Abstract
The impact of dietary lipid sources on nutrient metabolism and reproductive development is a critical focus in aquaculture broodstock nutrition. Previous studies have demonstrated that fish oil supplementation modulates the expression of genes involved in steroid hormone synthesis, glucose, and lipid metabolism promoting ovarian development in female Scatophagus argus (spotted scat). However, the effects of fish oil on hepatic function at the protein level remain poorly characterized. In this study, female S. argus were fed diets containing 8% fish oil (FO, experimental group) or 8% soybean oil (SO, control group) for 60 days. Comparative proteomic analysis of liver tissue identified significant differential protein expression between groups. The FO group exhibited upregulation of lipid metabolism-related proteins, including COMM domain-containing protein 1 (Commd1), tetraspanin 8 (Tspan8), myoglobin (Mb), transmembrane protein 41B (Tmem41b), stromal cell-derived factor 2-like protein 1 (Sdf2l1), and peroxisomal biogenesis factor 5 (Pex5). Additionally, glucose metabolism-associated proteins, such as Sdf2l1 and non-POU domain-containing octamer-binding protein (Nono), were elevated in the FO group. Moreover, proteins linked to inflammation and antioxidant responses, including G protein-coupled receptor 108 (Gpr108), protein tyrosine phosphatase non-receptor type 2 (Ptpn2), Pex5, p120 catenin (Ctnnd1), tripartite motif-containing protein 16 (Trim16), and aquaporin 11 (Aqp11), were elevated in the FO group, while proteins involved in oxidative stress, such as reactive oxygen species modulator 1 (Romo1), cathepsin A (Ctsa), and Cullin 4A (Cul4a), were downregulated. These proteomic findings align with prior transcriptomic data, indicating that dietary fish oil enhances hepatic lipid metabolism, mitigates oxidative stress, and strengthens antioxidant capacity. Furthermore, these hepatic adaptations may synergistically support ovarian maturation in S. argus. This study provides novel proteomic-level evidence supporting the role of fish oil in modulating hepatic lipid and energy metabolism, thereby elucidating the role of fish oil in optimizing hepatic energy metabolism and redox homeostasis to influence reproductive processes, advancing our understanding of n-3 long-chain polyunsaturated fatty acids (n-3 LC-PUFA) in teleost liver physiology.
Key Contribution:
This study provides proteomic evidence that fish oil supplementation modulates hepatic lipid metabolism and enhances antioxidant capacity in Scatophagus argus.
1. Introduction
The advancement of compound aquafeeds necessitates enhanced fundamental investigations into nutritional metabolism. Research priorities should emphasize (1) the strategic supplementation of functional lipid derivatives and (2) targeted modulation of core metabolic pathways governing broodstock energy homeostasis, enabling the holistic refinement of aquafeed nutrient architecture [1,2]. Specialized commercial diets for marine fish broodstock are rarely produced because of the relatively small quantities required. For most marine fish species, the broodstock diets are typically formulated based on the nutritional requirements of the diets used for the farmed fish reared for meat, where growth rate is the primary consideration. Pellet size and fish meal content are also typically increased for marine fish broodstock. In practice, hatcheries also improve broodstock nutrition by supplementing fresh marine by-products in combination with commercial feeds [3]. Common natural feeds include squid, cuttlefish, mussels, krill, trash fish, and small crustaceans. However, these unprocessed marine products often carry the risks of disease transmission, including parasites, bacteria, and viruses [4]. The experimental organism (Scatophagus argus), a euryhaline teleost, exhibits distinct ecological adaptations and osmoregulatory mechanisms compared to marine species [5], necessitating species-specific physiological considerations in aquaculture research. Nutritional enhancement for the broodstock is crucial, but challenging, and more research related to nutritional physiology is required to optimize dietary strategies. Both mammals and fish research have identified numerous nutrient-sensing genes and proteins in the ovaries, liver, and blood, whose expression is influenced by dietary nutrition factors [6,7,8,9]. These elements form a regulatory network linking nutritional status to reproductive capacity, thereby influencing ovarian development.
Fish oil exerts significant influence on ovarian development by providing essential nutrients, enhancing ovarian function, and regulating hormone levels, including estrogen and progestin [10]. Contributions include improved ovarian blood circulation and the promotion of follicular development [10,11,12,13]. Indirect support for ovarian health is achieved through the optimization of key organs such as the liver, intestines, and pituitary gland [14].
The relationship between fish oil and vitellogenin (Vtg) is particularly significant. The n-3 long-chain polyunsaturated fatty acids (PUFAs) found in fish oil have been shown to promote Vtg synthesis while concurrently modulating the endocrine system to elevate hormone levels, thereby indirectly enhancing Vtg production [15]. As a key component of egg yolk, Vtg provides essential nutritional and structural support to oocytes, playing an indispensable role in ovarian development. By directly stimulating Vtg synthesis and indirectly regulating endocrine function and optimizing organ performance, fish oil exerts a positive and multifaceted influence on ovarian development [15]. A study further revealed that dietary plant oils modulate steroidogenesis and upregulate key reproductive genes (cyp19a1a, hsd3b) in Ompok bimaculatus, leading to improved broodstock performance through enhanced egg production and fertilization rates [16]. These findings demonstrate the capacity of plant-based lipids to improve reproductive outcomes in aquaculture species. Amid rising global aquaculture demands and ecological pressures from overfishing, reducing reliance on fish oil has become imperative. Plant oils exhibit notable cost-effectiveness compared to marine-derived lipids, making their strategic partial substitution in aquafeeds critically significant for advancing sustainable aquaculture practices [2].
The spotted scat (S. argus), belonging to the family Scatophagidae of the order Perciformes, is a commercially valuable fish species in South and Southeast Asia, including China. It inhabits the South China Sea, Taiwan Strait, and East China Sea. Renowned for its high protein content, delicate texture, and unique flavor, S. argus enjoys a strong market demand. Its vibrant coloration and adaptability also make it popular in the ornamental fish trade. Recent nutritional studies demonstrate that diets containing 6% fish oil promote ovarian development in S. argus [17]. Research on its reproductive endocrinology [18,19,20], sex determination and differentiation [21,22], genetic breeding [23], and environmental adaptations [5,17] is also emerging. Ensuring the health of female broodstock by providing high-quality nutrition to promote ovarian development is a key goal in S. argus aquaculture. Our previous work demonstrated that fish oil-enriched diets significantly promote ovarian development in S. argus. A comparative transcriptomic analysis of S. argus liver tissue revealed differential effects between fish oil and soybean oil dietary regimens [18]. Fish oil supplementation enhanced hepatic lipid metabolic efficiency through the coordinated regulation of lipid homeostasis genes (fasn, acox1, apob) involved in synthesis, catabolism, and transport processes. Simultaneously, it upregulated mitochondrial bioenergetic regulators (ppara, cpt1a, ucp2) governing energy transduction pathways, thereby optimizing hepatic energy allocation. Notably, fish oil demonstrated pleiotropic regulatory effects on reproductive endocrine genes (star, cyp19a1, vtg) associated with steroidogenesis and oogenesis [18]. In this study, we applied proteomics to investigate dietary fish oil influences S. argus. Our findings aim to advance the understanding of animal nutrition and support the development of sustainable marine aquaculture practices.
2. Materials and Methods
2.1. Animals
The experimental subjects were two-year-old female S. argus purchased from Hengda Aquaculture Base in Zhuhai, Guangdong Province, China. The experimental cohort exhibited an initial mean body weight of 242.83 g (±16.10 SD) with corresponding total length measurements of 19.48 cm (±0.36 SD). S. argus were transported to the Donghaidao Experimental Base of Guangdong Ocean University, where S. argus was acclimated for two weeks before the feeding trial commenced. The study protocol was approved by the Ethics Committee of Guangdong Ocean University, and all animal procedures were conducted following relevant Chinese guidelines for laboratory animal use.
2.2. Experimental Design
Experimental fishes were reared in an open-air concrete pond (12 m × 5 m × 2 m) with a salinity of 8‰ and a water depth of approximately 1.6 m. Water was exchanged by 10–20% every 3 to 7 days, depending on water quality. An aerator was used continuously, with additional aeration from 10:00 pm to 8:00 am and for one hour before and after feeding to ensure adequate dissolved oxygen levels. Water quality was further stabilized through the regular addition of lactic acid bacteria and vitamin C to improve fish immunity. This study comprised two groups, a fish oil (FO, experimental group) group and a soybean oil (SO, control group) group, each housed in separate 15 m × 5 m × 2 m net cages set within the main pond. After the acclimation period, 100 female were randomly assigned to these groups (50 fish per group). The experimental design comprised duplicate treatment groups, each stocked with 25 broodfish in dedicated net cages (5 m × 3.5 m × 1.8 m). Comparative lipid compositions of the dietary oil formulations (with fatty acid profiles analyzed by Tongwei Company, China) are detailed in Table 1. The FO group received a diet containing 8% fish oil, and the SO group received a diet with 8% soybean oil, with diet formulations provided in Table 2. The feeding regimen administered S. argus a daily ration equivalent to 2.0–2.8% body weight, delivered twice daily (06:00 and 18:00 h). Following standardized protocols, the SO group was designated as the control, with experimental parameters derived from prior transcriptomic investigations to ensure methodological consistency with established research frameworks [10,18].

Table 1.
The lipid profile of fish oil and soybean oil in experimental feed.

Table 2.
Formulation and actual nutritional composition of experimental diets.
2.3. Growth Measurements and Sampling
Following a 60-day feeding period, body weight, length, viscera weight, liver weight, and gonad weight were recorded for each fish. The condition factor (CF), viscerosomatic index (VSI), hepatosomatic index (HSI), and gonadosomatic index (GSI) were calculated using the following formulas:
Condition Factor (CF) = Body weight (g)/(Body length (cm))3 × 100%
Viscerosomatic Index (VSI, %) = Viscera weight (g)/Body weight (g) × 100%
Hepatosomatic Index (HSI, %) = Liver weight (g)/Body weight (g) × 100%
Gonadosomatic Index (GSI, %) = Gonad weight (g)/Body weight (g) × 100%
2.4. Liver Protein Extraction and Peptide Digestion
Three fish from each of the FO and SO groups were randomly selected, and their livers were dissected, immediately flash-frozen in liquid nitrogen, and stored at −80 °C for protein extraction. Liver samples were lysed with SDT lysis buffer (4% Sodium Dodecyl Sulfate [SDS], 100 mM Tris-HCl, pH 7.6, 0.1 M Dithiothreitol [DTT]) to extract proteins. Protein concentration was determined using a BCA Protein Assay Kit (Bio-Rad, Hercules, CA, USA). For each sample, 20 µg of protein was digested using the filter-aided sample preparation (FASP) method [24]. DTT was added to a final concentration of 40 mM, and samples were mixed at 600 rpm at 37 °C for 1.5 h. After cooling to room temperature, iodoacetamide (IAA) was added to a final concentration of 20 mM to block reduced cysteine residues, and the samples were incubated in the dark for 30 min. The filters were washed three times with 100 µL UA buffer (8 M Urea in 0.1 M Tris-HCl, pH 8.5), followed by two washes with 100 µL 25 mM NH4HCO3. Trypsin was added at a 1:50 enzyme-to-protein ratio (wt/wt), and digestion proceeded overnight at 37 °C. Peptides were desalted using C18 cartridges (Empore™ SPE C18, standard density, 7 mm bed I.D., 3 mL, Sigma, St. Louis, MO, USA), vacuum-dried, and reconstituted in 40 µL 0.1% formic acid. Peptide concentrations were estimated at 280 nm by UV absorbance based on an extinction coefficient of 1.1 for 0.1% solution, considering the frequency of tryptophan and tyrosine residues in vertebrate proteins.
