunomodulatory Responses in Broiler Gut and Reduces Disease Pathogenesis Following Challenge. Microorganisms 2019, 7, 268. [Google Scholar] [CrossRef] [PubMed]
- Blajman, J.E.; Olivero, C.A.; Fusari, M.L.; Zimmermann, J.A.; Rossler, E.; Berisvil, A.P.; Romero Scharpen, A.; Astesana, D.M.; Soto, L.P.; Signorini, M.L.; et al. Impact of Lyophilized Lactobacillus salivarius DSPV 001P Administration on Growth Performance, Microbial Translocation, and Gastrointestinal Microbiota of Broilers Reared under Low Ambient Temperature. Res. Vet. Sci. 2017, 114, 388–394. [Google Scholar] [CrossRef]
- Shim, Y.H.; Ingale, S.L.; Kim, J.S.; Kim, K.H.; Seo, D.K.; Lee, S.C.; Chae, B.J.; Kwon, I.K. A Multi-Microbe Probiotic Formulation Processed at Low and High Drying Temperatures: Effects on Growth Performance, Nutrient Retention and Caecal Microbiology of Broilers. Br. Poult. Sci. 2012, 53, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.; Chang, S.Y.; Bogere, P.; Won, K.H.; Choi, J.Y.; Choi, Y.J.; Lee, H.K.; Hur, J.; Park, B.Y.; Kim, Y.; et al. Beneficial Roles of Probiotics on the Modulation of Gut Microbiota and Immune Response in Pigs. PLoS ONE 2019, 14, e0220843. [Google Scholar] [CrossRef] [PubMed]
- Pupa, P.; Apiwatsiri, P.; Sirichokchatchawan, W.; Pirarat, N.; Maison, T.; Koontanatechanon, A.; Prapasarakul, N. Use of Lactobacillus plantarum (Strains 22F and 25F) and Pediococcusacidilactici (Strain 72N) as Replacements for Antibiotic-Growth Promotants in Pigs. Sci. Rep. 2021, 11, 12028. [Google Scholar] [CrossRef] [PubMed]
- Chiang, M.L.; Chen, H.C.; Chen, K.N.; Lin, Y.C.; Lin, Y.T.; Chen, M.J. Optimizing Production of Two Potential Probiotic Lactobacilli Strains Isolated from Piglet Feces as Feed Additives for Weaned Piglets. Asian-Australas. J. Anim. Sci. 2015, 28, 1163. [Google Scholar] [CrossRef]
- Chang, S.Y.; Belal, S.A.; Kang, D.R.; Choi, Y.I.; Kim, Y.H.; Choe, H.S.; Heo, J.Y.; Shim, K.S. Influence of Probiotics-Friendly Pig Production on Meat Quality and Physicochemical Characteristics. Korean J. Food Sci. Anim. Resour. 2018, 38, 403. [Google Scholar] [CrossRef]
- Choi, J.Y.; Shinde, P.L.; Ingale, S.L.; Kim, J.S.; Kim, Y.W.; Kim, K.H.; Kwon, I.K.; Chae, B.J. Evaluation of Multi-Microbe Probiotics Prepared by Submerged Liquid or Solid Substrate Fermentation and Antibiotics in Weaning Pigs. Livest. Sci. 2011, 138, 144–151. [Google Scholar] [CrossRef]
- Izuddin, W.I.; Loh, T.C.; Foo, H.L.; Samsudin, A.A.; Humam, A.M.; Postbiotic, L. Plantarum RG14 Improves Ruminal Epithelium Growth, Immune Status and Upregulates the Intestinal Barrier Function in Post-Weaning Lambs. Sci. Rep. 2019, 9, 9938. [Google Scholar] [CrossRef]
- Casper, D.P.; Hultquist, K.M.; Acharya, I.P. Lactobacillus plantarum GB LP-1 as a Direct-Fed Microbial for Neonatal Calves. J. Dairy Sci. 2021, 104, 5557–5568. [Google Scholar] [CrossRef]
- Zhao, C.; Men, X.; Dang, Y.; Zhou, Y.; Ren, Y. Probiotics Mediate Intestinal Microbiome and Microbiota-Derived Metabolites Regulating the Growth and Immunity of Rainbow Trout (Oncorhynchus mykiss). Microbiol. Spectr. 2023, 11, e03980-22. [Google Scholar] [CrossRef]
- Akbari, H.; Shekrabi, S.P.H.; Soltani, M.; Mehrgan, M.S. Effects of Potential Probiotic Enterococcus casseliflavus (EC-001) on Growth Performance, Immunity, and Resistance to Aeromonas hydrophila Infection in Common Carp (Cyprinus carpio). Probiotics Antimicrob. Proteins 2021, 13, 1316–1325. [Google Scholar] [CrossRef]
- Madreseh, S.; Ghaisari, H.R.; Hosseinzadeh, S. Effect of Lyophilized, Encapsulated Lactobacillus fermentum and Lactulose Feeding on Growth Performance, Heavy Metals, and Trace Element Residues in Rainbow Trout (Oncorhynchus mykiss) Tissues. Probiotics Antimicrob. Proteins 2018, 11, 1257–1263. [Google Scholar] [CrossRef] [PubMed]
