Pore Fractal and Structure Analysis of Pore-Filling Chlorite in Continental Shales: A Case Study from the Qingshankou Formation in the Gulong Sag
Highlights
- The chlorite occurrence in Gulong shale is finely classified according to its pore fillers.
- The pore structure of unfilled chlorite is simpler compared to that of filled chlorite.
- Chlorite associated with organic matter and carbonate produces more complex pores during diagenesis.
- The classification of chlorite according to pore fillers suggests that the type of pore-filling material can influ-ence the overall characteristics of the shale's porosity.
- The simpler pore structure of unfilled chlorite could indicate less complex diagenetic processes or less influ-ence on shale's permeability.
- Chlorite associated with organic matter and carbonate could have a significant impact on the shale’s overall pore complexity, potentially affecting its storage and fluid flow properties.
Abstract
1. Introduction
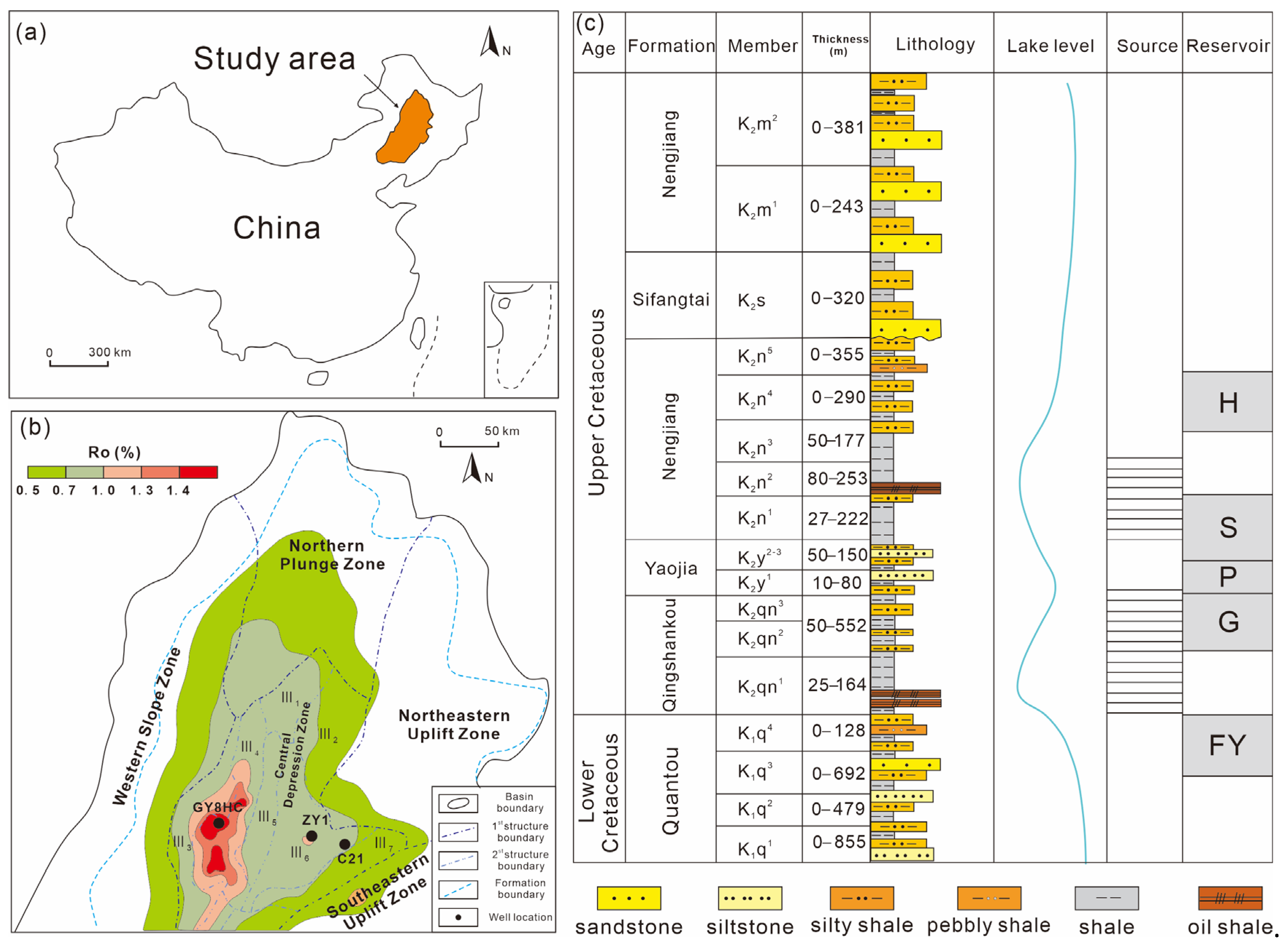
2. Geological Setting
3. Samples and Methods
3.1. Samples
3.2. Methods
- (1)
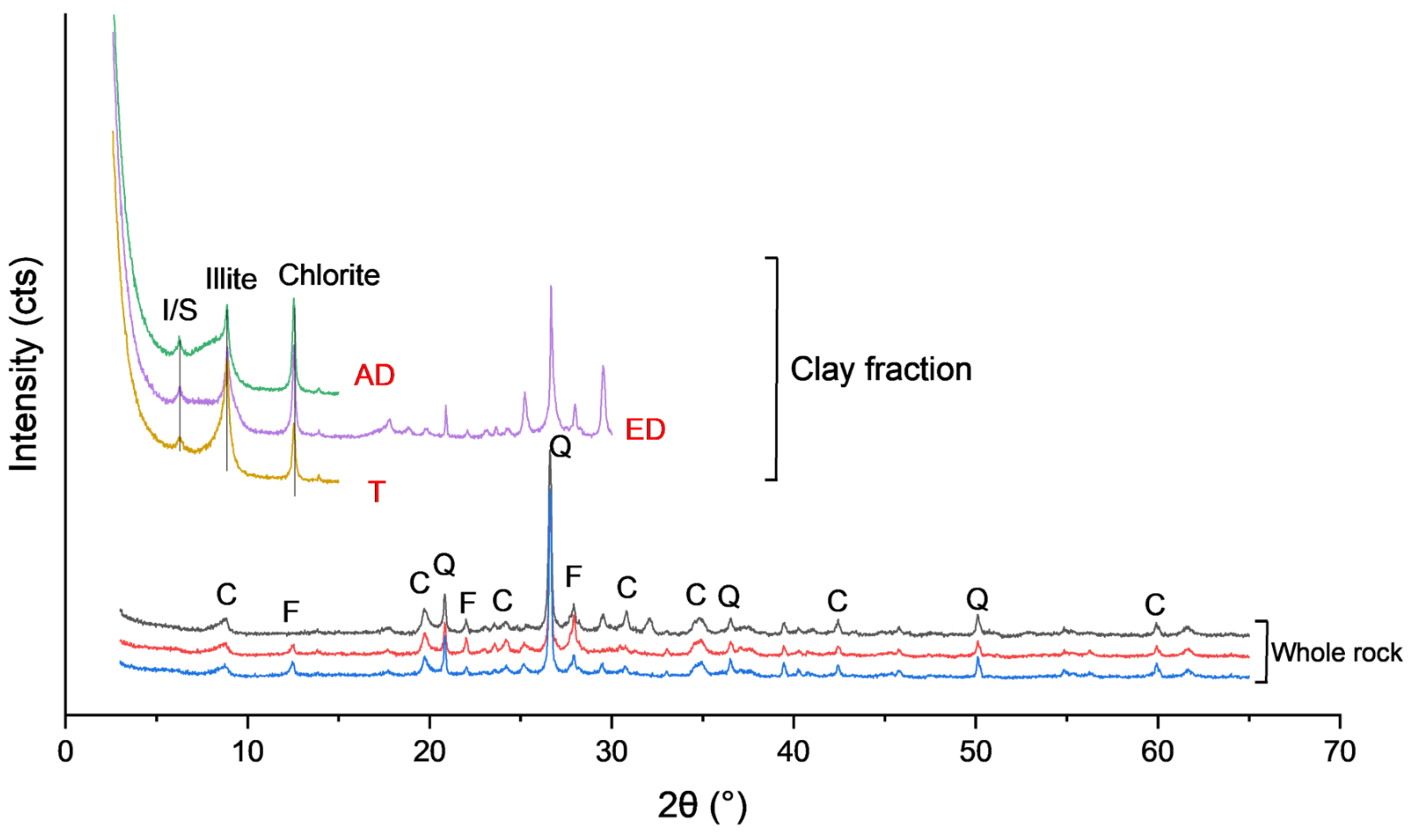
- X-ray diffraction (XRD)
- (2)
- Field-Emission Scanning Electron Microscopy (FE-SEM)
- (3)
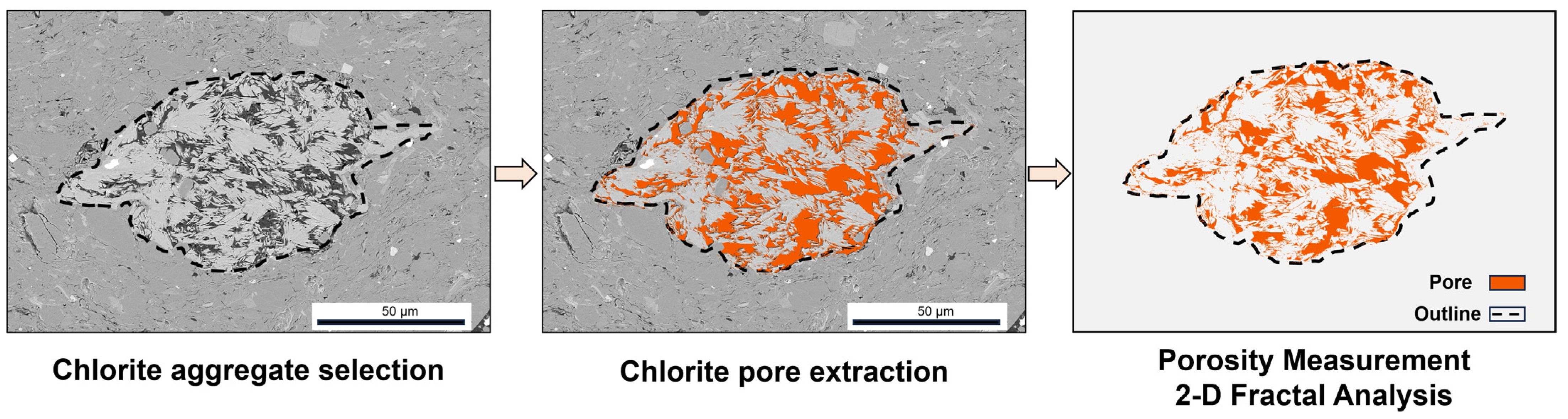
- Pore structure Measurement and Fractal Analysis
4. Results
4.1. Mineralogy
4.2. Chlorite Occurrence
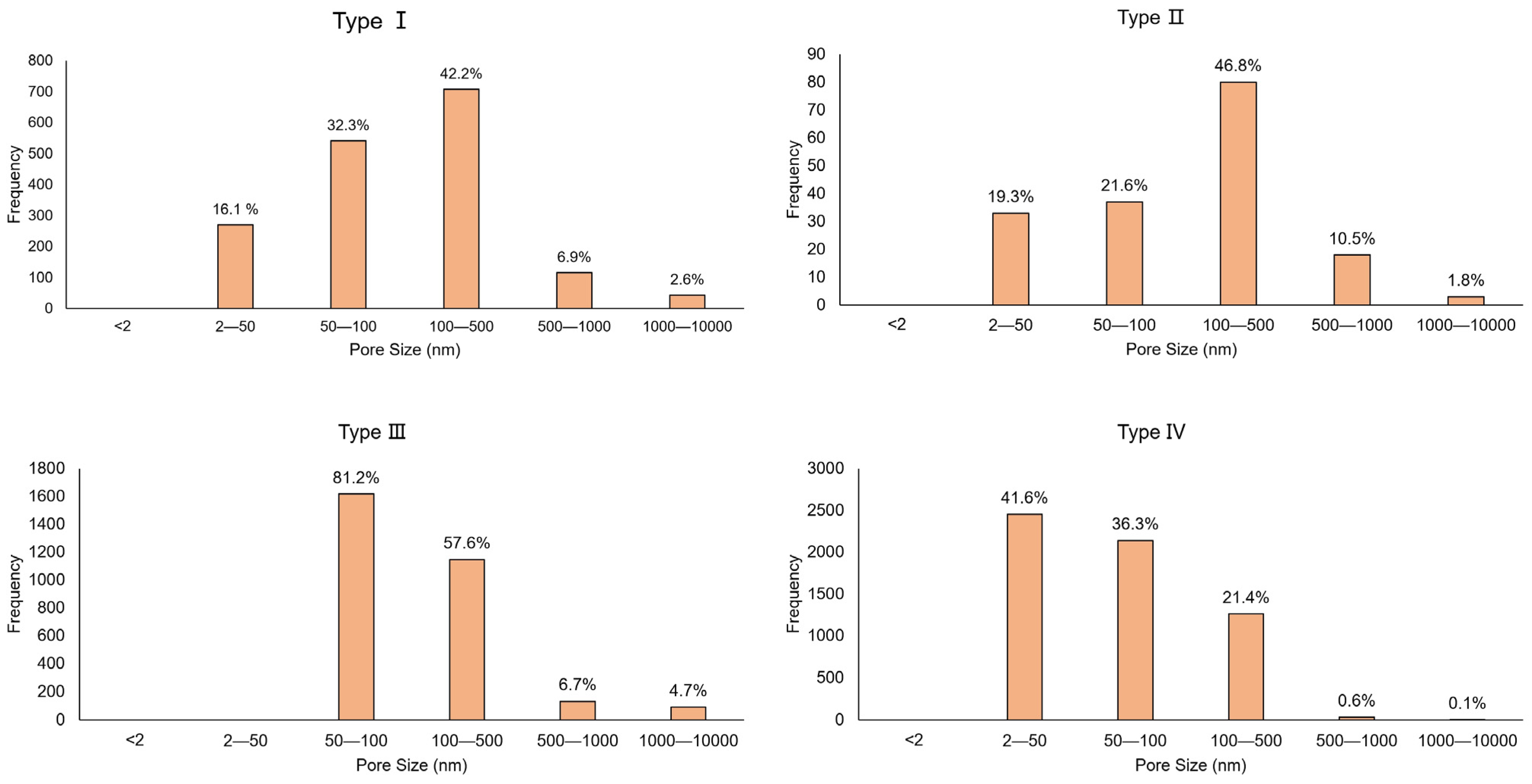
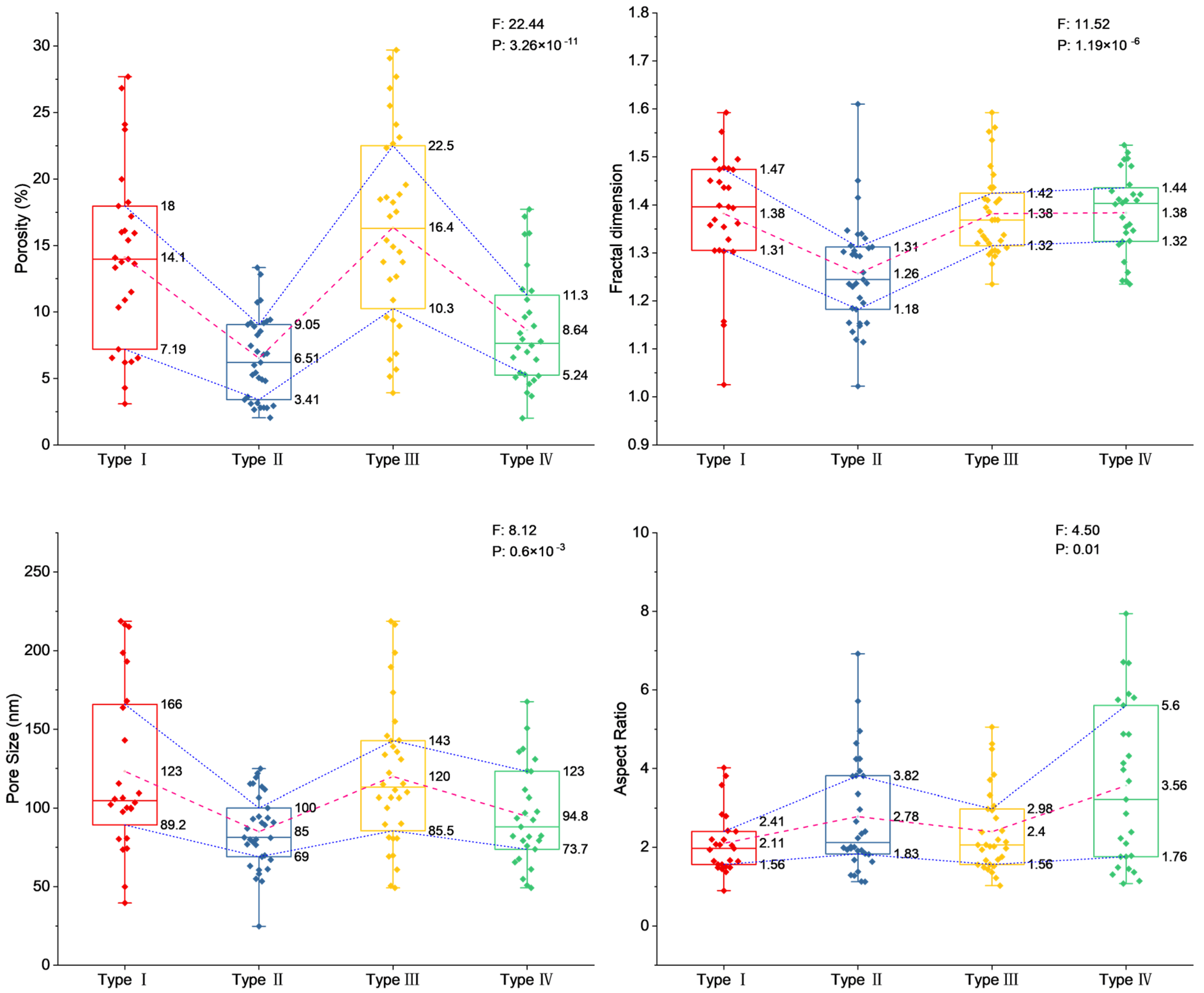
4.3. Distribution of Pore Structure and Fractal Dimension of Four Types of Chlorite
5. Discussion
5.1. Genesis of Pore-Filling Chlorite
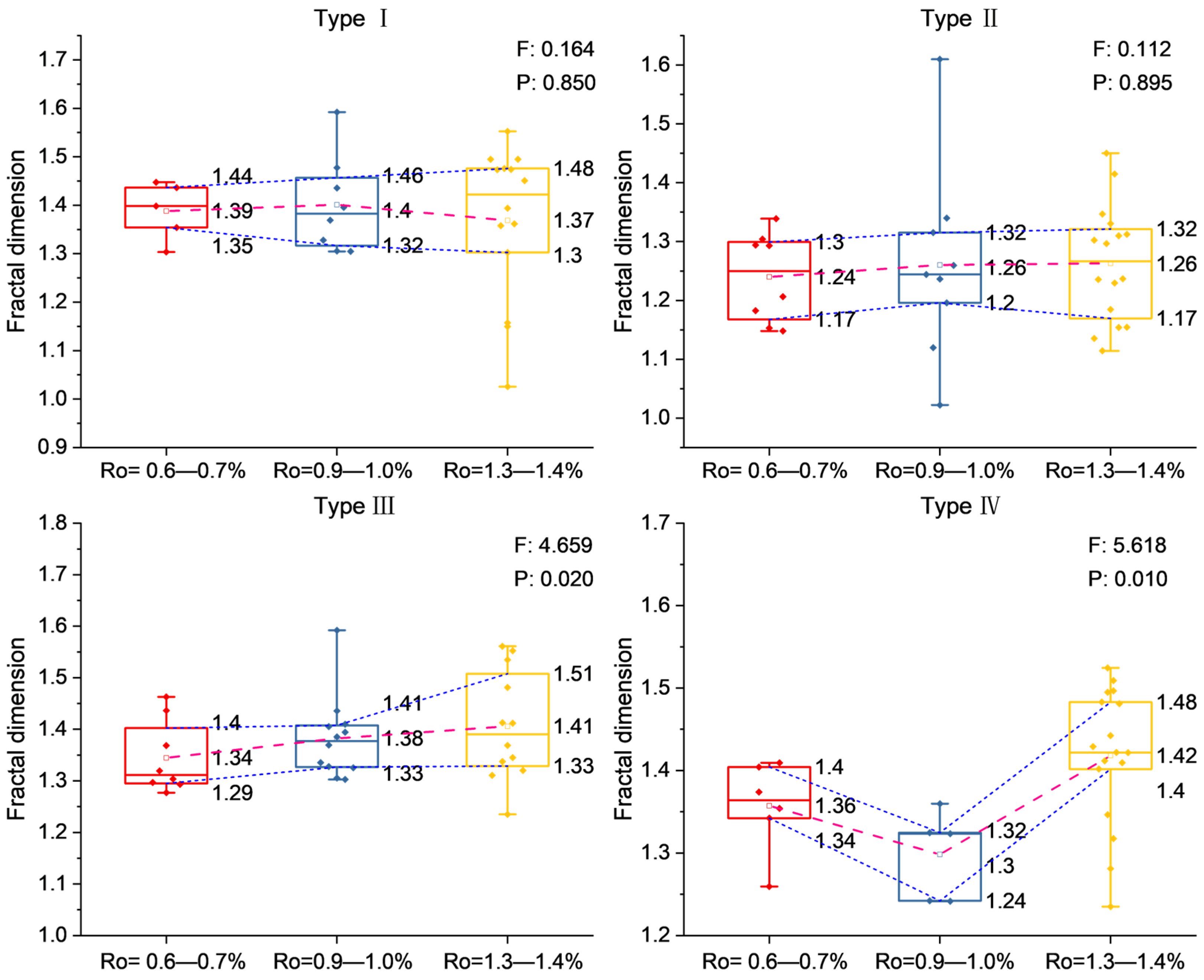
5.2. Evolution of Pore Structure and Fractal Dimension for Pore-Filling Chlorite
