Abstract
The present research examined the impact of L-glutamic acid (Glu) supplementation on the growth performance, muscle composition, gene expression correlated with muscle growth, and intestinal health of largemouth bass. There were 525 fish in total, which were distributed randomly into five groups. Each group had three replicates, and each replicate consisted of 35 fish. Groups with control and experimental diets were assigned glutamic acid amounts of 0.2%, 0.4%, 0.6%, and 0.8%. The findings demonstrated that glutamic acid supplementation enhanced growth performance, feed intake (FI), and condition factor (CF), with the best value being attained at 0.4% Glu. The mean muscle fiber area was increased and the muscle fiber density was decreased in the 0.6% Glu group. The levels of total amino acids and specific amino acids, such as glutamic acid, aspartic acid, leucine, valine, alanine, and glycine, were shown to be higher in the 0.6% Glu group. In the 0.6% Glu group, the mRNA expression levels of atrogin-1, murf-1, foxo3a, and 4e-bp1 were decreased compared to the control group. Conversely, the mRNA expression levels of myf5, myog, myod, s6k1, tor, akt, and pi3k were increased in the 0.6% Glu group compared to the control group. The 0.4% Glu group had higher intestinal amylase, lipase, and protease activities and greater villus height, villus width, and muscle thickness. In summary, Glu can support largemouth bass growth, muscular development, intestinal digestion, and absorption.
Key Contribution:
This study was conducted to investigate the effects of dietary HMB on growth performance, muscle development and intestinal health of largemouth bass and to explore the possible mechanisms.
1. Introduction
Recently, due to the progress of the economic landscape, there has been a growing demand for aquatic items among individuals [1]. The aquaculture sector has risen rapidly, with worldwide fish production rising from 2.6 million tons in 1970 to 87.5 million tons in 2020 [2]. As people’s demand for aquatic products continues to expand, various high-density, high-yield factory farming methods continue to innovate [3]. The increase in the total output of the aquaculture industry has made the competition in the aquaculture market more intense. People are eager to find ways to increase the output of aquatic products, enhance the health of aquatic products, and improve the meat quality of aquatic products [4].
In a previous study, amino acids, as the main component of proteins, undertake a variety of biological functions, including maintaining cell growth [5] and promoting the synthesis of physiological factors [6] and oxidation energy supply [7]. In the study of carnivorous fish, some amino acids can improve the growth performance of the fish, promote muscle development, improve muscle quality [8], improve intestinal digestion [9], and promote intestinal health [10]. L-glutamic acid (Glu) is a functional feed additive [11]. It can provide energy for animal tissues and participate in many physiological regulation processes [12]. Research has demonstrated that adding glutamate to the diet can enhance the growth performance of Ctenopharyngodon idella, enhance the antioxidant capacity of the intestinal tract, and facilitate digestion and absorption [13]. And studies have shown that glutamate can affect the growth, muscle development, and muscle quality of Ctenopharyngodon idellus by different regulation of protein metabolism, muscle development, and antioxidant-related genes [14]. In summary, glutamic acid as a functional additive has achieved good research results, but it has not been reported in the study of largemouth bass.
As a carnivorous freshwater fish, largemouth bass has high economic benefits [15], which can be well adapted to a variety of breeding modes [16]. It also has the advantages of rapid growth, delicious flavor, and is boneless between the muscles, which makes it popular in the Chinese market [17]. According to the statistics of the Fisheries Administration of the Ministry of Agriculture and Rural Affairs and the Chinese Aquatic Association, the output of largemouth bass reached 802,500 tons in 2022 [18]. Nevertheless, research is scarce about the impact of glutamic acid as a dietary supplement on the growth performance, intestinal health, and muscular growth and development of largemouth bass. Therefore, the present research was performed to examine the impacts of dietary glutamic acid on the developmental performance, intestinal health, and muscular development and growth of largemouth bass.
2. Generally Used Wording ID: Materials and Methods
2.1. Preparation of Experimental Diet
Five experimental diets were established, each containing equal amounts of nitrogen and lipids. The diets included fish meal, chicken meal, cottonseed meal, soybean meal, and soybean protein concentrate as the primary sources of protein. Fish oil and soybean oil were used as the primary sources of fat, while high-gluten flour served as the main source of sugar. Glutamic acid was added at 0.2%, 0.4%, 0.6%, and 0.8%. From McLean Biochemical Technology Co., Ltd Shanghai China., glutamic acid was acquired. After being crushed, the feed components were run through an 80-mesh sieve. The low-dose raw components were gradually pre-mixed before being combined in a V-type mixer for a thorough mixing. The oil-kneading machine was then filled with fish and soybean oils to begin the kneading process. After the oil was mixed, 30% water was added to the mixer to stir and mix, and then the SLX-80 twin-screw extruder (South China University of Technology, Guangzhou, China) was used to make a pellet feed with a particle size of 2.0 mm. The pellet feed was allowed to naturally cool to 55 °C before being wrapped in a bag and refrigerated at −20 °C for subsequent use (Table 1).

Table 1.
Formulation and composition of experimental diets (air-dry basis).
2.2. Experimental Design and Feeding Management
The feeding experiment was conducted in an indoor circulating water system at the Guangdong Academy of Agricultural Sciences’ Baiyun Experimental Base. Largemouth bass juveniles were purchased from Renzhi (Guangzhou China) Technology Co., Ltd. A total of 525 healthy fish, each weighing 8.00 ± 0.00 g, were selected and categorized into 5 groups. Each group had 3 repetitions, resulting in a total of 15 replicates. For 56 days, the fish were fed satiating meals twice per day (7:30 and 17:30). The ideal environmental conditions are natural light in summer, water temperature 27~31 °C, no heating equipment, ammonia nitrogen concentration below 0.20 mg/L, nitrite concentration below 0.01 mg/L, dissolved oxygen concentration above 5.0 mg/L, and pH levels between 7.8 and 8.2.
2.3. Sample Analysis
2.3.1. Evaluation of the Growth Performance and Physical Composition
Following the feeding experiment, the fish were deprived of food for 24 h. They were then weighed and numbered in each barrel, and the final average weight and survival rate were determined. From each replicate, a total of 10 fish were selected at random, and 3 of them were utilized to determine the overall body composition. Six fish were used for measuring body weight and body length, dissecting visceral mass, separating liver and weighing, and calculating body index. Additionally, 3 fish were picked from each cage. The foregut and liver were then separated and preserved in 4% paraformaldehyde. These samples were then maintained to section the intestinal and hepatic tissues.
2.3.2. Nutrient Composition of Diets and Muscle
The moisture content was evaluated by subjecting the sample to oven drying at a temperature of 105 °C until a consistent weight was achieved. The Kjeldahl method was employed to determine the protein content in both whole fish and muscle [19]. The ether Soxhlet extraction method was used to determine the crude fat content. The ash content was measured by subjecting the sample to combustion in a muffle furnace until a steady weight was achieved at a temperature of 550 °C [20].
2.3.3. Histological Analysis
One fish was randomly selected from each replicate, and the middle part of the dorsal muscle and the foregut were sliced on ice. The muscle and intestine sections were subjected to histological processing using the paraffin procedure and stained with the hematoxylin and eosin (H&E) staining method. The tissue was washed with a 0.01 mol/L PBS solution, treated with 4% paraformaldehyde for 10 min to fix it, slowly dehydrated in 75% ethanol, and finally embedded in paraffin and sliced into sections that were 5 μm thick. Finally, stained with eosin and hematoxylin, the intestinal and muscle tissues were observed under a microscope and photographed. The intestinal villus height, muscle thickness, villus width, muscle fiber density, muscle fiber number, and average muscle fiber area were measured [21].
