High-Performance Ceramics in Musculoskeletal Surgery: Current Use and Future Perspectives
Abstract
:1. Introduction
2. General Definition and Classification of High-Performance Ceramics
3. Ceramics in Musculoskeletal Surgery
3.1. Full-Thickness Ceramic Implant Components
3.1.1. General Introduction to Oxide Ceramics
3.1.2. Alumina (Al2O3)
3.1.3. Zirconia (Y-TZP)
3.1.4. Dispersion Ceramics
- Hardness HV10: 17 GPa;
- Fracture toughness: 7 MPa*m1/2 (vs. Al2O3: 4.3 MPa*m1/2);
- Compressive strength: 2600 MPa;
- Four-point bending strength: 440–800 MPa.
- Hardness HV10: 14 GPa;
- Compressive strength: 2100 MPa;
- Four-point bending strength: 820 MPa.
3.1.5. Pros
- tissue friendly and very well-tolerated by the organism;
- reduced risk of toxicity: compared to metal, ceramic particles are less cytotoxic to histiocytes and fibroblasts than metal particles at equivalent particle volumes [63];
- lower biofilm formation.
3.1.6. Cons
3.2. Ceramics as Coating or Finishing
4. Current Reasons for Using Ceramic Implants
4.1. General
4.2. Wear
4.3. Allergies
4.4. Biofilm Formation
5. Clinical Success
5.1. Full-Thickness Implants
5.1.1. Ceramic Elements
5.1.2. Metal-Free TKA
5.2. Ceramic Coatings
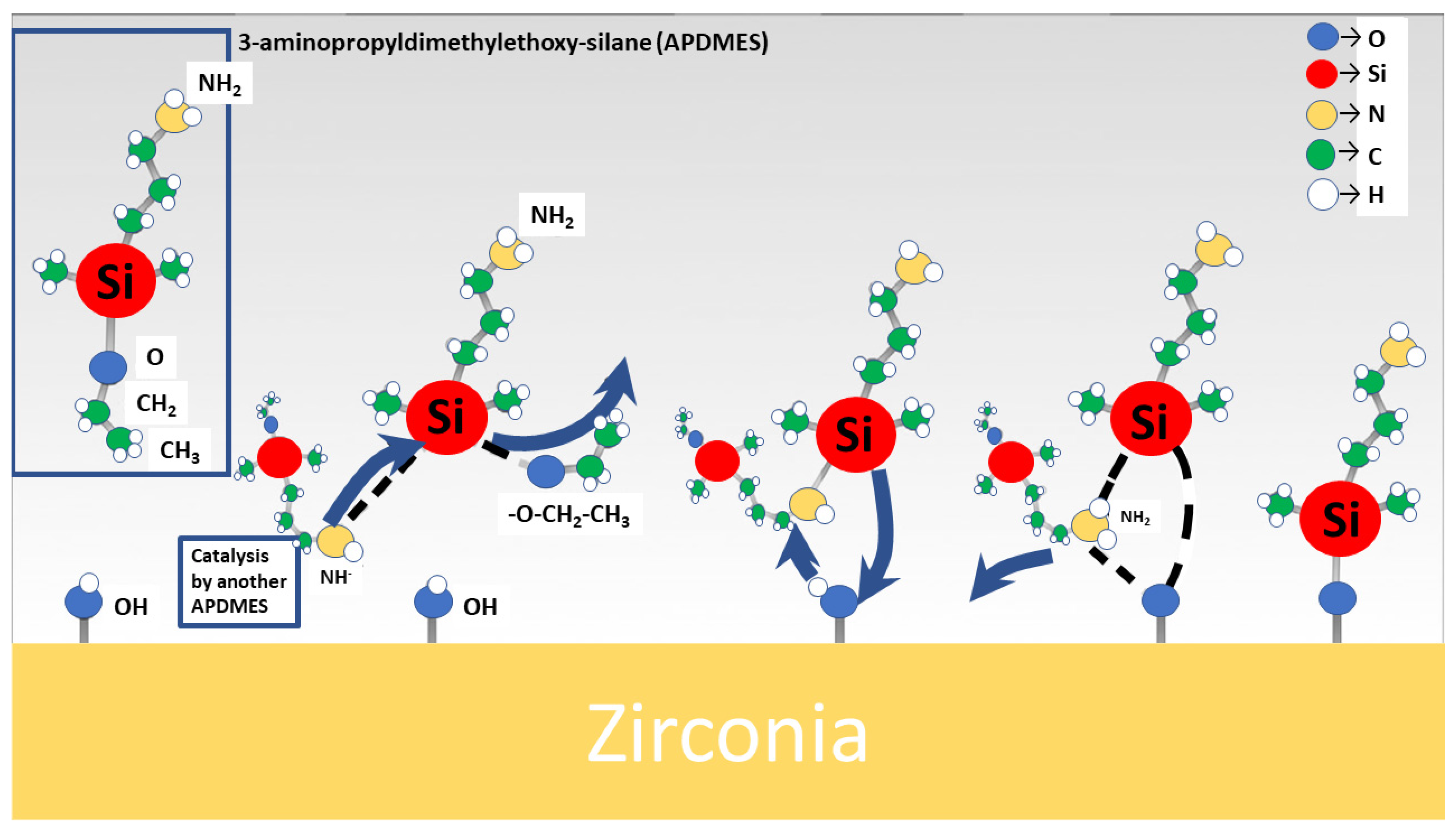
6. Biofunctionalisation of Ceramic Surfaces Using Biomolecules—An Alternative Approach
7. Considerations and Regulatory Obligations Related to the Use of High-Performance Bio Ceramics
8. Discussion
8.1. Wear and Ageing
- Detailed specifications and testing procedures;
- Processing techniques such as HIP (hot isostatic pressing/hot isostatic post-processing);
- Increased quality of ceramic granular.