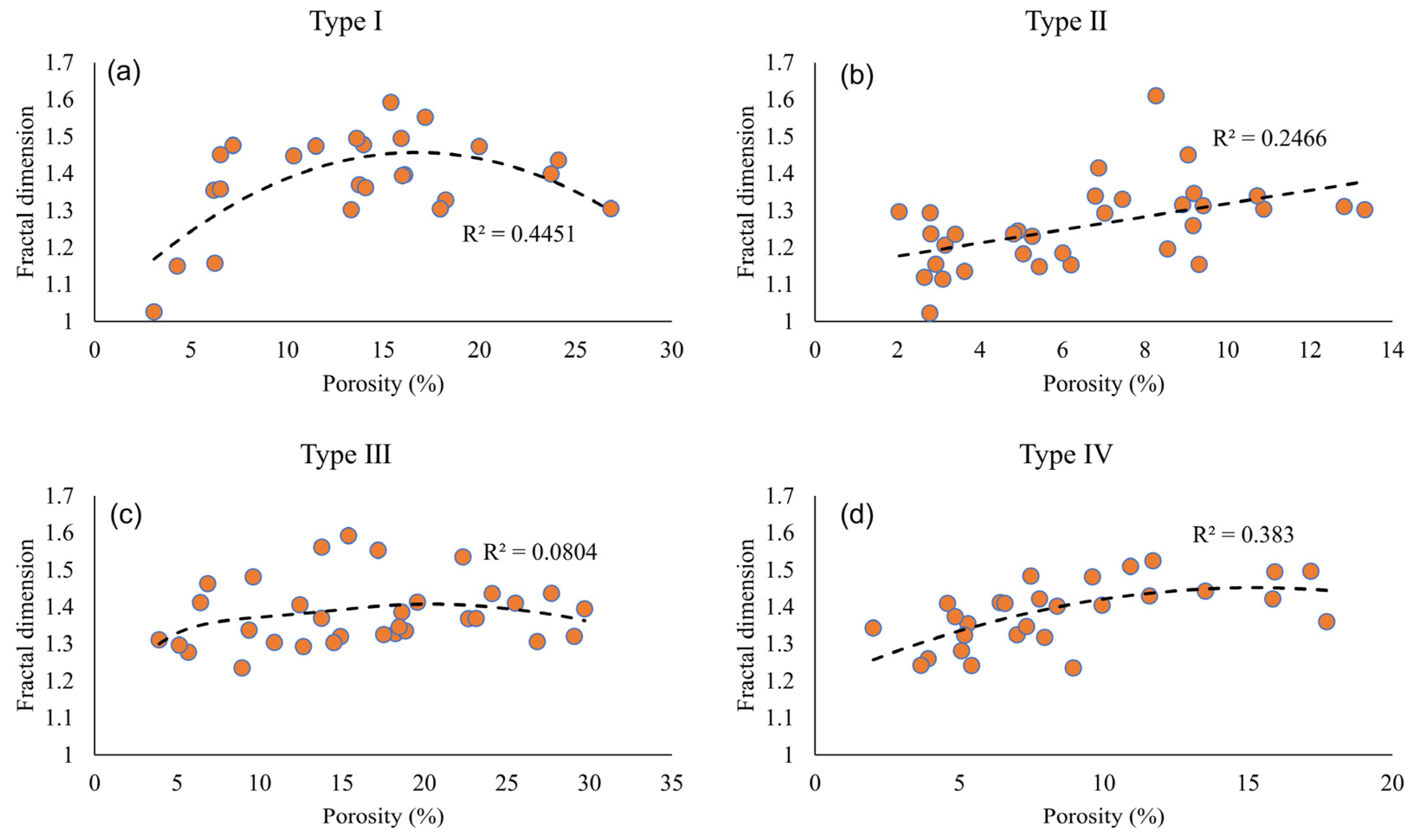
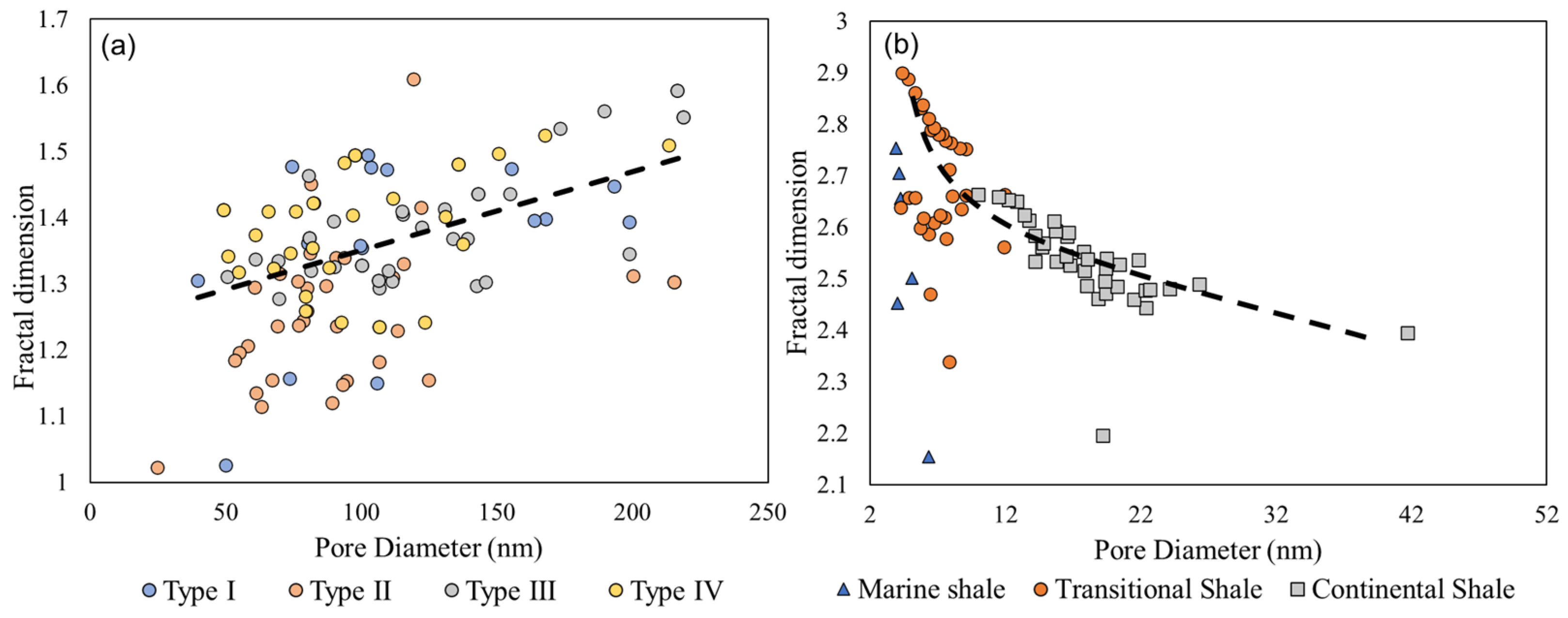
5.3. Pore Structure Versus Fractal Dimension of Pore-Filling Chlorite
5.4. Evolution Model of Pore-Filling Chlorite
5.5. Chlorite in Gulong Shale Reservoir Characteristics
6. Limitations and Future Research
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, L.; Cui, B.; Zhu, R.; Wang, R.; Feng, Z.; Li, B.; Zhang, J.; Gao, B.; Wang, Q.; Zeng, H.; et al. Shale oil enrichment evaluation and production law in Gulong Sag, Songliao Basin, NE China. Pet. Explor. Dev. 2023, 50, 505–519. [Google Scholar] [CrossRef]
- Wang, F.; Feng, Z.; Wang, X.; Zeng, H. Effect of organic matter, thermal maturity and clay minerals on pore formation and evolution in the Gulong Shale, Songliao Basin, China. Geoenergy Sci. Eng. 2023, 223, 211507. [Google Scholar] [CrossRef]
- Zhao, W.; Bian, C.; Li, Y.; Zhang, J.; He, K.; Liu, W.; Zhang, B.; Lei, Z.; Liu, C.; Zhang, J.; et al. Enrichment factors of movable hydrocarbons in lacustrine shale oil and exploration potential of shale oil in Gulong Sag, Songliao Basin, NE China. Pet. Explor. Dev. 2023, 50, 520–533. [Google Scholar] [CrossRef]
- Kang, Y.; Liu, K.; Zhu, R.; Yin, G.; Zhang, J.; Zhang, S. The evolution of clay mineral and its indication of hydrocarbons under overpressure: An example from the shale of the Qingshankou formation in the Gulong Sag. Pet. Sci. 2024, 21, 3867–3883. [Google Scholar] [CrossRef]
- Curtis, M.E.; Cardott, B.J.; Sondergeld, C.H.; Rai, C.S. Development of organic porosity in the Woodford Shale with increasing thermal maturity. Int. J. Coal Geol. 2012, 103, 26–31. [Google Scholar] [CrossRef]
- Nelson, P.H. Pore-throat sizes in sandstones, tight sandstones, and shales. Am. Assoc. Pet. Geol. Bull. 2009, 93, 329–340. [Google Scholar] [CrossRef]
