Abstract
For the first time, this work reports the seasonal influence on the chemical composition and antiradical capacity of Acmella oleracea floral essential oil, produced from a perennial herb of great nutritional and pharmacological importance in the Amazon region. The species was cultivated and the plantation was monitored from May to September 2022 between the rainy and dry seasons. The essential oils were obtained by hydrodistillation, analyzed by gas chromatography coupled with a mass spectrometer, and subjected to the free radical inhibition assay using the DPPH method. The highest oil yield (1.61%) occurred in May (rainy season), and the lowest (0.68%) occurred in September (dry season). Despite the difference in the oil yield between the rainy and dry seasons, no significant correlation with weather conditions (p > 0.05) occurred. During the collection period, the class of sesquiterpene hydrocarbons was predominant (16.35–46.01%). The main constituents of A. oleracea were E-caryophyllene (13.57–25.74%), caryophyllene oxide (0.88–31.72%), 1-pentadecene (5.42–16.58%), germacrene D (0.14–15.17%), and myrcene (1.08–11.99%), and a low concentration of its main bioactive spilanthol (0.66–5.2%) was also observed. The antiradical capacity was considered low, with inhibition of 7.96 to 7.53% of free radicals and a Trolox equivalence of 68.4 to 64.7 mg·ET/g. Although there were some changes in the levels of chemical components in A. oleracea essential oils, the species can be considered an alternative source of pharmacologically active compounds such as E-caryophyllene and caryophyllene oxide, in addition to presenting amounts of other bioactive molecules.
1. Introduction
Asteraceae, formerly known as Compositae, is the largest family of vascular plants, accounting for more than 10% of all angiosperms found today [1]. Asteraceae taxa are as old as dinosaurs; fossil discoveries indicate that the first Asteraceae appeared during the Cretaceous period around 85 million years ago, possibly on the South American continent [2].
Around 25,000 to 35,000 species represent this family, found on all continents, including Antarctica, and in different habitat types such as deserts, prairies, and mountainous regions [2,3]. In Brazil, 2215 species and 328 genera are reported, occurring throughout the Brazilian territory, such as in the Amazon and Cerrado [4].
The Acmella genus occurs in all regions of Brazil, represented by 17 species. Among these, Acmella oleracea (L.) R.K. Jansen (syn Spilanthes oleracea var.; Spilanthes oleracea L.; Spilanthes radicans Schrad. ex DC.), popularly known as “Jambu” and the “toothache plant”, is a perennial small herbaceous plant that has decumbent and erect glabrous branches, its petiolate leaves have shapes that vary from ovate to deltoid, and discoid capitula are characteristic of its inflorescences, which vary from red to golden yellow [5,6].
In traditional medicine, A. oleracea inflorescences are used to treat mouth and throat diseases and as an antiparasitic and antipyretic agent [5,7]. These effects are associated with several bioactive compounds found in the plant, such as isobutylamides and terpenoids, with anti-inflammatory, analgesic, and cytotoxic properties [8,9].
Essential oils (EOs), rich in mono- and sesquiterpenoids, act in the defense and plant communication mechanism. These substances, when extracted, present several biological activities, such as insecticidal, fungicidal, anti-inflammatory, and antitumor effects [10,11,12].
A. oleracea is cultivated in different countries and displays different chemotypes. In Italy, with a meso-temperate climate, E-caryophyllene (20.8%) and β-pinene (17.3%) were the main constituents [11]; in India, limonene (41%) and E-β-ocimene (20.39%) were more abundant [13]; and in southeastern Brazil, the EO obtained from inflorescences showed variation in E-caryophyllene (48.64–43.85%) and germacrene D (20.75–19.86%) [14].
Several factors can alter the chromatographic profile of essential oils, for example, some environmental aspects characteristic of each region, such as solar incidence, rainfall, variations in temperature and atmospheric pressure, and even atmospheric pollutants that influence the production of secondary metabolites in plants [15].
We hypothesized that the essential oil composition shows seasonal variation, which is important with respect to the biological activities and the ethnopharmacological uses of the plant. Therefore, given the medicinal importance of this species and the few studies of floral essential oils, this work aimed to evaluate the influence of Amazonian seasonality on the chemical composition and antiradical capacity of the essential oil from Acmella oleracea inflorescences.
2. Material and Methods
2.1. Plant Cultivation and Botanical Identification
A. oleracea samples were obtained in the Belém metropolitan region, in the municipality of Ananindeua, Pará, Brazil, under the geographic coordinates 1°20′52″ S, 48°23′10″ W, using a conventional A. oleracea cultivation protocol. Seeds of this species were planted in sandy clay soil and arranged in lines with a spacing of approximately 30 cm between each seedling [16]. Cultivation was carried out from May to October 2022. After approximately 40 days of growth, at the flowering stage, the inflorescences were harvested. The cultivation was monitored throughout the different Amazonian seasons (rainy, transition, and dry periods) with three bimonthly collections (May, July, and September) during the studied period. A voucher specimen was submitted to the “Drª Marlene Freitas da Silva” Herbarium at the Universidade do Estado do Pará (UEPA) under registration number MFS009837, and the specimen was registered in the National System for Management of Genetic Heritage and Associated Traditional Knowledge—SISGEN under registration number A984D4E.
