Bond Strength of Composite Resin to Bioceramic Cements: An In Vitro Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Evaluation of Shear Bond Strength
2.2. Evaluation of Mechanical Failure
- (a)
- Adhesive: fracture occurring between the composite resin and the bioceramic cement, with no composite resin remnants on the bioceramic surface.
- (b)
- Cohesive: fracture within the composite resin, with resin remnants covering most of the bioceramic surface.
- (c)
- Mixed: a combination of adhesive and cohesive failure, with some areas of the bioceramic showing composite resin remnants and others without.
2.3. Statistical Analysis
3. Results
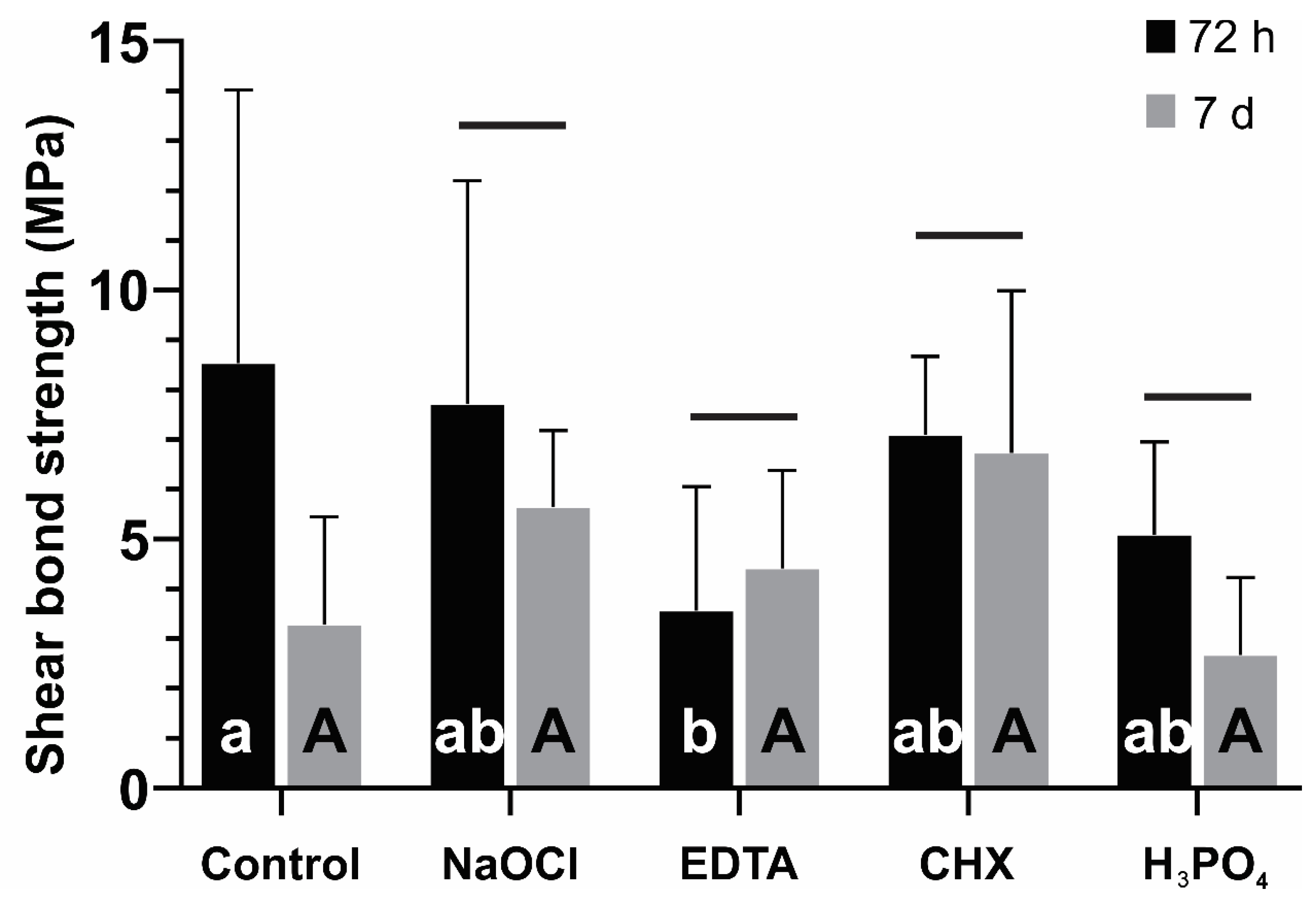
3.1. Evaluation of Shear Bond Strength
3.2. Evaluation of Mode of Failure
4. Discussion
5. Conclusions
- ○
- Adequate setting time: When the bioceramic material has a sufficient setting time, it does not significantly impact the SBS.