8.2. Allergies
8.3. Implant Fixation and Handling of Bio Ceramics
8.4. Established Use of Bio Ceramics in Arthroplasty
8.5. Modification of Ceramic Surfaces
8.6. Non-Oxide Ceramics as an Alternative
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Felson, D.T. Epidemiology of Hip and Knee Osteoartrritis. Epidemiol. Rev. 1988, 10, 1–28. [Google Scholar] [CrossRef]
- Allen, K.D.; Thoma, L.M.; Golightly, Y.M. Epidemiology of osteoarthritis. Osteoarthr. Cartil. 2022, 30, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Lespasio, M.J.; Sultan, A.A.; Piuzzi, N.S.; Khlopas, A.; Husni, M.E.; Muschler, G.F.; Mont, M.A. Hip Osteoarthritis: A Primer. Perm. J. 2018, 22, 17–84. [Google Scholar] [CrossRef] [PubMed]
- Quicke, J.G.; Conaghan, P.G.; Corp, N.; Peat, G. Osteoarthritis year in review 2021: Epidemiology & therapy. Osteoarthr. Cartil. 2022, 30, 196–206. [Google Scholar]
- Tong, L.; Yu, H.; Huang, X.; Shen, J.; Xiao, G.; Chen, L.; Wang, H.; Xing, L.; Chen, D. Current understanding of osteoarthritis pathogenesis and relevant new approaches. Bone Res. 2022, 10, 60. [Google Scholar] [CrossRef]
- Bergschmidt, P.; Bader, R.; Ganzer, D.; Hauzeur, C.; Lohmann, C.; Rüther, W.; Tigani, D.; Rani, N.; Prats, F.L.; Zorzi, C.; et al. Ceramic femoral components in total knee arthroplasty—Two year follow-up results of an international prospective multi-centre study. Open Orthop. J. 2012, 6, 172–178. [Google Scholar] [CrossRef]
- Statistisches Bundesamt. Die 20 Häufigsten Operationen Insgesamt (OPS 5): Vollstationär Behandelte Patientinnen und Patienten in Krankenhäusern 2020. Available online: https://www.destatis.de/DE/Themen/Gesellschaft-Umwelt/Gesundheit/Krankenhaeuser/Tabellen/drg-operationen-insgesamt.html;jsessionid=057935135CAAA80FFCBBC2BDF33DA20E.live741?view=main[Print] (accessed on 27 December 2023).