2.3.4. Analysis of Muscle Amino Acid Content
The analysis revealed the presence of fifteen different amino acids, namely arginine (Arg), lysine (Lys), histidine (His), phenylalanine (Phe), tyrosine (Tyr), leucine (Leu), isoleucine (Ile), methionine (Met), valine (Val), alanine (Ala), glycine (Gly), glutamic acid (Glu), serine (Ser), threonine (Thr), and asparagine (Asp). The mobile phase A was 40 mmol/L sodium dihydrogen phosphate (pH = 7.8); the detection signal was UV 339 nm, fluorescence (EX = 266 nm, EM = 305 nm). Acetonitrile, methanol, and water comprised the mobile phase B; the ratios were 45, 45, and 10, respectively. Every experimental technique was carried out strictly in line with the standard instructions; the measured findings deviate by less than 10% of the arithmetic mean value, and the correlation coefficient of every amino acid’s linear regression equation is >0.99.
2.3.5. Determination of Intestinal Enzyme Activity
The foregut was immediately frozen in a refrigerator at −80 °C. After weighing, the samples were homogenized with a high-speed tissue homogenizer and centrifuged with a refrigerated centrifuge (at 3000 r/min for 15 min at 4 °C). Trypsin activity was determined using r-toluenesul-phonyl-l-arginine methyl esther as a substrate in 0.05-mol/LTriseHCl buffer, (pH = 9.0). Amylase was assayed using 1% solublestarch as a substrate in 0.02 mol/L phosphate buffer, (pH = 8.0). Lipase activity was measured at 405 nm by the rate of methyl-resorufin formation.
2.3.6. Real-Time Quantitative PCR Analysis
The protein–nucleic acid approach was utilized to ascertain the total RNA concentration after largemouth bass muscle was treated with Trizol reagent to extract RNA. The process of reverse transcription was employed to convert the total RNA into complementary DNA (cDNA) using the TaKaRa reverse transcription kit (Takara, Tokyo, Japan) [22]. Primer Premier 5.0 software was used for primer design, cDNA sequences from Gen Bank or published papers, and primer sequences from Wang et al. (2021) [23] (Table 2). Using β-actin as an internal reference, the 2−ΔΔCt technique was used to calculate the relative expression of the gene based on the control group.

Table 2.
Primer sequences of target genes used in real-time quantitative PCR.
2.3.7. Statistical Analysis
Using SPSS 26.0, a one-way ANOVA was run on each result, and then the Tukey multiple comparisons procedure was applied. The statistical significance threshold was set at p < 0.05, and the data were presented as mean ± SD.
3. Results
3.1. Growth Performance
Table 3 displays growth performance and body indexes. Compared to the control group, the FBW, SGR, and WGR of the 0.2% and 0.4% Glu groups were considerably greater (p < 0.05). Significant differences were seen between the FCR of the 0.2% and 0.4% Glu groups and that of the control group (p < 0.05). Additionally, there was no statistically significant difference in the VSI between the groups, and the HSIs of the 0.4%, 0.6%, and 0.8% Glu groups were all considerably greater than those of the control group (p > 0.05).

Table 3.
Effects of dietary Glu supplementation on growth performance of largemouth bass.
3.2. Body Composition
Table 4 displays the body composition of the largemouth bass. The levels of protein, fat, moisture, and ash content were not significantly different across the groups (p > 0.05).

Table 4.
Effects of dietary Glu on body composition of largemouth bass.
3.3. Muscle Amino Acid Composition and Inosine Monophosphate Content
Table 5 displays the inosine monophosphate concentration and amino acid composition of largemouth bass muscle. There was a significant difference between the crude protein levels, total amino acid content, and inosine monophosphate amounts of the 0.2%, 0.4%, 0.6%, and 0.8% Glu groups and the control group (p < 0.05). The crude protein level of the 0.6% Glu group was the highest, while the total amino acid and inosine monophosphate contents were the highest at 0.4% Glu. The levels of glutamic acid, aspartic acid, and tyrosine in the 0.4% Glu and 0.6% Glu groups were considerably greater than those in the control group (p < 0.05). After adding glutamic acid, the contents of phenylalanine, alanine, and glycine were also increased. The levels of serine, threonine, valine, methionine, isoleucine, leucine, and lysine in Glu solutions with concentrations of 0.2%, 0.4%, 0.6%, and 0.8% were considerably greater than those in the control group (p < 0.05).

Table 5.
Effects of dietary Glu on muscle amino acid composition of largemouth bass.
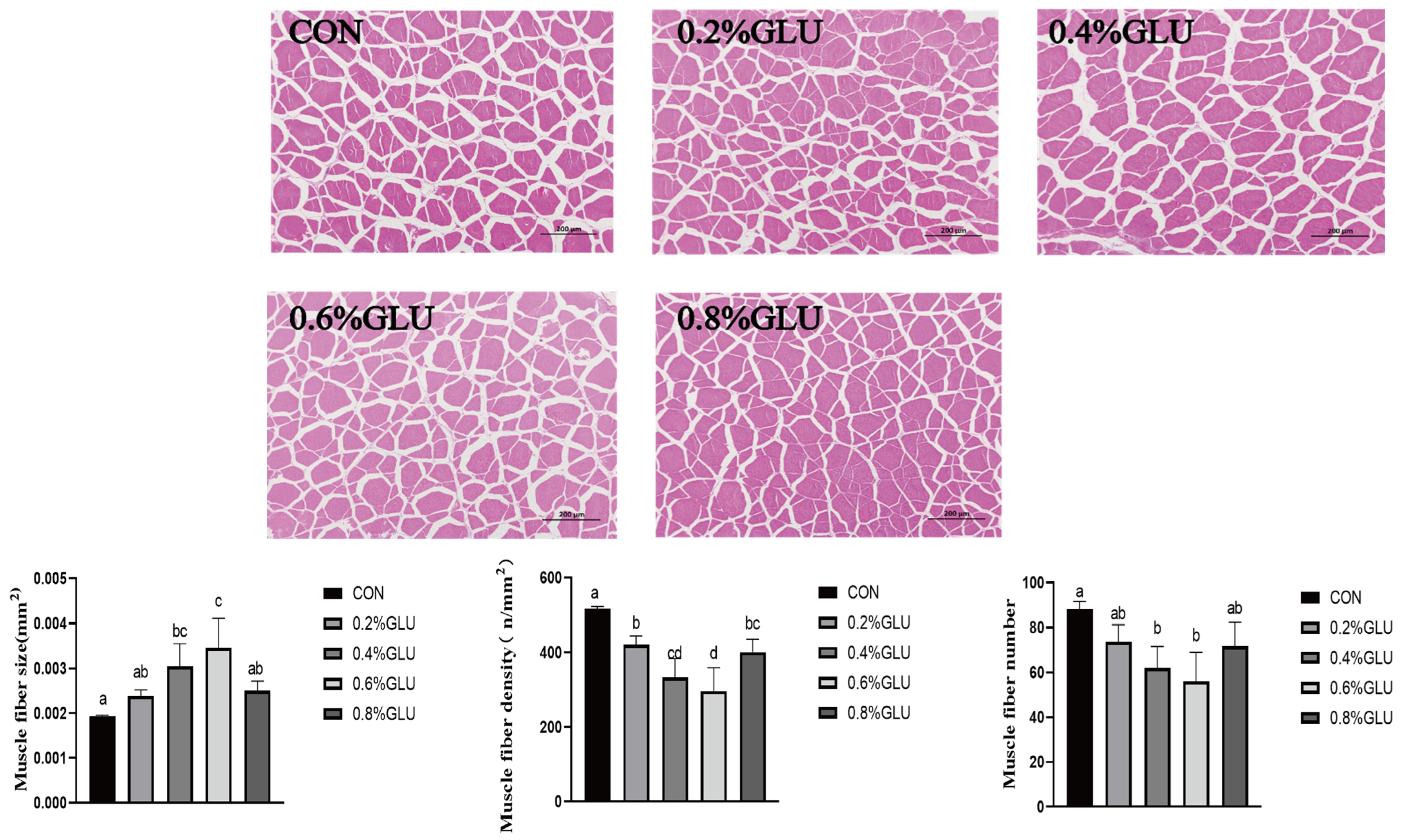
3.4. Proximate Composition and Histological Traits of Muscle Fibers
Muscle sections are shown in Figure 1. In comparison to the control group, the muscle fiber size of the 0.4% and 0.6% Glu groups was substantially larger (p < 0.05). In comparison to the control group, the muscle fiber density and number of the 0.6% and 0.7% Glu groups were much lower (p < 0.05).