- ○
- Effect of EDTA and H3PO4: The application of EDTA as an irrigating solution or the use of H3PO4 on the surface of Biodentine® reduces the SBS.
- ○
- Surface treatment consideration: Proper surface treatment of Biodentine® is crucial to ensure the success of endodontic and restorative treatments.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Silva, L.A.B.; Pieroni, K.A.M.G.; Nelson-Filho, P.; Silva, R.A.B.; Hernandéz-Gatón, P.; Lucisano, M.P.; Paula-Silva, F.W.G.; de Queiroz, A.M. Furcation Perforation: Periradicular Tissue Response to Biodentine as a Repair Material by Histopathologic and Indirect Immunofluorescence Analyses. J. Endod. 2017, 43, 1137–1142. [Google Scholar] [CrossRef]
- Septodont. Biodentine Active Biosilicate Technology. 2022. Available online: http://www.septodont.co.uk/sites/uk/files/201608/brochure%20Biodentine%20HD%20UK.pdf (accessed on 20 August 2024).
- Kaur, M.; Singh, H.; Dhillon, J.S.; Batra, M.; Saini, M. MTA versus Biodentine: Review of Literature with a Comparative Analysis. J. Clin. Diagn. Res. 2017, 11, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Malkondu, Ö.; Kazandağ, M.K.; Kazazoğlu, E. A Review on Biodentine, a Contemporary Dentine Replacement and Repair Material. Biomed. Res. Int. 2014, 2014, 160951. [Google Scholar] [CrossRef] [PubMed]
- Laurent, P.; Camps, J.; About, I. Biodentine Induces TGF-β1 Release from Human Pulp Cells and Early Dental Pulp Mineralization. Int. Endod. J. 2012, 45, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Bachoo, I.K.; Seymour, D.; Brunton, P. A Biocompatible and Bioactive Replacement for Dentine: Is This a Reality? The Properties and Uses of a Novel Calcium-Based Cement. Br. Dent. J. 2013, 214, E5. [Google Scholar] [CrossRef] [PubMed]
- Koubi, G.; Colon, P.; Franquin, J.-C.; Hartmann, A.; Richard, G.; Faure, M.-O.; Lambert, G. Clinical Evaluation of the Performance and Safety of a New Dentine Substitute, Biodentine, in the Restoration of Posterior Teeth-A Prospective Study. Clin. Oral Investig. 2013, 17, 243–249. [Google Scholar] [CrossRef]
- Al-Rayesse, R.; Al-Jabban, O.; Eid, A.; Kabtoleh, A.; Addiego, F.; Mancino, D.; Haikel, Y.; Kharouf, N. Influence of Bioceramic Cements on the Quality of Obturation of the Immature Tooth: An In Vitro Microscopic and Tomographic Study. Bioengineering 2024, 11, 213. [Google Scholar] [CrossRef]
- Ashi, T.; Mancino, D.; Hardan, L.; Bourgi, R.; Zghal, J.; Macaluso, V.; Al-Ashkar, S.; Alkhouri, S.; Haikel, Y.; Kharouf, N. Physicochemical and Antibacterial Properties of Bioactive Retrograde Filling Materials. Bioengineering 2022, 9, 624. [Google Scholar] [CrossRef]
- Mishra, S.; Taneja, S.; Bhalla, V.K.; Rathore, A. Outcome of Novel Pulp Capping Modalities after Full Pulpotomy in Teeth Diagnosed with Irreversible Pulpitis: A Prospective Randomized Clinical Trial. J. Conserv. Dent. Endod. 2024, 27, 205–213. [Google Scholar] [CrossRef]
- Agarwal, N.S.; Singh, S.; Chandrasekhar, P.; Kulkarni, G.; Podar, R. Conservative Nonsurgical Approach for Management of a Case of Type II Dens in Dente. Case Rep. Dent. 2024, 2024, 8843758. [Google Scholar] [CrossRef]