- Heimke, G.; Leyen, S.; Willmann, G. Knee arthoplasty: Recently developed ceramics offer new solutions. Biomaterials 2002, 23, 1539–1551. [Google Scholar] [CrossRef]
- Rahaman, M.N.; Yao, A.; Bal, B.S.; Garino, J.P.; Ries, M.D. Ceramics for prosthetic hip and knee joint replacement. J. Am. Ceram. Soc. 2007, 90, 1965–1988. [Google Scholar] [CrossRef]
- Emery, D.F.; Clarke, H.J.; Grover, M.L. Stanmore total hip replacement in younger patients: Review of a group of patients under 50 years of age at operation. J. Bone Jt. Surg. Br. Vol. 1997, 79, 240–246. [Google Scholar] [CrossRef]
- Denkena, B.; Köhler, J.; Turger, A.; Helmecke, P.; Correa, T.A.; Hurschler, C. Manufacturing Conditioned Wear of All-Ceramic Knee Prostheses; Gottfried Wilhelm Leibniz Universität Hannover, Technische Informationsbibliothek (TIB): Hannover, Germany, 2013. [Google Scholar]
- Ó Doinn, T.; Broderick, J.M. Biomaterials in Total Joint Arthroplasty: 2. In Arthroplasty; Zorzi, A., Toumi, H., Lespessailles, E., Eds.; IntechOpen: Rijeka, Croatia, 2022. [Google Scholar]
- Hembus, J.; Rößler, L.; Springer, A.; Frank, M.; Klinder, A.; Bader, R.; Zietz, C.; Enz, A. Experimental Investigation of Material Transfer on Bearings for Total Hip Arthroplasty—A Retrieval Study on Ceramic and Metallic Femoral Heads. J. Clin. Med. 2022, 11, 3946. [Google Scholar] [CrossRef]
- Moghadasi, K.; Isa, M.S.M.; Ariffin, M.A.; Raja, S.; Wu, B.; Yamani, M.; bin Muhamad, M.R.; Yusof, F.; Jamaludin, M.F.; bin Ab Karim, M.S.; et al. A review on biomedical implant materials and the effect of friction stir based techniques on their mechanical and tribological properties. J. Mater. Res. Technol. 2022, 17, 1054–1121. [Google Scholar] [CrossRef]
- Oral, E.; Muratoglu, O.K. Radiation cross-linking in ultra-high molecular weight polyethylene for orthopaedic applications. Nucl. Instrum. Methods Phys. Res. B 2007, 265, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Partridge, T.; Baker, P.N.; Jameson, S.S.; Mason, J.; Reed, M.R.; Deehan, D.J. Conventional Versus Highly Cross-Linked Polyethylene in Primary Total Knee Replacement: A Comparison of Revision Rates Using Data from the National Joint Registry for England, Wales, and Northern Ireland. J. Bone Jt. Surg. Am. Vol. 2019, 102, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Packer, L. Protective role of vitamin E in biological systems. Am. J. Clin. Nutr. 1991, 53, 1050S–1055S. [Google Scholar] [CrossRef]
- Grimberg, A.; Jansson, V.; Lützner, J.; Melsheimer, O.; Morlock, M.; Steinbrück, A. The German Arthroplasty Registry (EPRD): Annual Report 2021; EPRD Deutsche Endoprothesenregister gGmbH: Berlin, Germany, 2021; ISBN 978-3-9817673-8-4. [Google Scholar]
- Grimberg, A.; Jansson, V.; Lützner, J.; Melsheimer, O.; Morlock, M.; Steinbrück, A. Endoprothesenregister Deutschland (EPRD)—Jahresbericht 2021. Available online: https://www.eprd.de/fileadmin/user_upload/Dateien/Publikationen/Berichte/Jahresbericht2021_2021-10-25_F.pdf (accessed on 21 October 2022).
- Bergschmidt, P.; Bader, R.; Kluess, D.; Zietz, C.; Mittelmeier, W. The all-ceramic knee endoprosthesis—The gap between expectation and experience with ceramic implants. In Seminars in Arthroplasty; WB Saunders: Philadelphia, PA, USA, 2012; pp. 262–267. [Google Scholar]
- Sundfeldt, M.; Carlsson, L.V.; Johansson, C.B.; Thomsen, P.; Gretzer, C. Aseptic loosening, not only a question of wear: A review of different theories. Acta Orthop. 2006, 77, 177–197. [Google Scholar] [CrossRef]
- Cherian, J.J.; Jauregui, J.J.; Banerjee, S.; Pierce, T.; Mont, M.A. What Host Factors Affect Aseptic Loosening After THA and TKA? Clin. Orthop. Relat. Res. 2015, 473, 2700–2709. [Google Scholar] [CrossRef]
- Goodnough, L.H.; Finlay, A.K.; Huddleston, J.I.; Goodman, S.B.; Maloney, W.J.; Amanatullah, D.F. Obesity Is Independently Associated With Early Aseptic Loosening in Primary Total Hip Arthroplasty. J. Arthroplast. 2018, 33, 882–886. [Google Scholar] [CrossRef] [PubMed]
- Münger, P.; Röder, C.; Ackermann-Liebrich, U.; Busato, A. Patient-related risk factors leading to aseptic stem loosening in total hip arthroplasty: A case-control study of 5,035 patients. Acta Orthop. 2006, 77, 567–574. [Google Scholar] [CrossRef]
- Radunovic, A.; Popovic, Z.; Matic, A.; Vulovic, M. 12 Application of ceramic components in knee arthroplasties. In Advanced Ceramics and Applications; Gadow, R., Vojislav, V., Mitic, A., Eds.; De Gruyter: Berlin, Germany, 2021; pp. 155–164. [Google Scholar]
- Langer, G. Ceramic Tibial Plateau of the 70s. In Proceedings of the Bioceramics in Joint Arthroplasty: Proceedings 7th International BIOLOX Symposium, Stuttgart, Germany, 15–16 March 2002. [Google Scholar]
- Chevalier, J.; Gremillard, L. Ceramics for medical applications: A picture for the next 20 years. J. Eur. Ceram. Soc. 2009, 29, 1245–1255. [Google Scholar] [CrossRef]
- Saccomanno, M.F.; Sircana, G.; Masci, G.; Cazzato, G.; Florio, M.; Capasso, L.; Passiatore, M.; Autore, G.; Maccauro, G.; Pola, E. Allergy in total knee replacement surgery: Is it a real problem? World J. Orthop. 2019, 10, 63–70. [Google Scholar] [CrossRef]
- Whiteside, L.A. Clinical results of revision TKA in patients with presumed metal and cement allergy. J. Arthroplast. 2022, 37, S250–S257. [Google Scholar] [CrossRef]
- Roy, M.; Bandyopadhyay, A.; Bose, S. Ceramics in bone grafts and coated implants. In Materials for Bone Disorders; Elsevier: Amsterdam, The Netherlands, 2017; pp. 265–314. [Google Scholar]
- Peter Brehm GmbH. Knieendoprothese BPK-S Integration Ceramic: Neues Kniegelenk Trotz Unverträglichkeit. Available online: https://www.peter-brehm.ch/knieendoprothese-bpk-s-integration-ceramic.html (accessed on 1 July 2022).
