2D/2D Heterojunctions of Layered TiO2 and (NH4)2V3O8 for Sunlight-Driven Methylene Blue Degradation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Sample Preparation
2.3. Characterization Methods
2.4. Photocatalytic Degradation
3. Results and Discussion
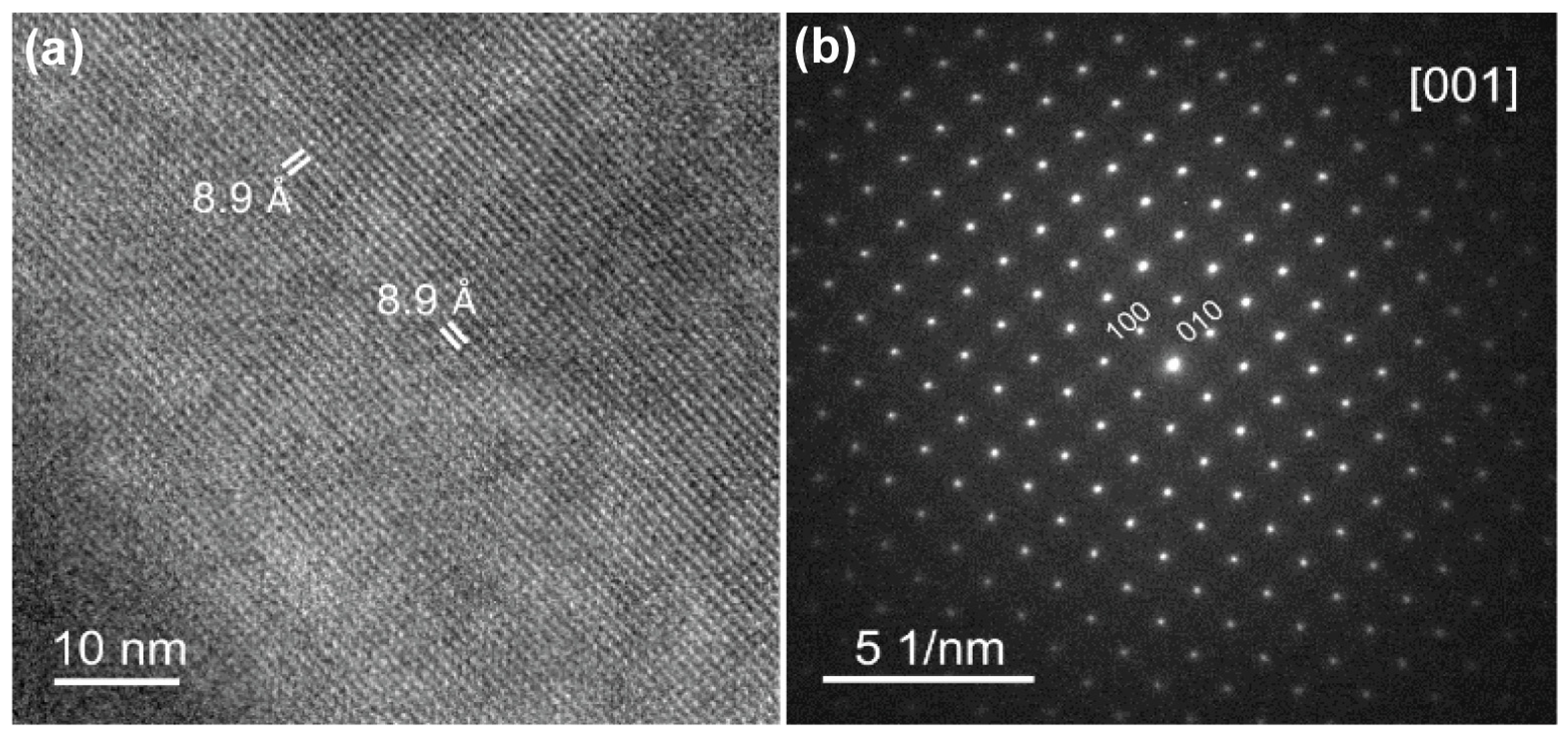
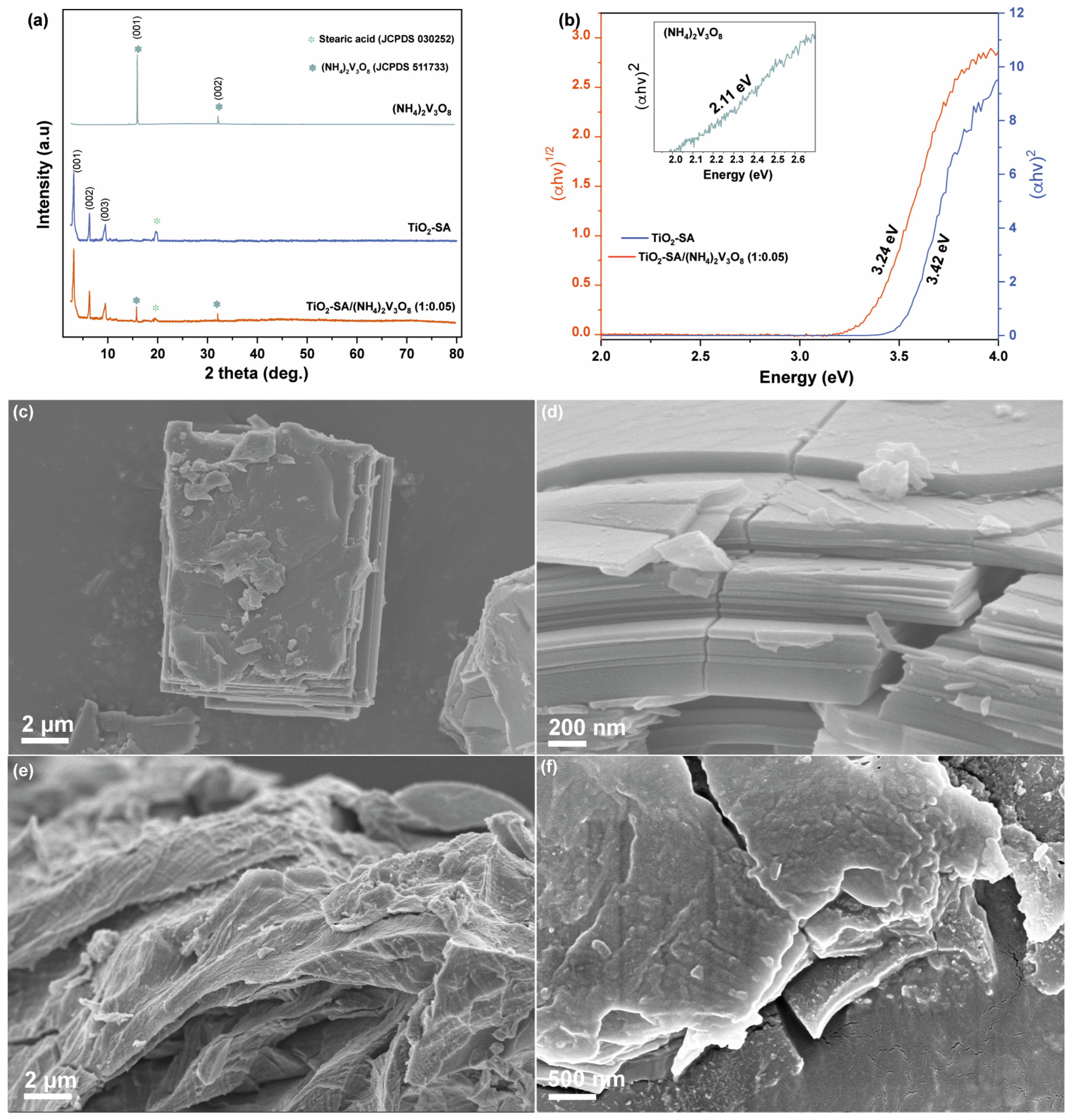
3.1. Synthesis and Characterization of the Precursors of the Photocatalytic System
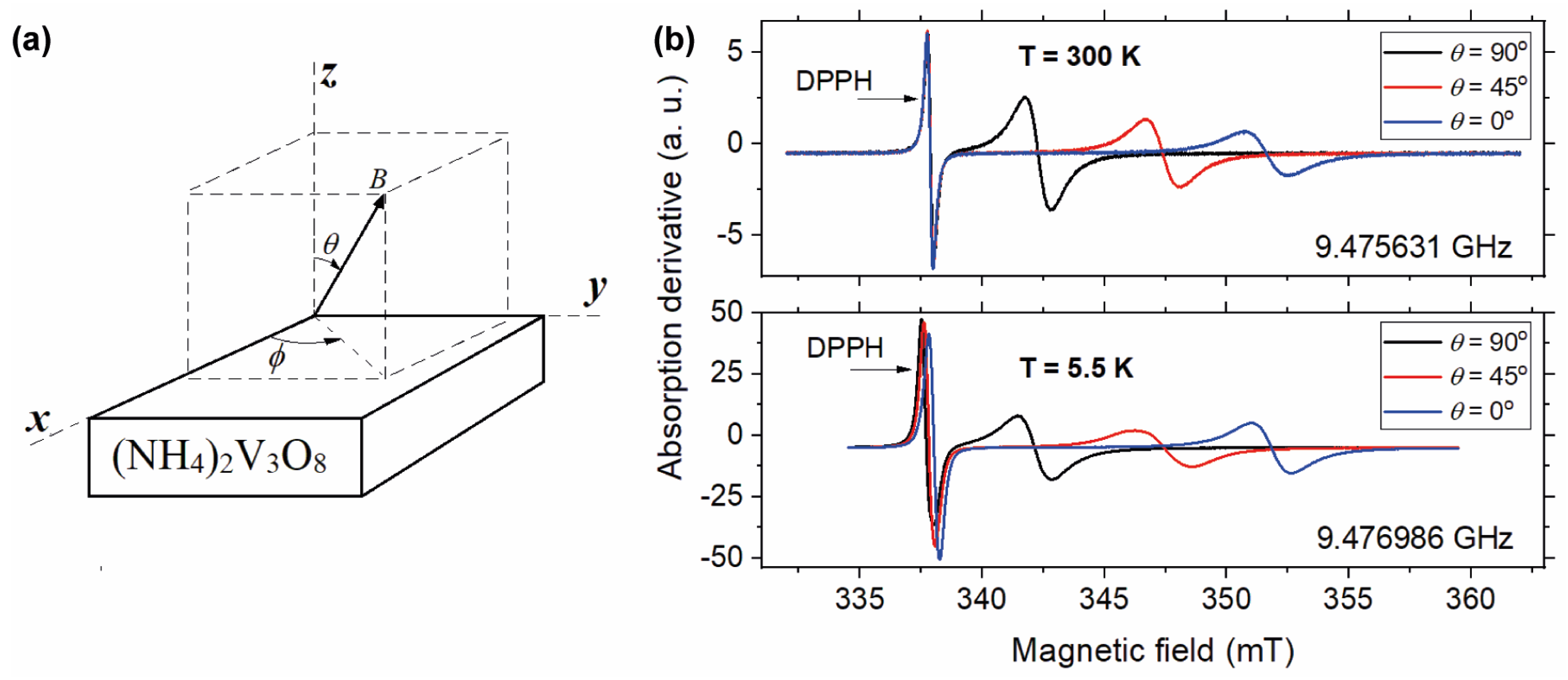
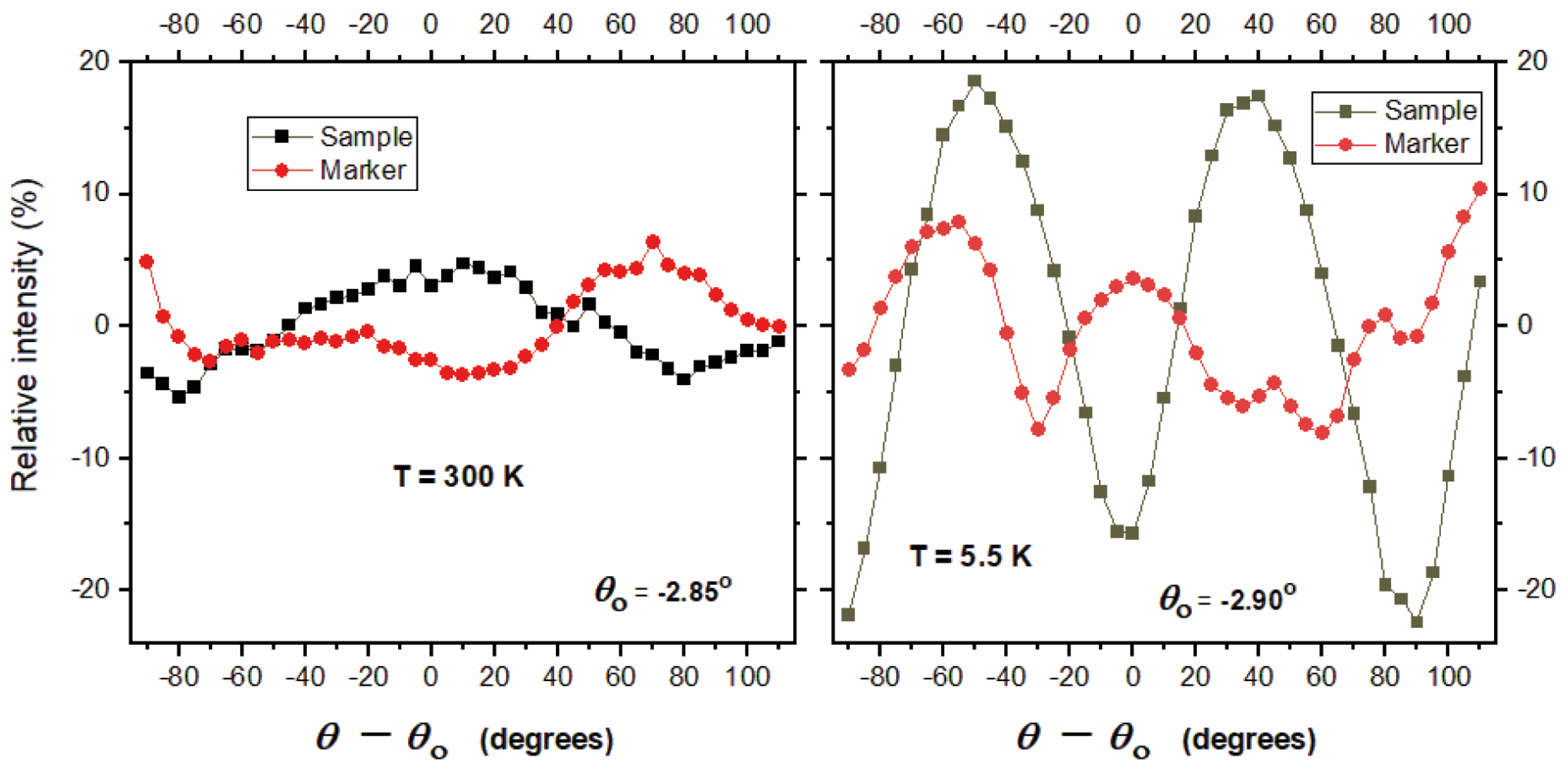
3.2. Electron Paramagnetic Resonance Studies of the Electronic Structure of Vanadium in the Crystal Structure of (NH4)2V3O8
3.3. Characterization Heterojunction TiO2–SA/(NH4)2V3O8
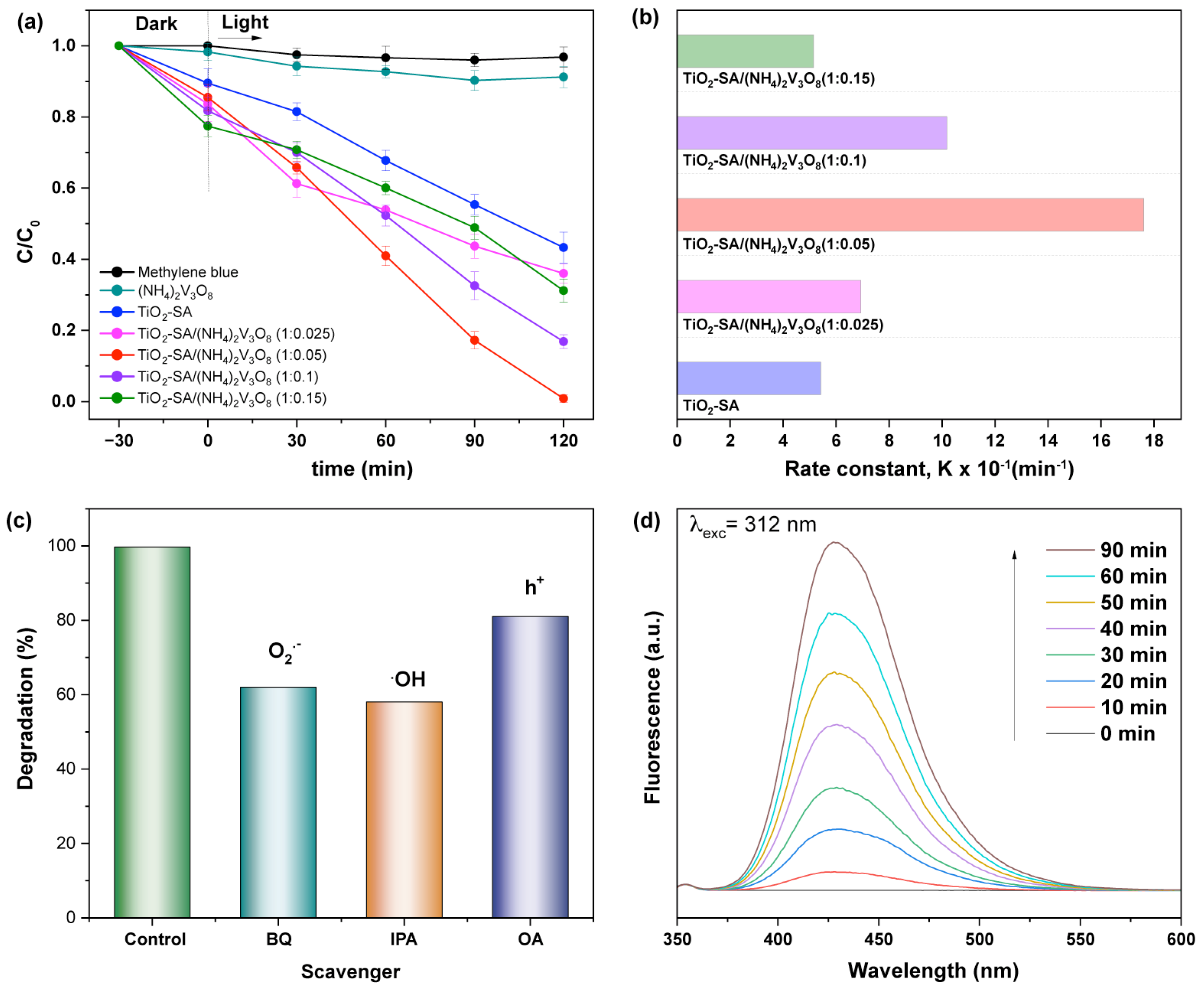
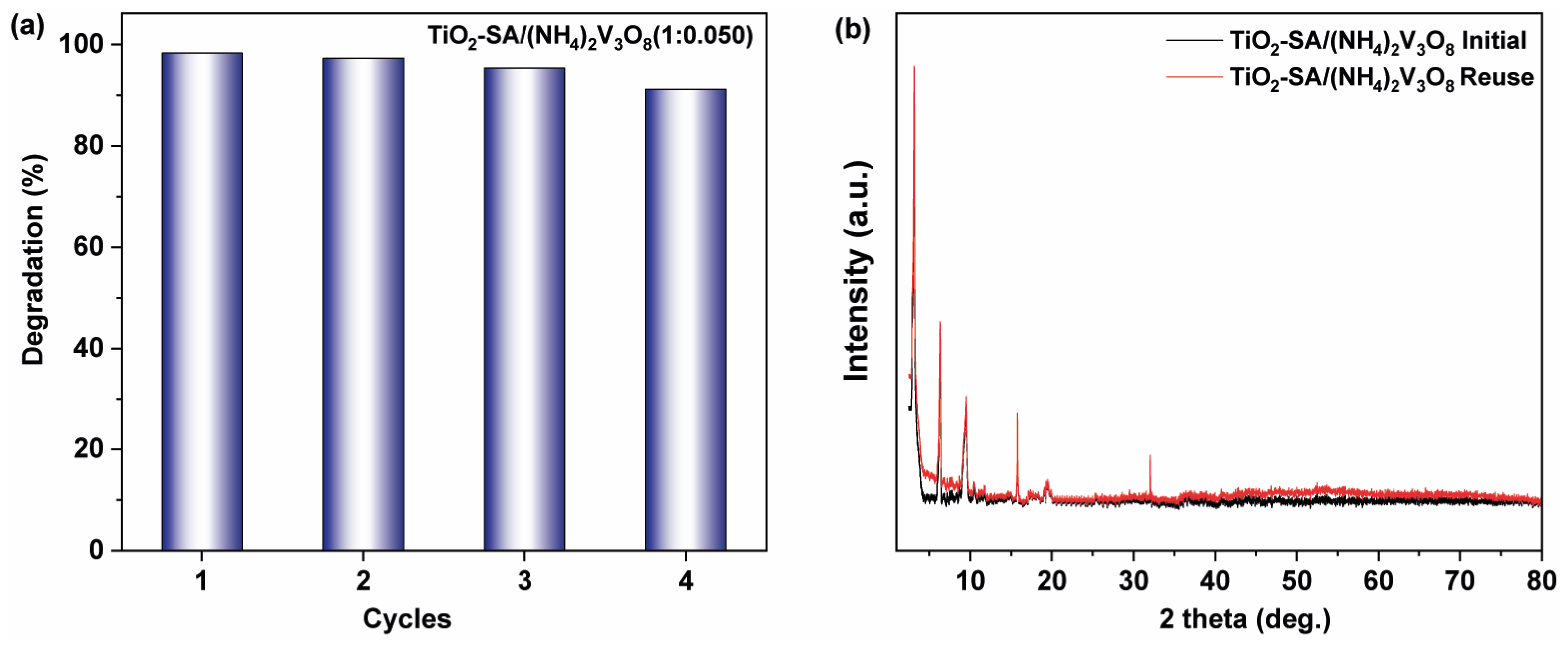
3.4. Photocatalytic Properties of TiO2–SA/(NH4)2V3O8 Composites
3.5. Degradation Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Velempini, T.; Prabakaran, E.; Pillay, K. Recent Developments in the Use of Metal Oxides for Photocatalytic Degradation of Pharmaceutical Pollutants in Water—A Review. Mater. Today Chem. 2021, 19, 100380. [Google Scholar] [CrossRef]
- Lebeau, B.; Jonas, F.; Gaudin, P.; Bonne, M.; Blin, J.-L. Dyes Depollution of Water Using Porous TiO2-Based Photocatalysts. Environ. Nanotechnol. 2020, 4, 35–92. [Google Scholar] [CrossRef]
- Moridon, S.N.F.; Arifin, K.; Yunus, R.M.; Minggu, L.J.; Kassim, M.B. Photocatalytic Water Splitting Performance of TiO2 Sensitized by Metal Chalcogenides: A Review. Ceram. Int. 2022, 48, 5892–5907. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S. Semiconductor Heterojunction Photocatalysts: Design, Construction, and Photocatalytic. Chem. Soc. Rev. 2014, 43, 5234–5244. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, M.; Li, D.; Fang, F.; Huang, W. Engineering Self-Doped Surface Defects of Anatase TiO2 Nanosheets for Enhanced Photocatalytic Efficiency. Appl. Surf. Sci. 2021, 540, 148330. [Google Scholar] [CrossRef]