2.5. LC-MS Data Acquisition for Proteomic Analysis
Peptides were separated on an Easy nLC nano-flow HPLC system. Mobile phase A was 0.1% formic acid in water, and mobile phase B was 0.1% formic acid in acetonitrile (84% acetonitrile). The analytical column (Thermo Scientific EASY column, 10 cm, ID 75 µm, 3 µm, C18-A2) was equilibrated with 95% buffer A, and samples were loaded onto a trap column (Thermo Scientific Acclaim PepMap100, 100 µm × 2 cm, nanoViper C18) with an autosampler. Peptides were eluted at a flow rate of 300 nL/min and analyzed on a Q-Exactive series mass spectrometer in positive ion mode. The MS scan range was set at 300–1800 m/z, with a primary MS resolution of 70,000 (at 200 m/z), an AGC target of 1 × 106, and a maximum injection time of 50 ms. Dynamic exclusion was set to 60 s. Each full scan was followed by 20 MS2 scans (HCD) with an isolation window of 2 m/z, a resolution of 17,500 (at 200 m/z), normalized collision energy of 30 eV, and an underfill ratio of 0.1%.
MaxQuant software (version 1.5.3.17) [25] was used for database search and quantitative analysis. The false discovery rate (FDR) for peptide and protein identification was set to 0.01, and protein abundance was calculated based on LFQ intensity. Differentially expressed proteins (DEPs) were identified based on a fold-change (FC) > 1.5 or <0.67 with a t-test p-value <0.05, in accordance with previously published criteria [26].
The GO (Gene Ontology) annotation of target proteins was performed using Blast2GO (BioBam Bioinformatics S.L., Valencia, Spain), including steps for sequence alignment (Blast), term mapping, annotation, and additional annotation via InterProScan. Structural domain annotation was derived from Pfam entries using the ProScan software package (version 5.1-44.0) integrated with InterPro database. KEGG (Kyoto Encyclopedia of Genes and Genomes) pathway annotation was performed via the KAAS (KEGG Automatic Annotation Server), and GO or KEGG pathway enrichment analyses were conducted by comparing distributions in the target and background protein sets using Fisher’s Exact Test.
3. Results
3.1. Effect of Fish Oil on Growth Parameters
During the experiment, no mortality was observed among the fish. Three fish were randomly selected from each group as experimental materials. Key growth indicators, including the CF, GSI, HSI, and VSI, showed no significant differences between the two dietary groups (Table 3).

Table 3.
Effects of fish oil vs. soybean oil on growth parameters of female S. argus.
3.2. Comparative Proteomic Analysis of Fish Fed Fish Oil and Soybean Oil Diets
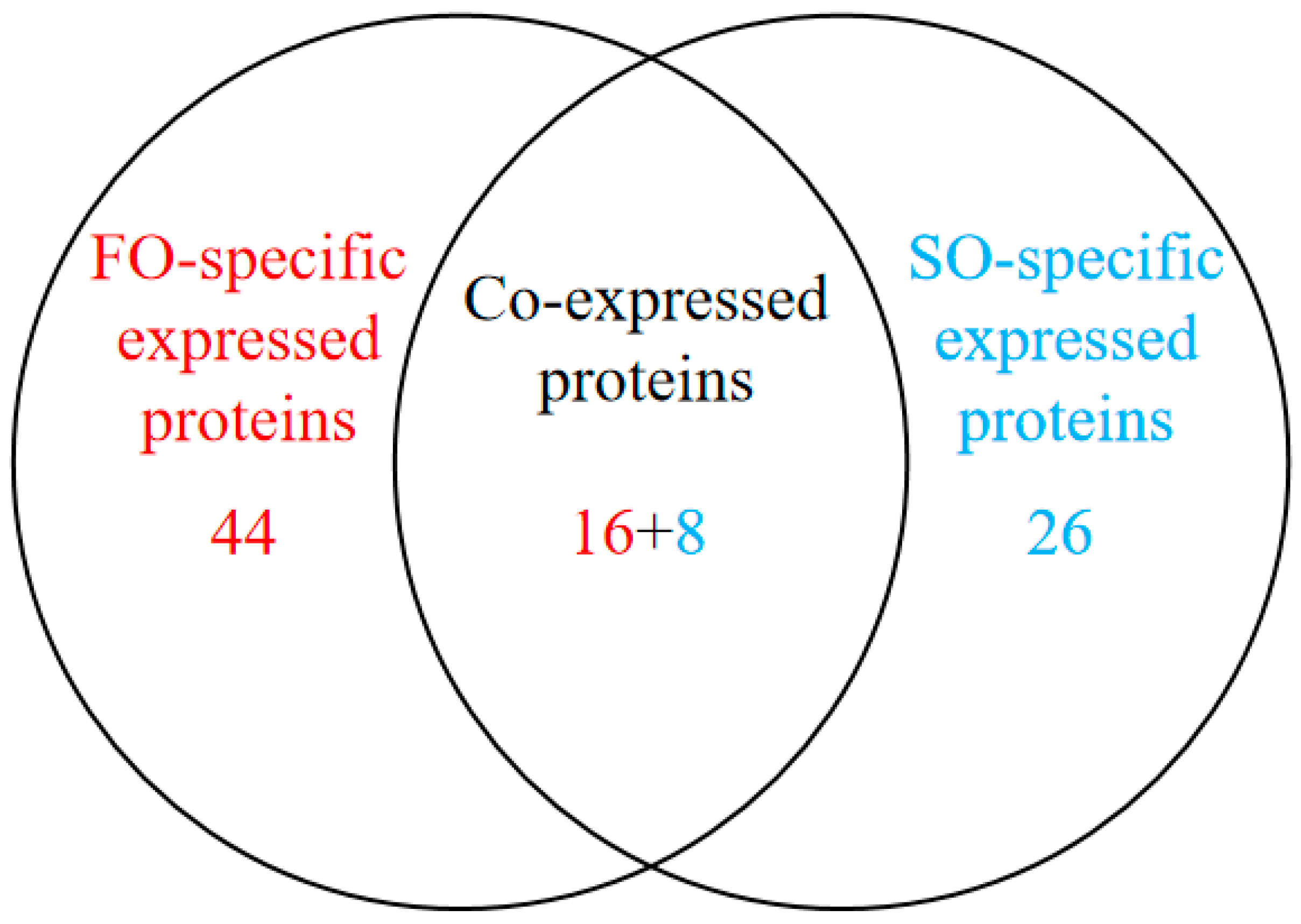
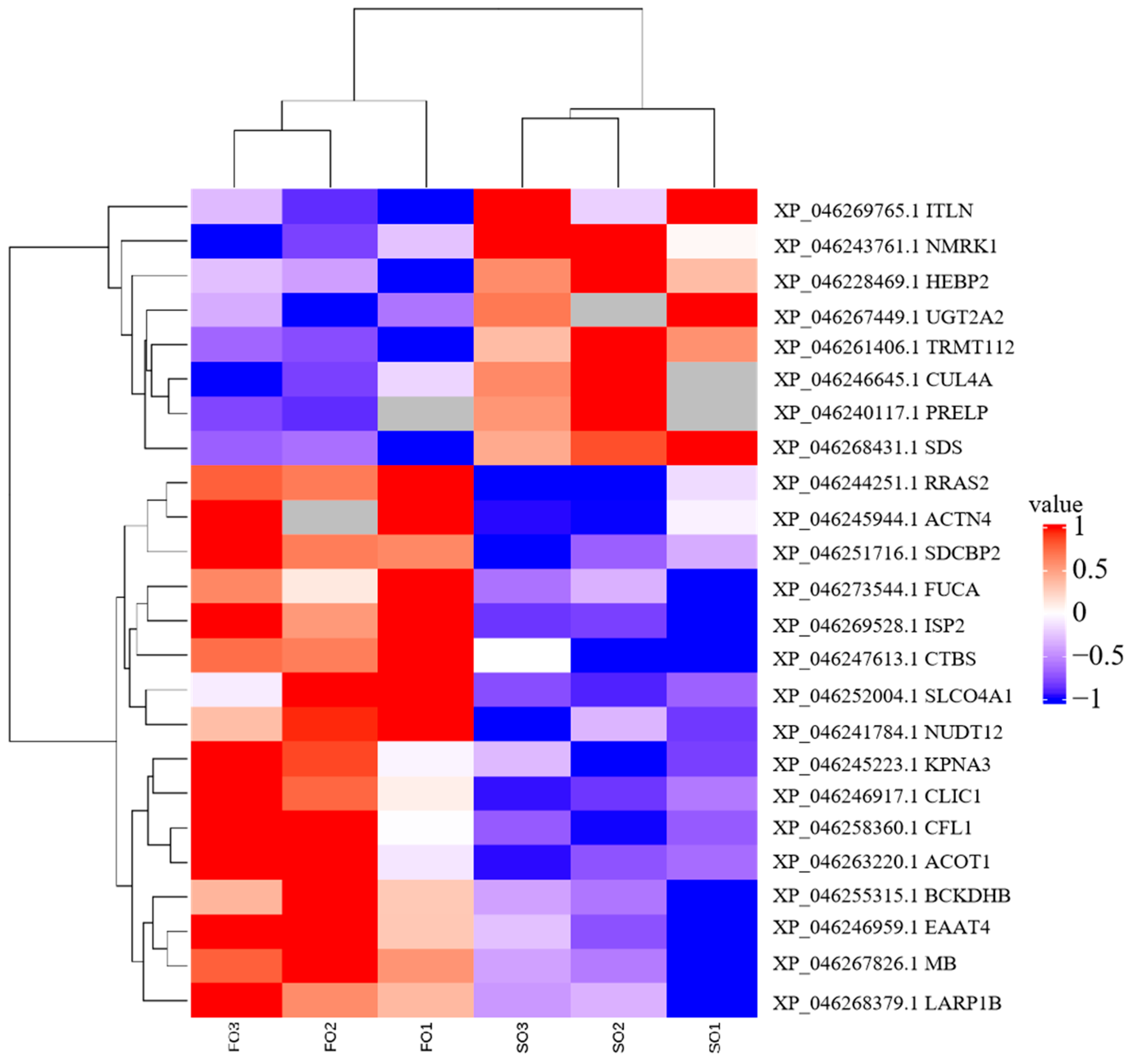
To investigate the effects of the FO group and the SO group diets on hepatic protein expression in S. argus, a label-free LC-MS/MS proteomic approach was employed. The analysis identified a total of 29,304 peptides corresponding to 3112 proteins. Of these, 3000 proteins were expressed in the FO group, and 2961 proteins were expressed in the SO group, with 2887 proteins shared between the two groups. A total of 94 DEPs were identified (Figure S1, Table S1). Among these, 16 proteins were upregulated, and 8 were downregulated in the FO group compared to the SO group. Interestingly, 44 proteins were uniquely expressed in the FO group, while 26 proteins were exclusively detected in the SO group (Table 4 and Table S2, Figure 1). Hierarchical clustering analysis revealed similar protein expression patterns within each group, while significant differences were observed between the two groups, indicating that dietary lipid sources influenced hepatic protein expression profiles (Figure 2).

Table 4.
Differentially expressed proteins between FO and SO based on expression difference analysis results.