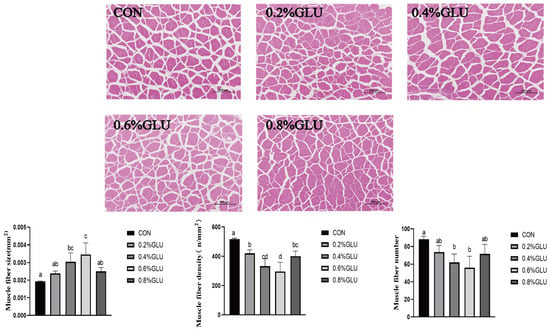
Figure 1.
Effects of dietary Glu on muscle histology of largemouth bass. Means in the same row with different superscripts are significantly different (mean ± SD; ANOVA, p < 0.05; n = 3).
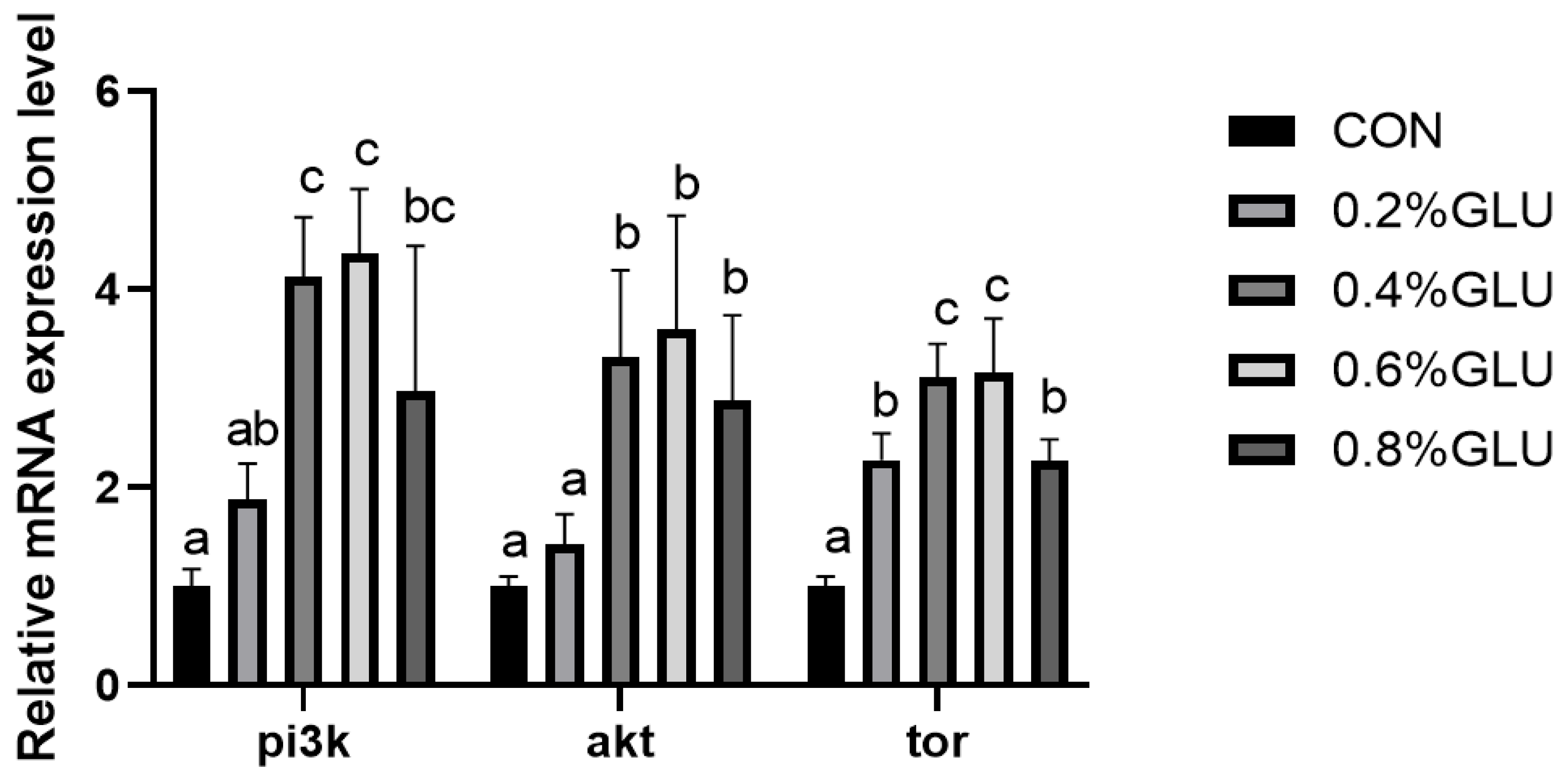
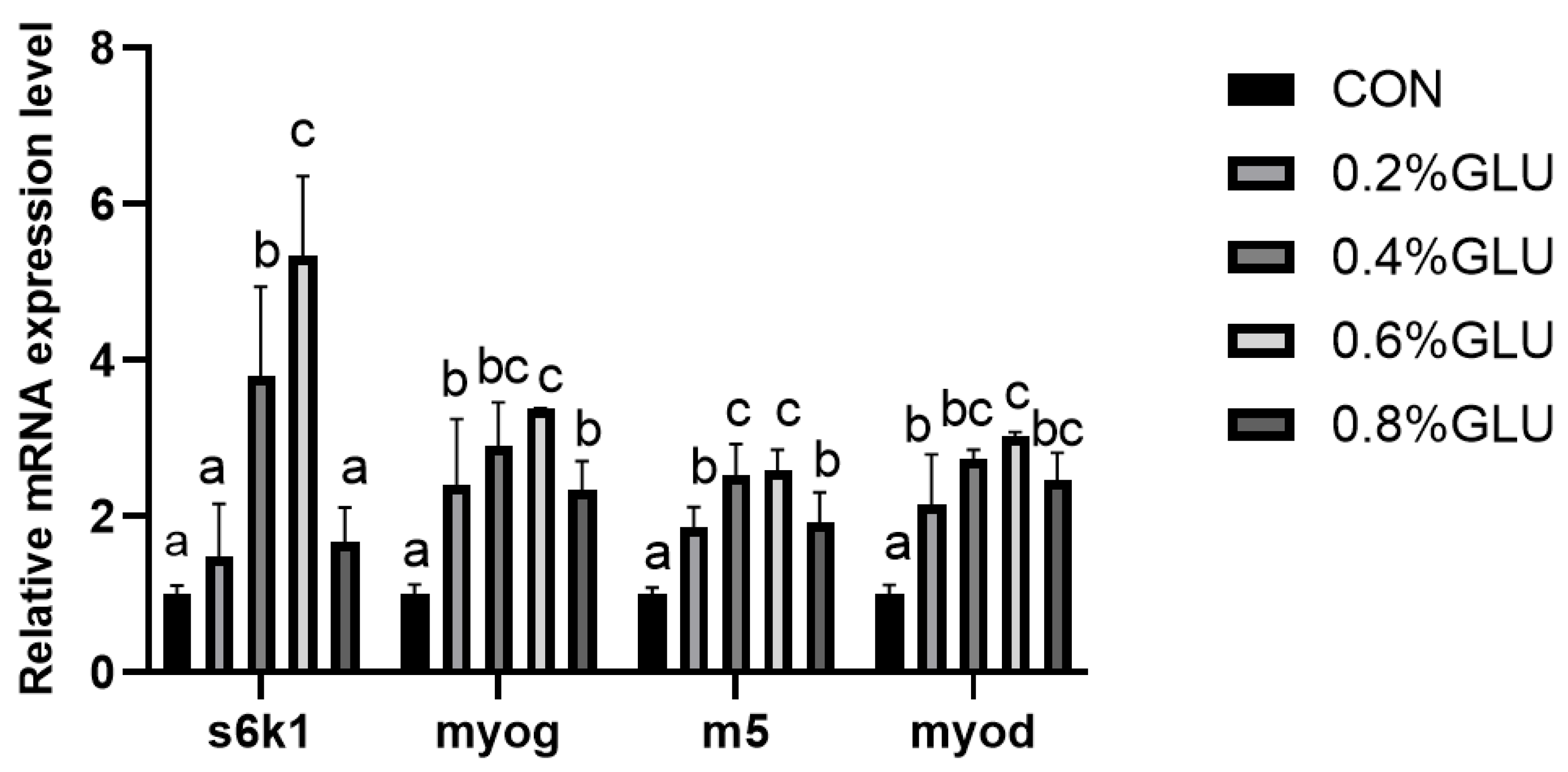
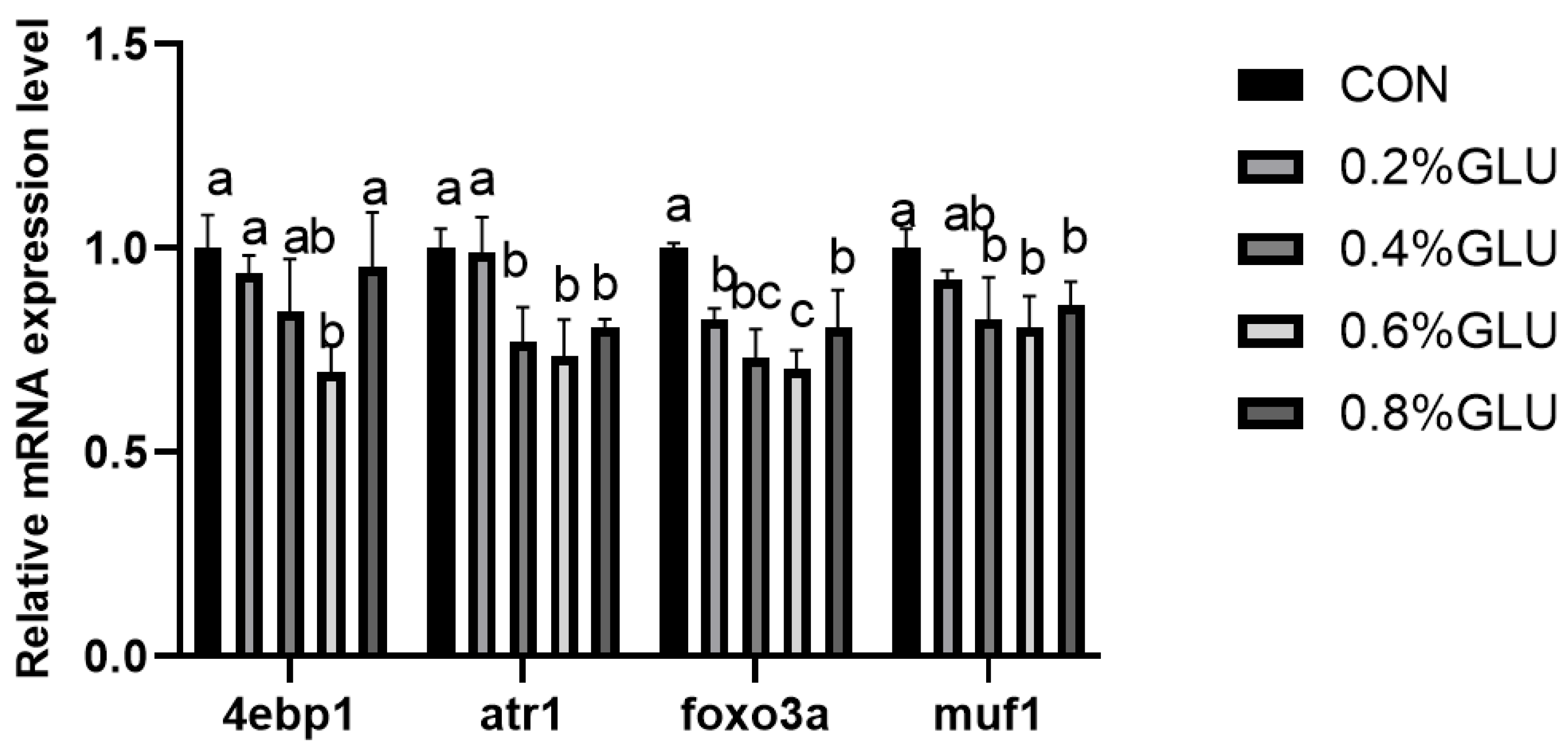
3.5. Muscle Growth and Development-Related Gene Expression
pi3k, akt, and tor mRNA expression levels in the 0.4% Glu, 0.6% Glu, and 0.8% Glu groups were considerably greater than those in the control group (Figure 2, Figure 3 and Figure 4). In comparison to the control group, the mRNA expression levels of s6k1, myod, myog, and myf5 in the 0.4% and 0.6% Glu groups were considerably higher (p < 0.05). 4e-bp1, foxo3a, murf-1, and atrogin-1 had significantly reduced mRNA expression levels in the 0.6% Glu group compared to the control group (p < 0.05).
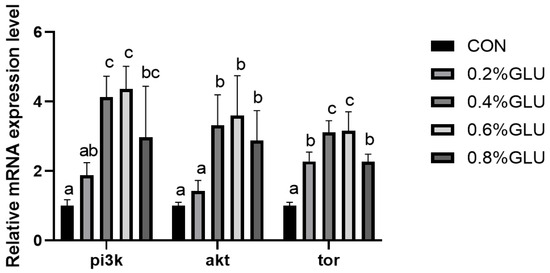
Figure 2.
Effects of dietary Glu supplementation on the relative mRNA expression levels of phosphatidyl inositol 3 kinase (pi3k), protein kinase B (akt), and target of rapamycin (tor) in the muscle of largemouth bass. Means in the same row with different superscripts are significantly different (mean ± SD; ANOVA, p < 0.05; n = 3).