- Baig, U.; Uddin, M.K.; Sajid, M. Surface Modification of TiO2 Nanoparticles Using Conducting Polymer Coating: Spectroscopic, Structural, Morphological Characterization and Interaction with Dye Molecules. Mater. Today Commun. 2020, 25, 101534. [Google Scholar] [CrossRef]
- Gu, S.; Liu, X.; Wang, H.; Liu, Z.; Xing, H.; Yu, L. Preparation and Characterization of TiO2 Photocatalytic Composites Supported by Blast Furnace Slag Fibres for Wastewater Degradation. Ceram. Int. 2023, 49, 5180–5188. [Google Scholar] [CrossRef]
- Lozano, H.; Devis, S.; Aliaga, J.; Alegría, M.; Guzmán, H.; Villarroel, R.; Benavente, E.; González, G. Two-Dimensional Titanium Dioxide—Surfactant Photoactive Supramolecular Networks: Synthesis, Properties, and Applications for the Conversion of Light Energy. Int. J. Mol. Sci. 2022, 23, 4006. [Google Scholar] [CrossRef]
- Benavente, E.; Maldonado, C.; Devis, S.; Diaz, L.; Lozano, H.; Sotomayor-Torres, C.; González, G. A Hybrid Organic-Inorganic Layered TiO2 Based Nanocomposite for Sunlight Photocatalysis. RSC Adv. 2016, 6, 18538–18541. [Google Scholar] [CrossRef]
- Ismail, A.A.; Bahnemann, D.W.; Robben, L.; Yarovyi, V.; Wark, M. Palladium Doped Porous Titania Photocatalysts: Impact of Mesoporous Order and Crystallinity. Chem. Mater. 2010, 22, 108–116. [Google Scholar] [CrossRef]
- Khan, H.; Shah, M.U.H. Modification Strategies of TiO2 Based Photocatalysts for Enhanced Visible Light Activity and Energy Storage Ability: A Review. J. Environ. Chem. Eng. 2023, 11, 111532. [Google Scholar] [CrossRef]
- Le, T.K.; Kang, M.; Kim, S.W. A Review on the Optical Characterization of V2O5 Micro-Nanostructures. Ceram. Int. 2019, 45, 15781–15798. [Google Scholar] [CrossRef]
- Li, Y.; Chen, S.; Duan, W.; Nan, Y.; Ding, D.; Xiao, G. Research Progress of Vanadium Pentoxide Photocatalytic Materials. RSC Adv. 2023, 13, 22945–22957. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.M.; Shad, N.A.; Javed, Y.; Khan, S.B.; Zhang, Z.; Amin, N.; Zhai, H. Preparation and Characterization of Vanadium Pentoxide (V2O5) for Photocatalytic Degradation of Monoazo and Diazo Dyes. Surf. Interfaces 2020, 19, 100502. [Google Scholar] [CrossRef]
