0.98(K0.5Na0.5)NbO3–0.02(Bi0.5Na0.5)(Zr0.85Sn0.15)O3 Single Crystals Grown by the Seed-Free Solid-State Crystal Growth Method and Their Characterization
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EU-Directive 2002/95/EC: Restriction of the Use of Certain Hazardous Substances in Electrical and Electronic Equipment (RoHS). Off. J. Eur. Union 2003, 46, 19–23.
- Rödel, J.; Jo, W.; Seifert, K.T.; Anton, E.M.; Granzow, T.; Damjanovic, D. Perspective on the development of lead-free piezoceramics. J. Am. Ceram. Soc. 2009, 96, 1153–1177. [Google Scholar] [CrossRef]
- Bell, A.J.; Deubzer, O. Lead-free piezoelectrics—The environmental and regulatory issues. MRS Bull. 2018, 43, 581–587. [Google Scholar] [CrossRef]
- Lv, X.; Zhu, J.; Xiao, D.; Zhang, X.-X.; Wu, J. Emerging new phase boundary in potassium sodium-niobate based ceramics. Chem. Soc. Rev. 2020, 49, 671–707. [Google Scholar] [CrossRef] [PubMed]
- Wu, J. Perovskite lead-free piezoelectric ceramics. J. Appl. Phys. 2020, 127, 190901. [Google Scholar] [CrossRef]
- Zhou, X.; Xue, G.; Luo, H.; Bowen, C.R.; Zhang, D. Phase structure and properties of sodium bismuth titanate lead-free piezoelectric ceramics. Prog. Mater. Sci. 2021, 122, 100836. [Google Scholar] [CrossRef]
- Hong, C.-H.; Kim, H.-P.; Choi, B.-Y.; Han, H.-S.; Son, J.S.; Ahn, C.W.; Jo, W. Lead-free piezoceramics—Where to move on? J. Mater. 2016, 2, 1–24. [Google Scholar] [CrossRef]
- Wang, X.; Wu, J.; Xiao, D.; Cheng, X.; Zheng, T.; Zhang, B.; Lou, X.; Zhu, J. Large d33 in (K,Na)(Nb,Ta,Sb)O3-(Bi,Na,K)ZrO3 lead-free ceramics. J. Mater. Chem. A 2014, 2, 4122–4126. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, Y.; Fan, P.; Marwat, M.A.; Ma, W.; Liu, K.; Liu, H.; Xie, B.; Wang, K.; Koruza, J. Temperature-insensitive electric-field-induced strain and enhanced piezoelectric properties of <001> textured (K,Na)NbO3-based lead-free piezoceramics. Acta Mater. 2018, 156, 389–398. [Google Scholar] [CrossRef]
- Gao, L.; Dursun, S.; Gurdal, A.E.; Hennig, E.; Zhang, S.; Randall, C.A. Atmospheric controlled processing enabling highly textured NKN with enhanced piezoelectric performance. J. Eur. Ceram. Soc. 2019, 39, 963–972. [Google Scholar] [CrossRef]
- Koruza, J.; Liu, H.; Höfling, M.; Zhang, M.-H.; Veber, P. (K,Na)NbO3-based piezoelectric single crystals: Growth methods, properties, and applications. J. Mater. Res. 2020, 35, 990–1016. [Google Scholar] [CrossRef]