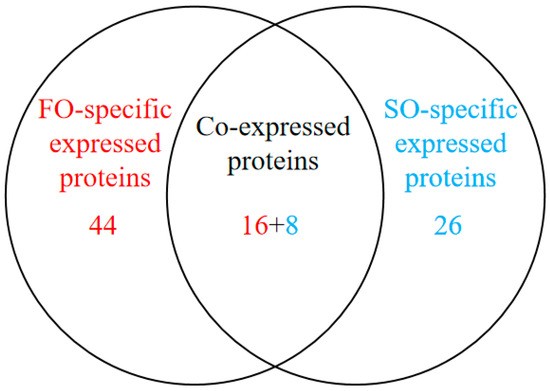
Figure 1.
Venn diagram of differentially expressed proteins between FO and SO groups based on differential expression analysis.
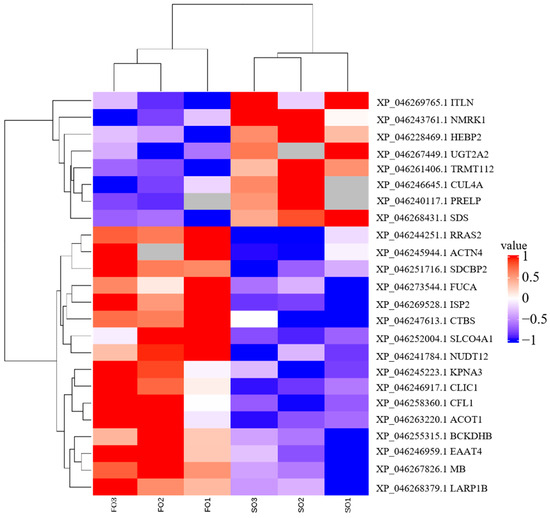
Figure 2.
Cluster heatmap displaying distinct protein expression profiles between FO and SO groups.
To delineate the functional ontology and pathway architecture of differentially expressed proteins (DEPs), functional enrichment analysis was conducted (Table S3). The subsequent KEGG-based pathway mapping of DEPs revealed conserved metabolic signatures (Table S4). The systematic interrogation of proteomic divergence was structured across three dimensions: differentially co-regulated proteins, SO cohort-specific proteoforms, and FO group-enriched protein clusters.
3.2.1. Co-Expressed Proteins
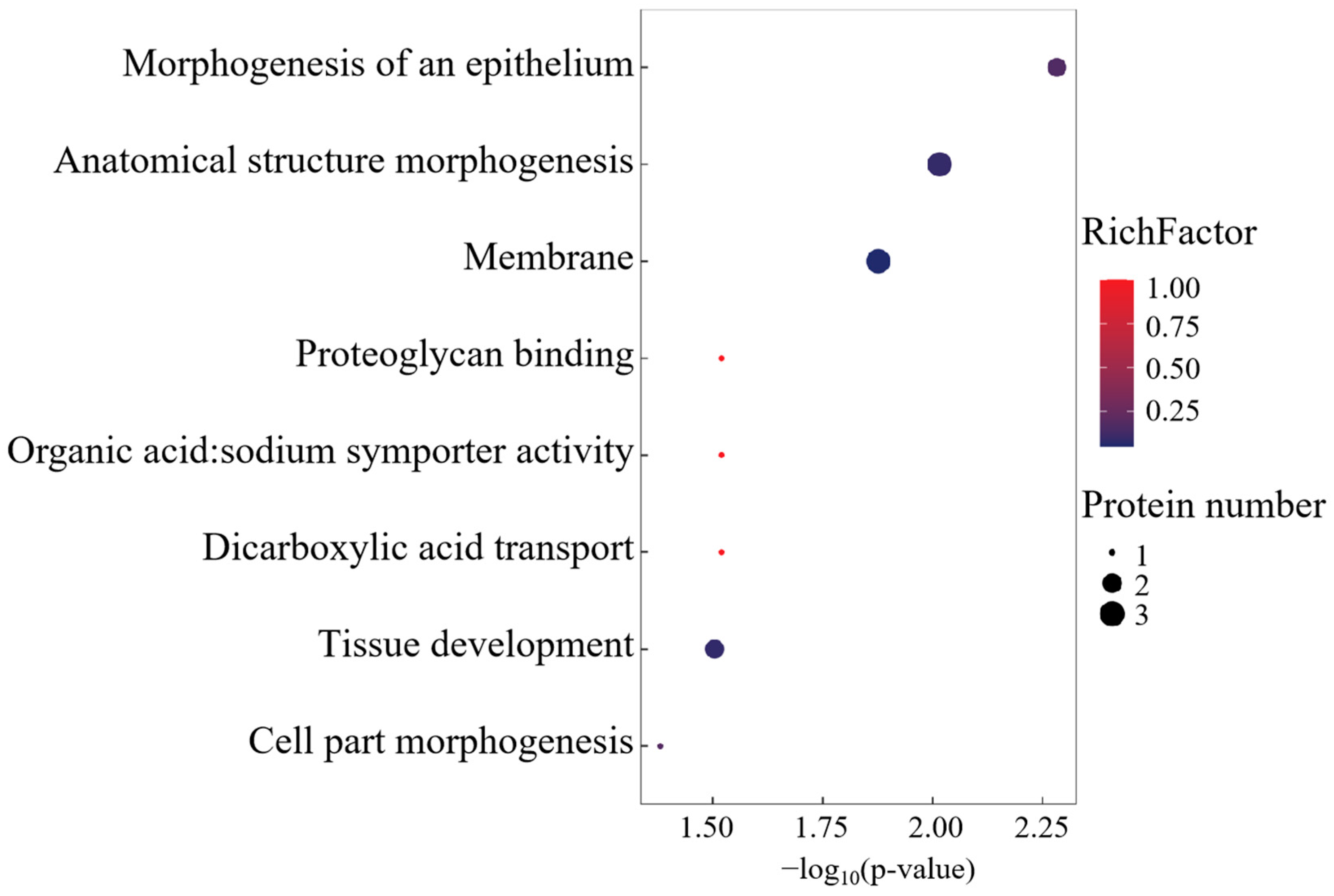
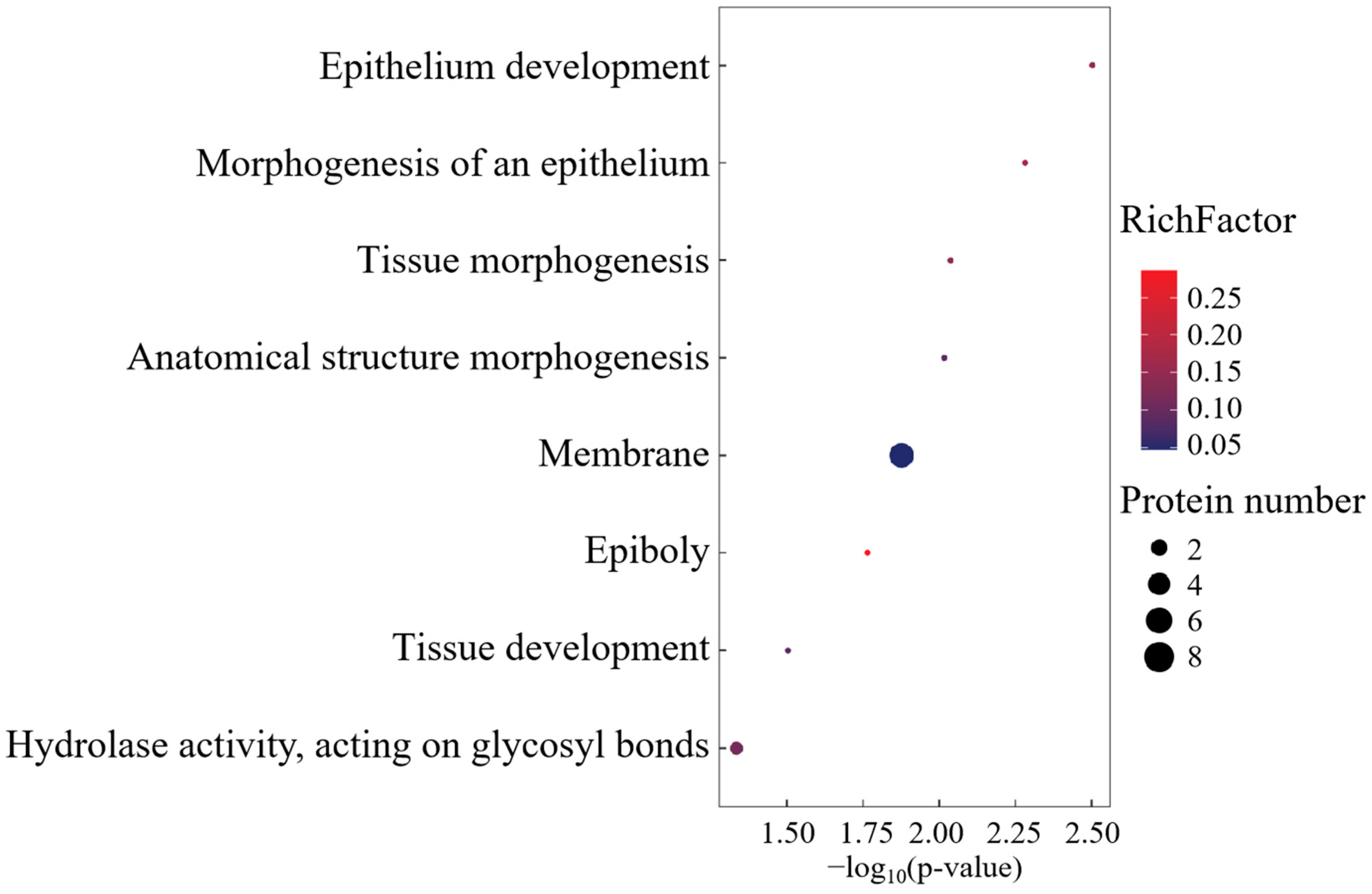
Gene Ontology (GO) enrichment analysis delineated a functional repertoire comprising 29 biological process ontologies, 5 molecular recognition domains, and 1 membrane-associated cellular compartment (Figure 3). The BP annotations were centered on morphogenetic regulation, transmembrane transport dynamics, and developmental patterning. MF enrichment highlighted dicarboxylate transmembrane transport machinery and proteoglycan binding affinities, while the singular CC term corresponded to membrane system architecture.
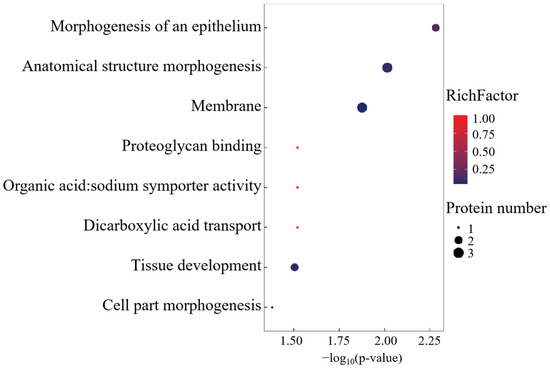
Figure 3.
GO enrichment analysis of differentially co-expressed proteins.
KEGG pathway mapping revealed conserved metabolic modules governing mitochondrial autophagy, cofactor biogenesis, peroxisomal redox regulation, and lipid homeostasis (Figure 4). Quantitative proteomics demonstrated marked overexpression of ACTN4, Cofilin-1, and RRAS2 in the FO cohort versus SO controls (p < 0.05), as well as three cytoskeletal regulators functioning as structural scaffolds in actin filament reorganization. Mechanistically, these effectors coordinate cellular morphogenesis through the spatiotemporal modulation of actomyosin contractility and membrane mechanotransduction pathways.