- Jayaraj, S.K.; Sadishkumar, V.; Arun, T.; Thangadurai, P. Enhanced Photocatalytic Activity of V2O5 Nanorods for the Photodegradation of Organic Dyes: A Detailed Understanding of the Mechanism and Their Antibacterial Activity. Mater. Sci. Semicond. Process 2018, 85, 122–133. [Google Scholar] [CrossRef]
- Nadolska, M.; Szkoda, M.; Trzciński, K.; Niedziałkowski, P.; Ryl, J.; Mielewczyk-Gryń, A.; Górnicka, K.; Prześniak-Welenc, M. Insight into Potassium Vanadates as Visible-Light-Driven Photocatalysts: Synthesis of V(IV)-Rich Nano/Microstructures for the Photodegradation of Methylene Blue. Inorg. Chem. 2022, 61, 9433–9444. [Google Scholar] [CrossRef]
- Guo, J.; Liang, J.; Yuan, X.; Jiang, L.; Zeng, G.; Yu, H.; Zhang, J. Efficient Visible-Light Driven Photocatalyst, Silver (Meta)Vanadate: Synthesis, Morphology and Modification. Chem. Eng. J. 2018, 352, 782–802. [Google Scholar] [CrossRef]
- Sadeghzadeh-Attar, A. Enhanced Photocatalytic Hydrogen Evolution by Novel Nb-Doped SnO2/V2O5 Heteronanostructures under Visible Light with Simultaneous Basic Red 46 Dye Degradation. J. Asian Ceram. Soc. 2020, 8, 662–676. [Google Scholar] [CrossRef]
- Mkhalid, I.A.; Ismail, A.A.; Hussein, M.A.; Al thomali, R.H.M. Visible-Light-Induced V2O5 -TiO2 Photocatalysts with High Photocatalytic Ability for Degradation of Tetracycline. Opt. Mater. 2023, 135, 113263. [Google Scholar] [CrossRef]
- Benavente, E.; González, G.; Cifuentes, N.; Sotomayor-Torres, C.; Aliaga, J. Enhancement Photocatalytic Activity of the Heterojunction of Two-Dimensional Hybrid Semiconductors ZnO/V2O5. Catalysts 2018, 8, 374. [Google Scholar] [CrossRef]
- Navas, D.; Donoso, J.P.; Magon, C.; Sotomayor-Torres, C.M.; Moreno, M.; Lozano, H.; Benavente, E.; González, G. Ammonium Hexadeca-Oxo-Heptavanadate Microsquares. A New Member in the Family of the V7O16 Mixed-Valence Nanostructures. New J. Chem. 2019, 43, 17548–17556. [Google Scholar] [CrossRef]