2.2. Climate Data Collection
Climatological data on precipitation, temperature, relative humidity, and insolation were monitored in parallel to the species’ cultivation period and accessed on 9 February 2023 (INMET, https://portal.inmet.gov.br/, accessed on 30 April 2024) [17]. Insolation, precipitation, humidity, and temperature data were recorded using the A-201 automatic station located in the city of Belém, State of Pará, Brazil (approximately 11 km in a straight line from the cultivation site of the studied species), equipped with a Vaisala system, model MAWS 301 (Vaisala Corporation, Helsinki, Finland).
2.3. Drying and Processing of Botanical Material
The inflorescence samples were sent to the Natural Products Chemistry Laboratory “LaQuiProN” belonging to the State University of Pará—UEPA and dried at room temperature for seven days. The inflorescences were crushed with a processor mixer and immediately subjected to hydrodistillation [18].
2.4. Essential Oil Extraction
Masses of 100 g of dried inflorescence samples were subjected to hydrodistillation in a modified Clevenger apparatus for 3 h in duplicate, equipped with a refrigeration system for condensation of water at 10–15 °C. The essential oils obtained were centrifuged for 5 min at 3000 rpm and centrifuged again with anhydrous sodium sulfate (Na2SO4) for dehydration, then subsequently stored in amber vials at 5 °C and sent for analysis [19].
2.5. Essential Oil Yield Calculation
The essential oil yields (%) of the plant samples were calculated from moisture-free biomass through the relationship between mass, oil, and moisture, as demonstrated in the equation below:
Residual moisture determination of plant samples was carried out using a Gehaka® moisture tester, model IV 2000 (Gehaka, São Paulo, São Paulo, Brazil), by infrared drying until a constant weight was achieved concomitantly with extraction.
2.6. Chemical Composition Analysis
Gas chromatography coupled with mass spectrometry (GC-MS) and gas chromatography coupled with a flame ionization detector (GC-FID) were used to analyze the composition of A. oleracea essential oils. A Shimadzu Model QP 2010 ultra instrument (Shimadzu, Tokyo, Japan) equipped with an Rtx-5MS fused silica capillary column (30 m, 0.25 mm; 0.25 μm film thickness) as the stationary phase was used (Restek, Bellefonte, PA, USA). Helium gas was adjusted to 1.0 mL/min at 57.5 kPa as the carrier gas. The injection of oil samples into the instrument was of the split type (proportion 1:20), with 1 μL of n-hexane solution (5 μL oil, 500 μL of n-hexane); the injector and interface temperatures were 250 °C; the programmed oven temperature was 60 to 240 °C (3 °C/min), followed by a 10 min isotherm; electron ionization mass spectrometry (EIMS) was run at 70 eV; and the ion source temperature was 200 °C.
The mass spectra were obtained using automatic scanning, with a fragment mass in the 35–400 m/z range. Identification of essential oil components was based on a comparison of the mass spectra and retention indices presented by the samples with those from the commercial libraries FFNSC-2 [20] and Adams [21]. The retention indices of volatile constituents were calculated using the linear equation of Van Den Dool and Kratz [22], using a homologous series of hydrocarbons (C8–C40, Sigma-Aldrich, St. Louis, MO, USA) under the same chromatographic conditions.
GC-FID analysis was performed on a Shimadzu QP-2010 instrument (Shimadzu, Tokyo, Japan) equipped with an FID detector under the same conditions described above, except hydrogen was used as the carrier gas. The percentage composition of the oil sample was calculated from the GC-FID peak areas. Analyses were performed in triplicate.
2.7. Analysis of Antiradical Capacity by DPPH Radical Scavenging
The evaluation of antiradical capacity was carried out using the DPPH• (1,1-diphenyl-2-picrylhydrazyl) free radical scavenging method. Stock solutions of essential oils were prepared at a concentration of 10 mg/mL in ethanol; aliquots of these solutions (50 μL) were added to 1900 μL of DPPH solution in ethanol with an absorbance of 0.65 at 517 nm. A volume of 50 µL of 0.5% (m/m) Tween 20 was used as a solubilizer.
The reaction media were incubated in cuvettes for 120 min. The control was carried out under the same conditions, but with the replacement of the essential oil solution with ethanol. Absorbances were measured at 30 min intervals for 2 h at 517 nm in a UV–vis spectrophotometer (K37-UVVIS, KASV, Pinhais, Paraná, Brazil). The standard antioxidant Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) was used as a positive control at concentrations of 60, 120, and 240 μg/mL under the same reaction conditions and without the presence of essential oil, and the linear regression line equation was [Trolox] = 4.2997. (inhibition), r = 0.9995 [23].