- Chen, S.; Han, Y.; Fu, C.; Zhang, H.; Zhu, Y.; Zuo, Z. Micro and nano-size pores of clay minerals in shale reservoirs: Implication for the accumulation of shale gas. Sediment. Geol. 2016, 342, 180–190. [Google Scholar] [CrossRef]
- Kennedy, M.J.; Löhr, S.C.; Fraser, S.A.; Baruch, E.T. Direct evidence for organic carbon preservation as clay-organic nanocomposites in a Devonian black shale; from deposition to diagenesis. Earth Planet. Sci. Lett. 2014, 388, 59–70. [Google Scholar] [CrossRef]
- Pichevin, L.; Bertrand, P.; Boussafir, M.; Disnar, J.-R. Organic matter accumulation and preservation controls in a deep sea modern environment: An example from Namibian slope sediments. Org. Geochem. 2004, 35, 543–559. [Google Scholar] [CrossRef]
- Zhao, T.; Xu, S.; Hao, F. Differential adsorption of clay minerals: Implications for organic matter enrichment. Earth-Sci. Rev. 2023, 246, 104598. [Google Scholar] [CrossRef]
- Ji, L.; Zhang, T.; Milliken, K.L.; Qu, J.; Zhang, X. Experimental investigation of main controls to methane adsorption in clay-rich rocks. Appl. Geochem. 2012, 27, 2533–2545. [Google Scholar] [CrossRef]
- Beaufort, D.; Rigault, C.; Billon, S.; Billault, V.; Inoue, A.; Inoue, S.; Patrier, P. Chlorite and chloritization processes through mixed-layer mineral series in low-temperature geological systems—A review. Clay Miner. 2015, 50, 497–523. [Google Scholar] [CrossRef]
- Patrier, P.; Beaufort, D.; Azzam, F.; Blaise, T.; Portier, E.; Brigaud, B.; Clerc, S. New insights on diagenetic chlorite and its source material in turbiditic sandstones of contrasted reservoir quality in the Lower Cretaceous Agat formation (Duva oil and gas field, northern Norwegian North Sea). Mar. Pet. Geol. 2023, 152, 106221. [Google Scholar] [CrossRef]