- Chen, K.; Xu, G.; Yang, D.; Wang, X.; Li, J. Dielectric and piezoelectric properties of lead-free 0.95(Na0.5K0.5)NbO3–0.05LiNbO3 crystals grown by the Bridgman method. J. Appl. Phys. 2007, 101, 044103. [Google Scholar] [CrossRef]
- Tian, H.; Hu, C.; Meng, X.; Tan, P.; Zhou, Z.; Li, J.; Yang, B. Top-Seeded Solution Growth and Properties of K1–xNaxNbO3 Crystals. Cryst. Growth Des. 2015, 15, 1180–1185. [Google Scholar] [CrossRef]
- Liu, H.; Koruza, J.; Veber, P.; Rytz, D.; Maglione, M.; Rödel, J. Orientation-dependent electromechanical properties of Mn-doped (Li,Na,K)(Nb,Ta)O3 single crystals. Appl. Phys. Lett. 2016, 109, 152902. [Google Scholar] [CrossRef]
- Tian, H.; Hu, C.; Meng, X.; Zhou, Z.; Shi, G. Dielectric, piezoelectric, and elastic properties of K0.8Na0.2NbO3 single crystals. J. Mater. Chem. C 2015, 3, 9609–9614. [Google Scholar] [CrossRef]
- Zhu, B.P.; Zhu, Y.H.; Yang, J.; Ou-Yang, J.; Yang, X.F.; Li, Y.X.; Wei, W. New Potassium Sodium Niobate Single Crystal with Thickness-independent High-performance for Photoacoustic Angiography of Atherosclerotic Lesion. Sci. Rep. 2016, 6, 8. [Google Scholar] [CrossRef]
- Tsuchida, K.; Kakimoto, K.-i.; Kagomiya, I. Effect of Crystal Growth Direction on Domain Structure of Mn-Doped (Na,K)NbO3 Crystal. Jpn. J. Appl. Phy. 2013, 52, 09KD02. [Google Scholar] [CrossRef]
- Inagaki, Y.; Kakimoto, K.-i.; Kagomiya, I. Crystal growth and ferroelectric property of Na0.5K0.5NbO3 and Mn-doped Na0.5K0.5NbO3 crystals grown by floating zone method. J. Eur. Ceram. Soc. 2010, 30, 301–306. [Google Scholar] [CrossRef]
- Zhou, H.; Deng, H.; Liu, X.; Yan, H.; Zhao, X.; Luo, H.; Xu, J. Dielectric and piezoelectric properties of lead-free (K0.44Na0.46)NbO3-0.5%MnO2 single crystals grown by the TSSG method. Ceram. Int. 2016, 42, 15327–15331. [Google Scholar] [CrossRef]
- Saravanan, R.; Rajesh, D.; Rajasekaran, S.V.; Perumal, R.; Chitra, M.; Jayavel, R. Crystal structure, dielectric properties of (K0.5Na0.5)NbO3 single crystal grown by flux method using B2O3 flux. Cryst. Res. Technol. 2013, 48, 22–28. [Google Scholar] [CrossRef]
- Xu, L.P.; Jiang, K.; Zhang, J.Z.; Xu, G.S.; Hu, Z.G.; Chu, J.H. Phase transitions and thermotropic phase boundaries in MnO2-doped (K0.5Na0.5)NbO3-0.05LiNbO3 single crystals: Raman scattering evidence at elevated temperatures. Appl. Phys. Lett. 2015, 106, 122901. [Google Scholar] [CrossRef]
- Popovič, A.; Bencze, L.; Koruza, J.; Malič, B. Vapour pressure and mixing thermodynamic properties of the KNbO3–NaNbO3 system. RSC Adv. 2015, 5, 76249–76256. [Google Scholar] [CrossRef]