The calculation of the percentage of inhibition of DPPH• radicals (IDPPH) for the samples was calculated according to the following equation:
where AbsA and AbsB are the sample and control absorbances (blank), respectively. The calculation of antiradical capacity equivalent to Trolox (TE) was carried out using the equation below:
where “Ab” and “Aa” are the absorbances of the negative control and the sample, respectively, “a” is the angular coefficient of the curve, and “D” is the dilution factor [24].
2.8. Statistical Analysis
The Pearson correlation coefficients (r) for the relationship between climatic parameters (precipitation, temperature, humidity, and insolation) and the yield and chemical composition of essential oils were calculated using the free Minitab software version 390 (Minitab Inc., State College, PA, USA). The Tukey test was performed using the GraphPad Prism software, version 5.0.
3. Results and Discussion
3.1. Seasonal Conditions and Essential Oil Yield
The average precipitation values ranged from 116.6 mm (July) to 408.1 mm (May), the average temperature ranged from 26.3 °C (May) to 27.5 °C (September), the relative humidity of the air presented a variation from 77.8% (September) to 89% (May), and the average insolation ranged from 141.2 h (May) to 244.3 h (September) (Figure 1).
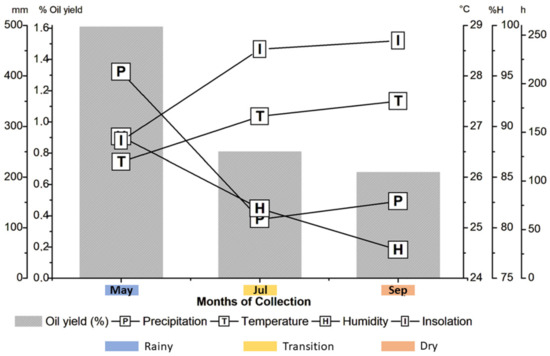
Figure 1.
Correlation of Acmella oleracea essential oil yields with climatic parameters.
According to climatological data, May was the rainy season, September was the dry season, and July was the month of a transition period between the two seasons. The Amazon has a hot and humid climate with only two seasons, the Amazonian winter and Amazonian summer, and with a rainy period (February–May) and a less rainy period (September–November); the other months are considered transition periods [25].
The inflorescences from the dry month (Sep) presented the lowest oil yield (0.68%), and those from the rainy month (May) presented the highest (1.61%).
According to Pearson’s correlation analysis (r), precipitation (r = 0.94) and humidity (r = 0.98) showed a strong and positive correlation with the yield but were not statistically significant factors (p > 0.05). Regarding other climatic conditions, the oil yield showed a strong and negative correlation with temperature (r = −0.99) and insolation (r = −0.99) but no statistical significance. Overall, despite the differences in oil production between the dry and rainy seasons, essential oil yields did not show statistically significant differences (p > 0.05) with any of the climatic conditions.
3.2. Seasonal Conditions and Chemical Composition
In total, 56 chemical constituents were identified in the EOs of mature inflorescences of A. oleracea during the collection period, representing an average of 82.44% of the total chemical composition of the oils (chromatograms showed in Appendix A). The class of sesquiterpene hydrocarbons was predominant (16.35–46.01%), followed by other classes of chemical constituents (11.36–29.51%). Monoterpene hydrocarbons, monoterpenes, and oxygenated sesquiterpenes showed low average amounts (<13.4%) (Table 1).

Table 1.
The chemical composition of essential oils from inflorescences of Acmella oleracea analyzed in the rainy (MAY), transition (JUL), and dry (SEP) seasons.
The sesquiterpene hydrocarbon E-caryophyllene was the main constituent of the oils, with a content of 13.57% in May, rising to 25.74% in July. Four other chemical components also presented relevant concentrations (>10%), such as caryophyllene oxide, which ranged from 0.88% (September) to 31.72% (May), 1-pentadecene from 5.42% (September) to 16.58% (May), germacrene D from 0.14% (May) to 15.17% (September), and myrcene from 1.08% (July) to 11.99% (September). Despite its minor amounts, spilanthol also stood out, which varied from 0.66% (May) to 5.2% (July) (Figure 2).
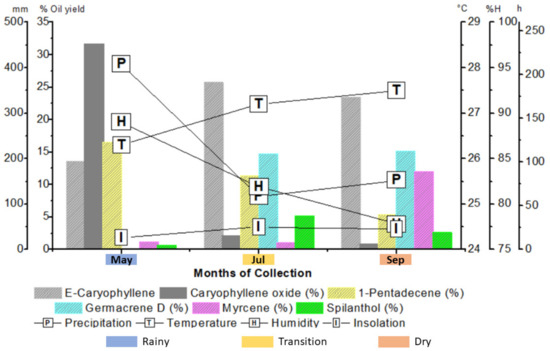
Figure 2.
Relationship between seasonal parameters of precipitation (P), temperature (T), humidity (H), and insolation (I) and Acmella oleracea main chemical components.
Some Asteraceae species have demonstrated variations in their chemical compositions in seasonal studies; for example, Achillea fragrantissima Sch.Bip. presents different chemical profiles in winter (santolina alcohol 5.31%, camphor 4.3%, and cedrene 9.01%) and in summer (α-cubebene 17.1%, spathulenol 1.54%, and globulol 5.2%) [26].