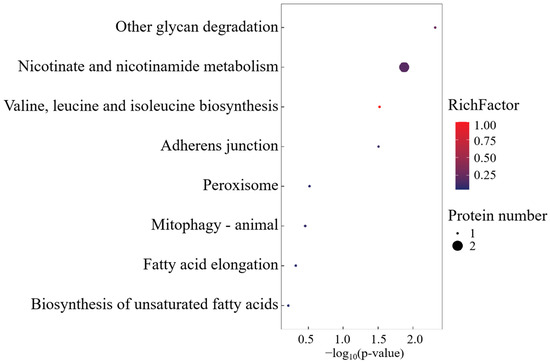
Figure 4.
KEGG enrichment analysis of differentially co-expressed proteins.
3.2.2. SO-Specific Expressed Proteins
Gene Ontology analysis of SO cohort-specific proteoforms identified seven biological process modules, one catalytic domain (glycoside hydrolase activity), and one organellar compartment (Figure 5). The singular molecular function ontology exclusively mapped to α-amylase-mediated glycosidic bond hydrolysis, establishing α-Amylase as the signature enzyme governing this functional axis.
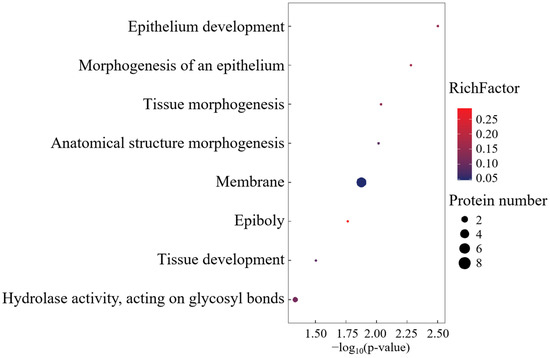
Figure 5.
GO enrichment analysis of SO group-specific expressed proteins.
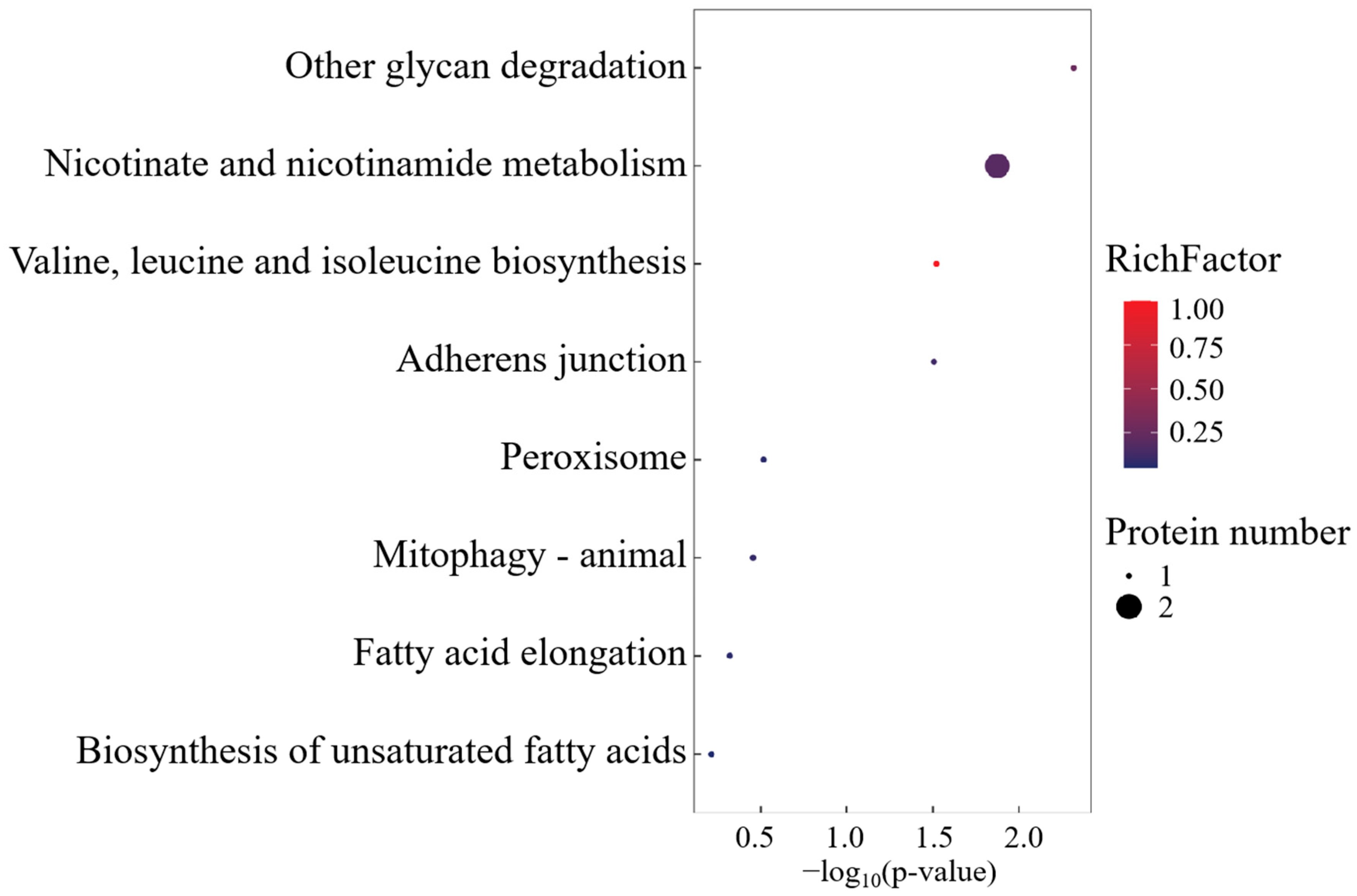
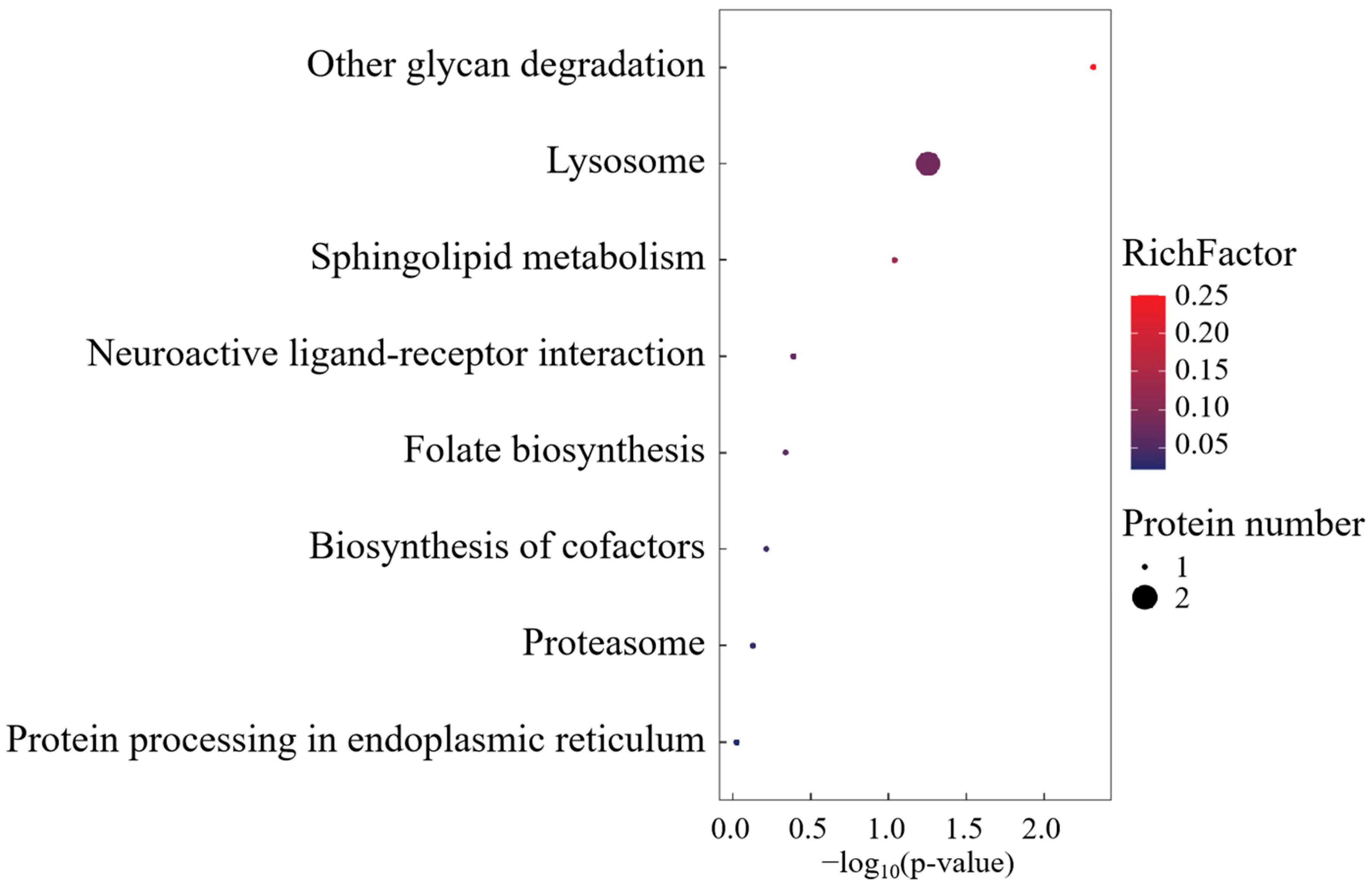
KEGG pathway mapping revealed conserved metabolic networks spanning glycan catabolism, proteostatic regulation, and lipid metabolic flux (Figure 6). Mechanistically, the differential overexpression of CTSA within lysosomal processing pathways was functionally prioritized, implicating its mechanistic role in biomacromolecule turnover through lysosome-mediated substrate channeling.
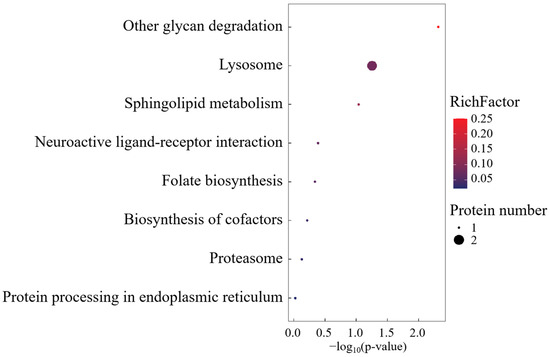
Figure 6.
KEGG enrichment analysis of SO group-specific expressed proteins.