- Kang, S.J.L.; Park, J.H.; Ko, S.Y.; Lee, H.Y. Solid-State Conversion of Single Crystals: The Principle and the State-of-the-Art. J. Am. Ceram. Soc. 2015, 98, 347–360. [Google Scholar] [CrossRef]
- Yang, J.; Fu, Z.; Yang, Q.; Li, Y.; Liu, Y. Effect of seeds and sintering additives on (K,Na,Li)NbO3 lead-free single crystals grown by a solid-state crystal growth method. J. Ceram. Soc. Jpn. 2016, 124, 365–369. [Google Scholar] [CrossRef]
- Jiang, M.; Randall, C.A.; Guo, H.; Rao, G.; Tu, R.; Gu, Z.; Cheng, G.; Liu, X.; Zhang, J.; Li, Y. Seed-Free Solid-State Growth of Large Lead-Free Piezoelectric Single Crystals: (Na1/2K1/2)NbO3. J. Am. Ceram. Soc. 2015, 98, 2988–2996. [Google Scholar] [CrossRef]
- Ahn, C.-W.; Lee, H.-Y.; Han, G.; Zhang, S.; Choi, S.-Y.; Choi, J.-J.; Kim, J.-W.; Yoon, W.-H.; Choi, J.-H.; Park, D.-S.; et al. Self-Growth of Centimeter-Scale Single Crystals by Normal Sintering Process in Modified Potassium Sodium Niobate Ceramics. Sci. Rep. 2015, 5, 17656. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, F.; Yang, Q.; Liu, Z.; Li, Y.; Liu, Y.; Zhang, Q. Large piezoelectric properties in KNN-based lead-free single crystals grown by a seed-free solid-state crystal growth method. Appl. Phys. Lett. 2016, 108, 182904. [Google Scholar] [CrossRef]
- Ahn, C.-W.; Rahman, A.; Ryu, J.; Choi, J.-J.; Kim, J.-W.; Yoon, W.-H.; Choi, J.-H.; Park, D.-S.; Hahn, B.-D. Composition Design for Growth of Single Crystal by Abnormal Grain Growth in Modified Potassium Sodium Niobate Ceramics. Cryst. Growth Des. 2016, 16, 6586–6592. [Google Scholar] [CrossRef]
- Hao, C.Y.; Gu, Z.F.; Cheng, G.; Li, L.; Zhang, J.W.; Song, J.G.; Yan, Y.F.; Jiang, M.H. Composition design and electrical property of a pure KxNa1−xNbO3 single crystal fabricated by the seed-free solid-state crystal growth. J. Mater. Sci. Mater. Electron. 2017, 28, 18357–18365. [Google Scholar] [CrossRef]
- Rahman, A.; Cho, K.-H.; Ahn, C.-W.; Ryu, J.; Choi, J.-J.; Kim, J.-W.; Yoon, W.-H.; Choi, J.-H.; Park, D.-S.; Hahn, B.-D. A composition design rule for crystal growth of centimeter scale by normal sintering process in modified potassium sodium niobate ceramics. J. Eur. Ceram. Soc. 2018, 38, 1416–1420. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, J.; Rao, G.; Li, D.; Randall, C.A.; Li, T.; Peng, B.; Li, L.; Gu, Z.; Liu, X.; et al. Ultrahigh piezoelectric coefficient of a lead-free K0.5Na0.5NbO3-based single crystal fabricated by a simple seed-free solid-state growth method. J. Mater. Chem. C 2019, 7, 14845–14854. [Google Scholar] [CrossRef]