- Fernández, L.; Martínez, R.; Pérez, M.; Arroyo, R.; Rodríguez, J.M. Characterization of Lactobacillus rhamnosus MP01 and Lactobacillus plantarum MP02 and Assessment of Their Potential for the Prevention of Gastrointestinal Infections in an Experimental Canine Model. Front. Microbiol. 2019, 10, 1117. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Huang, W.; Hou, Q.; Kwok, L.Y.; Laga, W.; Wang, Y.; Ma, H.; Sun, Z.; Zhang, H. Oral Administration of Compound Probiotics Improved Canine Feed Intake, Weight Gain, Immunity and Intestinal Microbiota. Front. Immunol. 2019, 10, 666. [Google Scholar] [CrossRef] [PubMed]
- Fusi, E.; Rizzi, R.; Polli, M.; Cannas, S.; Giardini, A.; Bruni, N.; Marelli, S.P. Effects of Lactobacillus acidophilus D2/CSL (CECT 4529) Supplementation on Healthy Cat Performance. Vet. Rec. Open 2019, 6, e000368. [Google Scholar] [CrossRef]
- Rodrigues, B.M.; Olivo, P.M.; Osmari, M.P.; Vasconcellos, R.S.; Ribeiro, L.B.; Bankuti, F.I.; Pozza, M.S.S. Microencapsulation of Probiotic Strains by Lyophilization Is Efficient in Maintaining the Viability of Microorganisms and Modulation of Fecal Microbiota in Cats. Int. J. Microbiol. 2020, 2020, 1293481. [Google Scholar] [CrossRef]
- Passot, S.; Cenard, S.; Douania, I.; Tréléa, I.C.; Fonseca, F. Critical Water Activity and Amorphous State for Optimal Preservation of Lyophilised Lactic Acid Bacteria. Food Chem. 2012, 132, 1699–1705. [Google Scholar] [CrossRef]
- Carvalho, A.S.; Silva, J.; Ho, P.; Teixeira, P.; Malcata, F.X.; Gibbs, P. Relevant Factors for the Preparation of Freeze-Dried Lactic Acid Bacteria. Int. Dairy J. 2004, 14, 835–847. [Google Scholar] [CrossRef]
- Fonseca, F.; Cenard, S.; Passot, S. Freeze-Drying of Lactic Acid Bacteria. Methods Mol. Biol. 2015, 1257, 477–488. [Google Scholar] [CrossRef]
- Santivarangkna, C.; Kulozik, U.; Foerst, P. Alternative Drying Processes for the Industrial Preservation of Lactic Acid Starter Cultures. Biotechnol. Prog. 2007, 23, 302–315. [Google Scholar] [CrossRef]
- Moreira, M.T.C.; Martins, E.; Perrone, Í.T.; de Freitas, R.; Queiroz, L.S.; de Carvalho, A.F. Challenges Associated with Spray Drying of Lactic Acid Bacteria: Understanding Cell Viability Loss. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3267–3283. [Google Scholar] [CrossRef]
- Wang, N.; Fu, N.; Chen, X.D. The Extent and Mechanism of the Effect of Protectant Material in the Production of Active Lactic Acid Bacteria Powder Using Spray Drying: A Review. Curr. Opin. Food Sci. 2022, 44, 100807. [Google Scholar] [CrossRef]
- Peighambardoust, S.H.; GolshanTafti, A.; Hesari, J. Application of Spray Drying for Preservation of Lactic Acid Starter Cultures: A Review. Trends Food Sci. Technol. 2011, 22, 215–224. [Google Scholar] [CrossRef]
- Golowczyc, M.A.; Silva, J.; Abraham, A.G.; De Antoni, G.L.; Teixeira, P. Preservation of Probiotic Strains Isolated from Kefir by Spray Drying. Lett. Appl. Microbiol. 2010, 50, 7–12. [Google Scholar] [CrossRef]
- Kim, S.S.; Bhowmik, S.R. Survival of Lactic Acid Bacteria during Spray Drying of Plain Yogurt. J. Food Sci. 1990, 55, 1008–1010. [Google Scholar] [CrossRef]
- Gardiner, G.E.; O’Sullivan, E.; Kelly, J.; Auty, M.A.E.; Fitzgerald, G.F.; Collins, J.K.; Ross, R.P.; Stanton, C. Comparative Survival Rates of Human-Derived Probiotic Lactobacillus paracasei and L. salivarius Strains during Heat Treatment and Spray Drying. Appl. Environ. Microbiol. 2000, 66, 2605–2612. [Google Scholar] [CrossRef] [PubMed]
- Mauriello, G.; Aponte, M.; Andolfi, R.; Moschetti, G.; Villani, F. Spray-Drying of Bacteriocin-Producing Lactic Acid Bacteria. J. Food Prot. 1999, 62, 773–777. [Google Scholar] [CrossRef]