- Hamadouche, M.; Sedel, L. Ceramics in orthopaedics. J. Bone Jt. Surg. Br. Vol. 2000, 82, 1095–1099. [Google Scholar] [CrossRef]
- Salmang, H.; Scholze, H. (Eds.) Keramik; 6., verb. u. erw. Aufl.; Springer: Berlin, Germany, 1982. [Google Scholar]
- Kumar, P.; Dehiya, B.S.; Sindhu, A. Bioceramics for hard tissue engineering applications: A review. Int. J. Appl. Eng. Res 2018, 13, 2744–2752. [Google Scholar]
- Ben-Nissan, B.; Choi, A.H.; Cordingley, R. 10—Alumina ceramics. In Bioceramics and Their Clinical Applications: Woodhead Publishing Series in Biomaterials; Kokubo, T., Ed.; Woodhead Publishing: Cambridge, UK, 2008; pp. 223–242. ISBN 978-1-84569-204-9. [Google Scholar]
- Kamboj, N.; Ressler, A.; Hussainova, I. Bioactive Ceramic Scaffolds for Bone Tissue Engineering by Powder Bed Selective Laser Processing: A Review. Materials 2021, 14, 5338. [Google Scholar] [CrossRef] [PubMed]
- Australian Orthopaedic Association National Joint Replacement Registry. Hip, Knee & Shoulder Arthroplasty: 2021 Annual Report; AOA: Adelaide, Australia, 2021. [Google Scholar]
- Godavitarne, C.; Robertson, A.; Peters, J.; Rogers, B. Biodegradable materials. Orthop. Trauma 2017, 31, 316–320. [Google Scholar] [CrossRef]
- Wuisman, P.I.J.M.; Smit, T.H. Bioresorbable polymers: Heading for a new generation of spinal cages. Eur. Spine J. 2006, 15, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Bathaei, M.J.; Istif, E.; Beker, L. A Review of Bioresorbable Implantable Medical Devices: Materials, Fabrication, and Implementation. Adv. Healthc. Mater. 2020, 9, e2000790. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Brånemark, P.-I.; Hansson, H.-A.; Lindström, J. Osseointegrated titanium implants: Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop. Scand. 1981, 52, 155–170. [Google Scholar] [CrossRef] [PubMed]
- James, L. Osseointegration: Its Mechanism and Recent Updates. J. Dent. Res. 2022, 4, 1. [Google Scholar]
- Albrektsson, T.; Johansson, C. Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 2001, 10, S96–S101. [Google Scholar] [PubMed]
- Zarb, G.A.; Albrektsson, T. Osseointegration: A requiem for the periodontal ligament. Int. J. Periodontics Restor. Dent. 1991, 11, 88–91. [Google Scholar]
- Mavrogenis, A.F.; Dimitriou, R.; Parvizi, J.; Babis, G.C. Biology of implant osseointegration. J. Musculoskelet. Neuronal Interact. 2009, 9, 61–71. [Google Scholar]
- Kripal, K. Endosseous integration. EC Dent. Sci. 2017, 12, 87–98. [Google Scholar]
- Davies, J.E. Understanding peri-implant endosseous healing. J. Dent. Educ. 2003, 67, 932–949. [Google Scholar] [CrossRef]
- Chen, S.; Guo, Y.; Liu, R.; Wu, S.; Fang, J.; Huang, B.; Li, Z.; Chen, Z.; Chen, Z. Tuning surface properties of bone biomaterials to manipulate osteoblastic cell adhesion and the signaling pathways for the enhancement of early osseointegration. Colloids Surf. B Biointerfaces 2018, 164, 58–69. [Google Scholar] [CrossRef]
- Khalili, A.A.; Ahmad, M.R. A Review of Cell Adhesion Studies for Biomedical and Biological Applications. Int. J. Mol. Sci. 2015, 16, 18149–18184. [Google Scholar] [CrossRef] [PubMed]
- Janiszewska, M.; Primi, M.C.; Izard, T. Cell adhesion in cancer: Beyond the migration of single cells. J. Biol. Chem. 2020, 295, 2495–2505. [Google Scholar] [CrossRef]
- Harjunpää, H.; Llort Asens, M.; Guenther, C.; Fagerholm, S.C. Cell Adhesion Molecules and Their Roles and Regulation in the Immune and Tumor Microenvironment. Front. Immunol. 2019, 10, 1078. [Google Scholar] [CrossRef]
- Campbell, H.K.; Maiers, J.L.; DeMali, K.A. Interplay between tight junctions & adherens junctions. Exp. Cell Res. 2017, 358, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Green, K.J.; Jaiganesh, A.; Broussard, J.A. Desmosomes: Essential contributors to an integrated intercellular junction network. F1000Research 2019, 8, 2150. [Google Scholar] [CrossRef]
- Ghayor, C.; Bhattacharya, I.; Guerrero, J.; Özcan, M.; Weber, F.E. 3D-Printed HA-Based Scaffolds for Bone Regeneration: Microporosity, Osteoconduction and Osteoclastic Resorption. Materials 2022, 15, 1433. [Google Scholar] [CrossRef]