- Morimoto, T.; Shimono, S.; Ishii, K. Growth of large unitary (K,Na)NbO3 single crystals using a seed-free solid-state crystal growth method by adjusting the B-site excess ratios and additional Bi2O3. Jpn. J. Appl. Phy. 2020, 59, SP1001. [Google Scholar] [CrossRef]
- Chung, S.Y.; Yoon, D.Y.; Kang, S.J.L. Effects of donor concentration and oxygen partial pressure on interface morphology and grain growth behavior in SrTiO3. Acta Mater. 2002, 50, 3361–3371. [Google Scholar] [CrossRef]
- Fisher, J.G.; Kang, S.J.L. Microstructural changes in (Na0.5K0.5)NbO3 ceramics sintered in various atmospheres. J. Eur. Ceram. Soc. 2009, 29, 2581–2588. [Google Scholar] [CrossRef]
- Heo, Y.H.; Jeon, S.C.; Fisher, J.G.; Choi, S.Y.; Hur, K.H.; Kang, S.J.L. Effect of step free energy on delayed abnormal grain growth in a liquid phase-sintered BaTiO3 model system. J. Eur. Ceram. Soc. 2011, 31, 755–762. [Google Scholar] [CrossRef]
- Ahn, C.W.; Nahm, S.; Karmarkar, M.; Viehland, D.; Kang, D.H.; Bae, K.S.; Priya, S. Effect of CuO and MnO2 on sintering temperature, microstructure, and piezoelectric properties of 0.95(K0.5Na0.5)NbO3–0.05BaTiO3 ceramics. Mater. Lett. 2008, 62, 3594–3596. [Google Scholar]
- Jo, W.; Hwang, N.M.; Kim, D.Y. Effect of Crystal Shape on the Grain Growth during Liquid Phase Sintering of Ceramics. J. Korean Ceram. Soc. 2006, 43, 728–733. [Google Scholar]
- Fisher, J.G.; Kang, S.J.L. Strategies and practices for suppressing abnormal grain growth during liquid phase sintering. J. Am. Ceram. Soc. 2019, 102, 717–735. [Google Scholar] [CrossRef]
- Fisher, J.G.; Benčan, A.; Godnjavec, J.; Kosec, M. Growth behaviour of potassium sodium niobate single crystals grown by solid-state crystal growth using K4CuNb8O23 as a sintering aid. J. Eur. Ceram. Soc. 2008, 28, 1657–1663. [Google Scholar] [CrossRef]
- Uwiragiye, E.; Pham, T.L.; Fisher, J.G.; Lee, J.S.; Lee, B.W.; Ko, J.H.; Kim, H.P.; Jo, W. Sintering Aid-Assisted Growth of 0.95(K0.5Na0.5)NbO3–0.05(Bi0.5Na0.5)(Zr0.85Sn0.15)O3 Single Crystals by the Solid-State Crystal Growth Method and Their Characterization. Phys. Status Solidi A 2022, 219, 2100875. [Google Scholar] [CrossRef]
- Gou, Q.; Zhu, J.; Wu, J.; Li, F.; Jiang, L.; Xiao, D. Microstructure and electrical properties of (1−x)K0.5Na0.5NbO3–xBi0.5Na0.5Zr0.85Sn0.15O3 lead-free ceramics. J. Alloys Compd. 2018, 730, 311–317. [Google Scholar] [CrossRef]
