Reduction of Lead Sulfate Using Fine-Grained Carbon-Bearing Materials †
1. Introduction
2. Research Methodology
- Heating the sample to temperatures of 900, 925, 950, 9750 and 1000 °C at a rate of 20 °C/min;
- Isothermal heating of the sample at the chosen temperature for 30 min;
- Cooling the sample to a temperature of 700 °C.
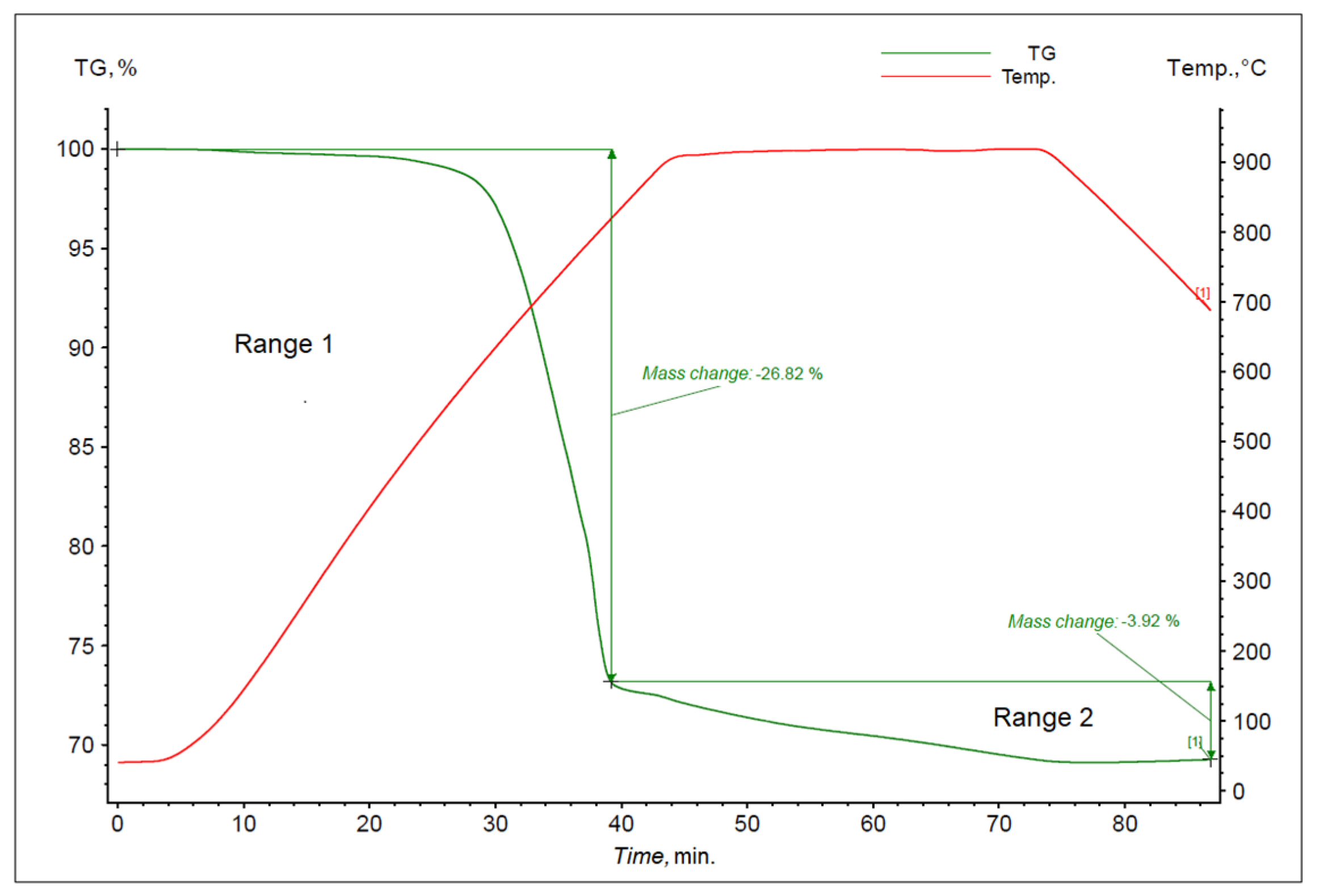
3. Results and Discussion
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arnout, S.; Nagels, E.; Blanpain, B. Thermodynamics of Lead Recycling. In Proceedings of the European Metallurgical Conference EMC 2011, Düsseldorf, Germany, 26–29 June 2011. [Google Scholar]
- Idoine, N.E.; Raycraft, E.R.; Hobbs, S.F.; Everett, P.; Evans, E.J.; Mills, A.J.; Currie, D.; Horn, S.; Shaw, R.A. World Mineral Production 2018–2022; British Geological Survey: Keyworth, Nottingham, 2024; ISBN 978-0-85272-800-0. [Google Scholar]
- Rand, D.A.J. Valve-Regulated Lead-Acid Batteries, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2004; ISBN 978-0-444-50746-4. [Google Scholar]
- Quirijnen, L. How to Implement Efficient Local Lead–Acid Battery Recycling. J. Power Sources 1999, 78, 267–269. [Google Scholar] [CrossRef]
- HSC Chemistry 2024. Available online: https://rawmarks.info/science/chemistry/ (accessed on 26 July 2024).
- Malecha, D.; Małecki, S.; Jarosz, P.; Kowalik, R.; Żabiński, P. Recovery of Pure Lead-Tin Alloy from Recycling Spent Lead-Acid Batteries. Materials 2023, 16, 5882. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Li, L.; Sun, X.; Yang, D.; Gao, L.; Liu, J.; Kumar, R.V.; Yang, J. Preparation of Basic Lead Oxide from Spent Lead Acid Battery Paste via Chemical Conversion. Hydrometallurgy 2012, 117–118, 24–31. [Google Scholar] [CrossRef]
- Jie, X.; Yao, Z.; Wang, C.; Qiu, D.; Chen, Y.; Zhang, Y.; Ma, B.; Gao, W. Progress in Waste Lead Paste Recycling Technology from Spent Lead–Acid Battery in China. J. Sustain. Metall. 2022, 8, 978–993. [Google Scholar] [CrossRef]
- Ning, P.; Pan, J.-Q.; Li, X.; Zhou, Y.; Chen, J.-F.; Wang, J.-X. Accelerated Desulphurization of Waste Lead Battery Paste in a High-Gravity Rotating Packed Bed. Chem. Eng. Process. Process Intensif. 2016, 104, 148–153. [Google Scholar] [CrossRef]