- Ma, P.J.; Lin, C.Y.; Zhang, S.Q.; Dong, C.M.; Xu, Y.F. Formation of chlorite rims and the impact of pore-lining chlorite on reservoir quality: A case study from Shiqianfeng sandstones in upper Permian of Dongpu Depression, Bohai Bay Basin, eastern China. Aust. J. Earth Sci. 2017, 64, 825–839. [Google Scholar] [CrossRef]
- Worden, R.H.; Griffiths, J.; Wooldridge, L.J.; Utley, J.E.P.; Lawan, A.Y.; Muhammed, D.D.; Simon, N.; Armitage, P.J. Chlorite in sandstones. Earth-Sci. Rev. 2020, 204, 103105. [Google Scholar] [CrossRef]
- He, W.; Liu, B.; Sun, M.; Wang, L.; Zhang, J.; Yasin, Q.; Tian, S.; Gao, S.; Ukaomah, C.F. Pore types, genesis, and evolution model of lacustrine oil-prone shale: A case study of the Cretaceous Qingshankou Formation, Songliao Basin, NE China. Sci. Rep. 2022, 12, 17210. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.D.; Wang, F.L.; Bai, X.F.; Feng, Z.H.; Shao, H.M.; Zeng, H.S.; Gao, B.; Wang, Y.C. Discovery of nano organo-clay complex pore-fractures in shale and its scientific significance: A case study of Cretaceous Qingshankou Formation shale, Songliao Basin, NE China. Pet. Explor. Dev. 2024, 51, 813–825. [Google Scholar] [CrossRef]
- Zhao, H.; Ling, K.; Qin, S.; Lei, M.; Wen, H. Modes of occurrence of lithium in black shale in the Nandan area, Guangxi, SW China: Implications for clay-type resources. Ore Geol. Rev. 2023, 157, 105409. [Google Scholar] [CrossRef]
- Li, H.; Liu, D.; Peng, P.; Wang, Q. Tectonic Impact on Reservoir Character of Chongqing and Its Neighbor Area. Nat. Gas Geosci. 2015, 26, 1705–1711. [Google Scholar]