The average temperature and relative humidity did not correlate significantly with the main constituents identified in A. oleracea essential oil (Table 2), indicating no significant link between these two environmental conditions and the chemical component amounts.

Table 2.
Correlation of the main components and seasonal parameters.
Pearson’s correlation coefficient (r) demonstrated a strong, negative, and statistically significant relationship (p < 0.05) between E-caryophyllene and precipitation (r = −0.99); therefore, the higher the rainfall rate, the lower the abundance of E-caryophyllene; in relation to insolation, it was possible to identify an insignificant correlation (p > 0.05), indicating that there is no interference of this seasonal variable in the content of this constituent.
E-Caryophyllene is a molecule with a complex bicyclic structure with a cyclobutane ring and a cyclononane ring, which presents the “E” configuration (Figure 3), as reported in some of the few studies on the chemical composition of A. oleracea essential oils. In southeastern Brazil, there are reports of significant levels of this constituent in inflorescences (43.85–48.64%) and leaves (33.61–59.29%) [14].
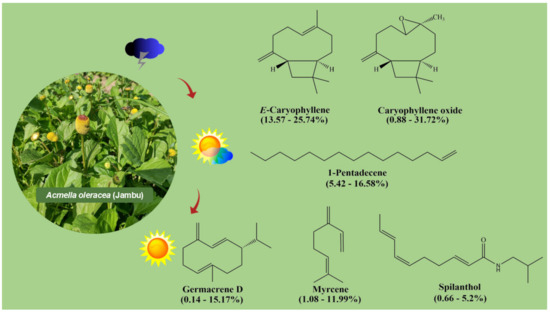
Figure 3.
Structures of the main components identified in essential oils from Acmella oleracea inflorescences.
Classified as a hydrocarbon sesquiterpene, E-caryophyllene has an active role in plant species as it helps plants defend against pathogenic bacteria [27]. In the human biological system, E-caryophyllene is part of the group of ligands that interact with type 2 cannabinoid receptors associated with the maintenance of several biological processes related to the immune system [28]. This constituent can induce immunomodulatory responses, aiding in anti-inflammatory effects and altering inflammatory cytokines, with a reduction in pro-inflammatory cytokines and an increase in anti-inflammatory cytokines [28], meaning it can be considered an important agent against inflammation.
On the other hand, caryophyllene oxide showed a strong, statistically significant (p < 0.05), and negative relationship with insolation (r = −0.99), demonstrating that the greater the exposure of A. oleracea to sunshine, the lower the yield of caryophyllene oxide in its essential oil, while the other climatic parameters of precipitation and humidity did not demonstrate a significant correlation with this constituent (p > 0.05).
Caryophyllene oxide is an oxygenated sesquiterpenoid that presents an epoxide group external to the caryophyllane ring. It was reported to have a content of 0.7% in the entire plant in studies carried out in India [29], while in Italy, this compound presented an abundance of 10%, being one of the main constituents identified A. oleracea [11].
This sesquiterpenoid has demonstrated pharmacological potential, as it presents several biological activities, such as the induction of apoptosis in prostate cells with cancerous mutation. Its action is linked to depolarization of the mitochondrial membrane and release of the pro-apoptotic protein Bax of Bcl-xL proteins responsible for regulating homeostasis between apoptosis and cell proliferation, in addition to presenting low toxicity against healthy cells, and it can be considered a safe antiproliferative agent [30].
During the rainy period, there was a marked presence of caryophyllene oxide and a considerably lower content of E-caryophyllene, while with the arrival of the transition and dry periods, there was an inversion in these abundances.
Precipitation appears to be linked to the oxidation process of E-caryophyllene and the formation of its oxidized product, since with an increase in the caryophyllene oxide content, there is a decrease in the E-caryophyllene content. These findings may be explained by the biosynthetic pathway. E-caryophyllene and caryophyllene oxide belong to the same pathway through the caryophyllyl cation. Caryophyllene oxide is a metabolic product of E-caryophyllene oxidation, meaning that the increase in the caryophyllene oxide content implies a decreasing E-caryophyllene content [31]. These variations in relation to the levels of these two constituents influenced by the rainy and dry periods in the Amazon have already been demonstrated in Myrtaceae specimens [31]. Moreover, E-caryophyllene and caryophyllene oxide have a robust wooden odor and are used as cosmetic and food additives. These two natural compounds are approved as flavorings by the Food and Drug Administration (FDA) and European Food Safety Authority (EFSA) and used in a mixture, as they often occur in plants. In medical practice, the application of the mixture of E-caryophyllene and caryophyllene oxide in combination with the classical anticancer drugs could bring significant benefits, potentializing the efficacy of chemotherapeutics, eliciting a supplementary antineoplastic effect, and reducing refractory cancer pain [31].
Germacrene D is another constituent that obtained a strong, significant (p < 0.05) but positive correlation with insolation (r = 0.99); the other parameters did not influence the content of this component (p > 0.05). A report of an A. oleracea specimen from India showed a concentration of 10.8%, and in Italy, there were variations from 11.8 to 15.2% of this constituent [8,32].