3.2.3. FO-Specific Expressed Proteins
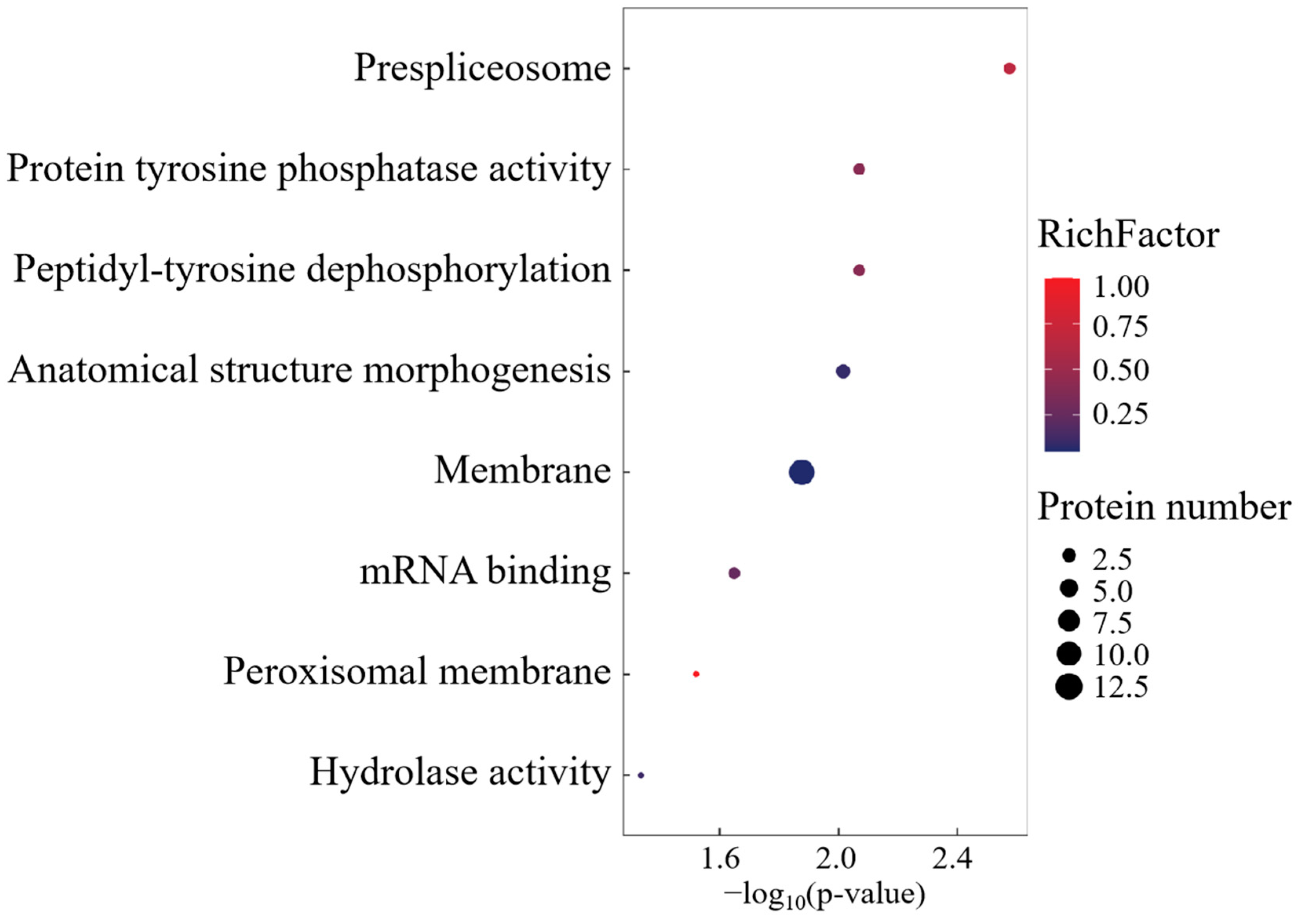
The Gene Ontology profiling of FO cohort-specific proteoforms revealed substantial enrichment across 26 biological process axes, 5 molecular recognition domains, and 16 macromolecular complexes (Figure 7). CC annotations predominated in spliceosomal machinery, cytoplasmic ribonucleoprotein granules, and membrane trafficking systems. Functional prioritization identified four cardinal effectors—GPR108, TSPAN8, SDF2L1, and TMEM41B—governing transmembrane signalosome assembly, intercellular adhesion dynamics, and autophagosome–lysosome coupling.
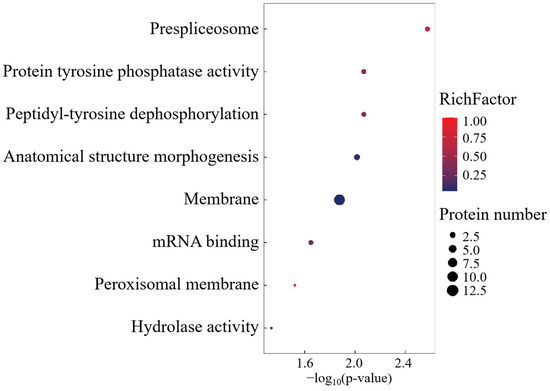
Figure 7.
GO enrichment analysis of FO group-specific expressed proteins.
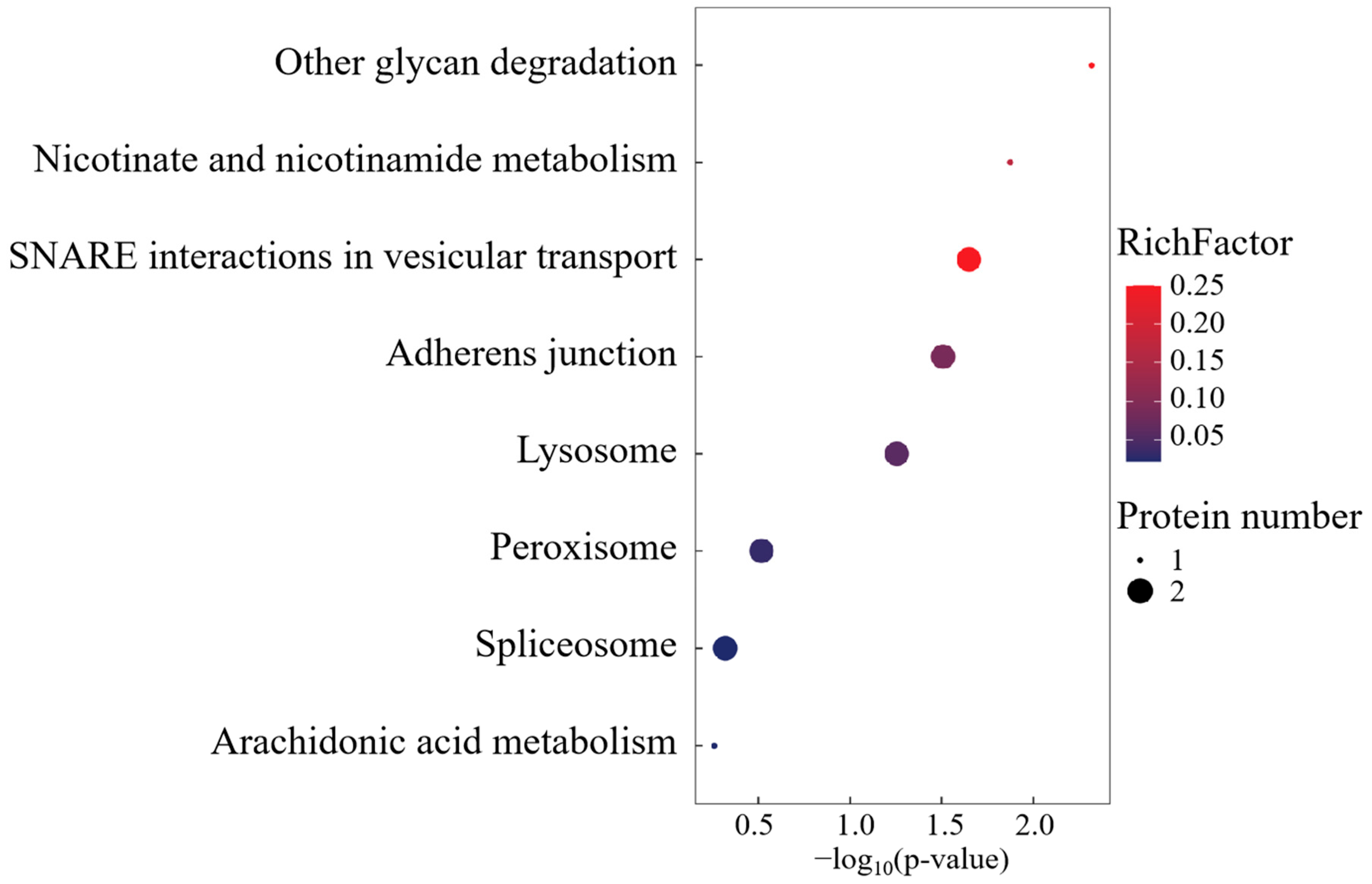
KEGG pathway deconvolution delineated three conserved regulatory modules: spliceosome-mediated RNA processing, transmembrane substrate flux, and lipid metabolic reprogramming (Figure 8). The FO-specific overexpression of peroxisomal docking receptor PEX5 emerged as a critical regulatory node, mechanistically positioned to orchestrate peroxisome biogenesis and redox homeostasis through matrix protein translocation. This peroxisomal signaling hub potentially modulates FO phenotypic adaptations via the coordinated regulation of lipid-derived signaling metabolites and oxidative stress buffering capacity.
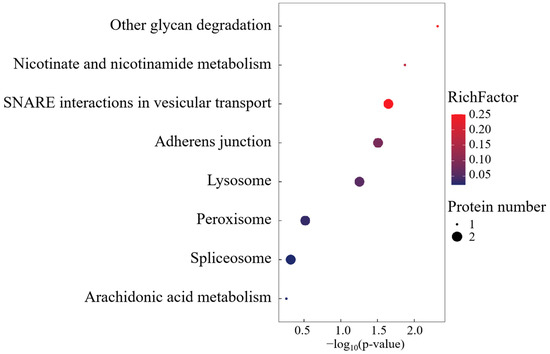
Figure 8.
KEGG enrichment analysis of FO group-specific expressed proteins.
4. Discussion
This study investigated the regulatory effects of fish oil and soybean oil diets on hepatic energy metabolism, lipid synthesis, and antioxidant stress in female S. argus by identifying protein-level evidence. From a proteomic perspective, the findings validate our team’s previous transcriptomic conclusions [18], elucidating that dietary fish oil promotes ovarian maturation in broodstock through the regulation of key metabolic pathways.
4.1. The Effect of Fish Oil on Growth Parameters
Throughout the 60-day feeding trial, S. argus in the SO group exhibited modest increases in growth parameters and the gonadosomatic index (GSI), though intergroup differences remained statistically non-significant (p > 0.05). Given experimental constraints including sampling stochasticity and individual genetic variability, these findings lack sufficient statistical confidence to substantiate the growth-promoting effects of soybean oil supplementation. Importantly, the observed growth performance parity aligns with prior transcriptomic evidence from this species [10,18] and corroborates findings from marine teleost models (Diplodus puntazzo [27], Rachycentron canadum [28]), where lipid source substitution in aquafeeds showed negligible growth modulation. Current data demonstrate that S. argus maintains normative growth under reduced fish oil regimens. However, the essentiality of dietary n-3 LC-PUFA cannot be conclusively dismissed, given residual n-3 LC-PUFA provision from minimal fishmeal content in SO formulations. This evidence base suggests the biological feasibility of partial fish oil substitution with plant-derived lipids in S. argus aquaculture, while highlighting the necessity for the methodologically rigorous exploration of substitution gradients to establish optimal replacement thresholds.
4.2. Effects of Fish Oil on Liver Metabolism
Fish oil significantly modulates the expression of proteins involved in hepatic lipid metabolism in S. argus. Proteomic analysis revealed the upregulation of several key proteins, including Commd1, TSPAN8, Ptges2, Sdf2l1, Nono, Tmem41b, and Pex5, all of which are critical for lipid synthesis and metabolism.