- Iliev, V.; Pavlov, D. The Influence of PbO Modification on the Kinetics of the 4PbO×PbSO4 Lead-Acid Battery Paste Formation. J. Appl. Electrochem. 1979, 9, 555–562. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, J.; Wu, X.; Hu, Y.; Yu, W.; Wang, J.; Dong, J.; Li, M.; Liang, S.; Hu, J.; et al. A Critical Review on Secondary Lead Recycling Technology and Its Prospect. Renew. Sustain. Energy Rev. 2016, 61, 108–122. [Google Scholar] [CrossRef]
- Ramus, K.; Hawkins, P. Lead/Acid Battery Recycling and the New Isasmelt Process. J. Power Sources 1993, 42, 299–313. [Google Scholar] [CrossRef]
- Sun, Z.; Cao, H.; Zhang, X.; Lin, X.; Zheng, W.; Cao, G.; Sun, Y.; Zhang, Y. Spent Lead-Acid Battery Recycling in China—A Review and Sustainable Analyses on Mass Flow of Lead. Waste Manag. 2017, 64, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cao, J.; Chen, Z.; Yu, J.; Zhang, J.; Chen, B.; Wu, L.; Zhou, S.; Rao, Y. Improving the Performance of Recovered Lead Oxide Powder from Waste Lead Paste as Active Material for Lead-acid Battery. Int. J. Energy Res. 2022, 46, 14268–14282. [Google Scholar] [CrossRef]
- Niesler, M.; Stecko, J.; Blacha, L.; Oleksiak, B. Application of Fine-Grained Coke Breeze Fractions in the Process of Iron Ore Sintering. Metalurgija 2014, 53, 37–39. [Google Scholar]
- Siwiec, G.; Oleksiak, B.; Vaskova, I.; Burdzik, R. A Study on Reduction of Copper Slag from the Flash Furnace with the Use of Anthracite Dust. Metalurgija 2014, 53, 343–345. [Google Scholar]
- Labaj, J.; Slowikowski, M.; Zymla, W.; Lipart, J. The Research on Reactivity of Alternative Carbon Reducers. Metalurgija 2013, 52, 68–70. [Google Scholar]
- Matuła, T.; Siwiec, G. Reduction of Lead Oxide by Fine-Grained Carbonaceous Materials. Arch. Metall. Mater. 2019, 64, 647–652. [Google Scholar] [CrossRef]
- Kala, S.; Mishra, A. Battery Recycling Opportunity and Challenges in India. Mater. Today Proc. 2021, 46, 1543–1556. [Google Scholar] [CrossRef]
- Zakiyya, H.; Distya, Y.D.; Ellen, R. A Review of Spent Lead-Acid Battery Recycling Technology in Indonesia: Comparison and Recommendation of Environment-Friendly Process. IOP Conf. Ser. Mater. Sci. Eng. 2018, 288, 012074. [Google Scholar] [CrossRef]