- Parmar, S.; Aggarwal, N.; Gupta, H.; Marwaha, J.; Pundir, P.; Saini, R. Comparative Evaluation of the Push-Out Bond Strength of Glass Ionomer Cement, Mineral Trioxide Aggregate, Biodentine, and Endosequence Root Repair Material in Repair of Furcation Perforations: An In Vitro Study. J. Pharm. Bioallied Sci. 2024, 16, S552–S554. [Google Scholar] [CrossRef] [PubMed]
- Villat, C.; Tran, V.X.; Pradelle-Plasse, N.; Villat, C.; Tran, V.X.; Pradelle-Plasse, N.; Ponthiaux, P.; Wenger, F.; Grosgogeat, B.; Colon, P. Impedance Methodology: A New Way to Characterize the Setting Reaction of Dental Cements. Dent. Mater. 2010, 26, 1127–1132. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, J.; Sorrentino, F.; Damidot, D. Investigation of the Hydration and Bioactivity of Radiopacified Tricalcium Silicate Cement, Biodentine and MTA Angelus. Dent. Mater. 2013, 29, 580–593. [Google Scholar] [CrossRef] [PubMed]
- Grech, L.; Mallia, B.; Camilleri, J. Investigation of the Physical Properties of Tricalcium Silicate Cement-Based Root-End Filling Materials. Dent. Mater. 2013, 29, e20–e28. [Google Scholar] [CrossRef]
- Ercan, E.; Ozekinci, T.; Atakul, F.; Gül, K. Antibacterial Activity of 2% Chlorhexidine Gluconate and 5.25% Sodium Hypochlorite in Infected Root Canal: In Vivo Study. J. Endod. 2004, 30, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Hülsmann, M.; Heckendorff, M.; Lennon, A. Chelating Agents in Root Canal Treatment: Mode of Action and Indications for Their Use. Int. Endod. J. 2003, 36, 810–830. [Google Scholar] [CrossRef]
- Guneser, M.B.; Akbulut, M.B.; Eldeniz, A.U. Effect of Various Endodontic Irrigants on the Push-out Bond Strength of Biodentine and Conventional Root Perforation Repair Materials. J. Endod. 2013, 39, 380–384. [Google Scholar] [CrossRef]
- Camilleri, J. Investigation of Biodentine as Dentine Replacement Material. J. Dent. 2013, 41, 600–610. [Google Scholar] [CrossRef]
- Hashem, D.F.; Foxton, R.; Manoharan, A.; Watson, T.F.; Banerjee, A. The Physical Characteristics of Resin Composite–Calcium Silicate Interface as Part of a Layered/Laminate Adhesive Restoration. Dent. Mater. 2014, 30, 343–349. [Google Scholar] [CrossRef]
- Kharouf, N.; Reitzer, F. Est-il possible de coller sur les biocéramique? Le point due vue du chercheur. CLINIC 2024, 45, 300–303. [Google Scholar]
- Cengiz, E.; Ulusoy, N. Microshear Bond Strength of Tri-Calcium Silicate-Based Cements to Different Restorative Materials. J. Adhes. Dent. 2016, 18, 231–237. [Google Scholar] [CrossRef]
- ISO/TS 11405:2015; Dentistry-Testing of Adhesion to Tooth Structure. International Organizazion for Standardization: Geneva, Switzerland, 2015.
- Ishibe, M.; Raigrodski, A.J.; Flinn, B.D.; Chung, K.H.; Spiekerman, C.; Winter, R.R. Shear bond strengths of pressed and layered veneering ceramics to high-noble alloy and zirconia cores. J. Prosthet. Dent. 2011, 106, 29–37. [Google Scholar] [CrossRef] [PubMed]
- ISO 9917-1:2007; Dentistry—Water-Based Cements. International Organizazion for Standardization: Geneva, Switzerland, 2007.
- Kaup, M.; Dammann, C.H.; Schäfer, E.; Dammaschke, T. Shear Bond Strength of Biodentine, ProRoot MTA, Glass Ionomer Cement and Composite Resin on Human Dentine Ex Vivo. Head Face Med. 2015, 11, 16. [Google Scholar] [CrossRef] [PubMed]
- ISO 6876:2012; Dentistry—Root Canal Sealing Materials. International Organizazion for Standardization: Geneva, Switzerland, 2012.