Germacrene D is a sesquiterpene with ecological importance, as it is produced by Asteraceae species to modulate the defense against aphid attack through the attraction of predatory ladybugs, in addition to being one of the main attractants for sensitive pollinators such as moths [33].
The 1-pentadecene, myrcene, and spilanthol levels did not show a statistically significant correlation with environmental conditions (p > 0.05). This is important since 1-pentadecene has been described for its insecticidal capacity, myrcene for its anticancer and insecticidal properties, and spilanthol for its analgesic, anti-inflammatory, insecticidal, and anesthetic actions [9,34,35,36,37].
Although unusual, the presence of 1-pentadecene in essential oils is not without precedence; 1-pentadecene (25.2%) and 2-tridecanone (30.1%) were the major constituents of Acmella radicans (Asteraceae) essential oil [38]. Thus, 1-pentadecene is biosynthesized via the acetate pathway (fatty acid biosynthesis) followed by modification [39]. Moreover, floral essential oils often contain long-chain hydrocarbons like pentacosane (40.17%, Himantoglossum robertianum) and tricosane (27.76%, Ophrys sphegodes) [40].
Despite variations in content between populations, A. oleracea appears to have typical chemical markers of its essential oil, with occurrences of the same constituents in studies in different locations. The biosynthesis and accumulation of secondary metabolites in a plant are affected by extrinsic factors such as light, temperature, and soil conditions, as well as intrinsic factors related to genetic aspects such as gene expression in a given species [41].
As previously reported, plants depend on volatile compounds for survival and environmental adaptation under different climatic conditions and their abiotic stresses. Inflorescences release volatile compounds to attract pollinators, and they can also produce and release chemical constituents of essential oils to resist pathogens and to repel herbivores and attract their natural adversaries. As a result, it is possible to have a variety of physiological and metabolic relationships that can be designed, requiring individual innovative studies so that they can be understood, described, and presented [42].
3.3. Seasonal Conditions and Antiradical Capacity
Essential oils from A. oleracea inflorescences showed low antiradical capacity against DPPH. The percentage of radical inhibition ranged from 7.96% ± 0.1 to 7.53% ± 0.3, and the Trolox equivalence from 68.4 mg·TE/g to 64.7 mg·TE/g. Using the Tukey test, the periods studied did not show a statistically significant difference (p > 0.05) (Table 3).

Table 3.
Antiradical capacity of Acmella oleracea essential oils.
No studies reporting the antiradical capacity of A. oleracea essential oils were found. However, the hydroethanolic extract of the leaves of this species also showed weak antiradical capacity against DPPH (IC50 of 130.0 µg/mL) [43]. Likewise, extracts of this plant obtained with ethyl acetate and methanol showed moderate antiradical capacity against these radicals (IC50 of 216 and 223 ug/mL, respectively) [44], and there is a report of the inability of isolated E-caryophyllene to eliminate DPPH radicals [45].
The potential action of a given antiradical compound against the DPPH radical is related to the availability of hydroxyl groups and the structural conformation of the molecule tested. Small molecules appear to have greater access to the radical’s unpaired electron and may have greater antiradical capacity when compared to larger molecules [46,47,48].
It was observed during the studied period that A. oleracea inflorescences presented significant levels of molecules with larger structures, such as sesquiterpene hydrocarbons (16.35–46.01%), and low concentrations of smaller molecules with available hydroxyl groups, such as oxygenated monoterpenes (0–0.56%), which could justify the low antioxidant capacity of this species in the assay against DPPH. Moreover, the absence of phenolic compounds, rather than molecular size, is the most important factor. Furthermore, myrcene, E-caryophyllene, and caryophyllene oxide are not effective antioxidants [45,49,50,51].
This study, which identified the terpenes known for their biological activities, has significant implications. The DPPH assay conclusively demonstrated the inefficiency of A. oleracea against this type of radical despite the changes in the chemical composition of the oil, which had a predominance of caryophyllene compounds. This finding shows that a high concentration of E-caryophyllene or caryophyllene oxide is not sufficient to modify the antiradical results.
4. Conclusions
The results presented in this study demonstrate that the chemical composition of Acmella oleracea essential oil is influenced by climatic parameters, precipitation, and insolation. This plant can be considered an alternative source of sesquiterpenes, such as E-caryophyllene, in different seasons and a producer of caryophyllene oxide in the rainy season.
Although the species has a low antiradical potential and no correlation with seasonal data, it was possible to determine how the aspects of the different Amazonian seasons can directly influence the chemical composition of A. oleracea essential oil. This demonstrates the versatility of this small herb in producing different secondary metabolites with potential application prospects, mainly in the medical sector.
Author Contributions
Conceptualization, P.L.B.F.; formal analysis, L.B.J., J.A.C.d.A., J.K.R.d.S. and R.H.V.M.; writing—original draft preparation, L.B.J.; writing—review and editing, P.L.B.F. and W.N.S. project administration, P.L.B.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors are grateful to the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for providing scholarships to L.B.J., and Universidade do Estado do Pará (UEPA) for providing postdoctoral scholarships to P.L.B.F.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A
Figure A1.