- Li, M.; Liu, J.; Han, W. Recycling and Management of Waste Lead-Acid Batteries: A Mini-Review. Waste Manag. Res. 2016, 34, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Díaz, G.; Andrews, D. Placid—A Clean Process for Recycling Lead from Batteries. JOM 1996, 48, 29–31. [Google Scholar] [CrossRef]
- Liliu, Q.; Liu, C. Current Status of Battery Recycling and Technology. J. Phys. Conf. Ser. 2023, 2608, 012011. [Google Scholar] [CrossRef]
- Matuła, T.; Siwiec, G. Thermogravimetric (TG) Studies of the Reaction of Lead Oxide with Lead Sulphide. Metalurgija 2023, 62, 279–281. [Google Scholar]

| Substrates | Temperature, °C | Total Mass Change, % |
|---|---|---|
| PbSO4 + anthracite dust | 900 | −30.74 |
| 925 | −31.77 | |
| 950 | −32.71 | |
| 975 | −32.11 | |
| 1000 | −37.17 |
| Substrates | Sample Form | Temperature, °C | Total Mass Change, % |
|---|---|---|---|
| PbSO4 + anthracite dust | loose | 900 | −30.74 |
| agglomerate | −29.69 | ||
| loose | 1000 | −37.17 | |
| agglomerate | −18.88 | ||
| PbSO4 + flotoconcentrate | loose | 900 | −41.30 |
| agglomerate | −38.34 | ||
| loose | 1000 | −41.66 | |
| agglomerate | −43.22 | ||
| PbSO4 + coke breeze | loose | 900 | −39.51 |
| agglomerate | −33.59 | ||
| loose | 1000 | −39.67 | |
| agglomerate | −26.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siwiec, G.; Matuła, T. Reduction of Lead Sulfate Using Fine-Grained Carbon-Bearing Materials. Proceedings 2024, 108, 9. https://doi.org/10.3390/proceedings2024108009
Siwiec G, Matuła T. Reduction of Lead Sulfate Using Fine-Grained Carbon-Bearing Materials. Proceedings. 2024; 108(1):9. https://doi.org/10.3390/proceedings2024108009
Chicago/Turabian StyleSiwiec, Grzegorz, and Tomasz Matuła. 2024. "Reduction of Lead Sulfate Using Fine-Grained Carbon-Bearing Materials" Proceedings 108, no. 1: 9. https://doi.org/10.3390/proceedings2024108009
APA StyleSiwiec, G., & Matuła, T. (2024). Reduction of Lead Sulfate Using Fine-Grained Carbon-Bearing Materials. Proceedings, 108(1), 9. https://doi.org/10.3390/proceedings2024108009