- Abyzov, A.M. Aluminum Oxide and Alumina Ceramics (review). Part 1. Properties of Al2O3 and Commercial Production of Dispersed Al2O3. Refract. Ind. Ceram. 2019, 60, 24–32. [Google Scholar] [CrossRef]
- ISO 6474-1:2019; Implants for Surgery—Ceramic Materials—Part 1: Ceramic Materials Based on High-Purity Alumina. International Organization for Standardization: Geneva, Switzerland, 2019; pp. 1–9.
- Piconi, C.; Maccauro, G. Zirconia as a ceramic biomaterial. Biomaterials 1999, 20, 1–25. [Google Scholar] [CrossRef]
- Hannink, R.H.J.; Kelly, P.M.; Muddle, B.C. Transformation Toughening in Zirconia-Containing Ceramics. J. Am. Ceram. Soc. 2000, 83, 461–487. [Google Scholar] [CrossRef]
- Maccauro, G.; Iommetti, P.R.; Raffaelli, L.; Manicone, P.F. Alumina and Zirconia Ceramic for Orthopaedic and Dental Devices: 15. In Biomaterials; Pignatello, R., Ed.; IntechOpen: Rijeka, Croatia, 2011. [Google Scholar]
- Kirsten, A.; Begand, S.; Oberbach, T.; Telle, R.; Fischer, H. Subcritical crack growth behavior of dispersion oxide ceramics. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 95, 202–206. [Google Scholar] [CrossRef]
- Technische Keramik GmbH. ATZ Keramik. Available online: https://tkc-keramik.de/werkstoffe/oxidkeramik/atz-aluminiumoxidverstaerktes-zirkoniumoxid/ (accessed on 30 June 2022).
- Hallab, N.J.; Jacobs, J.J. Orthopedic applications. In Biomaterials Science; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1079–1118. [Google Scholar]
- Bal, B.S.; Garino, J.; Ries, M.; Oonishi, H. Ceramic bearings in total knee arthroplasty. J. Knee Surg. 2007, 20, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Gadow, R.; Mitic, V.V. (Eds.) Advanced Ceramics and Applications; De Gruyter: Berlin, Germany; Boston, MA, USA, 2021; ISBN 9783110627992. [Google Scholar]
- Bos, I.; Willmann, G. Morphologic characteristics of periprosthetic tissues from hip prostheses with ceramic-ceramic couples: A comparative histologic investigation of 18 revision and 30 autopsy cases. Acta Orthop. Scand. 2001, 72, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.-C.; Kim, R.H. Incidence of Modern Alumina Ceramic and Alumina Matrix Composite Femoral Head Failures in Nearly 6 Million Hip Implants. J. Arthroplast. 2017, 32, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Haddouti, E.-M.; Welle, K.; Burger, C.; Kabir, K.; Schildberg, F.A. Local Cellular Responses to Metallic and Ceramic Nanoparticles from Orthopedic Joint Arthroplasty Implants. Int. J. Nanomed. 2020, 15, 6705. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.J.; Shanbhag, A.; Glant, T.T.; Black, J.; Galante, J.O. Wear debris in total joint replacements. JAAOS-J. Am. Acad. Orthop. Surg. 1994, 2, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Jasty, M.; Bragdon, C.R.; Lee, K.; Hanson, A.; Harris, W.H. Surface damage to cobalt-chrome femoral head prostheses. J. Bone Jt. Surg. Br. Vol. 1994, 76, 73–77. [Google Scholar] [CrossRef]
- Bauer, T.W.; Schils, J. The pathology of total joint arthroplasty. II. Mechanisms of implant failure. Skelet. Radiol. 1999, 28, 483–497. [Google Scholar] [CrossRef] [PubMed]
- Skinner, H.B. Ceramic bearing surfaces. Clin. Orthop. Relat. Res. 1999, 369, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Bergschmidt, P.; Bader, R.; Mittelmeier, W. Metal hypersensitivity in total knee arthroplasty: Revision surgery using a ceramic femoral component—A case report. Knee 2012, 19, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Solarino, G.; Piconi, C.; de Santis, V.; Piazzolla, A.; Moretti, B. Ceramic total knee arthroplasty: Ready to go? Joints 2017, 5, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Bader, R.; Bergschmidt, P.; Fritsche, A.; Ansorge, S.; Thomas, P.; Mittelmeier, W. Alternative materials and solutions in total knee arthroplasty for patients with metal allergy. Der. Orthop. 2008, 37, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Australian Orthopaedic Association. National Joint Replacement Registry—Annual Report. Available online: https://aoanjrr.sahmri.com/annual-reports-2016 (accessed on 27 December 2023).