- O’Dwyer, C.; Navas, D.; Lavayen, V.; Benavente, E.; Santa Ana, M.A.; González, G.; Newcomb, S.B.; Sotomayor Torres, C.M. Nano-Urchin: The Formation and Structure of High-Density Spherical Clusters of Vanadium Oxide Nanotubes. Chem. Mater. 2006, 18, 3016–3022. [Google Scholar] [CrossRef]
- Benavente, E.; Aliaga, J.; González, G. Chapter 14: Vanadium Oxides in Photocatalysis, Including Bare Oxides and VOx-Based Organic-Inorganic Nanocomposites. In Vanadium Catalysis; Sutradhar, M., Pombeiro, A.J.L., da Silva, J.A.L., Eds.; Royal Society of Chemistry: London, UK, 2020; pp. 340–373. [Google Scholar] [CrossRef]
- Liu, G.; Greedan, J.E. Magnetic Properties of Fresnoite-Type Vanadium Oxides: A2V3O8 (A = K, Rb, NH4). J. Solid. State Chem. 1995, 114, 499–505. [Google Scholar] [CrossRef]
- Lumsden, M.D.; Nagler, S.E.; Sales, B.C.; Tennant, D.A.; McMorrow, D.F.; Lee, S.H.; Park, S. Magnetic Excitation Spectrum of the Square Lattice S = 1/2 Heisenberg Antiferromagnet K2V3O8. Phys. Rev. B 2006, 74, 214424. [Google Scholar] [CrossRef]
- Rai, R.C.; Cao, J.; Musfeldt, J.L.; Singh, D.J.; Wei, X.; Jin, R.; Zhou, Z.X.; Sales, B.C.; Mandrus, D. Magnetodielectric Effect in the S=1/2 Quasi-Two-Dimensional Antiferromagnet K2V3O8. Phys. Rev. B 2006, 73, 075112. [Google Scholar] [CrossRef]
- Xu, G.; He, H.; Wan, H.; Liu, R.; Zeng, X.; Sun, D.; Huang, X.; Wang, H. Facile Synthesis and Lithium Storage Performance of (NH4)2V3O8 Nanoflakes. J. Appl. Electrochem. 2016, 46, 879–885. [Google Scholar] [CrossRef]
- Pang, H.; Song, Q.; Tian, P.; Cheng, J.; Zou, N.; Ning, G. Non-Hydrothermal Synthesis of (NH4)2V3O8 Hierarchical Flowers and Their Conversion into V2O5 for Lithium Ion Battery. Mater. Lett. 2016, 171, 5–9. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, J.; Wang, Q.; Zhang, S.; Hu, T.; Meng, C. One-Step Hydrothermal Preparation of (NH4)2V3O8/Carbon Composites and Conversion to Porous V2O5 Nanoparticles as Supercapacitor Electrode with Excellent Pseudocapacitive Capability. Appl. Surf. Sci. 2017, 423, 728–742. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, H.; Liu, J.H.; Gao, Y.; Cao, X.; Zhan, C.; Wang, Y.; Wang, S.; Chou, S.L.; Dou, S.X.; et al. Vanadium-Based Cathodes for Aqueous Zinc-Ion Batteries: Mechanism, Design Strategies and Challenges. Energy Storage Mater. 2022, 50, 21–46. [Google Scholar] [CrossRef]