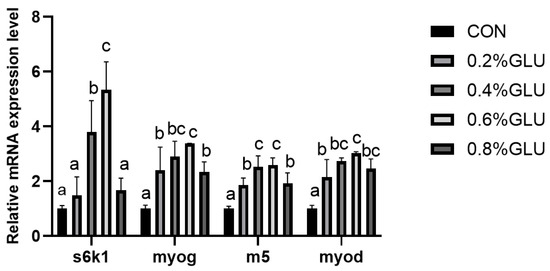
Figure 3.
Relative mRNA expression level of 70 kDa ribosomal protein S6 kinase 1 (S6K1), myoblast determination protein (myod), myogenin (myog), myogenic factor 5 (myf5) in the muscle of largemouth bass fed Glu diets. Means in the same row with different superscripts are significantly different (mean ± SD; ANOVA, p < 0.05; n = 3).
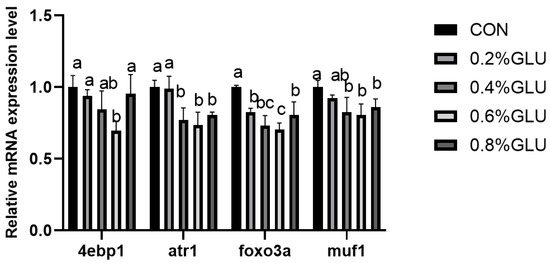
Figure 4.
Relative mRNA expression level of eukaryotic translation initiation factor 4E-binding protein 1 (4e-bp1), forkhead boxO3a (foxo3a), muscle-specific RING finger protein 1 (murfF-1), and atrogin-1 in the muscle of largemouth bass fed Glu diets. Means in the same row with different superscripts are significantly different (mean ± SD; ANOVA, p < 0.05; n = 3).
3.6. Intestinal Enzyme Activity
Protease, lipase, and amylase activity in the 0.2% and 0.4% Glu groups were significantly higher than those in the control group (p < 0.05). The highest concentrations of lipase, protease, and amylase were found in the 0.6%, 0.4%, and 0.6% Glu groups, respectively (Table 6).

Table 6.
Effects of dietary glutamate on intestinal villus height, villus width, and muscle thickness of largemouth bass.