- Odabas, M.E.; Bani, M.; Tirali, R.E. Shear Bond Strengths of Different Adhesive Systems to Biodentine. Sci. World J. 2013, 2013, 1–5. [Google Scholar] [CrossRef]
- Mustafa, R.M.; Al-Nasrawi, S.J.; Aljdaimi, A.I. The Effect of Biodentine Maturation Time on Resin Bond Strength When Aged in Artificial Saliva. Int. J. Dent. 2020, 2020, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kayahan, M.B.; Neekofar, M.H.; Kazanda, M. Effect of Acid-Etching Procedure on Selected Physical Properties of Mineral Trioxide Aggregate. Int. Endod. J. 2009, 42, 1004–1014. [Google Scholar] [CrossRef] [PubMed]
- Kayahan, M.B.; Nekoofar, M.H.; McCann, A.; Kazanda, M. Effect of Acid Etching Procedures on the Compressive Strength of 4 Calcium Silicate–Based Endodontic Cements. J. Endod. 2013, 39, 1646–1648. [Google Scholar] [CrossRef]
- Yan, P.; Peng, B.; Fan, B.; Fan, M.; Bian, Z. The Effects of Sodium Hypochlorite (5.25%), Chlorhexidine (2%), and Glyde File Prep on the Bond Strength of MTA-Dentin. J. Endod. 2006, 32, 58–60. [Google Scholar] [CrossRef]
- Lee, Y.L.; Lin, F.H.; Wang, W.H.; Ritchie, H.H. Effects of EDTA on the hydration mechanism of mineral trioxide aggregate. J. Dent. Res. 2007, 86, 534–538. [Google Scholar] [CrossRef]
- Aggarwal, V.; Jain, A.; Kabi, D. In vitro evaluation of effect of various endodontic solutions on selected physical properties of white mineral trioxide aggregate. Aust. Endod. J. 2011, 37, 61–64. [Google Scholar] [CrossRef]
- Ballal, V.; Marques, J.N.; Campos, C.N.; Gudipaneni, R.; Yeraballi, D. Effects of chelating agent and acids on Biodentine. Aust. Dent. J. 2018, 63, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Vichi, A.; Margvelashvili, M.; Goracci, C.; Papacchini, F.; Ferrari, M. Bonding and sealing ability of a new self-adhering flowable composite resin in class I restorations. Clin. Oral Investig. 2013, 17, 1497–1506. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.; Rizk, M.; Hoch, M.; Stansbury, J.; Gladys, S. Bonding performance of self-adhesive flowable composites to enamel, dentin and a nano-hybrid composite. Odontology. 2018, 106, 171–180. [Google Scholar] [CrossRef]
- David, C.; De Cardoso, G.C.; Insolan, C.P.; Melo, T.; Flores-Sahagun, T.H.; Cuevas-Suárez, C.E. Bond strength of self-adhesive flowable composite resins to dental tissues: A systematic review and meta-analysis of in vitro studies. J. Prosthet. Dent. 2021, 128, 1–10. [Google Scholar] [CrossRef]
- Taschner, M.; Nato, F.; Mazzoni, A.; Breschi, L.; Petschelt, A.; Breschi, L. Role of preliminary etching for one-step self-etch adhesives. Eur. J. Oral Sci. 2010, 118, 517–524. [Google Scholar] [CrossRef]
- Altunsoy, M.; Tanrıver, M.; Ok, E.; Kucukyilmaz, E. Shear bond strength of a self-adhering flowable composite and a flowable base composite to mineral trioxide aggregate, calcium-enriched mixture cement, and Biodentine. J. Endod. 2015, 41, 1691–1695. [Google Scholar] [CrossRef] [PubMed]
- Hardan, L.; Mancino, D.; Bourgi, R.; Alvarado-Orozco, A.; Rodriguez-Vilchis, L.E.; Flores-Ledesma, A.; Cuevas-Suarez, C.E.; Lukomska-Szymanska, M.; Eid, A.; Danhache, M.L.; et al. Bond Strength of Adhesive Systems to Calcium Silicate-Based Materials: A Systematic Review and Meta-Analysis of In Vitro Studies. Gels 2022, 8, 311. [Google Scholar] [CrossRef]