- Tymczyszyn, E.E.; Díaz, R.; Pataro, A.; Sandonato, N.; Gómez-Zavaglia, A.; Disalvo, E.A. Critical Water Activity for the Preservation of Lactobacillus bulgaricus by Vacuum Drying. Int. J. Food Microbiol. 2008, 128, 342–347. [Google Scholar] [CrossRef]
- King, V.A.E.; Su, J.T. Dehydration of Lactobacillus acidophilus. Process Biochem. 1993, 28, 47–52. [Google Scholar] [CrossRef]
- Savedboworn, W.; Noisumdang, C.; Arunyakanon, C.; Kongcharoen, P.; Phungamngoen, C.; Rittisak, S.; Charoen, R.; Phattayakorn, K. Potential of Protein-Prebiotic as Protective Matrices on the Storage Stability of Vacuum-Dried Probiotic Lactobacillus casei. LWT 2020, 131, 109578. [Google Scholar] [CrossRef]
- Santivarangkna, C.; Wenning, M.; Foerst, P.; Kulozik, U. Damage of Cell Envelope of Lactobacillus helveticus during Vacuum Drying. J. Appl. Microbiol. 2007, 102, 748–756. [Google Scholar] [CrossRef]
- Santivarangkna, C.; Naumann, D.; Kulozik, U.; Foerst, P. Protective Effects of Sorbitol during the Vacuum Drying of Lactobacillus helveticus: An FT-IR Study. Ann. Microbiol. 2010, 60, 235–242. [Google Scholar] [CrossRef]
- Foerst, P.; Kulozik, U.; Schmitt, M.; Bauer, S.; Santivarangkna, C. Storage Stability of Vacuum-Dried Probiotic Bacterium Lactobacillus paracasei F19. Food Bioprod. Process. 2012, 90, 295–300. [Google Scholar] [CrossRef]
- Toledo, N.; Ferrer, J.; Bórquez, R. Drying and Storage Stability of a Probiotic Strain Incorporated into a Fish Feed Formulation. Dry. Technol. 2010, 28, 508–516. [Google Scholar] [CrossRef]
- Zhang, Y.; Goh, K.L.; Ng, Y.L.; Chow, Y.; Wang, S.; Zivkovic, V. Process Intensification in Micro-Fluidized Bed Systems: A Review. Chem. Eng. Process.-Process Intensif. 2021, 164, 108397. [Google Scholar] [CrossRef]
- Wang, G.; Luo, L.; Dong, C.; Zheng, X.; Guo, B.; Xia, Y.; Tao, L.; Ai, L. Polysaccharides Can Improve the Survival of Lactiplantibacillus plantarum Subjected to Freeze-Drying. J. Dairy Sci. 2021, 104, 2606–2614. [Google Scholar] [CrossRef]
- Londoño, L.; Ramírez Toro, C.; Bolívar, G.A. Effect of Drying on the Viability of the Probiotic Bacterium Lactobacillus plantarum Lab9 (CPQBA 144-09 DRM 03) Impregnated in the Feed for Tilapia (Oreochromis sp.). Aquac. Res. 2015, 48, 646–654. [Google Scholar] [CrossRef]
- Wirunpan, M.; Savedboworn, W.; Wanchaitanawong, P. Survival and Shelf Life of Lactobacillus lactis 1464 in Shrimp Feed Pellet after Fluidized Bed Drying. Agric. Nat. Resour. 2016, 50, 1–7. [Google Scholar] [CrossRef][Green Version]
- Ren, H.; Zentek, J.; Vahjen, W. Optimization of Production Parameters for Probiotic Lactobacillus Strains as Feed Additive. Molecules 2019, 24, 3286. [Google Scholar] [CrossRef]
- Reddy, K.B.P.K.; Awasthi, S.P.; Madhu, A.N.; Prapulla, S.G. Role of Cryoprotectants on the Viability and Functional Properties of Probiotic Lactic Acid Bacteria during Freeze Drying. Food Biotechnol. 2009, 23, 243–265. [Google Scholar] [CrossRef]
- Abd-Talib, N.; Mohd-Setapar, S.H.; Khamis, A.K.; Nian-Yian, L.; Aziz, R. Survival of Encapsulated Probiotics through Spray Drying and Non-Refrigerated Storage for Animal Feeds Application. Agric. Sci. 2013, 4, 78–83. [Google Scholar] [CrossRef]
- Reddy, K.B.P.K.; Madhu, A.N.; Prapulla, S.G. Comparative Survival and Evaluation of Functional Probiotic Properties of Spray-Dried Lactic Acid Bacteria. Int. J. Dairy Technol. 2009, 62, 240–248. [Google Scholar] [CrossRef]
- Bucio, A.; Hartemink, R.; Schrama, J.W.; Verreth, J.; Rombouts, F.M. Survival of Lactobacillus plantarum 44a after Spraying and Drying in Feed and during Exposure to Gastrointestinal Tract Fluids In Vitro. J. Gen. Appl. Microbiol. 2005, 51, 221–227. [Google Scholar] [CrossRef][Green Version]
- Piyadeatsoontorn, S.; Taharnklaew, R.; Upathanpreecha, T.; Sornplang, P. Encapsulating Viability of Multi-Strain Lactobacilli as Potential Probiotic in Pigs. Probiotics Antimicrob. Proteins 2018, 11, 438–446. [Google Scholar] [CrossRef]