- Ren, T.Z.; Yuan, Z.Y.; Zou, X. Crystal Growth of Mixed-Valence Ammonium Vanadates. Cryst. Res. Technol. 2007, 42, 317–320. [Google Scholar] [CrossRef]
- Popov, I.S.; Zakharova, G.S.; Liu, Y.; Enyashin, A.N. Relative Stability, Electronic and Structural Properties in the Family of NH4V3O7 Polymorphs from First Principles Calculations. Comput. Theor. Chem. 2015, 1070, 9–13. [Google Scholar] [CrossRef]
- Theobald, F.R.; Theobald, J.G.; Vedrine, J.C.; Clad, R.; Renard, J. Crystal Growth, Structure, Electron Paramagnetic Resonance and Magnetic Properties of (NH4)2V3O8. J. Phys. Chem. Solids 1984, 45, 581–587. [Google Scholar] [CrossRef]
- Paraskevopoulou, A.; Pandis, P.; Argirusis, C.; Sourkouni, G. Sonochemical Synthesis of Indium Nitride Nanoparticles and Photocatalytic Composites with Titania. Ceramics 2024, 7, 478–490. [Google Scholar] [CrossRef]
- Bubacz, K.; Kusiak-Nejman, E.; Tryba, B.; Morawski, A.W. Investigation of OH Radicals Formation on the Surface of TiO2/N Photocatalyst at the Presence of Terephthalic Acid Solution. Estimation of Optimal Conditions. J. Photochem. Photobiol. A Chem. 2013, 261, 7–11. [Google Scholar] [CrossRef]
- Livage, J. Hydrothermal Synthesis of Nanostructured Vanadium Oxides. Materials 2010, 3, 4175–4195. [Google Scholar] [CrossRef] [PubMed]
- Baran, E.J. Structural and Spectroscopic Studies Related to Vanadium Chemistry and Biochemistry. Coord. Chem. Rev. 2024, 502, 215549. [Google Scholar] [CrossRef]
- Krumeich, F.; Muhr, H.J.; Niederberger, M.; Bieri, F.; Schnyder, B.; Nesper, R. Morphology and Topochemical Reactions of Novel Vanadium Oxide Nanotubes. J. Am. Chem. Soc. 1999, 121, 8324–8331. [Google Scholar] [CrossRef]