- Bergschmidt, P.; Ellenrieder, M.; Bader, R.; Kluess, D.; Finze, S.; Schwemmer, B.; Mittelmeier, W. Prospective comparative clinical study of ceramic and metallic femoral components for total knee arthroplasty over a five-year follow-up period. Knee 2016, 23, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Kreve, S.; Dos Reis, A.C. Effect of surface properties of ceramic materials on bacterial adhesion: A systematic review. J. Esthet. Restor. Dent. 2022, 34, 461–472. [Google Scholar] [CrossRef]
- Rimondini, L. Bacteria and Biofilm Formation on Biomaterials. Insights. 2022. Available online: https://www.ceramtec-medical.com/fileadmin/user_upload/Medical/Bilder/Infocenter/CeraNews/CeraNews_Issue_2022_1/lay_CeraNews_122_INSIGHTS_EN_IMPLANT_MATERIAL_Rimondini_06.pdf (accessed on 27 December 2023).
- Treccani, L. Interactions Between Surface Material and Bacteria: From Biofilm Formation to Suppression. In Surface-Functionalized Ceramics: For Biotechnological and Environmental Applications; Treccani, L., Meder, F., Eds.; Wiley: Hoboken, NJ, USA, 2023; pp. 283–335. [Google Scholar]
- Salomoni, A.; Tucci, A.; Esposito, L.; Stamenkovic, I. Forming and sintering of multiphase bioceramics. J. Mater. Sci. Mater. Med. 1994, 5, 651–653. [Google Scholar] [CrossRef]
- Begand, S.; Glien, W.; Oberbach, T. ATZ—A New Material with a High Potential in Joint Replacement. Key Eng. Mater. 2005, 284–286, 983–986. [Google Scholar] [CrossRef]
- Piconi, C. Bioinert ceramics: State-of-the-art. Key Eng. Mater. 2017, 758, 3–13. [Google Scholar] [CrossRef]
- Odegaard, K.S.; Torgersen, J.; Elverum, C.W. Structural and biomedical properties of common additively manufactured biomaterials: A concise review. Metals 2020, 10, 1677. [Google Scholar] [CrossRef]
- Papannagari, R.; Hines, G.; Sprague, J. Long-Term Wear Performance of an Advanced Bearing Technology for TKA. Available online: https://www.ors.org/transactions/57/1141.pdf (accessed on 27 December 2023).