Commd1, known for its role in cholesterol homeostasis and LDL receptor regulation [29,30], was significantly upregulated in the fish oil group. This suggests that fish oil enhances cholesterol and LDL synthesis in S. argus. Similarly, the upregulation of TSPAN8 further supports the role of fish oil in promoting hepatic lipid metabolism [31]. Ptges2, associated with cholesterol synthesis and arachidonic acid metabolism [32,33], showed significant upregulation, indicating the activation of metabolic pathways in S. argus. Additionally, the upregulation of Sdf2l1, which is essential for glucose and lipid homeostasis [34], suggests that fish oil enhances energy absorption to meet the metabolic demands of diverse biological processes. Nono, a regulator of pre-mRNA processing for genes involved in glucose and lipid metabolism [35], was also upregulated, further confirming the role of fish oil in augmenting hepatic lipid metabolism. Tmem41b, involved in lipid droplet and lipoprotein formation [36,37], and Pex5, associated with lipid catabolism and energy regulation [38], were significantly upregulated, highlighting the influence of fish oil on lipid transport mechanisms and energy metabolism in S. argus.
These findings underscore that fish oil rich in DHA and EPA enhances hepatic lipid metabolism, improves lipid transport, and increases energy availability. These results align with observations in other fish species, where dietary fish oil has been shown to enhance lipid metabolism [39,40,41,42,43,44]. Notably, plant oil substitution failed to elicit significant transcriptional modulation of core metabolic regulators in Dicentrarchus labrax hepatic and intestinal tissues, encompassing lipid trafficking mediators (FABP, ApoB/A1/A4, MTP), triglyceride biosynthetic machinery (DGAT), a cholesterol homeostasis gatekeeper (HMGCR), glucose transport systems (GLUT2), and glycolytic/gluconeogenic enzymes (GK, PK, G6Pase, PEPCK) [45]. This phenomenon may be attributed to interspecies differences in the utilization of essential fatty acids, suggesting that nutrient-specific feed formulations need to be customized according to species-specific requirements [2].
4.3. Effects of Fish Oil on Energy Metabolism Balance, Anti-Inflammatory, and Antioxidant Properties in the Liver
Fish oil also regulates proteins involved in energy metabolism balance, anti-inflammatory responses, and antioxidant properties in the liver of S. argus, suggesting a protective role of fish oil in maintaining liver health.
Regarding anti-inflammatory mechanisms, the significant upregulation of proteins such as p120-Catenin and Trim16 was observed. These proteins play central roles in regulating inflammatory responses [46,47,48,49,50,51,52,53,54], indicating that fish oil alleviates liver inflammation by activating anti-inflammatory signaling pathways. These findings corroborate with prior research on the anti-inflammatory properties of fish oil in Acanthopagrus schlegelii [41], Ctenopharyngodon idella [40], and Larimichthys crocea [55], reinforcing its potential as a liver health enhancer.
In terms of antioxidant effects, the upregulation of Aqp11 and Pex5 was identified, highlighting the role of fish oil in mitigating oxidative stress and improving redox homeostasis in the liver [56,57,58,59,60,61,62,63]. These results are consistent with studies on Salmo salar [43] and Larimichthys crocea [55], where fish oil reduced the risk of oxidative damage and maintained liver cell integrity.
For inflammation protection, this study revealed that fish oil significantly upregulated proteins such as Commd1, Gpr108, and Ptpn2 [64,65,66,67,68,69]. The upregulation of these proteins may further strengthen the liver’s anti-inflammatory capacity, providing solid support for maintaining liver homeostasis. This observation is in line with mRNA-level results seen in Ctenopharyngodon idella [40]. Additionally, the downregulation of Romo1 and CatA could be indicative of reduced oxidative stress and inflammation in the liver [70,71,72]. Notably, empirical evidence indicates that plant oil substitution in Micropterus salmoides aquafeeds significantly augments systemic antioxidant capacity [39]. This metabolic adaptation likely reflects phylogenetic divergence in n-3 LC-PUFA utilization thresholds, emphasizing the necessity for species-centric nutritional optimization in precision feed formulation [2].
Notably, fish oil significantly increased the expression of myoglobin, a protein involved in oxygen transport and storage [73,74,75]. This upregulation suggests enhancing the hepatic oxygen supply to meet metabolic demands across diverse biological processes.
Proteomic analysis demonstrates that fish oil improves lipid metabolism, maintains energy homeostasis, and enhances antioxidant capacity in the liver, providing robust evidence for its regulatory role in hepatic function and support of systemic metabolic activities.
4.4. Gene-Protein Co-Expression Trends
Comparisons with previous transcriptomic data revealed no clear co-expression trends between genes and proteins [18]. This observation is consistent with recent studies, highlighting that mRNA and protein expression correlations can be relatively low due to factors such as differences in mRNA half-life, post-translational modifications, and translation efficiency. For instance, mRNA stability is significantly lower than protein stability, and abundance levels between the two can vary dramatically [76]. Environmental factors, experimental conditions, and sample variability may also contribute to these discrepancies.
5. Conclusions
Proteomic analysis in this study highlights the positive effects of dietary fish oil on hepatic lipid metabolism, energy balance, and antioxidant capacity in female S. argus. While no differentially expressed proteins directly associated with gonadal development were identified in this study, we provide protein-level evidence supporting the hypothesis that fish oil modulates hepatic metabolism, thereby promoting reproductive regulation and gonadal development [10,18]. Further research is warranted to elucidate the precise mechanisms underlying these effects, particularly through integrated multi-omics approaches.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/fishes10030128/s1: Figure S1: Venn diagram showing the overlap of identified proteins between the two groups; Table S1: Comprehensive protein identification results; Table S2: Identification details for differentially expressed proteins; Table S3: GO functional annotation for all differentially expressed proteins; Table S4: KEGG pathway analysis of all differentially expressed proteins.
Author Contributions
J.H.: investigation, data curation, visualization, and writing—original draft; H.M.: writing—review and editing; D.J.: conceptualization, supervision, and writing—review and editing; T.W.: resources; Z.L.: investigation; Y.H.: resources; G.S.: resources; C.Z.: resources; G.L.: conceptualization and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 32473155), the Guangdong Science and Technology Department Project (Grant No. 2023B0202010005), the Guangdong Provincial Key Laboratory of Aquatic Animal Disease Control and Healthy culture (PBEA2024YB06), and the Zhanjiang Science and Technology Project (Grant No. 2022A01046).
Institutional Review Board Statement
The experimental protocols for this research were approved by the Animal Research Ethics Committee of Guangdong Ocean University (201903004). The study did not involve any endangered or protected species.
Informed Consent Statement
Not applicable.
Data Availability Statement
The mass spectrometry proteomic data have been deposited to the ProteomeXchange Consortium (https://proteomecentral.proteomexchange.org (accessed on 5 February 2025) via the iProX partner repository [77,78] with dataset identifier PXD060497.
Conflicts of Interest
The author Yucong Hong was employed by the company Guangdong Yuequn Marine Biotechnology Co., Ltd., Jieyang, China. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Tocher, D.R. Omega-3 long-chain polyunsaturated fatty acids and aquaculture in perspective. Aquaculture 2015, 449, 94–107. [Google Scholar] [CrossRef]
- Glencross, B.D. Exploring the nutritional demand for essential fatty acids by aquaculture species. Rev. Aquacult. 2009, 1, 71–124. [Google Scholar] [CrossRef]
- Dadras, H.; Dzyuba, B.; Cosson, J.; Golpour, A.; Siddique, M.A.M.; Linhart, O. Effect of water temperature on the physiology of fish spermatozoon function: A brief review. Aquacult. Res. 2017, 48, 729–740. [Google Scholar] [CrossRef]
- Reilly, A.; Kaferstein, F. Food safety and products from aquaculture. J. Appl. Microbiol. 1998, 85 (Suppl. 1), 249S–257S. [Google Scholar] [CrossRef] [PubMed]
- Gui, L.; Zhang, P.P.; Liang, X.M.; Su, M.L.; Wu, D.; Zhang, J.B. Adaptive responses to osmotic stress in kidney-derived cell lines from Scatophagus argus, a euryhaline fish. Gene 2016, 583, 134–140. [Google Scholar] [CrossRef]
- Bertucci, J.I.; Blanco, A.M.; Sundarrajan, L.; Rajeswari, J.J.; Velasco, C.; Unniappan, S. Nutrient Regulation of Endocrine Factors Influencing Feeding and Growth in Fish. Front. Endocrinol. 2019, 10, 83. [Google Scholar] [CrossRef]
- Sung, Y.; Yu, Y.C.; Han, J.M. Nutrient sensors and their crosstalk. Exp. Mol. Med. 2023, 55, 1076–1089. [Google Scholar] [CrossRef]
- Templeman, N.M.; Murphy, C.T. Regulation of reproduction and longevity by nutrient-sensing pathways. J. Cell Biol. 2018, 217, 93–106. [Google Scholar] [CrossRef]
- Scaramuzzi, R.J.; Brown, H.M.; Dupont, J. Nutritional and Metabolic Mechanisms in the Ovary and Their Role in Mediating the Effects of Diet on Folliculogenesis: A Perspective. Reprod. Domest. Anim. 2010, 45, 32–41. [Google Scholar] [CrossRef]