- Pérez-Benítez, A.; Bernès, S. Redetermination of Diammonium Trivanadate, (NH4)2V3O8. IUCrdata 2020, 5, x200488. [Google Scholar] [CrossRef]
- Zakharova, G.S.; Enyashin, A.N.; Podval’naya, N.V.; Zhuravlev, N.A.; Kuznetsov, M.V.; Gorodetsky, R.S.; Liu, Y.; Zhu, Q. Structural, Electronic Properties of Microscale (NH4)2V3O8 Fabricated Using a Novel Preparation Method. J. Phys. Chem. Solids 2017, 101, 58–64. [Google Scholar] [CrossRef]
- Infrared and Raman Characteristic Group Frequencies. Tables and Charts George Socrates, 3rd ed.; John Wiley and Sons, Ltd.: Chichester, UK, 2001. [Google Scholar]
- Czamara, K.; Majzner, K.; Pacia, M.Z.; Kochan, K.; Kaczor, A.; Baranska, M. Raman Spectroscopy of Lipids: A Review. J. Raman Spectrosc. 2015, 46, 4–20. [Google Scholar] [CrossRef]
- Jokisaari, J.R.; Bayerl, D.; Zhang, K.; Xie, L.; Nie, Y.; Schlom, D.G.; Kioupakis, E.; Graham, G.W.; Pan, X. Polarization-Dependent Raman Spectroscopy of Epitaxial TiO2(B) Thin Films. Chem. Mater. 2015, 27, 7896–7902. [Google Scholar] [CrossRef]
- Alegría, M.; Aliaga, J.; Ballesteros, L.; Sotomayor-Torres, C.; González, G.; Benavente, E. Layered Nanocomposite 2D-TiO2 with Cu2O Nanoparticles as an Efficient Photocatalyst for 4-Chlorophenol Degradation and Hydrogen Evolution. Top Catal. 2020, 64, 167–180. [Google Scholar] [CrossRef]
- Narayana, M.; Kevan, L. Reinterpretation of the State of Paramagnetic Vanadium Species in Aluminophosphates. J. Phys. C Solid State Phys. 1983, 16, L863. [Google Scholar] [CrossRef]
- Davidson, A.; Che, M. Temperature-Induced Diffusion of Probe Vanadium (IV) Ions into the Matrix of Titanium Dioxide as Investigated by ESR Techniques. J. Phys. Chem. 1992, 96, 9909–9915. [Google Scholar] [CrossRef]
- Jakes, P.; Eichel, R.A. Characterization of Tetravalent Vanadium Functional Centres in Metal Oxides Derived from a Spin-Hamiltonian Analysis. Mol. Phys. 2012, 110, 277–282. [Google Scholar] [CrossRef]
- Ravikumar, R.V.S.S.N.; Rajagopal Reddy, V.; Chandrasekhar, A.V.; Reddy, B.J.; Reddy, Y.P.; Rao, P.S. Tetragonal Site of Transition Metal Ions Doped Sodium Phosphate Glasses. J. Alloys Compd. 2002, 337, 272–276. [Google Scholar] [CrossRef]