- Martins, E.; Cnossen, D.C.; Silva, C.R.J.; Vakarelova, M.; Carvalho, A.F. Short communication: Effect of Lactose on the Spray Drying of Lactococcus lactis in Dairy Matrices. J. Dairy Sci. 2019, 102, 9763–9766. [Google Scholar] [CrossRef]
- Crowe, J.H.; Hoekstra, F.A.; Crowe, L.M. Anhydrobiosis. Annu. Rev. Physiol. 1992, 54, 579–599. [Google Scholar] [CrossRef] [PubMed]
- Leslie, S.B.; Israeli, E.; Lighthart, B.; Crowe, J.H.; Crowe, L.M. Trehalose and Sucrose Protect Both Membranes and Proteins in Intact Bacteria during Drying. Appl. Environ. Microbiol. 1995, 61, 3592–3597. [Google Scholar] [CrossRef] [PubMed]
- Santivarangkna, C.; Higl, B.; Foerst, P. Protection Mechanisms of Sugars during Different Stages of Preparation Process of Dried Lactic Acid Starter Cultures. Food Microbiol. 2008, 25, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Lodato, P.; Segovia de Huergo, M.; Buera, M.P. Viability and Thermal Stability of a Strain of Saccharomyces cerevisiae Freeze-Dried in Different Sugar and Polymer Matrices. Appl. Microbiol. Biotechnol. 1999, 52, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Sosa, N.; Gerbino, E.; Golowczyc, M.A.; Schebor, C.; Gómez-Zavaglia, A.; Tymczyszyn, E.E. Effect of Galacto-Oligosaccharides: Maltodextrin Matrices on the Recovery of Lactobacillus plantarum after Spray-Drying. Front. Microbiol. 2016, 7, 584. [Google Scholar] [CrossRef]
- Romano, N.; Schebor, C.; Mobili, P.; Gómez-Zavaglia, A. Role of Mono- and Oligosaccharides from FOS as Stabilizing Agents during Freeze-Drying and Storage of Lactobacillus delbrueckii subsp. bulgaricus. Food Res. Int. 2016, 90, 251–258. [Google Scholar] [CrossRef]
- Guowei, S.; Yang, X.; Li, C.; Huang, D.; Lei, Z.; He, C. Comprehensive Optimization of Composite Cryoprotectant for Saccharomyces boulardii during Freeze-Drying and Evaluation of Its Storage Stability. Prep. Biochem. Biotechnol. 2019, 49, 846–857. [Google Scholar] [CrossRef] [PubMed]
- Bayram, Ö.A.; Bayram, M.; Tekin, A.R. Spray Drying of Sumac Flavour Using Sodium Chloride, Sucrose, Glucose and Starch as Carriers. J. Food Eng. 2005, 69, 253–260. [Google Scholar] [CrossRef]
- Fu, S.; Miao, S.; Ma, X.; Ding, T.; Ye, X.; Liu, D. Inhibition of Lactose Crystallisation in the Presence of Galacto-Oligosaccharide. Food Hydrocoll. 2019, 88, 127–136. [Google Scholar] [CrossRef]
- Golowczyc, M.; Vera, C.; Santos, M.; Guerrero, C.; Carasi, P.; Illanes, A.; Gómez-Zavaglia, A.; Tymczyszyn, E. Use of Whey Permeate Containing in Situ Synthesised Galacto-Oligosaccharides for the Growth and Preservation of Lactobacillus plantarum. J. Dairy Res. 2013, 80, 374–381. [Google Scholar] [CrossRef]
- Hugo, A.A.; Bruno, F.; Golowczyc, M.A. Whey Permeate Containing Galacto-Oligosaccharides as a Medium for Biomass Production and Spray Drying of Lactobacillus plantarum CIDCA 83114. LWT Food Sci. Technol. 2016, 69, 185–190. [Google Scholar] [CrossRef]
- Craig, J.M. Food Intolerance in Dogs and Cats. J. Small Anim. Pract. 2019, 60, 77–85. [Google Scholar] [CrossRef]
- Khoramnia, A.; Abdullah, N.; Liew, S.L.; Sieo, C.C.; Ramasamy, K.; Ho, Y.W. Enhancement of Viability of a Probiotic Lactobacillus Strain for Poultry during Freeze-Drying and Storage Using the Response Surface Methodology. Anim. Sci. J. 2011, 82, 127–135. [Google Scholar] [CrossRef]
- Teixeira, P.C.; Castro, M.H.; Malcata, F.X.; Kirby, R.M. Survival of Lactobacillus delbrueckii ssp. bulgaricus Following Spray-Drying. J. Dairy Sci. 1995, 78, 1025–1031. [Google Scholar] [CrossRef]
- Desmond, C.; Stanton, C.; Fitzgerald, G.F.; Collins, K.; Paul Ross, R. Environmental Adaptation of Probiotic Lactobacilli towards Improvement of Performance during Spray Drying. Int. Dairy J. 2002, 12, 183–190. [Google Scholar] [CrossRef]