- Wang, T.; Liu, Z.L.; Li, G.L.; Mustapha, U.F.; Ndandala, C.B.; Shi, H.J.; Zhu, C.H.; Chen, H.P.; Huang, Y.; Jiang, D.N. Ovary transcriptomic analysis reveals regulation effects of dietary fish oil on hormone, lipid, and glucose metabolism in female adult spotted scat (Scatophagus argus). Front. Mar. Sci. 2022, 9, 935968. [Google Scholar] [CrossRef]
- Peng, S.M.; Gao, Q.X.; Shi, Z.H.; Zhang, C.J.; Wang, J.G.; Yin, F.; Zhang, Y.L. Effect of dietary n 3 LC-PUFAs on plasma vitellogenin, sex steroids, and ovarian steroidogenesis during vitellogenesis in female silver pomfret (Pampus argenteus) broodstock. Aquaculture 2015, 444, 93–98. [Google Scholar] [CrossRef]
- Zhang, M.Z.; Li, G.L.; Zhu, C.H.; Deng, S.P. Effects of fish oil on ovarian development in spotted scat (Scatophagus argus). Anim. Reprod. Sci. 2013, 141, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Cai, Y.; Shao, Y.; Zhang, X.; Li, N.; Zhang, H.; Liu, Z. Fish Oil Ameliorates High-Fat Diet Induced Male Mouse Reproductive Dysfunction via Modifying the Rhythmic Expression of Testosterone Synthesis Related Genes. Int. J. Mol. Sci. 2018, 19, 1325. [Google Scholar] [CrossRef]
- Erez, I.; Serbester, U. Effects of prenatal fish oil supplementation on the development and performance of female kids after weaning. PLoS ONE 2024, 19, e0310220. [Google Scholar] [CrossRef] [PubMed]
- Bemanian, V.; Male, R.; Goksoyr, A. The aryl hydrocarbon receptor-mediated disruption of vitellogenin synthesis in the fish liver: Cross-talk between AHR- and ERalpha-signalling pathways. J. Hepatol. 2004, 3, 2. [Google Scholar] [CrossRef]
- Mandal, S.C.; Tripathy, P.S.; Khatei, A.; Devi, N.C.; Biswas, P.; Sundaray, J.K.; Hoque, F.; Parhi, J. Effect of vegetable oil on ovarian steroidogenesis- A transcriptome approach to understand molecular mechanisms of hypothalamus pituitary and gonad axis (HPG) in Ompok bimaculatus. PLoS ONE 2024, 19, e0309311. [Google Scholar] [CrossRef] [PubMed]
- Li, G.L.; Zhang, M.Z.; Deng, S.P.; Chen, H.P.; Zhu, C.H. Effects of temperature and fish oil supplementation on ovarian development and foxl2 mRNA expression in spotted scat Scatophagus argus. J. Fish Biol. 2015, 86, 248–260. [Google Scholar] [CrossRef]
- Wang, T.; Jiang, D.N.; Shi, H.J.; Mustapha, U.F.; Deng, S.P.; Liu, Z.L.; Li, W.X.; Chen, H.P.; Zhu, C.H.; Li, G.L. Liver Transcriptomic Analysis of the Effects of Dietary Fish Oil Revealed a Regulated Expression Pattern of Genes in Adult Female Spotted Scat (Scatophagus argus). Front. Mar. Sci. 2021, 8, 784845. [Google Scholar] [CrossRef]
- Jiang, D.N.; Li, J.T.; Tao, Y.X.; Chen, H.P.; Deng, S.P.; Zhu, C.H.; Li, G.L. Effects of melanocortin-4 receptor agonists and antagonists on expression of genes related to reproduction in spotted scat, Scatophagus argus. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2017, 187, 603–612. [Google Scholar] [CrossRef]
- Mandal, B.; Kailasam, M.; Bera, A.; Sukumaran, K.; Hussain, T.; Makesh, M.; Thiagarajan, G.; Vijayan, K.K. Gonadal recrudescence and annual reproductive hormone pattern of captive female Spotted Scats (Scatophagus argus). Anim. Reprod. Sci. 2020, 213, 106273. [Google Scholar] [CrossRef]
- Mustapha, U.F.; Assan, D.; Huang, Y.Q.; Li, G.L.; Jiang, D.N. High Polymorphism in the Dmrt2a Gene Is Incompletely Sex-Linked in Spotted Scat, Scatophagus argus. Animals 2022, 12, 613. [Google Scholar] [CrossRef] [PubMed]
- He, F.X.; Jiang, D.N.; Huang, Y.Q.; Mustapha, U.F.; Yang, W.; Cui, X.F.; Tian, C.X.; Chen, H.P.; Shi, H.J.; Deng, S.P.; et al. Comparative transcriptome analysis of male and female gonads reveals sex-biased genes in spotted scat (Scatophagus argus). Fish Physiol. Biochem. 2019, 45, 1963–1980. [Google Scholar] [CrossRef]
- Mandal, B.; Kailasam, M.; Bera, A.; Sukumaran, K.; Hussain, T.; Biswas, G.; Vijayan, K.K. Standardization of oocyte size during artificial fertilization and optimization of stocking density during indoor larval and outdoor nursery rearing of captive spotted scat (Scatophagus argus) for a viable juvenile production system. Aquaculture 2021, 534, 736262. [Google Scholar] [CrossRef]
- Wisniewski, J.R.; Zougman, A.; Nagaraj, N.; Mann, M. Universal sample preparation method for proteome analysis. Nat. Methods 2009, 6, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.; Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008, 26, 1367–1372. [Google Scholar] [CrossRef]
- Li, M.; Ren, C.; Zhou, S.; He, Y.; Guo, Y.; Zhang, H.; Liu, L.; Cao, Q.; Wang, C.; Huang, J.; et al. Integrative proteome analysis implicates aberrant RNA splicing in impaired developmental potential of aged mouse oocytes. Trends Analyt. Chem. 2021, 20, e13482. [Google Scholar] [CrossRef]
- Piedecausa, M.A.; Mazón, M.J.; García, B.G.; Hernández, M.D. Effects of total replacement of fish oil by vegetable oils in the diets of sharpsnout seabream (Diplodus puntazzo). Aquaculture 2007, 263, 211–219. [Google Scholar] [CrossRef]
- Trushenski, J.; Schwarz, M.; Lewis, H.; Laporte, J.; Delbos, B.; Takeuchi, R.; Sampaio, L.A. Effect of replacing dietary fish oil with soybean oil on production performance and fillet lipid and fatty acid composition of juvenile cobia Rachycentron canadum. Aquacult. Nutr. 2011, 17, E437–E447. [Google Scholar] [CrossRef]
- Bartuzi, P.; Billadeau, D.D.; Favier, R.; Rong, S.X.; Dekker, D.; Fedoseienko, A.; Fieten, H.; Wijers, M.; Levels, J.H.; Huijkman, N.; et al. CCC- and WASH-mediated endosomal sorting of LDLR is required for normal clearance of circulating LDL. Nat. Commun. 2016, 7, 10961. [Google Scholar] [CrossRef]
- Fedoseienko, A.; Wijers, M.; Wolters, J.C.; Dekker, D.; Smit, M.; Huijkman, N.; Kloosterhuis, N.; Klug, H.; Schepers, A.; van Dijk, K.W.; et al. The COMMD Family Regulates Plasma LDL Levels and Attenuates Atherosclerosis Through Stabilizing the CCC Complex in Endosomal LDLR Trafficking. Circ. Res. 2018, 122, 1648–1660. [Google Scholar] [CrossRef]
- Champy, M.F.; Le Voci, L.; Selloum, M.; Peterson, L.B.; Cumiskey, A.M.; Blom, D. Reduced body weight in male Tspan8-deficient mice. Int. J. Obes. 2011, 35, 605–617. [Google Scholar] [CrossRef]
- Wang, W.T.; Shi, Z.Y.; Zhang, R.H.; Yu, J.J.; Wang, C.Y.; Hou, J.A.; Sun, J.; Liu, Y.H.; Qin, K.R.; Liu, Y.; et al. Liver proteomics analysis reveals abnormal metabolism of bile acid and arachidonic acid in Chinese hamsters with type 2 diabetes mellitus. J. Proteom. 2021, 239, 104186. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Liu, X.; Xiang, Y.; Hou, Z.; He, K.; Zhong, G.; Hu, J.; Cai, D.; Liu, Y.; Ren, J.; et al. Inhibiting cholesterol de novo synthesis promotes hepatocellular carcinoma progression by upregulating prostaglandin E synthase 2-mediated arachidonic acid metabolism under high fatty acid conditions. Cancer Sci. 2024, 115, 477–489. [Google Scholar] [CrossRef]
- Sasako, T.; Ohsugi, M.; Kubota, N.; Itoh, S.; Okazaki, Y.; Terai, A.; Kubota, T.; Yamashita, S.; Nakatsukasa, K.; Kamura, T.; et al. Hepatic Sdf2l1 controls feeding-induced ER stress and regulates metabolism. Nat. Commun. 2019, 10, 947. [Google Scholar] [CrossRef] [PubMed]
- Benegiamo, G.; Mure, L.S.; Erikson, G.; Le, H.D.; Moriggi, E.; Brown, S.A.; Panda, S. The RNA-Binding Protein NONO Coordinates Hepatic Adaptation to Feeding. Cell Metab. 2018, 27, 404–418. [Google Scholar] [CrossRef]
- Morita, K.; Hama, Y.; Izume, T.; Tamura, N.; Ueno, T.; Yamashita, Y.; Sakamaki, Y.; Mimura, K.; Morishita, H.; Shihoya, W.; et al. Genome-wide CRISPR screen identifies TMEM41B as a gene required for autophagosome formation. J. Cell Biol. 2018, 217, 3817–3828. [Google Scholar] [CrossRef]
- Huang, D.; Xu, B.L.; Liu, L.; Wu, L.Z.; Zhu, Y.G.; Ghanbarpour, A.; Wang, Y.W.; Chen, F.J.; Lyu, J.; Hu, Y.T.; et al. TMEM41B acts as an ER scramblase required for lipoprotein biogenesis and lipid homeostasis. Cell Metab. 2021, 33, 1655–1670. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Ji, Y.; Jeon, Y.G.; Han, J.S.; Han, K.H.; Lee, J.H.; Lee, G.; Jang, H.; Choe, S.S.; Baes, M.; et al. Spatiotemporal contact between peroxisomes and lipid droplets regulates fasting-induced lipolysis via PEX5. Nat. Commun. 2020, 11, 578. [Google Scholar] [CrossRef]
- Gong, Y.; Chen, S.W.; Wang, Z.J.; Li, W.F.; Xie, R.T.; Zhang, H.T.; Huang, X.X.; Chen, N.S.; Li, S.L. Dietary lipid sources affect growth performance, lipid deposition, antioxidant capacity and inflammatory response of largemouth bass (Micropterus salmoides). Fish Shellfish Immunol. 2024, 150, 109635. [Google Scholar] [CrossRef]
- Huang, X.C.; Sun, J.; Bian, C.C.; Ji, S.H.; Ji, H. Docosahexaenoic acid lessens hepatic lipid accumulation and inflammation via the AMP-activated protein kinase and endoplasmic reticulum stress signaling pathways in grass carp (Ctenopharyngodon idella). Food Funct. 2022, 13, 1846–1859. [Google Scholar] [CrossRef]
- Shen, Y.D.; Li, X.J.; Bao, Y.G.; Zhu, T.T.; Wu, Z.X.; Yang, B.Q.; Jiao, L.F.; Zhou, Q.C.; Jin, M. Lipid metabolic disorders and physiological stress caused by a high-fat diet have lipid source-dependent effects in juvenile black seabream Acanthopagrus schlegelii. Fish Physiol. Biochem. 2022, 48, 955–971. [Google Scholar] [CrossRef]
- Roy, J.; Vigor, C.; Vercauteren, J.; Reversat, G.; Zhou, B.Q.; Surget, A.; Larroquet, L.; Lanuque, A.; Sandres, F.; Terrier, F.; et al. Characterization and modulation of brain lipids content of rainbow trout fed with 100% plant based diet rich in omega-3 long chain polyunsaturated fatty acids DHA and EPA. Biochimie 2020, 178, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Hall, J.R.; Caballero-Solares, A.; Eslamloo, K.; Taylor, R.G.; Parrish, C.C.; Rise, M.L. Liver Transcriptome Profiling Reveals That Dietary DHA and EPA Levels Influence Suites of Genes Involved in Metabolism, Redox Homeostasis, and Immune Function in Atlantic Salmon (Salmo salar). Mar. Biotechnol. 2020, 22, 263–284. [Google Scholar] [CrossRef] [PubMed]