- Joy, P.A.; Vasudevan, S. Magnetism and Spin Dynamics in MnPS3 and Pyridine Intercalated MnPS3: An Electron Paramagnetic Resonance Study. J. Chem. Phys. 1993, 99, 4411–4422. [Google Scholar] [CrossRef]
- Murphy, A.B. Band-Gap Determination from Diffuse Reflectance Measurements of Semiconductor Films, and Application to Photoelectrochemical Water-Splitting. Sol. Energy Mater. Sol. Cells 2007, 91, 1326–1337. [Google Scholar] [CrossRef]
- Lee, D.E.; Moru, S.; Reddy, K.P.; Jo, W.K.; Tonda, S. 2D/2D BiOIO3/g-C3N4 S-Scheme Hybrid Heterojunction with Face-to-Face Interfacial Contact for Effective Photocatalytic H2 Production and Norfloxacin Degradation. J. Mater. Sci. Technol. 2023, 148, 19–30. [Google Scholar] [CrossRef]
- Yong, X.; Schoonen, M.A.A. The Absolute Energy Positions of Conduction and Valence Bands of Selected Semiconducting Minerals. Am. Mineral. 2000, 85, 543–556. [Google Scholar] [CrossRef]
- Hu, Y.; Li, D.; Zheng, Y.; Chen, W.; He, Y.; Shao, Y.; Fu, X.; Xiao, G. BiVO4/TiO2 Nanocrystalline Heterostructure: A Wide Spectrum Responsive Photocatalyst towards the Highly Efficient Decomposition of Gaseous Benzene. Appl. Catal. B 2011, 104, 30–36. [Google Scholar] [CrossRef]
- Gurulakshmi, M.; Selvaraj, M.; Selvamani, A.; Vijayan, P.; Sasi Rekha, N.R.; Shanthi, K. Enhanced Visible-Light Photocatalytic Activity of V2O 5/S-TiO2 Nanocomposites. Appl. Catal. A Gen. 2012, 449, 31–46. [Google Scholar] [CrossRef]
- Wang, Y.; Su, Y.R.; Qiao, L.; Liu, L.X.; Su, Q.; Zhu, C.Q.; Liu, X.Q. Synthesis of One-Dimensional TiO2/V2O5 Branched Heterostructures and Their Visible Light Photocatalytic Activity towards Rhodamine B. Nanotechnology 2011, 22, 225702. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, X.; Zhao, Q.; Ke, J.; Zhang, D. Novel V2O5/BiVO4/TiO2 Nanocomposites with High Visible-Light-Induced Photocatalytic Activity for the Degradation of Toluene. J. Phys. Chem. C 2014, 118, 10113–10121. [Google Scholar] [CrossRef]