3.7. Intestinal Tissue Structure
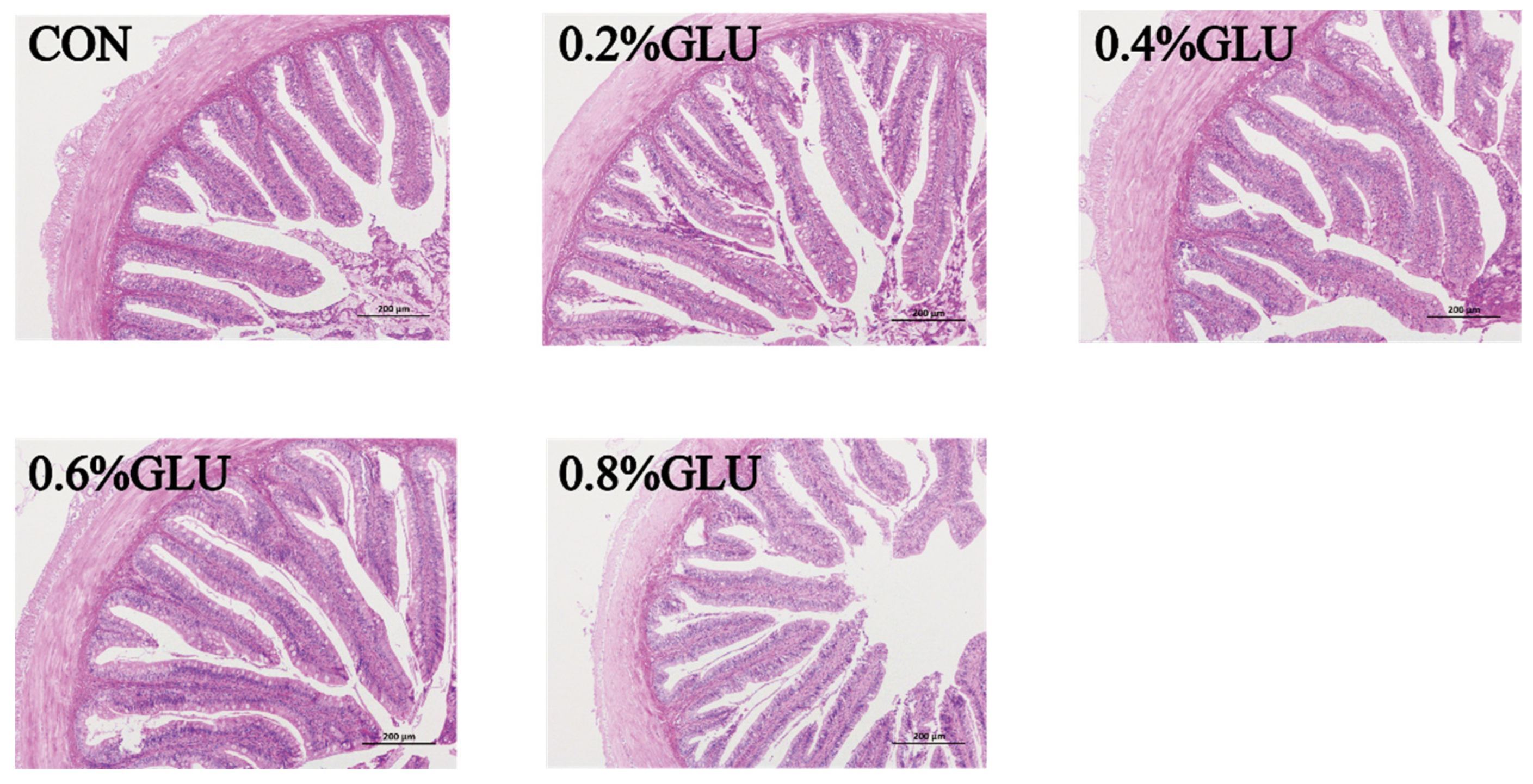
Compared to the control group, the villus height of the 0.4% Glu group was substantially higher (p < 0.05). The width of the 0.6% Glu group was substantially greater than that of the control group (p < 0.05). The thickness of the intestinal walls did not significantly differ across the groups (Table 7 and Figure 5).

Table 7.
Effects of dietary glutamate on intestinal villus height, villus width, and muscle layer thickness of largemouth bass after 8 weeks of feeding.
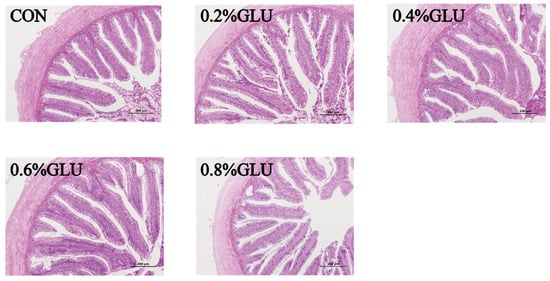
Figure 5.
Effects of dietary Glu supplementation on intestinal morphology of largemouth bass.
4. Discussion
This research shows that adding glutamic acid to largemouth bass can improve the growth performance of largemouth bass. Additionally, it boosted their feed intake and condition factor. This was similar to the results of Jian carp (Cyprinus carpio var. Jian) with dietary glutamate levels of 68.4 and 83.4 g/kg [24]. Administration of 0.25% of glutamic acid to Ctenopharyngodon idellus also had the same effect [14]. In recent years, more and more studies have shown that non-essential amino acids such as glutamic acid, glycine [25], and proline [26] can also promote the growth of fish, thereby enhancing the growth performance of fish. The potential cause for the enhancement in growth performance in largemouth bass due to glutamic acid supplementation may be attributed to the observed increase in meal intake. This glutamic acid experiment was carried out in summer and achieved results such as improving fish growth performance.
In this study, the addition of glutamic acid to the feed can increase the crude protein content of largemouth bass. In a prior study, it was found that adding 4% glutamic acid boosted the protein content of Sparus aurata [27]. Similarly, adding 3.2% glutamic acid to the diet of triploid crucian carp resulted in the same finding. The protein level in aquatic animals is strongly linked to protein turnover, which is a key element influencing muscle growth and the quality of meat [28]. The important gene-level pathway is the pi3k/akt signaling pathway [29]. mTOR phosphorylation can be enhanced by the control of protein phosphorylation in the pi3k/akt pathway [30]. In addition, s6k1 and 4e-bp1 are downstream targets of tor signal transduction [31]. Two downstream effectors can be directly phosphorylated by tor, which stimulates the start and translation of protein transcription and controls cell activity to control protein synthesis. Conversely, foxo3a functions as a downstream effector of the pi3k signaling pathway and is essential for the degradation of proteins [32]. Dephosphorylation of foxo3a is induced by inhibition of akt phosphorylation, and dephosphorylated foxo3a enters the nucleus upon activation by stimulating the production of muscle ring-finger gene 1 (murf1) and muscle atrophy Fbox (mafbx/atrogin-1), causing the myofibrillar protein to degrade [33]. The mRNA expression of s6k1, tor, akt, pi3k, myod, myog, and myf5 was elevated in this study by dietary glutamate. Atrogin-1, murf-1, foxo3a, and 4e-bp1 all had downregulated mRNA expression levels. The findings demonstrated that by stimulating the pi3k/akt pathway, Glu might increase the synthesis of muscle protein and decrease its decomposition.
Precursors of flavor compounds include glutamic acid, aspartic acid, leucine, and valine. These compounds are strongly linked to the development of muscle flavor [34]. The sweet amino acids alanine and glycine may help to increase the sweetness of fish during chewing [35]. In this study, the inclusion of glutamic acid in the meal raised the amount of umami amino acids, including aspartic acid, leucine, valine, alanine, and glycine, as well as the overall amino acid content of the largemouth bass muscle. This is consistent with the result of adding 3.2% Glu to crucian carp. Inosine monophosphate is an important flavor substance in fish muscle [36]. The inclusion of glutamic acid in the food resulted in an elevation of inosine monophosphate levels in the muscle tissue of largemouth bass observed in this study.