- Miao, S.; Mills, S.; Stanton, C.; Fitzgerald, G.F.; Roos, Y.; Ross, R.P. Effect of Disaccharides on Survival during Storage of Freeze Dried Probiotics. Dairy Sci. Technol. 2008, 88, 19–30. [Google Scholar] [CrossRef]
- Higl, B.; Kurtmann, L.; Carlsen, C.U.; Ratjen, J.; Först, P.; Skibsted, L.H.; Kulozik, U.; Risbo, J. Impact of Water Activity, Temperature, and Physical State on the Storage Stability of Lactobacillus paracasei ssp. Paracasei Freeze-Dried in a Lactose Matrix. Biotechnol. Prog. 2007, 23, 794–800. [Google Scholar] [CrossRef]
- Tymczyszyn, E.E.; Sosa, N.; Gerbino, E.; Hugo, A.; Gómez-Zavaglia, A.; Schebor, C. Effect of Physical Properties on the Stability of Lactobacillus bulgaricus in a Freeze-Dried Galacto-Oligosaccharides Matrix. Int. J. Food Microbiol. 2012, 155, 217–221. [Google Scholar] [CrossRef]
- Alves, N.N.; Messaoud, G.B.; Desobry, S.; Costa, J.M.C.; Rodrigues, S. Effect of Drying Technique and Feed Flow Rate on Bacterial Survival and Physicochemical Properties of a Non-Dairy Fermented Probiotic Juice Powder. J. Food Eng. 2016, 189, 45–54. [Google Scholar] [CrossRef]
- Brizuela, N.S.; Arnez-Arancibia, M.; Semorile, L.; Bravo-Ferrada, B.M.; Tymczyszyn, E.E. Whey Permeate as a Substrate for the Production of Freeze-Dried Lactiplantibacillus plantarumto Be Used as a Malolactic Starter Culture. World J. Microbiol. Biotechnol. 2021, 37, 115. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, P.; Castro, H.; Kirby, R. Evidence of Membrane Lipid Oxidation of Spray-Dried Lactobacillus bulgaricus during Storage. Lett. Appl. Microbiol. 1996, 22, 34–38. [Google Scholar] [CrossRef]
- Carlsen, C.U.; Kurtmann, L.; Brüggemann, D.A.; Hoff, S.; Risbo, J.; Skibsted, L.H. Investigation of Oxidation in Freeze-Dried Membranes Using the Fluorescent Probe C11-BODIPY581/591. Cryobiology 2009, 58, 262–267. [Google Scholar] [CrossRef]
- Potts, M. Desiccation Tolerance of Prokaryotes. Microbiol. Rev. 1994, 58, 755–805. [Google Scholar] [CrossRef]
- Bravo-Ferrada, B.M.; Gómez-Zavaglia, A.; Semorile, L.; Tymczyszyn, E.E. Effect of the Fatty Acid Composition of Acclimated Oenological Lactobacillus plantarum on the Resistance to Ethanol. Lett. Appl. Microbiol. 2015, 60, 155–161. [Google Scholar] [CrossRef]
- Shao, Y.; Gao, S.; Guo, H.; Zhang, H. Influence of Culture Conditions and Preconditioning on Survival of Lactobacillus delbrueckii subspecies bulgaricus ND02 during Lyophilization. J. Dairy Sci. 2014, 97, 1270–1280. [Google Scholar] [CrossRef]
- Wood, J.M.; Bremer, E.; Csonka, L.N.; Kraemer, R.; Poolman, B.; Van der Heide, T.; Smith, L.T. Osmosensing and Osmoregulatory Compatible Solute Accumulation by Bacteria. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2001, 130, 437–460. [Google Scholar] [CrossRef]
- Yang, H.; He, M.; Wu, C. Cross Protection of Lactic Acid Bacteria during Environmental Stresses: Stress Responses and Underlying Mechanisms. LWT 2021, 144, 111203. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, X.; Wang, K.; Jiang, J.; Zeng, J.; Zhang, L.; Gong, P. Enhancing Spray-Drying Tolerance of Lactobacillus bulgaricus via Non-Sporeforming Dormancy Induction. Innov. Food Sci. Emerg. Technol. 2023, 84, 103309. [Google Scholar] [CrossRef]
- Grześkowiak, L.; Isolauri, E.; Salminen, S.; Gueimonde, M. Manufacturing Process Influences Properties of Probiotic Bacteria. Br. J. Nutr. 2011, 105, 887–894. [Google Scholar] [CrossRef]
- Grześkowiak, L.; Endo, A.; Collado, M.C.; Pelliniemi, L.J.; Beasley, S.; Salminen, S. The Effect of Growth Media and Physical Treatments on the Adhesion Properties of Canine Probiotics. J. Appl. Microbiol. 2013, 115, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Grześkowiak, L.; Collado, M.C.; Beasley, S.; Salminen, S. Pathogen Exclusion Properties of Canine Probiotics Are Influenced by the Growth Media and Physical Treatments Simulating Industrial Processes. J. Appl. Microbiol. 2014, 116, 1308–1314. [Google Scholar] [CrossRef]