- Grätzel, M. Photoelectrochemical Cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Shao, G. Work Function and Electron Affinity of Semiconductors: Doping Effect and Complication Due to Fermi Level Pinning. Energy Environ. Mater. 2021, 4, 273–276. [Google Scholar] [CrossRef]
- Benavente, E.; Navas, D.; Devis, S.; Segovia, M.; Sotomayor-Torres, C.; González, G. Composites of Laminar Nanostructured ZnO and VOx-Nanotubes Hybrid as Visible Light Active Photocatalysts. Catalysts 2018, 8, 93. [Google Scholar] [CrossRef]
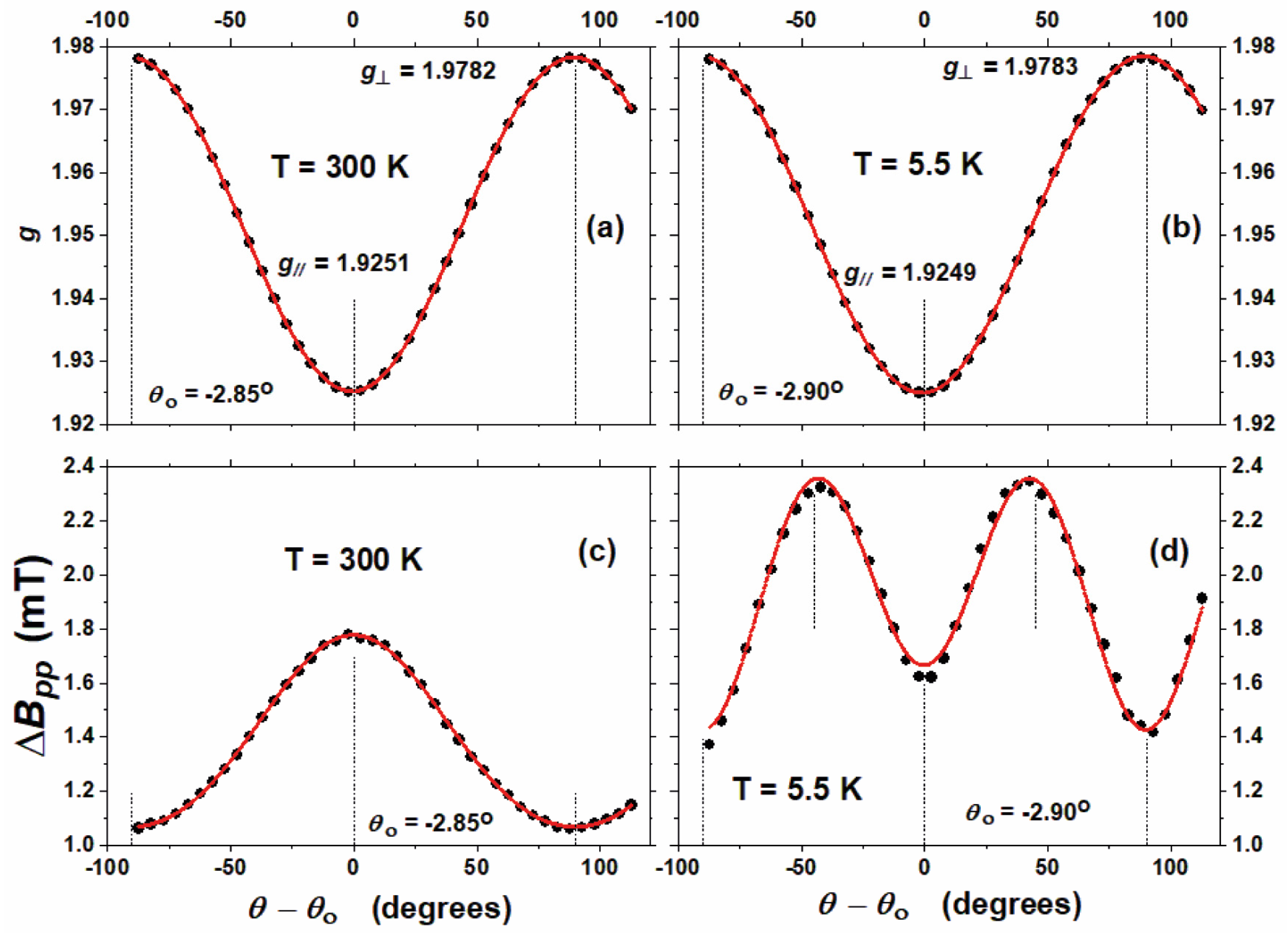

| Temperature | g⊥ | g‖ | A (mT) | B (mT) | C (mT) |
|---|---|---|---|---|---|
| 300 K | 1.9782 | 1.9251 | 1.751 | −0.779 | 0.0236 |
| 5.5 K | 1.9783 | 1.9249 | 2.022 | 0.838 | −0.359 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aliaga, J.; Alegria, M.; Donoso, J.P.; Magon, C.J.; Silva, I.D.A.; Lozano, H.; Molins, E.; Benavente, E.; González, G. 2D/2D Heterojunctions of Layered TiO2 and (NH4)2V3O8 for Sunlight-Driven Methylene Blue Degradation. Ceramics 2024, 7, 926-943. https://doi.org/10.3390/ceramics7030060
Aliaga J, Alegria M, Donoso JP, Magon CJ, Silva IDA, Lozano H, Molins E, Benavente E, González G. 2D/2D Heterojunctions of Layered TiO2 and (NH4)2V3O8 for Sunlight-Driven Methylene Blue Degradation. Ceramics. 2024; 7(3):926-943. https://doi.org/10.3390/ceramics7030060
Chicago/Turabian StyleAliaga, Juan, Matías Alegria, J. Pedro Donoso, Claudio J. Magon, Igor D. A. Silva, Harold Lozano, Elies Molins, Eglantina Benavente, and Guillermo González. 2024. "2D/2D Heterojunctions of Layered TiO2 and (NH4)2V3O8 for Sunlight-Driven Methylene Blue Degradation" Ceramics 7, no. 3: 926-943. https://doi.org/10.3390/ceramics7030060
APA StyleAliaga, J., Alegria, M., Donoso, J. P., Magon, C. J., Silva, I. D. A., Lozano, H., Molins, E., Benavente, E., & González, G. (2024). 2D/2D Heterojunctions of Layered TiO2 and (NH4)2V3O8 for Sunlight-Driven Methylene Blue Degradation. Ceramics, 7(3), 926-943. https://doi.org/10.3390/ceramics7030060