- Houston, S.J.S.; Karalazos, V.; Tinsley, J.; Betancor, M.B.; Martin, S.A.M.; Tocher, D.R.; Monroig, O. The compositional and metabolic responses of gilthead seabream (Sparus aurata) to a gradient of dietary fish oil and associated n-3 long-chain PUFA content. Br. J. Nutr. 2017, 118, 1010–1022. [Google Scholar] [CrossRef] [PubMed]
- Castro, C.; Corraze, G.; Panserat, S.; Oliva-Teles, A. Effects of fish oil replacement by a vegetable oil blend on digestibility, postprandial serum metabolite profile, lipid and glucose metabolism of European sea bass (Dicentrarchus labrax) juveniles. Aquacult. Nutr. 2015, 21, 592–603. [Google Scholar] [CrossRef]
- Perez-Moreno, M.; Davis, M.A.; Wong, E.; Pasolli, H.A.; Reynolds, A.B.; Fuchs, E. p120-Catenin mediates inflammatory responses in the skin. Cell 2006, 124, 631–644. [Google Scholar] [CrossRef]
- Perez-Moreno, M.; Song, W.M.; Pasolli, H.A.; Williams, S.E.; Fuchs, E. Loss of p120 catenin and links to mitotic alterations, inflammation, and skin cancer. Proc. Natl. Acad. Sci. USA 2008, 105, 15399–15404. [Google Scholar] [CrossRef]
- Smalley-Freed, W.G.; Efimov, A.; Burnett, P.E.; Short, S.P.; Davis, M.A.; Gumucio, D.L.; Washington, M.K.; Coffey, R.J.; Reynolds, A.B. p120-catenin is essential for maintenance of barrier function and intestinal homeostasis in mice. J. Clin. Investig. 2010, 120, 1824–1835. [Google Scholar] [CrossRef]
- Karayiannakis, A.J.; Syrigos, K.N.; Efstathiou, J.; Valizadeh, A.; Noda, M.; Playford, R.J.; Kmiot, W.; Pignatelli, M. Expression of catenins and E-cadherin during epithelial restitution in inflammatory bowel disease. J. Pathol. 1998, 185, 413–418. [Google Scholar] [CrossRef]
- Smalley-Freed, W.G.; Efimov, A.; Short, S.P.; Jia, P.L.; Zhao, Z.M.; Washington, M.K.; Robine, S.; Coffey, R.J.; Reynolds, A.B. Adenoma formation following limited ablation of p120-catenin in the mouse intestine. PLoS ONE 2011, 6, e19880. [Google Scholar] [CrossRef]
- Xu, H.G.; Gao, Z.; Ma, M.M.; Xu, J.J.; Xiao, L.; Wang, H.; Zhang, T.; Liu, X.; Xu, Y.M.; Zhang, X.L. P120-Catenin Mediates Intermittent Cyclic Mechanical Tension-Induced Inflammation in Chondrocytes. J. Cell. Biochem. 2017, 118, 4508–4516. [Google Scholar] [CrossRef]
- Hatakeyama, S. TRIM Family Proteins: Roles in Autophagy, Immunity, and Carcinogenesis. Trends Biochem. Sci. 2017, 42, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, X.; Lin, Z.B.; Yang, P.J.; Xu, H.; Duan, J.L.; Ruan, B.; Song, P.; Liu, J.J.; Yue, Z.S.; et al. Tripartite motif 16 ameliorates nonalcoholic steatohepatitis by promoting the degradation of phospho-TAK1. Cell Metab. 2021, 33, 1372–1388. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.Q.; Su, F.F.; Dong, Z.W.; Shi, Y.J.; Tian, X.L.; Cui, Z.S.; Li, J.X. TRIM16 exerts protective function on myocardial ischemia/reperfusion injury through reducing pyroptosis and inflammation via NLRP3 signaling. Biochem. Biophys. Res. Commun. 2022, 632, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.; Ding, Y.; Li, X.; Dong, X.; Mai, K.; Ai, Q. Nrf2 pathway in vegetable oil-induced inflammation of large yellow croaker (Larimichthys crocea). Fish Shellfish Immunol. 2022, 127, 778–787. [Google Scholar] [CrossRef]
- Frühbeck, G.; Balaguer, I.; Méndez-Giménez, L.; Valentí, V.; Becerril, S.; Catalán, V.; Gómez-Ambrosi, J.; Silva, C.; Salvador, J.; Calamita, G.; et al. Aquaporin-11 Contributes to TGF-β1-induced Endoplasmic Reticulum Stress in Human Visceral Adipocytes: Role in Obesity-Associated Inflammation. Cells 2020, 9, 1403. [Google Scholar] [CrossRef]
- Morishita, Y.; Matsuzaki, T.; Hara-Chikuma, M.; Andoo, A.; Shimono, M.; Matsuki, A.; Kobayashi, K.; Ikeda, M.; Yamamoto, T.; Verkman, A.; et al. Disruption of aquaporin-11 produces polycystic kidneys following vacuolization of the proximal tubule. Mol. Cell. Biol. 2005, 25, 7770–7779. [Google Scholar] [CrossRef]
- Rützler, M.; Rojek, A.; Damgaard, M.V.; Andreasen, A.; Fenton, R.A.; Nielsen, S. Temporal deletion of Aqp11 in mice is linked to the severity of cyst-like disease. Am. J. Physiol. Renal Physiol. 2017, 312, F343–F351. [Google Scholar] [CrossRef]
- Hoshino, Y.; Sonoda, H.; Nishimura, R.; Mori, K.; Ishibashi, K.; Ikeda, M. Involvement of the NADPH oxidase 2 pathway in renal oxidative stress in Aqp11-/- mice. Biochem. Biophys. Rep. 2019, 17, 169–176. [Google Scholar] [CrossRef]
- Rojek, A.; Füchtbauer, E.M.; Füchtbauer, A.; Jelen, S.; Malmendal, A.; Fenton, R.A.; Nielsen, S. Liver-specific Aquaporin 11 knockout mice show rapid vacuolization of the rough endoplasmic reticulum in periportal hepatocytes after amino acid feeding. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G501–G515. [Google Scholar] [CrossRef]
- Fransen, M.; Nordgren, M.; Wang, B.; Apanasets, O. Role of peroxisomes in ROS/RNS-metabolism: Implications for human disease. Biochim. Biophys. Acta Mol. Basis Dis. 2012, 1822, 1363–1373. [Google Scholar] [CrossRef] [PubMed]
- Antonenkov, V.D.; Grunau, S.; Ohlmeier, S.; Hiltunen, J.K. Peroxisomes Are Oxidative Organelles. Antioxid. Redox Signal 2010, 13, 525–537. [Google Scholar] [CrossRef]
- Yamashita, H.; Avraham, S.; Jiang, S.; London, R.; Van Veldhoven, P.P.; Subramani, S.; Rogers, R.A.; Avraham, H. Characterization of human and murine PMP20 peroxisomal proteins that exhibit antioxidant activity in vitro. J. Biol. Chem. 1999, 274, 29897–29904. [Google Scholar] [CrossRef] [PubMed]
- Vonk, W.I.M.; Wijmenga, C.; Berger, R.; van de Sluis, B.; Klomp, L.W.J. Cu,Zn superoxide dismutase maturation and activity are regulated by COMMD1. J. Biol. Chem. 2010, 285, 28991–29000. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Chan, L.; Bartuzi, P.; Melton, S.D.; Weber, A.; Ben-Shlomo, S.; Varol, C.; Raetz, M.; Mao, X.C.; Starokadomskyy, P.; et al. Copper Metabolism Domain-Containing 1 Represses Genes That Promote Inflammation and Protects Mice From Colitis and Colitis-Associated Cancer. Gastroenterology 2014, 147, 184–195. [Google Scholar] [CrossRef]
- Bartuzi, P.; Wijshake, T.; Dekker, D.C.; Fedoseienko, A.; Kloosterhuis, N.J.; Youssef, S.A.; Li, H.Y.; Shiri-Sverdlov, R.; Kuivenhoven, J.A.; de Bruin, A.; et al. A cell-type-specific role for murine Commd1 in liver inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2014, 1842, 2257–2265. [Google Scholar] [CrossRef]
- Zhang, L.L.; Li, L.H.; Li, Y.L.; Jiang, H.; Sun, Z.; Zang, G.Y.; Qian, Y.J.; Shao, C.; Wang, Z.Q. Disruption of COMMD1 accelerates diabetic atherosclerosis by promoting glycolysis. Diabetes Vasc. Dis. Res. 2023, 20, 14791641231159009. [Google Scholar] [CrossRef]
- Dong, D.F.; Zhou, H.S.; Na, S.Y.; Niedra, R.; Peng, Y.B.; Wang, H.J.; Seed, B.; Zhou, G.L. GPR108, an NF-κB activator suppressed by TIRAP, negatively regulates TLR-triggered immune responses. PLoS ONE 2018, 13, e0205303. [Google Scholar] [CrossRef]
- You-Ten, K.E.; Muise, E.S.; Itie, A.; Michaliszyn, E.; Wagner, J.; Jothy, S.; Lapp, W.S.; Tremblay, M.L. Impaired bone marrow microenvironment and immune function in T cell protein tyrosine phosphatase-deficient mice. J. Exp. Med. 1997, 186, 683–693. [Google Scholar] [CrossRef]
- Hwang, I.T.; Chung, Y.M.; Kim, J.J.; Chung, J.S.; Kim, B.S.; Kim, H.J.; Kim, J.S.; Do Yoo, Y. Drug resistance to 5-FU linked to reactive oxygen species modulator 1. Biochem. Biophys. Res. Commun. 2007, 359, 304–310. [Google Scholar] [CrossRef]
- Hohl, M.; Mayr, M.; Lang, L.S.; Nickel, A.G.; Barallobre-Barreiro, J.; Yin, X.K.; Speer, T.; Selejan, S.R.; Goettsch, C.; Erb, K.; et al. Cathepsin A contributes to left ventricular remodeling by degrading extracellular superoxide dismutase in mice. J. Biol. Chem. 2020, 295, 12605–12617. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, H.; Chen, X.; Wang, N.N.; Zhan, Y.F.; Huang, Z.Y.; Ruan, K.Y.; Qi, Q.L.; Deng, M.; Jiang, Y.X. A novel tRNA-derived fragment tRF-3023b suppresses inflammation in RAW264.7 cells by targeting Cul4a through NF-κB signaling. Func. Integr. Genom. 2024, 24, 9. [Google Scholar] [CrossRef] [PubMed]
- Endeward, V.; Gros, G.; Jürgens, K.D. Significance of myoglobin as an oxygen store and oxygen transporter in the intermittently perfused human heart: A model study. Cardiovasc. Res. 2010, 87, 22–29. [Google Scholar] [CrossRef]
- Godecke, A.; Flogel, U.; Zanger, K.; Ding, Z.; Hirchenhain, J.; Decking, U.K.; Schrader, J. Disruption of myoglobin in mice induces multiple compensatory mechanisms. Proc. Natl. Acad. Sci. USA 1999, 96, 10495–10500. [Google Scholar] [CrossRef]
- Grange, R.W.; Meeson, A.; Chin, E.; Lau, K.S.; Stull, J.T.; Shelton, J.M.; Williams, R.S.; Garry, D.J. Functional and molecular adaptations in skeletal muscle of myoglobin-mutant mice. Am. J. Physiol. Cell Physiol. 2001, 281, C1487–C1494. [Google Scholar] [CrossRef] [PubMed]
- Schwanhäusser, B.; Busse, D.; Li, N.; Dittmar, G.; Schuchhardt, J.; Wolf, J.; Chen, W.; Selbach, M. Global quantification of mammalian gene expression control. Nature 2011, 473, 337–342. [Google Scholar] [CrossRef]
- Ma, J.; Chen, T.; Wu, S.; Yang, C.; Bai, M.; Shu, K.; Li, K.; Zhang, G.; Jin, Z.; He, F.; et al. iProX: An integrated proteome resource. Nucleic Acids Res. 2019, 47, D1211–D1217. [Google Scholar] [CrossRef]
- Chen, T.; Ma, J.; Liu, Y.; Chen, Z.G.; Xiao, N.; Lu, Y.T.; Fu, Y.J.; Yang, C.Y.; Li, M.S.; Wu, S.F.; et al. iProX in 2021: Connecting proteomics data sharing with big data. Nucleic Acids Res. 2022, 50, D1522–D1527. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).