- Malic, B.; Jenko, D.; Bernard, J.; Cilensek, J.; Kosec, M. Synthesis and Sintering of (K,Na)NbO3 Based Ceramics. Mat. Res. Soc. Proc. 2002, 755, 83–88. [Google Scholar] [CrossRef]
- Lee, M. Characterization of Thin Films by X-Ray Diffraction. In X-ray Diffraction for Materials Research: From Fundamentals to Applications; Apple Academic Press Inc.: New York, NY, USA, 2016; pp. 182–223. [Google Scholar]
- Iamsasri, T.; Tutuncu, G.; Uthaisar, C.; Pojprapai, S.; Jones, J.L. Analysis methods for characterizing ferroelectric/ferroelastic domain reorientation in orthorhombic perovskite materials and application to Li-doped Na0.5K0.5NbO3. J. Mater. Sci. 2013, 48, 6905–6910. [Google Scholar] [CrossRef]
- Rubio-Marcos, F.; Fernandez, J.F.; Ochoa, D.A.; García, J.E.; Rojas-Hernandez, R.E.; Castro, M.; Ramajo, L. Understanding the piezoelectric properties in potassium-sodium niobate-based lead-free piezoceramics: Interrelationship between intrinsic and extrinsic factors. J. Eur. Ceram. Soc. 2017, 37, 3501–3509. [Google Scholar] [CrossRef]
- Ochoa, D.A.; Esteves, G.; Iamsasri, T.; Rubio-Marcos, F.; Fernández, J.F.; García, J.E.; Jones, J.L. Extensive domain wall contribution to strain in a (K,Na)NbO3-based lead-free piezoceramics quantified from high energy X-ray diffraction. J. Eur. Ceram. Soc. 2016, 36, 2489–2494. [Google Scholar] [CrossRef]
- Kang, S.J.L.; Lee, M.G.; An, S.M. Microstructural Evolution During Sintering with Control of the Interface Structure. J. Am. Ceram. Soc. 2009, 92, 1464–1471. [Google Scholar] [CrossRef]
- Kang, S.J.L.; Ko, S.Y.; Moon, S.Y. Mixed control of boundary migration and the principle of microstructural evolution. J. Ceram. Soc. Jpn. 2016, 124, 259–267. [Google Scholar] [CrossRef]
- Fisher, J.G.; Sim, S.-H.; Ðoàn, T.T.; Uwiragiye, E.; Mok, J.; Lee, J. Comparison of (K0.5Na0.5)NbO3 Single Crystals Grown by Seed-Free and Seeded Solid-State Single Crystal Growth. Materials 2023, 16, 3638. [Google Scholar] [CrossRef] [PubMed]
- Trodahl, H.J.; Klein, N.; Damjanovic, D.; Setter, N.; Ludbrook, B.; Rytz, D.; Kuball, M. Raman spectroscopy of (K,Na)NbO3 and (K,Na)1−xLixNbO3. Appl. Phys. Lett. 2008, 93, 262901. [Google Scholar] [CrossRef]
- Kakimoto, K.; Akao, K.; Guo, Y.P.; Ohsato, H. Raman scattering study of piezoelectric (Na0.5K0.5)NbO3-LiNbO3 ceramics. Jpn. J. Appl. Phy. 2005, 44, 7064–7067. [Google Scholar] [CrossRef]
- Zhou, Z.; Fang, L.; Xiong, Z.; Zhang, Y.; Liu, Y.; Liu, G.; Liu, Y.; He, R.; Han, T.; Li, J.; et al. Phase transition of potassium sodium niobate under high pressures. Appl. Phys. Lett. 2023, 123, 012904. [Google Scholar] [CrossRef]