- Markowiak, P.; Ślizewska, K. The Role of Probiotics, Prebiotics and Synbiotics in Animal Nutrition. Gut Pathog. 2018, 10, 21. [Google Scholar] [CrossRef]
- Khomayezi, R.; Adewole, D. Probiotics, Prebiotics, and Synbiotics: An Overview of Their Delivery Routes and Effects on Growth and Health of Broiler Chickens. Worlds Poult. Sci. J. 2022, 78, 57–81. [Google Scholar] [CrossRef]
- Torshizi, M.A.K.; Moghaddam, A.R.; Rahimi, S.; Mojgani, N. Assessing the Effect of Administering Probiotics in Water or as a Feed Supplement on Broiler Performance and Immune Response. Br. Poult. Sci. 2010, 51, 178–184. [Google Scholar] [CrossRef]
- Papizadeh, M.; Rohani, M.; Nahrevanian, H.; Javadi, A.; Pourshafie, M.R. Probiotic Characters of Bifidobacterium and Lactobacillus Are a Result of the Ongoing Gene Acquisition and Genome Minimization Evolutionary Trends. Microb. Pathog. 2017, 111, 118–131. [Google Scholar] [CrossRef]
- Kiepś, J.; Dembczyński, R. Current Trends in the Production of Probiotic Formulations. Foods 2022, 11, 2330. [Google Scholar] [CrossRef]
- Coman, M.M.; Cecchini, C.; Verdenelli, M.C.; Silvi, S.; Orpianesi, C.; Cresci, A. Functional Foods as Carriers for SYNBIO®®, a Probiotic Bacteria Combination. Int. J. Food Microbiol. 2012, 157, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Der Poel, A.F.B.v.; Abdollahi, M.R.; Cheng, H.; Colovic, R.; den Hartog, L.A.; Miladinovic, D.; Page, G.; Sijssens, K.; Smillie, J.F.; Thomas, M.; et al. Future Directions of Animal Feed Technology Research to Meet the Challenges of a Changing World. Anim. Feed Sci. Technol. 2020, 270, 114692. [Google Scholar] [CrossRef]
- Amerah, A.M.; Quiles, A.; Medel, P.; Sánchez, J.; Lehtinen, M.J.; Gracia, M.I. Effect of Pelleting Temperature and Probiotic Supplementation on Growth Performance and Immune Function of Broilers Fed Maize/Soy-Based Diets. Anim. Feed Sci. Technol. 2013, 180, 55–63. [Google Scholar] [CrossRef]
- Augustin, M.A.; Sanguansri, L. Challenges and Solutions to Incorporation of Nutraceuticals in Foods. Annu. Rev. Food Sci. Technol. 2015, 6, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.E.; Marco, M.L. Food Formats for Effective Delivery of Probiotics. Annu. Rev. Food Sci. Technol. 2010, 1, 65–85. [Google Scholar] [CrossRef]
- de Souza, E.L.; de Oliveira, K.Á.; de Oliveira, M.E. Influence of Lactic Acid Bacteria Metabolites on Physical and Chemical Food Properties. Curr. Opin. Food Sci. 2023, 49, 100981. [Google Scholar] [CrossRef]
- Meybodi, N.M.; Mortazavian, A.M.; Sohrabvandi, S.; da Cruz, A.G.; Mohammadi, R. Probiotic Supplements and Food Products: Comparison for Different Targets. Appl. Food Biotechnol. 2017, 4, 123–132. [Google Scholar] [CrossRef]
- Marcial-Coba, M.S.; Knøchel, S.; Nielsen, D.S. Low-Moisture Food Matrices as Probiotic Carriers. FEMS Microbiol. Lett. 2019, 366. [Google Scholar] [CrossRef]
- Gibson, G.R.; Probert, H.M.; Van Loo, J.; Rastall, R.A.; Roberfroid, M.B. Dietary Modulation of the Human Colonic Microbiota: Updating the Concept of Prebiotics. Nutr. Res. Rev. 2004, 17, 259–275. [Google Scholar] [CrossRef]
- Gibson, G.R.; Roberfroid, M.B. Dietary Modulation of the Human Colonic Microbiota: Introducing the Concept of Prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef]
- Ochoa, L.; Paniagua Michel, J.d.J.; Olmos-Soto, J. Complex Carbohydrates as a Possible Source of High Energy to Formulate Functional Feeds. Adv. Food Nutr. Res. 2014, 73, 259–288. [Google Scholar] [CrossRef] [PubMed]
- Arena, M.P.; Russo, P.; Capozzi, V.; Rascón, A.; Felis, G.E.; Spano, G.; Fiocco, D. Combinations of Cereal β-Glucans and Probiotics Can Enhance the Anti-Inflammatory Activity on Host Cells by a Synergistic Effect. J. Funct. Foods 2016, 23, 12–23. [Google Scholar] [CrossRef]