Muscle growth is related to the number and area of muscle fibers [37]. Diet plays an important role in the balance between muscle fiber hyperplasia and hypertrophy [38]. The study found that incorporating glutamic acid into the diet increased the average size of muscle fibers in largemouth bass and decreased muscle fiber density. This suggests that glutamic acid effectively stimulates muscle growth in largemouth bass. In short, the inclusion of glutamic acid in the meal resulted in an augmentation of the protein composition in the muscle of largemouth bass. This led to the stimulation of muscle growth and development, as well as an increase in the concentration of umami amino acids.
The gastrointestinal system is the primary site for the breakdown and assimilation of nutrients in fish [39]. The morphological structure of the digestive tract is an important basis for maintaining the growth status of largemouth bass [40]. The intestine is the main digestive organ of fish [41]. The integrity of the intestinal structure has a direct impact on the process of digesting and absorbing nutrients [42], as well as on the growth and development of fish [43]. Muscle layer thickness (MT) [44], villus height (VH), and villus width (VW) [45] are usually used to evaluate the intestinal digestion ability of animals [46]. The increase in villus height [47] and muscle layer thickness [48] can increase the surface area of the fish intestine and improve its ability to digest and absorb feed nutrients [49]. The study found that adding glutamic acid to the diet of largemouth bass elevated the thickness of the intestinal muscle, as well as the height and width of the villi. The activity of digestive enzymes is also crucial to the digestive capacity of largemouth bass [50]. After entering the body of largemouth bass, macromolecular nutrients such as protein and fatty acids need to be decomposed into small molecules by digestive enzymes in the digestive tract before they can be absorbed by the digestive tract [51]. As the first step to obtain nutrients, digestive enzyme activity is closely related to nutrient utilization efficiency [52]. The presence of lipase [53], protease [54], and amylase [55] in the fish gut can impact the process of absorbing and utilizing nutrients [56]. As a result, this has an impact on the proliferation and development of the fish [57]. These findings demonstrated that the inclusion of glutamic acid in the meal enhanced the enzymatic activity of amylase, protease, and lipase in the intestine of largemouth bass. The aforementioned findings suggest that including Glu in the meal enhances the digestive capacity and fosters intestinal well-being in largemouth bass. This is comparable to the effects of glutamic acid on Jian carp (Cyprinus carpio var. Jian) [24].
5. Conclusions
In summary, our findings showed that largemouth bass can benefit from Glu in terms of growth performance, muscular development, and intestinal digestion. Taking growth performance as the index, the optimum addition amount of Glu was 0.4%, and taking muscle development as the index, the optimum addition amount of Glu was 0.6%.
Author Contributions
Conceptualization, F.J., H.G. and X.L.; Methodology, F.J., X.L. and Z.Y.; Software, W.H., H.G., Z.Y. and M.S.; Validation, M.S.; Formal analysis, F.J., H.G., Z.Y., M.S. and Y.H.; Investigation, M.Z. and X.L.; Resources, W.H.; Data curation, M.S.; Writing—original draft, F.J.; Writing—review and editing, F.J.; Visualization, F.J.; Supervision, W.H. and Y.H.; Project administration, M.Z. and Y.H.; Funding acquisition, M.Z. and Y.H. All authors have read and agreed to the published version of the manuscript.
Funding
The research was supported by the innovation team project of Guangdong Provincial Department of Education, the innovation team of functional feed and animal immune regulation (2020KCXTD019), and the research and development of special feed for California bass (D122222L6).
Institutional Review Board Statement
The animal management procedures followed the guidelines of the Animal Care and Use Committee of Zhongkai Agricultural Engineering University (ethical approval number: ZHKUMO-2022-055).
Data Availability Statement
The datasets of the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
Author Wenqing Huang was employed by the company Guangzhou Fishteach Biotechnology. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Pincinato, R.B.M. Market aspects and external economic effects of aquaculture. Aquac. Econ. Manag. 2021, 25, 127–134. [Google Scholar] [CrossRef]
- Afewerki, S.; Asche, F.; Misund, B.; Thorvaldsen, T.; Tveteras, R. Innovation in the Norwegian aquaculture industry. Rev. Aquac. 2023, 15, 759–771. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, Y. Marine factory farming techniques and equipment. IOP Conf. Ser. Earth Environ. Sci. 2020, 615, 012013. [Google Scholar] [CrossRef]
- He, J.; Feng, P.; Lv, C.; Lv, M.; Ruan, Z.; Yang, H.; Ma, H.; Wang, R. Effect of a fish–rice co-culture system on the growth performance and muscle quality of tilapia (Oreochromis niloticus). Aquac. Rep. 2020, 17, 100367. [Google Scholar] [CrossRef]
- Yu, J.; Yang, H.; Wang, Z.; Dai, H.; Xu, L.; Ling, C. Effects of arginine on the growth performance, hormones, digestive organ development and intestinal morphology in the early growth stage of layer chickens. Ital. J. Anim. Sci. 2018, 17, 1077–1082. [Google Scholar] [CrossRef]
- Garcia, I.S.; Teixeira, S.A.; Costa, K.A.; Marques, D.B.D.; Rodrigues, G.d.A.; Costa, T.C.; Guimarães, J.D.; Otto, P.I.; Saraiva, A.; Ibelli, A.M.G.; et al. l-Arginine supplementation of gilts during early gestation modulates energy sensitive pathways in pig conceptuses. Mol. Reprod. Dev. 2020, 87, 819–834. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Liu, Z.; Zhou, Y.; Wei, H.; Zhang, X.; Xia, M.; Deng, Z.; Zou, Y.; Jiang, S.; Peng, J. Effect of oregano essential oil supplementation to a reduced-protein, amino acid-supplemented diet on meat quality, fatty acid composition, and oxidative stability of Longissimus thoracis muscle in growing-finishing pigs. Meat Sci. 2017, 133, 103–109. [Google Scholar] [CrossRef]
- Wu, Y.-Y.; Dai, Y.-J.; Xiao, K.; Wang, X.; Wang, M.-M.; Huang, Y.-Y.; Guo, H.-X.; Li, X.-F.; Jiang, G.-Z.; Liu, W.-B. Effects of different dietary ratio lysine and arginine on growth, muscle fiber development and meat quality of Megalobrama amblycephala. Aquac. Rep. 2022, 26, 101322. [Google Scholar] [CrossRef]
- Ding, L.; Chen, J.; He, F.; Chen, Q.; Li, Y.; Chen, W. Effects of dietary arginine supplementation on growth performance, antioxidant capacity, intestinal digestive enzyme activity, muscle transcriptome, and gut health of Siniperca chuatsi. Front. Mar. Sci. 2024, 10, 1305192. [Google Scholar] [CrossRef]
- Yu, Y.; Huang, D.; Zhang, L.; Chen, X.; Wang, Y.; Zhang, L.; Ren, M.; Liang, H. Dietary arginine levels affect growth performance, intestinal antioxidant capacity and immune responses in largemouth bass (Micropterus salmoides). Aquac. Rep. 2023, 32, 101703. [Google Scholar] [CrossRef]