Chromatograms of Acmella oleracea collected in this seasonal study.
References
- Kostić, A.Ž.; Janaćković, P.; Kolašinac, S.M.; Dajić Stevanović, Z.P. Balkans’ Asteraceae species as a source of biologically active compounds for the parmaceutical and food industry. Chem. Biodivers. 2020, 17, e2000097. [Google Scholar] [CrossRef] [PubMed]
- Mandel, J.R.; Dikow, R.B.; Siniscalchi, C.M.; Thapa, R.; Watson, L.E.; Funk, V.A. A fully resolved backbone phylogeny reveals numerous dispersals and explosive diversifications throughout the history of Asteraceae. Proc. Natl. Acad. Sci. USA 2019, 116, 14083–14088. [Google Scholar] [CrossRef]
- Smith, R.I.L.; Richardson, M. Fuegian plants in antarctica: Natural or anthropogenically assisted immigrants? Biol. Invasions 2011, 13, 1–5. [Google Scholar] [CrossRef]
- Flora e Funga do Brasil. Asteraceae in Flora e Funga do Brasil. Available online: https://floradobrasil.jbrj.gov.br/FB55 (accessed on 24 July 2024).
- Rondanelli, M.; Fossari, F.; Vecchio, V.; Braschi, V.; Riva, A.; Allegrini, P.; Petrangolini, G.; Iannello, G.; Faliva, M.A.; Peroni, G.; et al. Acmella oleracea for pain management. Fitoterapia 2020, 140, 104419. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, J. Acmella in Flora e Funga do Brasil. Jardim Botânico do Rio de Janeiro. Available online: https://floradobrasil.jbrj.gov.br/FB15913 (accessed on 28 June 2024).
- Khan, M.N.N.A.; Abidem, M. An overview on Spilanthues acmella. Int. J. Adv. Res. 2020, 8, 322–331. [Google Scholar] [CrossRef]
- Spinozzi, E.; Pavela, R.; Bonacucina, G.; Perinelli, D.R.; Cespi, M.; Petrelli, R.; Cappellacci, L.; Fiorini, D.; Scortichini, S.; Garzoli, S.; et al. Spilanthol-rich essential oil obtained by microwave-assisted extraction from Acmella oleracea (L.) R.K. Jansen and its nanoemulsion: Insecticidal, cytotoxic and anti-inflammatory activities. Ind. Crops Prod. 2021, 172, 114027. [Google Scholar] [CrossRef]
- Stein, R.; Berger, M.; Santana de Cecco, B.; Mallmann, L.P.; Terraciano, P.B.; Driemeier, D.; Rodrigues, E.; Beys-da-Silva, W.O.; Konrath, E.L. Chymase inhibition: A key factor in the anti-inflammatory activity of ethanolic extracts and spilanthol isolated from Acmella oleracea. J. Ethnopharmacol. 2021, 270, 113610. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Drenaggi, E.; Maggi, F. Insecticidal efficacy of the essential oil of jambú (Acmella oleracea (L.) R.K. Jansen) cultivated in central Italy against filariasis mosquito vectors, houseflies and moth pests. J. Ethnopharmacol. 2019, 229, 272–279. [Google Scholar] [CrossRef]
- do Nascimento, K.F.; Moreira, F.M.F.; Alencar Santos, J.; Kassuya, C.A.L.; Croda, J.H.R.; Cardoso, C.A.L.; Vieira, M.d.C.; Góis Ruiz, A.L.T.; Ann Foglio, M.; de Carvalho, J.E.; et al. Antioxidant, anti-inflammatory, antiproliferative and antimycobacterial activities of the essential oil of Psidium guineense Sw. and spathulenol. J. Ethnopharmacol. 2018, 210, 351–358. [Google Scholar] [CrossRef]
- Padhan, D.; Pattnaik, S.; Behera, A. Growth-arresting activity of Acmella essential oil and its isolated component d-limonene (1, 8 p-mentha diene) against Trichophyton rubrum (microbial type culture collection 296). Pharmacogn. Mag. 2017, 13, 555. [Google Scholar] [CrossRef]
- Borges, L.d.S.; Vieira, M.A.R.; Marques, M.O.M.; Vianello, F.; Lima, G.P.P. Influence of organic and mineral soil fertilization on essential oil of Spilanthes oleracea cv. Jambuarana. Am. J. Plant Physiol. 2012, 7, 135–142. [Google Scholar] [CrossRef]
- Gobbo-Neto, L.; Lopes, N.P. Plantas medicinais: Fatores de influência no conteúdo de metabólitos secundários. Quim. Nova 2007, 30, 374–381. [Google Scholar] [CrossRef]
- Silva, S.G.; Figueiredo, P.L.B.; Nascimento, L.D.; da Costa, W.A.; Maia, J.G.S.; Andrade, E.H.A. Planting and seasonal and circadian evaluation of a thymol-type oil from Lippia thymoides Mart. & Schauer. Chem. Cent. J. 2018, 12, 113. [Google Scholar] [CrossRef] [PubMed]
- INMET, Instituto Nacional de Metereologia, Brazilian Government. Available online: http://www.inmet.gov.br/portal (accessed on 23 July 2022).