- Rivera-Espinoza, Y.; Gallardo-Navarro, Y. Non-Dairy Probiotic Products. Food Microbiol. 2010, 27, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Marquart, L.; Cohen, E.A. Increasing Whole Grain Consumption. Food Sci. Nutr. 2005, 59, 24–32. [Google Scholar]
- Ramos, P.E.; Cerqueira, M.A.; Teixeira, J.A.; Vicente, A.A. Physiological Protection of Probiotic Microcapsules by Coatings. Crit. Rev. Food Sci. Nutr. 2018, 58, 1864–1877. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Toalá, J.E.; Garcia-Varela, R.; Garcia, H.S.; Mata-Haro, V.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Postbiotics: An Evolving Term within the Functional Foods Field. Trends Food Sci. Technol. 2018, 75, 105–114. [Google Scholar] [CrossRef]
- Sabahi, S.; Homayouni Rad, A.; Aghebati-Maleki, L.; Sangtarash, N.; Ozma, M.A.; Karimi, A.; Hosseini, H.; Abbasi, A. Postbiotics as the New Frontier in Food and Pharmaceutical Research. Crit. Rev. Food Sci. Nutr. 2022. online ahead of print. [Google Scholar] [CrossRef]
- de Almada, C.N.; Almada, C.N.; Martinez, R.C.R.; Sant’Ana, A.S. Paraprobiotics: Evidences on Their Ability to Modify Biological Responses, Inactivation Methods and Perspectives on Their Application in Foods. Trends Food Sci. Technol. 2016, 58, 96–114. [Google Scholar] [CrossRef]
- Wegh, C.A.M.; Geerlings, S.Y.; Knol, J.; Roeselers, G.; Belzer, C. Postbiotics and Their Potential Applications in Early Life Nutrition and Beyond. Int. J. Mol. Sci. 2019, 20, 4673. [Google Scholar] [CrossRef]
- Ooi, M.F.; Foo, H.L.; Loh, T.C.; Mohamad, R.; Rahim, R.A.; Ariff, A. A Refined Medium to Enhance the Antimicrobial Activity of Postbiotic Produced by Lactiplantibacillus plantarum RS5. Sci. Rep. 2021, 11, 7617. [Google Scholar] [CrossRef] [PubMed]
- Rad, A.H.; Abbasi, A.; Kafil, H.S.; Ganbarov, K. Potential Pharmaceutical and Food Applications of Postbiotics: A Review. Curr. Pharm. Biotechnol. 2020, 21, 1576–1587. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Duan, Y.; Dong, H.; Zhang, J. Effects of Dietary Lactobacillus plantarum in Different Treatments on Growth Performance and Immune Gene Expression of White Shrimp Litopenaeus vannamei under Normal Condition and Stress of Acute Low Salinity. Fish Shellfish Immunol. 2017, 62, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Loh, T.C.; Choe, D.W.; Foo, H.L.; Sazili, A.Q.; Bejo, M.H. Effects of Feeding Different Postbiotic Metabolite Combinations Produced by Lactobacillus plantarum Strains on Egg Quality and Production Performance, Faecal Parameters and Plasma Cholesterol in Laying Hens. BMC Vet. Res. 2014, 10, 149. [Google Scholar] [CrossRef] [PubMed]
- Kareem, K.Y.; Loh, T.C.; Foo, H.L.; Akit, H.; Samsudin, A.A. Effects of Dietary Postbiotic and Inulin on Growth Performance, IGF1 and GHR MRNA Expression, Faecal Microbiota and Volatile Fatty Acids in Broilers. BMC Vet. Res. 2016, 12, 163. [Google Scholar] [CrossRef]
- Díaz Cano, J.V.; Argente, M.J.; García, M.L. Effect of Postbiotic Based on Lactic Acid Bacteria on Semen Quality and Health of Male Rabbits. Animal 2021, 11, 1007. [Google Scholar] [CrossRef]
- Cuevas-González, P.F.; Liceaga, A.M.; Aguilar-Toalá, J.E. Postbiotics and Paraprobiotics: From Concepts to Applications. Food Res. Int. 2020, 136, 109502. [Google Scholar] [CrossRef]
- Chiu, Y.H.; Chiu, H.P.; Lin, M.Y. Synergistic Effect of Probiotic and Postbiotic on Attenuation of PM2.5-Induced Lung Damage and Allergic Response. J. Food Sci. 2023, 88, 513–522. [Google Scholar] [CrossRef]
- Vinderola, G.; Sanders, M.E.; Salminen, S. The Concept of Postbiotics. Foods 2022, 11, 1077. [Google Scholar] [CrossRef]