- Meraji, N.; Camilleri, J. Bonding over dentin replacement materials. J. Endod. 2017, 43, 1343–1349. [Google Scholar] [CrossRef]
- Elnaghy, A.M. Influence of acidic environment on properties of biodentine and white mineral trioxide aggregate: A comparative study. J. Endod. 2015, 40, 953–957. [Google Scholar] [CrossRef]


| Material | Manufacturer | Composition |
|---|---|---|
| Biodentine® | Septodont | Powder: tricalcium silicate, zirconium oxide, calcium oxide, calcium carbonate, and iron oxides. Liquid: calcium chloride, polycarboxylate, and purified water. |
| Dyad FlowTM | Kerr | Urethane dimethacrylate, Bisphenol A Ethoxylates Dimethacrylate, barium alumino silicate, silicon dioxide, camphorquinone, and dibutyl hydroxy toluene. |
| Clorox™ | Clorox™ | Sodium hypochlorite. |
| Consepsis™ | Ultradent | Chlorhexidine. |
| MD-Cleanser™ Smear layer removal | Metabiomed | Ethylenediaminetetraacetic acid. |
| Total etch | Ivoclar-Vivadent | Phosphoric acid. |
| 24 h Setting | 72 h Setting | |||||
|---|---|---|---|---|---|---|
| Group | Adhesive (%) | Cohesive (%) | Mixed (%) | Adhesive (%) | Cohesive (%) | Mixed (%) |
| Control | 60 | 40 | 0 | 100 | 0 | 0 |
| NaOCl | 40 | 20 | 40 | 80 | 0 | 20 |
| EDTA | 80 | 0 | 20 | 90 | 0 | 10 |
| CHX | 90 | 0 | 10 | 80 | 10 | 10 |
| H3PO4 | 100 | 0 | 0 | 100 | 0 | 0 |
| 24 h Setting | 72 h Setting | |||||
|---|---|---|---|---|---|---|
| Group | Adhesive (%) | Cohesive (%) | Mixed (%) | Adhesive (%) | Cohesive (%) | Mixed (%) |
| Control | 80 | 20 | 0 | 90 | 0 | 10 |
| NaOCl | 40 | 40 | 20 | 80 | 0 | 20 |
| EDTA | 50 | 0 | 50 | 70 | 0 | 30 |
| CHX | 80 | 10 | 10 | 20 | 20 | 60 |
| H3PO4 | 100 | 0 | 0 | 80 | 0 | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvarado-Orozco, A.; Hardan, L.; Bourgi, R.; Monjarás-Ávila, A.J.; Cuevas-Suárez, C.E.; Rodríguez-Vilchis, L.E.; Farrayeh, A.; Flores-Ferreyra, B.I.; Contreras-Bulnes, R.; Haikel, Y.; et al. Bond Strength of Composite Resin to Bioceramic Cements: An In Vitro Study. Ceramics 2024, 7, 1137-1146. https://doi.org/10.3390/ceramics7030074
Alvarado-Orozco A, Hardan L, Bourgi R, Monjarás-Ávila AJ, Cuevas-Suárez CE, Rodríguez-Vilchis LE, Farrayeh A, Flores-Ferreyra BI, Contreras-Bulnes R, Haikel Y, et al. Bond Strength of Composite Resin to Bioceramic Cements: An In Vitro Study. Ceramics. 2024; 7(3):1137-1146. https://doi.org/10.3390/ceramics7030074
Chicago/Turabian StyleAlvarado-Orozco, Alejandra, Louis Hardan, Rim Bourgi, Ana Josefina Monjarás-Ávila, Carlos Enrique Cuevas-Suárez, Laura Emma Rodríguez-Vilchis, Antoun Farrayeh, Blanca Irma Flores-Ferreyra, Rosalía Contreras-Bulnes, Youssef Haikel, and et al. 2024. "Bond Strength of Composite Resin to Bioceramic Cements: An In Vitro Study" Ceramics 7, no. 3: 1137-1146. https://doi.org/10.3390/ceramics7030074
APA StyleAlvarado-Orozco, A., Hardan, L., Bourgi, R., Monjarás-Ávila, A. J., Cuevas-Suárez, C. E., Rodríguez-Vilchis, L. E., Farrayeh, A., Flores-Ferreyra, B. I., Contreras-Bulnes, R., Haikel, Y., & Kharouf, N. (2024). Bond Strength of Composite Resin to Bioceramic Cements: An In Vitro Study. Ceramics, 7(3), 1137-1146. https://doi.org/10.3390/ceramics7030074