- Chang, J.Q.; Fan, X.D.; Jiang, Z.X.; Wang, X.M.; Chen, L.; Li, J.T.; Zhu, L.; Wan, C.X.; Chen, Z.X. Differential impact of clay minerals and organic matter on pore structure and its fractal characteristics of marine and continental shales in China. Appl. Clay Sci. 2022, 216, 13. [Google Scholar] [CrossRef]
- Cao, T.; Song, Z.; Luo, H.; Zhou, Y.; Wang, S. Pore system characteristics of the Permian transitional shale reservoir in the Lower Yangtze Region, China. J. Nat. Gas Geosci. 2016, 1, 383–395. [Google Scholar] [CrossRef]
- Li, G.; Qin, Y.; Li, G.; Wu, M.; Liu, H. Influence of reservoir properties on gas occurrence and fractal features of transitional shale from the Linxing area, Ordos Basin, China. Arab. J. Geosci. 2022, 15, 250. [Google Scholar] [CrossRef]
- Hou, L.; Wu, S.; Jing, Z.; Jiang, X.; Yu, Z.; Hua, G.; Su, L.; Yu, C.; Liao, F.; Tian, H. Effects of types and content of clay minerals on reservoir effectiveness for lacustrine organic matter rich shale. Fuel 2022, 327, 125043. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Mou, W.; Yan, C. Influences of clay minerals on physical properties of Chang 6 tight sandstone reservoir in Jiyuan area, Ordos Basin. Nat. Gas Geosci. 2017, 28, 1043–1053. [Google Scholar]
- Huang, S.J.; Xie, L.W.; Zhang, M.; Wu, W.-H.; Shen, L.C.; Liu, J. Formation mechanism of authigenic chlorite and relation to preservation of porosity in nonmarine Triassic reservoir sandstones, Ordos Basin and Sichuan Basin, China. J. Chengdu Univ. Technol. (Sci. Technol. Ed.) 2004, 31, 273–281. [Google Scholar]
- Lu, Z.; Qing, C. Geological factors affecting reservoir permeability of the Upper Shaximiao Formation in the Xinchang gas field, western Sichuan. Sediment. Geol. Tethyan Geol. 2001, 21, 57–63. [Google Scholar]