- Hou, Y.; Wu, G. l-Glutamate nutrition and metabolism in swine. Amino Acids 2018, 50, 1497–1510. [Google Scholar] [CrossRef]
- Hu, C.J.; Jiang, Q.Y.; Zhang, T.; Yin, Y.L.; Li, F.N.; Deng, J.P.; Wu, G.Y.; Kong, X.F. Dietary supplementation with arginine and glutamic acid modifies growth performance, carcass traits, and meat quality in growing-finishing pigs1. J. Anim. Sci. 2017, 95, 2680–2689. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hu, Y.; Zhou, X.-Q.; Zeng, X.-Y.; Feng, L.; Liu, Y.; Jiang, W.-D.; Li, S.-H.; Li, D.-B.; Wu, X.-Q.; et al. Effects of dietary glutamate supplementation on growth performance, digestive enzyme activities and antioxidant capacity in intestine of grass carp (Ctenopharyngodon idella). Aquac. Nutr. 2015, 21, 935–941. [Google Scholar] [CrossRef]
- Cai, Y.; He, L.; Cao, S.; Zeng, P.; Xu, L.; Luo, Y.; Tang, X.; Wang, Q.; Liu, Z.; He, Z.; et al. Insights into Dietary Different Co-Forms of Lysine and Glutamate on Growth Performance, Muscle Development, Antioxidation and Related Gene Expressions in Juvenile Grass Carp (Ctenopharyngodon idellus). Mar. Biotechnol. 2024, 26, 74–91. [Google Scholar] [CrossRef]
- Song, R.; Yao, X.; Jing, F.; Yang, W.; Wu, J.; Zhang, H.; Zhang, P.; Xie, Y.; Pan, X.; Zhao, L.; et al. Effects of Five Lipid Sources on Growth, Hematological Parameters, Immunity and Muscle Quality in Juvenile Largemouth Bass (Micropterus salmoides). Animals 2024, 14, 781. [Google Scholar] [CrossRef]
- Li, Y.; Qin, J.; Zheng, X.; Wang, Y. Production performance of largemouth bass Micropterus salmoides and water quality variation in monoculture, polyculture and integrated culture. Aquac. Res. 2019, 50, 423–430. [Google Scholar] [CrossRef]
- Zheng, Z.; Nie, Z.; Zheng, Y.; Tang, X.; Sun, Y.; Zhu, H.; Gao, J.; Xu, P.; Xu, G. Effects of Submerged Macrophytes on the Growth, Morphology, Nutritional Value, and Flavor of Cultured Largemouth Bass (Micropterus salmoides). Molecules 2022, 27, 927. [Google Scholar] [CrossRef]
- Xu, J.-M.; Gao, W.-R.; Liang, P.; Cai, G.-H.; Yang, H.-L.; Lin, J.-B.; Sun, Y.-Z. Pleurotus eryngii root waste and soybean meal co-fermented protein improved the growth, immunity, liver and intestinal health of largemouth bass (Micropterus salmoides). Fish. Shellfish. Immunol. 2024, 149, 109551. [Google Scholar] [CrossRef] [PubMed]
- Ido, A.; Ali, M.-F.-Z.; Takahashi, T.; Miura, C.; Miura, T. Growth of Yellowtail (Seriola quinqueradiata) Fed on a Diet Including Partially or Completely Defatted Black Soldier Fly (Hermetia illucens) Larvae Meal. Insects 2021, 12, 722. [Google Scholar] [CrossRef]
- Jastaniah, S.D.; Mansour, A.A.; Al-Tarawni, A.H.; El-Haroun, E.; Munir, M.B.; Saghir, S.A.M.; Abdul Kari, Z.; Téllez-Isaías, G.; Bottje, W.G.; Al-Farga, A.; et al. The effects of nano-curcumin on growth performance, feed utilization, blood biochemistry, disease resistance, and gene expression in European seabass (Dicentrarchus labrax) fingerlings. Aquac. Rep. 2024, 36, 102034. [Google Scholar] [CrossRef]
- Chen, M.; Li, Q.; Yang, L.; Lin, W.; Qin, Z.; Liang, S.; Lin, L.; Xie, X. Effects of diet containing germinated faba bean (Vicia faba L.) on the intestinal health and gut microbial communities of Nile tilapia (Oreochromis niloticus). Aquac. Rep. 2024, 36, 102053. [Google Scholar] [CrossRef]
- Che, M.; Lu, Z.; Liu, L.; Li, N.; Ren, L.; Chi, S. Dietary lysophospholipids improves growth performance and hepatic lipid metabolism of largemouth bass (Micropterus salmoides). Anim. Nutr. 2023, 13, 426–434. [Google Scholar] [CrossRef]
- Wang, W.; Yang, P.; He, C.; Chi, S.; Li, S.; Mai, K.; Song, F. Effects of dietary methionine on growth performance and metabolism through modulating nutrient-related pathways in largemouth bass (Micropterus salmoides). Aquac. Rep. 2021, 20, 100642. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, T.-R.; Li, Q.; Feng, L.; Liu, Y.; Jiang, W.-D.; Wu, P.; Zhao, J.; Zhou, X.-Q.; Jiang, J. Effect of dietary L-glutamate levels on growth, digestive and absorptive capability, and intestinal physical barrier function in Jian carp (Cyprinus carpio var. Jian). Anim. Nutr. 2020, 6, 198–209. [Google Scholar] [CrossRef]
- Xie, S.; Tian, L.; Niu, J.; Liang, G.; Liu, Y. Effect of N-acetyl cysteine and glycine supplementation on growth performance, glutathione synthesis, and antioxidative ability of grass carp, Ctenopharyngodon idella. Fish. Physiol. Biochem. 2017, 43, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Deng, K.; Zhang, W.; Mai, K. Interactions of dietary vitamin C and proline on growth performance, anti-oxidative capacity and muscle quality of large yellow croaker Larimichthys crocea. Aquaculture 2020, 528, 735558. [Google Scholar] [CrossRef]
- Caballero-Solares, A.; Viegas, I.; Salgado, M.C.; Siles, A.M.; Sáez, A.; Metón, I.; Baanante, I.V.; Fernández, F. Diets supplemented with glutamate or glutamine improve protein retention and modulate gene expression of key enzymes of hepatic metabolism in gilthead seabream (Sparus aurata) juveniles. Aquaculture 2015, 444, 79–87. [Google Scholar] [CrossRef]
- Wei, Z.; Zhuang, Y.; Liu, X.; Zou, D.; Mai, K.; Sun, Z.; Ye, C. Leucine promotes protein synthesis of juvenile white shrimp Litopenaeus vannamei through TOR signaling pathway. Aquaculture 2023, 564, 739060. [Google Scholar] [CrossRef]
- Zhu, X.; Ren, L.; Liu, J.; Chen, L.; Cheng, J.; Chu, W.; Zhang, J. Transcriptome analysis provides novel insights into the function of PI3K/AKT pathway in maintaining metabolic homeostasis of Chinese perch muscle. Aquac. Rep. 2021, 21, 100838. [Google Scholar] [CrossRef]
- Luo, C.; Zhao, S.; Dai, W.; Zheng, N.; Wang, J. Proteomic analyses reveal GNG12 regulates cell growth and casein synthesis by activating the Leu-mediated mTORC1 signaling pathway. Biochim. Et Biophys. Acta (BBA)—Proteins Proteom. 2018, 1866, 1092–1101. [Google Scholar] [CrossRef]
- Gao, Q.; Hou, B.; Yang, H.; Jiang, X. Distinct role of 4E-BP1 and S6K1 in regulating autophagy and hepatitis B virus (HBV) replication. Life Sci. 2019, 220, 1–7. [Google Scholar] [CrossRef]
- Li, J.; Long, H.; Cong, Y.; Gao, H.; Lyu, Q.; Yu, S.; Kuang, Y. Quercetin prevents primordial follicle loss via suppression of PI3K/Akt/Foxo3a pathway activation in cyclophosphamide-treated mice. Reprod. Biol. Endocrinol. 2021, 19, 63. [Google Scholar] [CrossRef]
- Liu, H.-W.; Chen, Y.-J.; Chang, Y.-C.; Chang, S.-J. Oligonol, a Low-Molecular Weight Polyphenol Derived from Lychee, Alleviates Muscle Loss in Diabetes by Suppressing Atrogin-1 and MuRF1. Nutrients 2017, 9, 1040. [Google Scholar] [CrossRef] [PubMed]
- Roobab, U.; Zeng, X.-A.; Ahmed, W.; Madni, G.M.; Manzoor, M.F.; Aadil, R.M. Effect of Pulsed Electric Field on the Chicken Meat Quality and Taste-Related Amino Acid Stability: Flavor Simulation. Foods 2023, 12, 710. [Google Scholar] [CrossRef]
- Dong, M.; Zhang, Y.-Y.; Huang, X.-H.; Xin, R.; Dong, X.-P.; Konno, K.; Zhu, B.-W.; Fisk, I.; Qin, L. Dynamic sensations of fresh and roasted salmon (Salmo salar) during chewing. Food Chem. 2022, 368, 130844. [Google Scholar] [CrossRef]