- Santos, P.V.L.; da Cruz, E.d.N.S.; Barroso, A.d.S.; Mourão, R.H.V.; Setzer, W.N.; da Silva, J.K.; do Nascimento, W.M.O.; da Costa, J.S.; Figueiredo, P.L.B. Chemometric analysis of the seasonal variation in the essential oil composition of Psidium acutangulum growing in the Brazilian Amazon. Biochem. Syst. Ecol. 2022, 105, 104528. [Google Scholar] [CrossRef]
- Maia, J.G.S.; Andrade, E.H.A. Database of the Amazon aromatic plants and their essential oils. Quim. Nova 2009, 32, 595–622. [Google Scholar] [CrossRef]
- Mondello, L. FFNSC 2: Flavors and Fragrances of Natural and Synthetic Compounds, Mass Spectral Database; John Wiley & Sons Inc: Hoboken, NJ, USA, 2011; ISBN 1118145836. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- van Den Dool, H.; Kratz, P. A generalization of the retention index system including linear temperature programmed gas—Liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Barros, L.d.S.P.; Santos da Cruz, E.d.N.; de Araújo Guimarães, B.; Setzer, W.N.; Veras Mourão, R.H.; do Rosário da Silva, J.K.; Silva da Costa, J.; Baia Figueiredo, P.L. Chemometric analysis of the seasonal variation in the essential oil composition and antioxidant activity of a new geraniol chemotype of Lippia alba (Mill.) N.E.Br. Ex Britton & P. Wilson from the Brazilian Amazon. Biochem. Syst. Ecol. 2022, 105, 104503. [Google Scholar] [CrossRef]
- Figueiredo, P.L.B.; Pinto, L.C.; da Costa, J.S.; da Silva, A.R.C.; Mourão, R.H.V.; Montenegro, R.C.; da Silva, J.K.R.; Maia, J.G.S. Composition, antioxidant capacity and cytotoxic activity of Eugenia uniflora L. chemotype-oils from the Amazon. J. Ethnopharmacol. 2019, 232, 30–38. [Google Scholar] [CrossRef]
- Loureiro, R.d.S.; Saraiva, J.M.; Saraiva, I.; Senna, R.C.; Fredó, A.S. Estudo dos eventos extremos de precipitação ocorridos em 2009 no Estado do Pará. Rev. Bras. Meteorol. 2014, 29, 83–94. [Google Scholar] [CrossRef]
- Elsharkawy, E.; Nahed, N.E.-D.M. Effect of seasonal variations on the yield of essential oil and antioxidant of Achillea fragrantissima (Forssk) Sch. Bip. African J. Biotechnol. 2018, 17, 892–897. [Google Scholar] [CrossRef][Green Version]
- Huang, M.; Sanchez-Moreiras, A.M.; Abel, C.; Sohrabi, R.; Lee, S.; Gershenzon, J.; Tholl, D. The Major volatile organic compound emitted from Arabidopsis thaliana flowers, the sesquiterpene (E)-β-caryophyllene, is a defense against a bacterial pathogen. New Phytol. 2012, 193, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, V.B.; Askari, V.R. A mechanistic review on immunomodulatory effects of selective type two cannabinoid receptor β-caryophyllene. BioFactors 2022, 48, 857–882. [Google Scholar] [CrossRef] [PubMed]
- Jirovetz, L.; Buchbauer, G.; Wobus, A.; Shafi, M.P.; Abraham, G.T. Essential oil analysis of Spilanthes acmella Murr. fresh plants from southern India. J. Essent. Oil Res. 2005, 17, 429–431. [Google Scholar] [CrossRef]
- Delgado, C.; Mendez-Callejas, G.; Celis, C. Caryophyllene oxide, the active compound isolated from leaves of Hymenaea courbaril L. (Fabaceae) with antiproliferative and apoptotic effects on PC-3 androgen-independent prostate cancer cell line. Molecules 2021, 26, 6142. [Google Scholar] [CrossRef]
- da Cruz, E.d.N.S.; Peixoto, L.d.S.; da Costa, J.S.; Mourão, R.H.V.; do Nascimento, W.M.O.; Maia, J.G.S.; Setzer, W.N.; da Silva, J.K.; Figueiredo, P.L.B. Seasonal variability of a caryophyllane chemotype essential oil of Eugenia patrisii Vahl occurring in the Brazilian Amazon. Molecules 2022, 27, 2417. [Google Scholar] [CrossRef]
- Baruah, R.N.; Leclercq, P.A. Characterization of the essential oil from flower heads of Spilanthes acmella. J. Essent. Oil Res. 1993, 5, 693–695. [Google Scholar] [CrossRef]
- Li, J.; Hu, H.; Chen, Y.; Xie, J.; Li, J.; Zeng, T.; Wang, M.; Luo, J.; Zheng, R.; Jongsma, M.A.; et al. Tissue specificity of (E)-β-farnesene and germacrene D accumulation in pyrethrum flowers. Phytochemistry 2021, 187, 112768. [Google Scholar] [CrossRef]