- Baier-Saip, J.A.; Ramos-Moor, E.; Cabrera, A.L. Raman study of phase transitions in KNbO3. Solid State Commun. 2005, 135, 367–372. [Google Scholar] [CrossRef]
- Buixaderas, E.; Nuzhnyy, D.; Gregora, I.; Kamba, S.; Berta, M.; Malic, B.; Kosec, M. Lattice Dynamics and Phase Transitions in KNbO3 and K0.5Na0.5NbO3 Ceramics. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2009, 56, 1843–1849. [Google Scholar] [CrossRef]
- Trung, D.T.; Uwiragiye, E.; Lan, T.T.; Fisher, J.G.; Lee, J.-S.; Mok, J.; Lee, J.; Naqvi, F.U.H.; Ko, J.-H. Growth of single crystals of (K1−xNax)NbO3 by the self-flux method and characterization of their phase transitions. Materials 2024. to be submitted. [Google Scholar]
- Hayes, W.; Loudon, R. Polar Vibrational Scattering. In Scattering of Light by Crystals; Dover Publications: Mineola, NY, USA, 2004; pp. 227–311. [Google Scholar]
- Powell, R.C. Symmetry and Lattice Vibrations. In Symmetry, Group Theory, and the Physical Properties of Crystals; Springer: New York, NY, USA, 2010; pp. 165–199. [Google Scholar]
- Sang, S.; Yuan, Z.; Zheng, L.; Sun, E.; Qi, X.; Zhang, R.; Jiang, X.; Li, S.; Du, J. Raman spectra of (K, Na)(Nb, Ta)O3 single crystal. J. Alloys Compd. 2017, 704, 804–808. [Google Scholar] [CrossRef]
- Xia, H.R.; Xia, H.R.; Chen, H.C.; Yu, H.; Wang, K.X.; Zhao, B.Y. Vibrational Spectra of a K0.30Na0.10Sr0.48Ba0.32Nb0.2O0.6 Single Crystal Studied by Raman and Infrared Reflectivity Spectroscopy. Phys. Status Solidi B 1998, 210, 47–59. [Google Scholar] [CrossRef]
- Klein, N.; Hollenstein, E.; Damjanovic, D.; Trodahl, H.J.; Setter, N.; Kuball, M. A study of the phase diagram of (K,Na,Li)NbO3 determined by dielectric and piezoelectric measurements, and Raman spectroscopy. J. Appl. Phys. 2007, 102, 014112. [Google Scholar] [CrossRef]
- Kodre, A.; Tellier, J.; Arčon, I.; Malič, B.; Kosec, M. Extended x-ray absorption fine structure study of phase transitions in the piezoelectric perovskite K0.5Na0.5NbO3. J. Appl. Phys. 2009, 105, 113528. [Google Scholar] [CrossRef]
- Tsuda, K.; Tanaka, M. Nanometer-scale local structural study of the paraelectric cubic phase of KNbO3 by convergent-beam electron diffraction. Jpn. J. Appl. Phy. 2017, 56, 10PB09. [Google Scholar] [CrossRef]
- Yoneda, Y.; Ohara, K.; Nagata, H. Local structure and phase transitions of KNbO3. Jpn. J. Appl. Phy. 2018, 57, 11UB07. [Google Scholar] [CrossRef]