- Wang, M.; Xue, H.; Tian, S.; Wilkins, R.W.T.; Wang, Z. Fractal characteristics of Upper Cretaceous lacustrine shale from the Songliao Basin, NE China. Mar. Pet. Geol. 2015, 67, 144–153. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Pan, Z.; Niu, X.; Yu, Y.; Meng, S. Pore structure and its fractal dimensions of transitional shale: A cross-section from east margin of the Ordos Basin, China. Fuel 2019, 241, 417–431. [Google Scholar] [CrossRef]
- Ma, X.; Guo, S.; Shi, D.; Zhou, Z.; Liu, G. Investigation of pore structure and fractal characteristics of marine-continental transitional shales from Longtan Formation using MICP, gas adsorption, and NMR (Guizhou, China). Mar. Pet. Geol. 2019, 107, 555–571. [Google Scholar] [CrossRef]
- Wang, X.; Hou, J.; Li, S.; Dou, L.; Song, S.; Kang, Q.; Wang, D. Insight into the nanoscale pore structure of organic-rich shales in the Bakken Formation, USA. J. Pet. Sci. Eng. 2020, 191, 107182. [Google Scholar] [CrossRef]
- Zhu, H.; Huang, C.; Ju, Y.; Bu, H.; Li, X.; Yang, M.; Chu, Q.; Feng, H.; Qiao, P.; Qi, Y.; et al. Multi-scale multi-dimensional characterization of clay-hosted pore networks of shale using FIBSEM, TEM, and X-ray micro-tomography: Implications for methane storage and migration. Appl. Clay Sci. 2021, 213, 106239. [Google Scholar] [CrossRef]
- Sun, L.; Liu, H.; He, W.; Li, G.; Zhang, S.; Zhu, R.; Jin, X.; Meng, S.; Jiang, H. An analysis of major scientific problems and research paths of Gulong shale oil in Daqing Oilfield, NE China. Pet. Explor. Dev. 2021, 48, 527–540. [Google Scholar] [CrossRef]
- SY/T 5163-2018; X-ray Diffraction Analysis Method of Clay Minerals and Common Non-Clay Minerals in Sedimentary Rocks. National Energy Administration: Beijing, China, 2018.