- Đukić, N.; Andrić, G.; Glinwood, R.; Ninkovic, V.; Andjelković, B.; Radonjić, A. The Effect of 1-pentadecene on Tribolium castaneum behaviour: Repellent or attractant? Pest Manag. Sci. 2021, 77, 4034–4039. [Google Scholar] [CrossRef]
- Bai, X.; Tang, J. Myrcene exhibits antitumor activity against lung cancer cells by inducing oxidative stress and apoptosis mechanisms. Nat. Prod. Commun. 2020, 15, 1934578X2096118. [Google Scholar] [CrossRef]
- Kadir, H.A.; Zakaria, M.B.; Kechil, A.A.; Azirun, M.D.S. Toxicity and electrophysiological effects of Spilanthes acmella Murr. extracts on Periplaneta americana L. Pestic. Sci. 1989, 25, 329–335. [Google Scholar] [CrossRef]
- Sun, J.S.; Feng, Y.; Wang, Y.; Li, J.; Zou, K.; Liu, H.; Hu, Y.; Xue, Y.; Yang, L.; Du, S.; et al. α-Pinene, caryophyllene and β-myrcene from Peucedanum terebinthaceum essential oil: Insecticidal and repellent effects on three stored-product insects. Rec. Nat. Prod. 2020, 14, 177–189. [Google Scholar] [CrossRef]
- Jirovetz, L.; Buchbauer, G.; Abraham, G.T.; Shafi, M.P. Chemical composition and olfactoric characterization of Acmella radicans (Jacq.) R.K. Jansen var. radicans from southern India. Flavour Fragr. J. 2006, 21, 88–91. [Google Scholar] [CrossRef]
- Ney, P.; Boland, W. Biosynthesis of 1-alkenes in higher plants. Eur. J. Biochem. 1987, 162, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Robustelli della Cuna, F.S.; Cortis, P.; Esposito, F.; De Agostini, A.; Sottani, C.; Sanna, C. Chemical composition of essential oil from four sympatric orchids in NW-Italy. Plants 2022, 11, 826. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef]
- Charoimek, N.; Phusuwan, S.; Petcharak, C.; Huanhong, K.; Prasad, S.K.; Junmahasathien, T.; Khemacheewakul, J.; Sommano, S.R.; Sunanta, P. Do abiotic stresses affect the aroma of damask roses? Plants 2023, 12, 3428. [Google Scholar] [CrossRef]
- de Araújo, I.F.; de Araújo, P.H.F.; Ferreira, R.M.A.; Sena, I.D.S.; Lima, A.L.; Carvalho, J.C.T.; Ferreira, I.M.; Souto, R.N.P. Larvicidal effect of hydroethanolic extract from the leaves of Acmella oleracea L. R. K. Jansen in Aedes aegypti and Culex quinquefasciatus. South Afr. J. Bot. 2018, 117, 134–140. [Google Scholar] [CrossRef]
- Wongsawatkul, O.; Prachayasittikul, S.; Isarankura-Na-Ayudhya, C.; Satayavivad, J.; Ruchirawat, S.; Prachayasittikul, V. Vasorelaxant and antioxidant activities of Spilanthes acmella Murr. Int. J. Mol. Sci. 2008, 9, 2724–2744. [Google Scholar] [CrossRef]
- Calleja, M.A.; Vieites, J.M.; Montero-Meterdez, T.; Torres, M.I.; Faus, M.J.; Gil, A.; Suárez, A. The Antioxidant effect of β-caryophyllene protects rat liver from carbon tetrachloride-induced fibrosis by inhibiting hepatic stellate cell activation. Br. J. Nutr. 2013, 109, 394–401. [Google Scholar] [CrossRef]
- Alves, C.Q.; David, J.M.; David, J.P.; Bahia, M.V.; Aguiar, R.M. Métodos para determinação de atividade antioxidante in vitro em substratos orgânicos. Quim. Nova 2010, 33, 2202–2210. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Pastor, J.; García, M.; Steinbauer, S.; Setzer, W.N.; Scull, R.; Gille, L.; Monzote, L. Combinations of ascaridole, carvacrol, and caryophyllene oxide against Leishmania. Acta Trop. 2015, 145, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Xanthis, V.; Fitsiou, E.; Voulgaridou, G.-P.; Bogadakis, A.; Chlichlia, K.; Galanis, A.; Pappa, A. Antioxidant and cytoprotective potential of the essential oil Pistacia lentiscus var. chia and its major components myrcene and α-pinene. Antioxidants 2021, 10, 127. [Google Scholar] [CrossRef] [PubMed]
- Ruberto, G.; Baratta, M.T. Antioxidant activity of selected essential oil components in two lipid model systems. Food Chem. 2000, 69, 167–174. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).