- Bencan, A.; Oveisi, E.; Hashemizadeh, S.; Veerapandiyan, V.K.; Hoshina, T.; Rojac, T.; Deluca, M.; Drazic, G.; Damjanovic, D. Atomic scale symmetry and polar nanoclusters in the paraelectric phase of ferroelectric materials. Nat. Commun. 2021, 12, 3509. [Google Scholar] [CrossRef] [PubMed]
- Uwiragiye, E.; Farooq, M.U.; Moon, S.H.; Pham, T.L.; Nguyen, D.T.; Lee, J.S.; Fisher, J.G. High performance lead-free piezoelectric 0.96(K0.48Na0.52)NbO3-0.03[Bi0.5(Na0.7K0.2Li0.1)0.5]ZrO3-0.01(Bi0.5Na0.5)TiO3 single crystals grown by solid state crystal growth and their phase relations. J. Eur. Ceram. Soc. 2017, 37, 4597–4607. [Google Scholar] [CrossRef]
- Fridkin, V.M. Thermodynamics of Ferroelectric Semiconductors. In Ferroelectric Semiconductors; Consultants Bureau: New York, NY, USA, 1980; pp. 1–21. [Google Scholar]
- Lines, M.E.; Glass, A.M. Macroscopics and Phenomenology. In Principles and Applications of Ferroelectrics and Related Materials; Oxford University Press: Oxford, UK, 2001; pp. 59–86. [Google Scholar]
- Rubio-Marcos, F.; Del Campo, A.; López-Juárez, R.; Romero, J.J.; Fernández, J.F. High spatial resolution structure of (K,Na)NbO3 lead-free ferroelectric domains. J. Mater. Chem. 2012, 22, 9714–9720. [Google Scholar] [CrossRef]
- Doan, T.T.; Fisher, J.G.; Lee, J.-S.; Tran, H.T.; Gao, J.; Mok, J.; Lee, J.; Benčan, A.; Dražić, G.; Junaid, S.B.; et al. Growth of KNbO3 Single Crystals by the Flux Method Using KBO2 as a Flux. Inorganics 2024, 12, 151. [Google Scholar] [CrossRef]
- Jin, L.; Li, F.; Zhang, S. Decoding the Fingerprint of Ferroelectric Loops: Comprehension of the Material Properties and Structures. J. Am. Ceram. Soc. 2014, 97, 1–27. [Google Scholar] [CrossRef]
- Yao, X.; Jiang, M.; Li, W.; Lu, H.; Li, L.; Rao, G. Effects of the calcined temperature of ingredients on the growth, structure and electrical properties of (K0.5Na0.5)NbO3-based single crystals. J. Mater. Sci. Mater. Electron. 2020, 31, 21971–21980. [Google Scholar] [CrossRef]
- Morimoto, T.; Shimono, S.; Yoshiichi, Y.; Kishimura, H.; Ishii, K. Conditions of large unitary (K, Na)NbO3 system single crystals for rapid solid-state crystal growth method. Jpn. J. Appl. Phys. 2023, 62, 035501. [Google Scholar] [CrossRef]
- Morimoto, T.; Shimono, S.; Yoshiichi, Y.; Kishimura, H.; Ishii, K. Effects of Cu addition on rapid solid-state crystal growth of (K,Na)NbO3 single crystals. Jpn. J. Appl. Phys. 2022, 61, SN1015. [Google Scholar] [CrossRef]