- Ackroff, K.; Sclafani, A. Flavor Preferences Conditioned by Dietary Glutamate. Adv. Nutr. 2016, 7, 845S–852S. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lin, F.; Lin, J.; Liu, X.; Yuan, Y.; Liu, G.; Ye, X. Effects of temperature on muscle growth and collagen deposition in zebrafish (Danio rerio). Aquac. Rep. 2022, 22, 100952. [Google Scholar] [CrossRef]
- Bao, S.-T.; Liu, X.-C.; Huang, X.-P.; Guan, J.-F.; Xie, D.-Z.; Li, S.-A.; Xu, C. Magnesium supplementation in high carbohydrate diets: Implications on growth, muscle fiber development and flesh quality of Megalobrama amblycephala. Aquac. Rep. 2022, 23, 101039. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, W.-D.; Zhang, J.-X.; Feng, L.; Wu, P.; Liu, Y.; Jiang, J.; Kuang, S.-Y.; Tang, L.; Peng, Y.; et al. Cinnamaldehyde improves the growth performance and digestion and absorption capacity in grass carp (Ctenopharyngodon idella). Fish. Physiol. Biochem. 2020, 46, 1589–1601. [Google Scholar] [CrossRef]
- Yang, W.; Wu, J.; Song, R.; Li, Z.; Jia, X.; Qian, P.; Zhang, H.; Zhang, P.; Xue, X.; Li, S.; et al. Effects of dietary soybean lecithin on growth performances, body composition, serum biochemical parameters, digestive and metabolic abilities in largemouth bass Micropterus salmoides. Aquac. Rep. 2023, 29, 101528. [Google Scholar] [CrossRef]
- Zhang, H.; Ding, Q.; Wang, A.; Liu, Y.; Teame, T.; Ran, C.; Yang, Y.; He, S.; Zhou, W.; Olsen, R.E.; et al. Effects of dietary sodium acetate on food intake, weight gain, intestinal digestive enzyme activities, energy metabolism and gut microbiota in cultured fish: Zebrafish as a model. Aquaculture 2020, 523, 735188. [Google Scholar] [CrossRef]
- Volatiana, J.A.; Wang, L.; Gray, N.; Tong, S.; Zhang, G.; Shao, Q. Tributyrin-supplemented high-soya bean meal diets of juvenile black sea bream, Acanthopagrus schlegelii: Study on growth performance and intestinal morphology and structure. Aquac. Res. 2020, 51, 135–146. [Google Scholar] [CrossRef]
- Lei, W.; Li, J.; Fang, P.; Wu, S.; Deng, Y.; Luo, A.; He, Z.; Peng, M. Effects of Dietary Bile Acids on Growth Performance, Lipid Deposition, and Intestinal Health of Rice Field Eel (Monopterus albus) Fed with High-Lipid Diets. Aquac. Nutr. 2023, 2023, 3321734. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.; El Asely, A.; Abd El-Naby, A.S.; Samir, F.; El-Ashram, A.; Sudhakaran, R.; Dawood, M.A.O. Growth performance, intestinal histomorphology and growth-related gene expression in response to dietary Ziziphus mauritiana in Nile tilapia (Oreochromis niloticus). Aquaculture 2019, 512, 734301. [Google Scholar] [CrossRef]
- Barca, A.; Abramo, F.; Nazerian, S.; Coppola, F.; Sangiacomo, C.; Bibbiani, C.; Licitra, R.; Susini, F.; Verri, T.; Fronte, B. Hermetia illucens for Replacing Fishmeal in Aquafeeds: Effects on Fish Growth Performance, Intestinal Morphology, and Gene Expression in the Zebrafish (Danio rerio) Model. Fishes 2023, 8, 127. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.; Li, L.; Yao, Y.; Chen, C.; Hong, Y.; Chai, Y.; Liu, W. Effects of Tannic Acid Supplementation of a High-Carbohydrate Diet on the Growth, Serum Biochemical Parameters, Antioxidant Capacity, Digestive Enzyme Activity, and Liver and Intestinal Health of Largemouth Bass, Micropterus salmoides. Aquac. Nutr. 2024, 2024, 6682798. [Google Scholar] [CrossRef] [PubMed]
- Xavier, M.J.; Navarro-Guillén, C.; Lopes, A.; Colen, R.; Teodosio, R.; Mendes, R.; Oliveira, B.; Valente, L.M.P.; Conceição, L.E.C.; Engrola, S. Effects of dietary curcumin in growth performance, oxidative status and gut morphometry and function of gilthead seabream postlarvae. Aquac. Rep. 2022, 24, 101128. [Google Scholar] [CrossRef]
- Chen, X.-C.; Huang, X.-Q.; Tang, Y.-W.; Zhang, L.; Lin, F. Effects of dietary nucleotides on growth performance, immune response, intestinal morphology and disease resistance of juvenile largemouth bass, Micropterus salmoides. J. Fish. Biol. 2022, 101, 204–212. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, M.; Feng, D.; Wang, S.; Li, M. Effects of dietary D-mannose supplementation on growth performance, intestinal digestive capacity, gut microbiota, and ammonia tolerance of largemouth bass Micropterus salmoides. Aquac. Rep. 2024, 36, 102054. [Google Scholar] [CrossRef]
- Wang, S.; Han, Z.; Turchini, G.M.; Wang, X.; Fang, Z.; Chen, N.; Xie, R.; Zhang, H.; Li, S. Effects of Dietary Phospholipids on Growth Performance, Digestive Enzymes Activity and Intestinal Health of Largemouth Bass (Micropterus salmoides) Larvae. Front. Immunol. 2022, 12, 827946. [Google Scholar] [CrossRef]
- Vogt, G. Synthesis of digestive enzymes, food processing, and nutrient absorption in decapod crustaceans: A comparison to the mammalian model of digestion. Zoology 2021, 147, 125945. [Google Scholar] [CrossRef] [PubMed]
- Dai, B.; Hou, Y.; Hou, Y.; Qian, L. Effects of multienzyme complex and probiotic supplementation on the growth performance, digestive enzyme activity and gut microorganisms composition of snakehead (Channa argus). Aquac. Nutr. 2019, 25, 15–25. [Google Scholar] [CrossRef]
- Fang, H.; Xie, J.; Liao, S.; Guo, T.; Xie, S.; Liu, Y.; Tian, L.; Niu, J. Effects of Dietary Inclusion of Shrimp Paste on Growth Performance, Digestive Enzymes Activities, Antioxidant and Immunological Status and Intestinal Morphology of Hybrid Snakehead (Channa maculata ♀ × Channa argus ♂). Front. Physiol. 2019, 10, 1027. [Google Scholar] [CrossRef] [PubMed]
- Lakwani, M.A.S.; Kenanoğlu, O.N.; Taştan, Y.; Bilen, S. Effects of black mustard (Brassica nigra) seed oil on growth performance, digestive enzyme activities and immune responses in rainbow trout (Oncorhynchus mykiss). Aquac. Res. 2022, 53, 300–313. [Google Scholar] [CrossRef]
- Huang, B.; Zhang, S.; Dong, X.; Chi, S.; Yang, Q.; Liu, H.; Tan, B.; Xie, S. Effects of fishmeal replacement by black soldier fly on growth performance, digestive enzyme activity, intestine morphology, intestinal flora and immune response of pearl gentian grouper (Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatus ♂). Fish. Shellfish. Immunol. 2022, 120, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Ruenkoed, S.; Nontasan, S.; Phudkliang, J.; Phudinsai, P.; Pongtanalert, P.; Panprommin, D.; Mongkolwit, K.; Wangkahart, E. Effect of dietary gamma aminobutyric acid (GABA) modulated the growth performance, immune and antioxidant capacity, digestive enzymes, intestinal histology and gene expression of Nile tilapia (Oreochromis niloticus). Fish. Shellfish. Immunol. 2023, 141, 109056. [Google Scholar] [CrossRef]
- Sokooti, R.; Chelemal Dezfoulnejad, M.; Javaheri Baboli, M.; Askary Sary, A.; Mabudi, H. The effects of probiotics-supplemented diets on Asian sea bass (Lates calcarifer): Growth performance, microbial flora, digestive enzymes activity, serum biochemical and non-specific immune indices. Aquac. Res. 2022, 53, 5500–5509. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).