- Peng, R.; Xie, H.; Ju, Y. Computation Method of Fractal Dimension for 2-D Digital Image. J. China Univ. Min. Technol. 2004, 33, 19–24. [Google Scholar]
- White, R.; Budarin, V.; Luque, R.; Clark, J.; Macquarrie, D. Tuneable porous carbonaceous materials from renewable resources. Chem. Soc. Rev. 2009, 38, 3401–3418. [Google Scholar] [CrossRef]
- Stricker, S.; Jones, S.J. Enhanced Porosity Preservation by Pore Fluid Overpressure and Chlorite Grain Coatings in the Triassic Skagerrak, Central Graben, North Sea, UK. In Conference on Reservoir Quality of Clastic and Carbonate Rocks-Analysis, Modelling and Prediction; Geological Soc Publishing House: London, UK, 2014. [Google Scholar] [CrossRef]
- Zhu, H.; Zhong, D.; Yao, J.; Sun, H.; Niu, X.; Liang, X.; You, Y.; Li, X. Alkaline diagenesis and its effects on reservoir porosity: A case study of Upper Triassic Chang 7 Member tight sandstone in Ordos Basin, NW China. Pet. Explor. Dev. 2015, 42, 56–65. [Google Scholar] [CrossRef]
- Webb, A.D.; Dickens, G.R.; Oliver, N.H.S. Carbonate alteration of the Upper Mount McRae Shale beneath the martite-microplaty hematite ore deposit at Mount Whaleback, Western Australia. Miner. Depos. 2004, 39, 632–645. [Google Scholar] [CrossRef]
- Griffiths, J.; Worden, R.H.; Wooldridge, L.J.; Utley, J.E.P.; Duller, R.A. Compositional variation in modern estuarine sands: Predicting major controls on sandstone reservoir quality. AAPG Bull. 2019, 103, 797–833. [Google Scholar] [CrossRef]
- Wunderlin, C.A.; Collo, G.; Ezpeleta, M.; Reinoso Carbonell, V.V.; Nóbile, J.C.; Ciccioli, P.L.; Faudone, S. Authigenic and detrital clay minerals as indicators of paleoenvironmental and postdepositional evolution in a Cretaceous–Cenozoic succession from Argentine Central Andes. Sediment. Geol. 2022, 437, 106179. [Google Scholar] [CrossRef]
- Zhu, R.; Li, M.; Yang, J.; Zhang, S.; Cai, Y.; Cao, Y.; Kang, Y. Advances and trends of fine-grained sedimentology. Oil Gas Geol. 2022, 43, 251–264. [Google Scholar]
- Wilson, M.J.; Shaldybin, M.V.; Wilson, L. Clay mineralogy and unconventional hydrocarbon shale reservoirs in the USA. I. Occurrence and interpretation of mixed-layer R3 ordered illite/smectite. Earth-Sci. Rev. 2016, 158, 31–50. [Google Scholar] [CrossRef]
- Goral, J.; Panja, P.; Deo, M.; Andrew, M.; Linden, S.; Schwarz, J.-O.; Wiegmann, A. Confinement Effect on Porosity and Permeability of Shales. Sci. Rep. 2020, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Zhu, R.; Cui, B.; Zhang, S.; Meng, Q.; Bai, B.; Feng, Z.; Lei, Z.; Wu, S.; He, K.; et al. The geoscience frontier of Gulong shale oil: Revealing the role of continental shale from oil generation to production. Engineering 2023, 28, 79–92. [Google Scholar] [CrossRef]
- Ye, C.; Yang, Y.; Fang, X.; Hong, H.; Wang, C.; Yang, R.; Zhang, W. Chlorite chemical composition change in response to the Eocene-Oligocene climate transition on the northeastern Tibetan Plateau. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2018, 512, 23–32. [Google Scholar] [CrossRef]
- Anjos, S.M.C.; De Ros, L.F.; Silva, C.M.A. Chlorite Authigenesis and Porosity Preservation in the Upper Cretaceous Marine Sandstones of the Santos Basin, Offshore Eastern Brazil. In Clay Mineral Cements in Sandstones; Wiley: Hoboken, NJ, USA, 1999; pp. 289–316. [Google Scholar] [CrossRef]
- Zhao, C.; Jiang, Y.; Yang, H.; Wang, L. The genesis of authigenic minerals and the porosity evolution of various lithologies and their implications for identifying high-quality reservoirs in the fourth member of Xujiahe Formation (Northeast-Sichuan Basin, China). J. Pet. Sci. Eng. 2022, 212, 110261. [Google Scholar] [CrossRef]
- Li, K.; Xi, K.; Cao, Y.; Niu, X.; Wu, S.; Feng, S.; You, Y. Chlorite authigenesis and its impact on reservoir quality in tight sandstone reservoirs of the Triassic Yanchang formation, southwestern Ordos basin, China. J. Pet. Sci. Eng. 2021, 205, 108843. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Zhang, Z.; Yao, S.; Zhang, H.; Zheng, G.; Luo, F.; Feng, L.; Liu, K.; Jiang, L. Recent techniques on analyses and characterizations of shale gas and oil reservoir. Energy Rev. 2024, 3, 100067. [Google Scholar] [CrossRef]
- Li, X.; Zhu, H.; Zhang, K.; Li, Z.; Yu, Y.; Feng, X.; Wang, Z. Pore characteristics and pore structure deformation evolution of ductile deformed shales in the Wufeng-Longmaxi Formation, southern China. Mar. Pet. Geol. 2021, 127, 104992. [Google Scholar] [CrossRef]
- Yang, F.; Ning, Z.; Wang, Q.; Kong, D.; Peng, K.; Xiao, L. Fractal Characteristics of Nanopore in Shales. Nat. Gas Geosci. 2014, 25, 618–623. [Google Scholar]
- Yang, R.; He, S.; Yi, J.; Hu, Q. Nano-scale pore structure and fractal dimension of organic-rich Wufeng-Longmaxi shale from Jiaoshiba area, Sichuan Basin: Investigations using FE-SEM, gas adsorption and helium pycnometry. Mar. Pet. Geol. 2016, 70, 27–45. [Google Scholar] [CrossRef]
- Fu, H.; Yan, D.; Yao, C.; Su, X.; Wang, X.; Wang, H.; Li, Y. Pore Structure and Multi-Scale Fractal Characteristics of Adsorbed Pores in Marine Shale: A Case Study of the Lower Silurian Longmaxi Shale in the Sichuan Basin, China. J. Earth Sci. 2022, 33, 1278–1290. [Google Scholar] [CrossRef]