- Fisher, J.G.; Benčan, A.; Holc, J.; Kosec, M.; Vernay, S.; Rytz, D. Growth of potassium sodium niobate single crystals by solid state crystal growth. J. Cryst. Growth 2007, 303, 487–492. [Google Scholar] [CrossRef]
- Fisher, J.G.; Bencan, A.; Kosec, M.; Vernay, S.; Rytz, D. Growth of dense single crystals of potassium sodium niobate by a combination of solid-state crystal growth and hot pressing. J. Am. Ceram. Soc. 2008, 91, 1503–1507. [Google Scholar] [CrossRef]


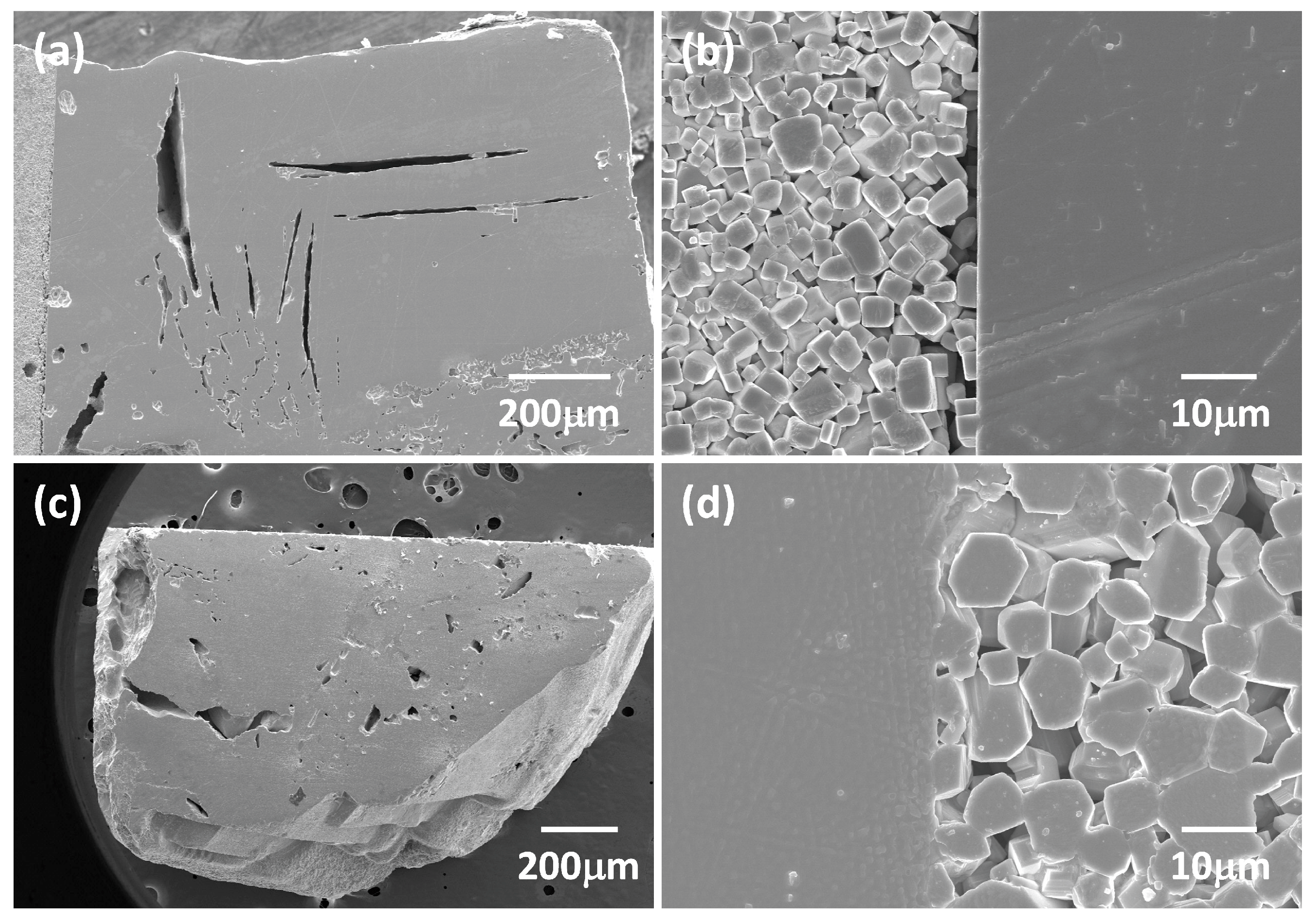



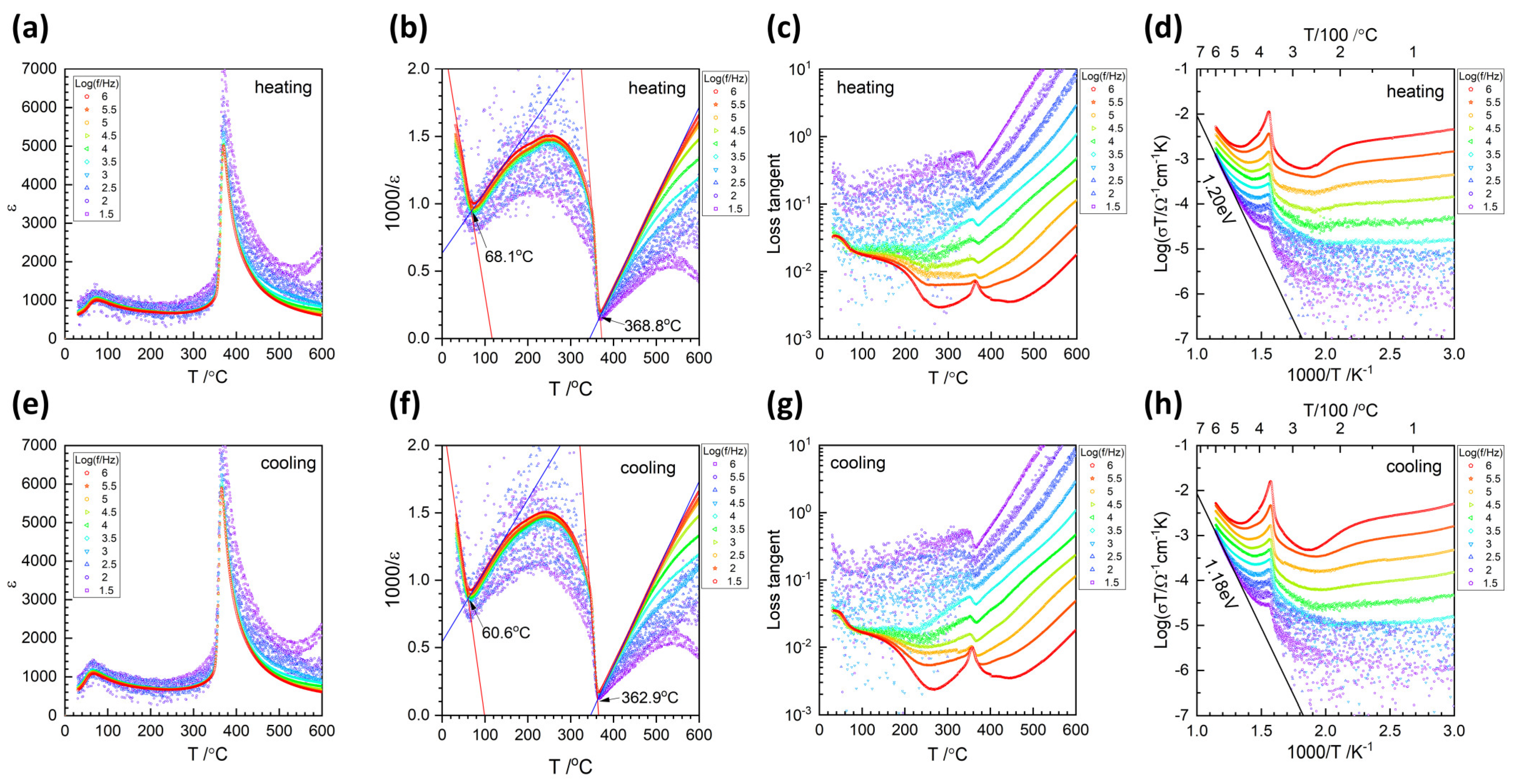


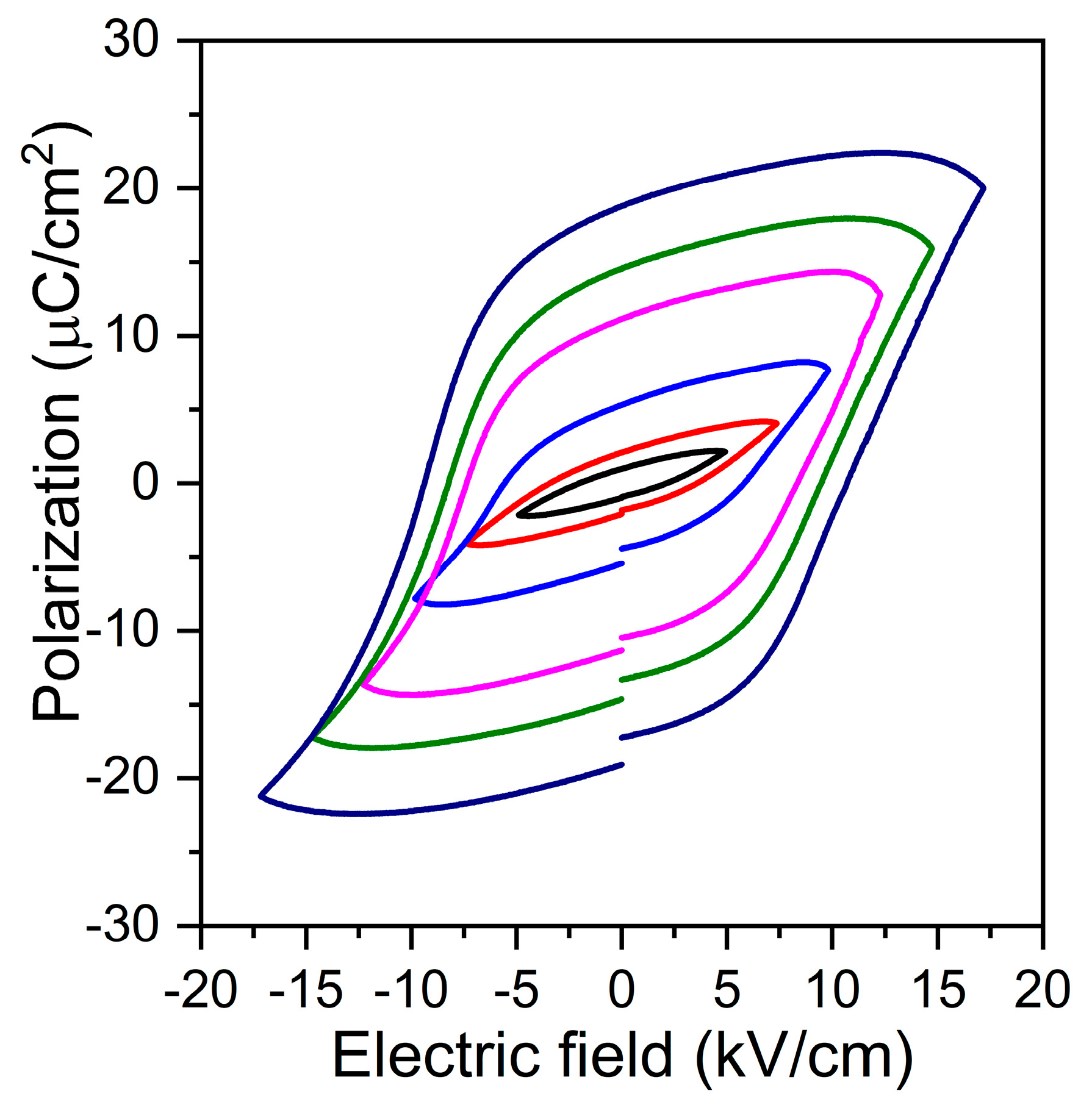
| Oxide | Single Crystal (mol %) | Matrix (mol %) | Nominal (mol %) |
|---|---|---|---|
| K2O | 22.16 ± 0.32 | 22.40 ± 0.43 | 24.26 |
| Na2O | 23.44 ± 0.34 | 24.09 ± 0.91 | 24.75 |
| Nb2O5 | 51.58 ± 0.23 | 50.83 ± 0.91 | 48.51 |
| Bi2O3 | 0.81 ± 0.07 | 0.72 ± 0.10 | 0.50 |
| ZrO2 | 1.69 ± 0.04 | 1.62 ± 0.16 | 1.68 |
| SnO2 | 0.32 ± 0.10 | 0.34 ± 0.05 | 0.30 |
| Measurement Equipment | Measurement Temperature (°C) | Relative Permittivity | Loss Tangent |
|---|---|---|---|
| TS1500 hot stage | 31 | 691 | 0.044 |
| CCR-400/200 cryostat | 25 | 448 | 0.049 |
| THMS600 cryostat | 25 | 619 | 0.028 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uwiragiye, E.; Pham, T.L.; Lee, J.-S.; Lee, B.-W.; Ko, J.-H.; Fisher, J.G. 0.98(K0.5Na0.5)NbO3–0.02(Bi0.5Na0.5)(Zr0.85Sn0.15)O3 Single Crystals Grown by the Seed-Free Solid-State Crystal Growth Method and Their Characterization. Ceramics 2024, 7, 840-857. https://doi.org/10.3390/ceramics7030055
Uwiragiye E, Pham TL, Lee J-S, Lee B-W, Ko J-H, Fisher JG. 0.98(K0.5Na0.5)NbO3–0.02(Bi0.5Na0.5)(Zr0.85Sn0.15)O3 Single Crystals Grown by the Seed-Free Solid-State Crystal Growth Method and Their Characterization. Ceramics. 2024; 7(3):840-857. https://doi.org/10.3390/ceramics7030055
Chicago/Turabian StyleUwiragiye, Eugenie, Thuy Linh Pham, Jong-Sook Lee, Byoung-Wan Lee, Jae-Hyeon Ko, and John G. Fisher. 2024. "0.98(K0.5Na0.5)NbO3–0.02(Bi0.5Na0.5)(Zr0.85Sn0.15)O3 Single Crystals Grown by the Seed-Free Solid-State Crystal Growth Method and Their Characterization" Ceramics 7, no. 3: 840-857. https://doi.org/10.3390/ceramics7030055
APA StyleUwiragiye, E., Pham, T. L., Lee, J.-S., Lee, B.-W., Ko, J.-H., & Fisher, J. G. (2024). 0.98(K0.5Na0.5)NbO3–0.02(Bi0.5Na0.5)(Zr0.85Sn0.15)O3 Single Crystals Grown by the Seed-Free Solid-State Crystal Growth Method and Their Characterization. Ceramics, 7(3), 840-857. https://doi.org/10.3390/ceramics7030055