- Nakamura, S.; Ito, H.; Nakamura, K.; Kuriyama, S.; Furu, M.; Matsuda, S. Long-term durability of ceramic tri-condylar knee implants: A minimum 15-year follow-up. J. Arthroplast. 2017, 32, 1874–1879. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.; Zhao, Y.; Li, Z.; Feng, B.; Weng, X. Clinical outcomes of ceramic femoral prosthesis in total knee arthroplasty: A systematic review. J. Orthop. Surg. Res. 2019, 14, 57. [Google Scholar] [CrossRef] [PubMed]
- Solarino, G.; Zagra, L.; Piazzolla, A.; Morizio, A.; Vicenti, G.; Moretti, B. Results of 200 Consecutive Ceramic-on-Ceramic Cementless Hip Arthroplasties in Patients Up to 50 Years of Age: A 5–24 Years of Follow-Up Study. J. Arthroplast. 2019, 34, S232–S237. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Oonishi, E.; Yasuda, T.; Nakagawa, Y. A new knee prosthesis with bisurface femoral component made of zirconia-ceramic (Report 2). Key Eng. Mater. 2004, 254–256, 607–610. [Google Scholar] [CrossRef]
- Nakamura, T.; Akagi, M.; Yasuda, T.; Nakagawa, Y.; Shimizu, M. A new knee prosthesis with bisurface femoral component made of zirconia-ceramic. Key Eng. Mater. 2002, 218–220, 563–566. [Google Scholar]
- Oonishi, H.; Fujita, H.; Itoh, S.; Kin, S.; Amino, H. Development and improvement of ceramic TKP for 19 years and clinical results. Key Eng. Mater. 2002, 218, 479–482. [Google Scholar] [CrossRef]
- Oonishi, H.; Kim, S.C.; Kyomoto, M.; Masuda, S.; Asano, T.; Clarke, I.C. Change in UHMWPE properties of retrieved ceramic total knee prosthesis in clinical use for 23 years. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 74, 754–759. [Google Scholar] [CrossRef]
- Oonishi, H.; Murata, N.; Saito, M.; Wakitani, S.; Imoto, K.; Kin, S.; Chen, Y.; Nakaya, H.; Tanaka, M.; Amino, H. 3 to 18 year clinical results of total knee replacement with ceramic components. Key Eng. Mater. 2001, 192–195, 999–1004. [Google Scholar] [CrossRef]
- Oonishi, H.; Oonishi, H.; Kim, S.C.; Kyomoto, M.; Iwamoto, M.; Masuda, S.; Ueno, M. Ceramic Total Knee Arthroplasty: Advanced Clinical Experiences of 26 Years. Semin. Arthroplast. 2006, 17, 134–140. [Google Scholar] [CrossRef]
- Breuer, R.; Fiala, R.; Trieb, K.; Rath, B. Prospective Mid-Term Results of a Completely Metal-Free Ceramic Total Knee Endoprosthesis: A Concise Follow-Up of a Previous Report. J. Arthroplast. 2021, 36, 3161–3167. [Google Scholar] [CrossRef]
- Meier, E.; Gelse, K.; Trieb, K.; Pachowsky, M.; Hennig, F.F.; Mauerer, A. First clinical study of a novel complete metal-free ceramic total knee replacement system. J. Orthop. Surg. Res. 2016, 11, 21. [Google Scholar] [CrossRef]
- Trieb, K. A novel ceramic tibial component is as safe as its metal counterpart. Biomed. Tech. 2018, 63, 327–332. [Google Scholar] [CrossRef]
- Laskin, R.S. An oxidized Zr ceramic surfaced femoral component for total knee arthroplasty. Clin. Orthop. Relat. Res. 2003, 416, 191–196. [Google Scholar] [CrossRef]
- Thienpont, E. Titanium niobium nitride knee implants are not inferior to chrome cobalt components for primary total knee arthroplasty at medium-term follow-up. Arch. Orthop. Trauma Surg. 2023, 143, 5269–5275. [Google Scholar] [CrossRef] [PubMed]
- Louwerens, J.K.G.; Hockers, N.; Achten, G.; Sierevelt, I.N.; Nolte, P.A.; van Hove, R.P. No clinical difference between TiN-coated versus uncoated cementless CoCrMo mobile-bearing total knee arthroplasty; 10-year follow-up of a randomized controlled trial. Knee Surg. Sports Traumatol. Arthrosc. 2021, 29, 750–756. [Google Scholar] [CrossRef] [PubMed]
- Turner, I.G. Ceramics and Glasses. In Biomedical Materials; Springer: Cham, Switzerland, 2021; pp. 101–137. [Google Scholar]
- Qu, H.; Fu, H.; Han, Z.; Sun, Y. Biomaterials for bone tissue engineering scaffolds: A review. RSC Adv. 2019, 9, 26252–26262. [Google Scholar] [CrossRef] [PubMed]
- Böke, F.; Labude, N.; Lauria, I.; Ernst, S.; Müller-Newen, G.; Neuss, S.; Fischer, H. Biological Activation of Bioinert Medical High-Performance Oxide Ceramics by Hydrolytically Stable Immobilization of c(RGDyK) and BMP-2. ACS Appl. Mater. Interfaces 2018, 10, 38669–38680. [Google Scholar] [CrossRef] [PubMed]