- Loh, T.C.; Foo, H.L.; Chang, H.M. Postbiotic Metabolites of Probiotics in Animal Feeding; Springer: Berlin/Heidelberg, Germany, 2021; pp. 179–190. [Google Scholar] [CrossRef]
| Drying Method | Production Cost * | Thermal Stress | Oxidative Stress | Large-Scale Production | ** Final Humidity |
|---|---|---|---|---|---|
| FD | ↑↑ | ↓↓ | ↓↓ | ↓ | ↓↓ |
| SD | ↓ | ↑ | ↑ | ↑↑ | ↓↓ |
| VD | ↓↓ | ↓ | ↓ | ↓ | ↑ |
| FBD | ↓ | ↑ | ↑ | ↑ | ↑ |
| AHD | ↓↓ | ↑↑↑ | ↑↑ | ↑↑ | ↓ |
| Drying Method | Strain | Protectant/Carriers or Feed Matrix | Animal Target | Storage | In vivo Study | Ref. |
|---|---|---|---|---|---|---|
| Freeze-drying | Lgb.agilis, Lgb.salivarius | SM/Suc/Tre | Broilers | 4 °C and RT | No | [95] |
| Lb. acidophilus, Lcb. casei, Lb. lactis, B. bifidum, E. faecium, S. cereviceae. | Tre/FOS Arabic gum SM | Cats | RT | Yes | [72] | |
| Lb. Johnsonii, Lb. mucosae | n.d. | Pigs | n.d. | Yes | [61] | |
| Lcb. casei | SM/Tre/SM/ Phytoglycogen | n.d. | 4 °C 12 days | No | [92] | |
| Lgb. salivarius | SM | Broilers | n.d. | Yes | [57] | |
| Lmb. Fermentum | SM/lactulose | Fish | n.d. | Yes | [68] | |
| Lb. acidophilus | SM/Suc/starch | Calves | n.d. | Yes | [51] | |
| Lpb.plantarum, Lgb. salivarius, P. acidilactici | SM/MD/FOS /lactose | n.d. | 4 °C 60 days | No | [96] | |
| Spray-drying | Lpb. plantarum | Arabic gum/gelatin/ Coconut oil/MD | n.d. | 25 °C | No | [97] |
| Lpb. plantarum, Lgb. salivarius, P. acidilactici | NFSM/MD | n.d | 4 and 30 °C 60 days | No | [98] | |
| Lb. acidophilus, Lcb. casei Lb. lactis, B. bifidum, E. faecium, S. crevisiae | Trehalose/FOS Arabic gum SM | Cats | RT | Yes | [72] | |
| Lpb. plantarum, P. acidilactici | double-coating with alginate and chitosan | Piglets | 6 months Temp.: n.d. | Yes | [60] | |
| Lpb. plantarum | On feed | Fish | 25 °C | No | [99] | |
| Lpb. plantarum Lpb. paraplantarum | Arabic gum/gelatin Coconut oil (SD) | Pig | 4 °C | No | [100] | |
| Air heat-drying | Lcb. casei | SM/Tre/SM/ Phytoglycogenon feed | n.d. | 4 °C 12 days | No | [92] |
| Lpb. plantarum | n.d. | Fish | 26 °C–75% RH | No | [93] | |
| Lb. acidophilus, B. subtilis S. cerevisiae, A. oryzae | Growth in Solid state fermentation | Broilers | n.d. | Yes | [58] | |
| Lb. acidophilus, B. subtilis S. cerevisiae, A. oryzae | Solid state fermentation | Pigs | n.d. | Yes | [63] | |
| Fluid bed-drying | Lcb. brevis | Mixed with feed | Fish | 4 and 20 °C 42 days | No | [90] |
| Lb. lactis | SM/MD/acacia gum MSG | Fish | 4, 30 °C 12 months | No | [94] | |
| Vacuum-drying | Lcb. brevis | Mixed with feed | Fish | 4 and 20 °C 42 days | No | [90] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moretti, A.F.; Brizuela, N.S.; Bravo-Ferrada, B.M.; Tymczyszyn, E.E.; Golowczyc, M.A. Current Applications and Future Trends of Dehydrated Lactic Acid Bacteria for Incorporation in Animal Feed Products. Fermentation 2023, 9, 742. https://doi.org/10.3390/fermentation9080742
Moretti AF, Brizuela NS, Bravo-Ferrada BM, Tymczyszyn EE, Golowczyc MA. Current Applications and Future Trends of Dehydrated Lactic Acid Bacteria for Incorporation in Animal Feed Products. Fermentation. 2023; 9(8):742. https://doi.org/10.3390/fermentation9080742
Chicago/Turabian StyleMoretti, Ana F., Natalia S. Brizuela, Bárbara M. Bravo-Ferrada, Emma E. Tymczyszyn, and Marina A. Golowczyc. 2023. "Current Applications and Future Trends of Dehydrated Lactic Acid Bacteria for Incorporation in Animal Feed Products" Fermentation 9, no. 8: 742. https://doi.org/10.3390/fermentation9080742
APA StyleMoretti, A. F., Brizuela, N. S., Bravo-Ferrada, B. M., Tymczyszyn, E. E., & Golowczyc, M. A. (2023). Current Applications and Future Trends of Dehydrated Lactic Acid Bacteria for Incorporation in Animal Feed Products. Fermentation, 9(8), 742. https://doi.org/10.3390/fermentation9080742