- Liu, B.; Wang, Y.; Tian, S.; Guo, Y.; Wang, L.; Yasin, Q.; Yang, J. Impact of thermal maturity on the diagenesis and porosity of lacustrine oil-prone shales: Insights from natural shale samples with thermal maturation in the oil generation window. Int. J. Coal Geol. 2022, 261, 104079. [Google Scholar] [CrossRef]
- Liu, B.; Wang, H.; Fu, X.; Bai, Y.; Bai, L.; Jia, M.; He, B. Lithofacies and depositional setting of a highly prospective lacustrine shale oil succession from the Upper Cretaceous Qingshankou Formation in the Gulong sag, northern Songliao Basin, northeast China. AAPG Bull. 2019, 103, 405–432. [Google Scholar] [CrossRef]
- Huang, D. Advances in hydrocarbon generation theory (I): Generation and evolution model for immature oils and hydrocarbon. J. Pet. Sci. Eng. 1999, 22, 121–130. [Google Scholar] [CrossRef]
- Vandenbroucke, M.; Largeau, C. Kerogen origin, evolution and structure. Org. Geochem. 2007, 38, 719–833. [Google Scholar] [CrossRef]
- Zhao, J.; Jin, Z.; Jin, Z.; Wen, X.; Geng, Y. Origin of authigenic quartz in organic-rich shales of the Wufeng and Longmaxi Formations in the Sichuan Basin, South China: Implications for pore evolution. J. Nat. Gas Sci. Eng. 2017, 38, 21–38. [Google Scholar] [CrossRef]
- Wang, S.W.; Li, X.J.; Xue, H.T.; Shen, Z.H.; Chen, L.Y. Fractal characteristics of shale pore structure and its influence on seepage flow. R. Soc. Open Sci. 2021, 8. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhang, W.; Liang, C.; Wang, Y.; Liu, H.; Chen, X. Basic characteristics and evaluation of shale oil reservoirs. Pet. Res. 2016, 1, 149–163. [Google Scholar] [CrossRef]
- Jiang, F.J.; Chen, D.; Chen, J.; Li, Q.W.; Liu, Y.; Shao, X.H.; Hu, T.; Dai, J.X. Fractal Analysis of Shale Pore Structure of Continental Gas Shale Reservoir in the Ordos Basin, NW China. Energy Fuels 2016, 30, 4676–4689. [Google Scholar] [CrossRef]
- Yang, F.; Ning, Z.; Liu, H. Fractal characteristics of shales from a shale gas reservoir in the Sichuan Basin, China. Fuel 2014, 115, 378–384. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, B.; Yuan, Q.; Huang, D.; Peng, H. Characterization, factors, and fractal dimension of pore structure of fly ash-based geopolymers. J. Mater. Res. Technol. 2023, 26, 3395–3407. [Google Scholar] [CrossRef]
- Liu, Z.; Han, J.; Yang, H.; Lv, J.; Dong, S. A new model for coal gas seepage based on fracture-pore fractal structure characteristics. Int. J. Rock Mech. Min. Sci. 2024, 173, 105626. [Google Scholar] [CrossRef]
- Medina, F.J.; Jausoro, I.; Floridia Addato, M.A.; Rodriguez, M.J.; Tomassini, F.G.; Caneiro, A. On the evaluation of Representative Elementary Area for porosity in shale rocks by Field Emission Scanning Electron Microscopy. Energy 2022, 253, 124141. [Google Scholar] [CrossRef]
- Arif, M.; Mahmoud, M.; Zhang, Y.; Iglauer, S. X-ray tomography imaging of shale microstructures: A review in the context of multiscale correlative imaging. Int. J. Coal Geol. 2021, 233, 103641. [Google Scholar] [CrossRef]
- Cosenza, P.; Prêt, D.; Fauchille, A.-L.; Hedan, S. Representative elementary area of shale at the mesoscopic scale. Int. J. Coal Geol. 2019, 216, 103316. [Google Scholar] [CrossRef]
- Saif, T.; Lin, Q.; Bijeljic, B.; Blunt, M.J. Microstructural imaging and characterization of oil shale before and after pyrolysis. Fuel 2017, 197, 562–574. [Google Scholar] [CrossRef]
- Goral, J.; Andrew, M.; Olson, T.; Deo, M. Correlative core- to pore-scale imaging of shales. Mar. Pet. Geol. 2020, 111, 886–904. [Google Scholar] [CrossRef]
- Kelly, S.; El-Sobky, H.; Torres-Verdín, C.; Balhoff, M.T. Assessing the utility of FIB-SEM images for shale digital rock physics. Adv. Water Resour. 2016, 95, 302–316. [Google Scholar] [CrossRef]
- Saif, T.; Lin, Q.; Butcher, A.R.; Bijeljic, B.; Blunt, M.J. Multi-scale multi-dimensional microstructure imaging of oil shale pyrolysis using X-ray micro-tomography, automated ultra-high resolution SEM, MAPS Mineralogy and FIB-SEM. Appl. Energy 2017, 202, 628–647. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, Y.; Zhu, R.; Liu, K.; Zhang, J.; Liu, C. Pore Fractal and Structure Analysis of Pore-Filling Chlorite in Continental Shales: A Case Study from the Qingshankou Formation in the Gulong Sag. Fractal Fract. 2025, 9, 266. https://doi.org/10.3390/fractalfract9040266
Kang Y, Zhu R, Liu K, Zhang J, Liu C. Pore Fractal and Structure Analysis of Pore-Filling Chlorite in Continental Shales: A Case Study from the Qingshankou Formation in the Gulong Sag. Fractal and Fractional. 2025; 9(4):266. https://doi.org/10.3390/fractalfract9040266
Chicago/Turabian StyleKang, Yuan, Rukai Zhu, Kouqi Liu, Jingya Zhang, and Chang Liu. 2025. "Pore Fractal and Structure Analysis of Pore-Filling Chlorite in Continental Shales: A Case Study from the Qingshankou Formation in the Gulong Sag" Fractal and Fractional 9, no. 4: 266. https://doi.org/10.3390/fractalfract9040266
APA StyleKang, Y., Zhu, R., Liu, K., Zhang, J., & Liu, C. (2025). Pore Fractal and Structure Analysis of Pore-Filling Chlorite in Continental Shales: A Case Study from the Qingshankou Formation in the Gulong Sag. Fractal and Fractional, 9(4), 266. https://doi.org/10.3390/fractalfract9040266