- Desante, G.; Labude, N.; Rütten, S.; Römer, S.; Kaufmann, R.; Zybała, R.; Jagiełło, J.; Lipińska, L.; Chlanda, A.; Telle, R.; et al. Graphene oxide nanofilm to functionalize bioinert high strength ceramics. Appl. Surf. Sci. 2021, 566, 150670. [Google Scholar] [CrossRef]
- Caravaca, C.; Shi, L.; Balvay, S.; Rivory, P.; Laurenceau, E.; Chevolot, Y.; Hartmann, D.; Gremillard, L.; Chevalier, J. Direct silanization of zirconia for increased biointegration. Acta Biomater. 2016, 46, 323–335. [Google Scholar] [CrossRef]
- Li, W.; Tan, S.; Xing, Y.; Liu, Q.; Li, S.; Chen, Q.; Yu, M.; Wang, F.; Hong, Z. cRGD Peptide-Conjugated Pyropheophorbide-a Photosensitizers for Tumor Targeting in Photodynamic Therapy. Mol. Pharm. 2018, 15, 1505–1514. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Deng, Y.; Feng, P.; Mao, Z.; Li, P.; Yang, B.; Deng, J.; Cao, Y.; Shuai, C.; Peng, S. Current progress in bioactive ceramic scaffolds for bone repair and regeneration. Int. J. Mol. Sci. 2014, 15, 4714–4732. [Google Scholar] [CrossRef] [PubMed]
- Zang, S.; Chang, S.; Shahzad, M.B.; Sun, X.; Jiang, X.; Yang, H. Ceramics-based Drug Delivery System: A Review and Outlook. Rev. Adv. Mater. Sci. 2019, 58, 82–97. [Google Scholar] [CrossRef]
- Chethan, K.N.; Shenoy, B.S.; Bhat, N.S. Role of different orthopedic biomaterials on wear of hip joint prosthesis: A review. Mater. Today Proc. 2018, 5, 20827–20836. [Google Scholar] [CrossRef]
- Cooke, F.W. Ceramics in orthopedic surgery. Clin. Orthop. Relat. Res. 1992, 276, 135–146. [Google Scholar] [CrossRef]
- Paione, C.M.; Baino, F. Non-Oxide Ceramics for Bone Implant Application: State-of-the-Art Overview with an Emphasis on the Acetabular Cup of Hip Joint Prosthesis. Ceramics 2023, 6, 994–1016. [Google Scholar] [CrossRef]
| Orthopaedic Biomaterial | Elastic Modulus (Young’s Modulus) (GPa) | Yield Strength (Elastic Limit) (MPa) |
|---|---|---|
| Al2O3 | 366 | ./. |
| Y-TZP | 201 | ./. |
| Cortical Bone # | ||
| Low strain | 15.2 | 114 |
| High strain | 40.8 | ./. |
| Ti6Al4V | 116 | 897–1034 |
| CoCr Alloys | 210–253 | 448–841 |
| UHMWPE | 0.5–1.3 | 20–30 |
| Materials | Applications |
|---|---|
| Alumina | Femoral heads and inserts for THA bearings Osteosynthetic devices [83] |
| Zirconia | Dental implants, dental blanks for CAD/CAM # Fixed partial dentures [83] VERILAST technology [84] |
| ZTA/ATZ | Femoral heads and inserts for THA; surfaces for TKA bearings; and components for disc replacements (in spine surgery) |
| Material | Product Name (Company) | Modification Technique |
|---|---|---|
| TiNbN | EFK Femur (OHST Medizical Technology, Rathenow, Germany), balanSys (Mathys, Bettlach, Switzerland), NitrX (Microport, Shanghai, China) | Physical Vapor Deposition (PVD) |
| TiN | Score AS (Amplitude, Valence, France), SensiTiN (Medacta, Castel San Pietro, Switzerland), Apex Knee (Corin, Cirencester, UK) | PVD |
| ZrN | AS Advanced Surface (Aesculap, Center Valley, PA, USA) | PVD |
| Oxidised ZrNb | Oxinium (Smith&Nephew, London, UK) | Oxidised by a heat treatment |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eschweiler, J.; Greven, J.; Rath, B.; Kobbe, P.; Modabber, A.; Hildebrand, F.; Migliorini, F.; Hofmann, U.K. High-Performance Ceramics in Musculoskeletal Surgery: Current Use and Future Perspectives. Ceramics 2024, 7, 310-328. https://doi.org/10.3390/ceramics7010020
Eschweiler J, Greven J, Rath B, Kobbe P, Modabber A, Hildebrand F, Migliorini F, Hofmann UK. High-Performance Ceramics in Musculoskeletal Surgery: Current Use and Future Perspectives. Ceramics. 2024; 7(1):310-328. https://doi.org/10.3390/ceramics7010020
Chicago/Turabian StyleEschweiler, Jörg, Johannes Greven, Björn Rath, Philipp Kobbe, Ali Modabber, Frank Hildebrand, Filippo Migliorini, and Ulf Krister Hofmann. 2024. "High-Performance Ceramics in Musculoskeletal Surgery: Current Use and Future Perspectives" Ceramics 7, no. 1: 310-328. https://doi.org/10.3390/ceramics7010020
APA StyleEschweiler, J., Greven, J., Rath, B., Kobbe, P., Modabber, A., Hildebrand, F., Migliorini, F., & Hofmann, U. K. (2024). High-Performance Ceramics in Musculoskeletal Surgery: Current Use and Future Perspectives. Ceramics, 7(1), 310-328. https://doi.org/10.3390/ceramics7010020








