Research of Characteristics of the Thermal Runaway Process of Full-Size Prefabricated Cabin Energy Storage System
Abstract
1. Instruction
2. Experimental Design
2.1. Test Materials and Equipment
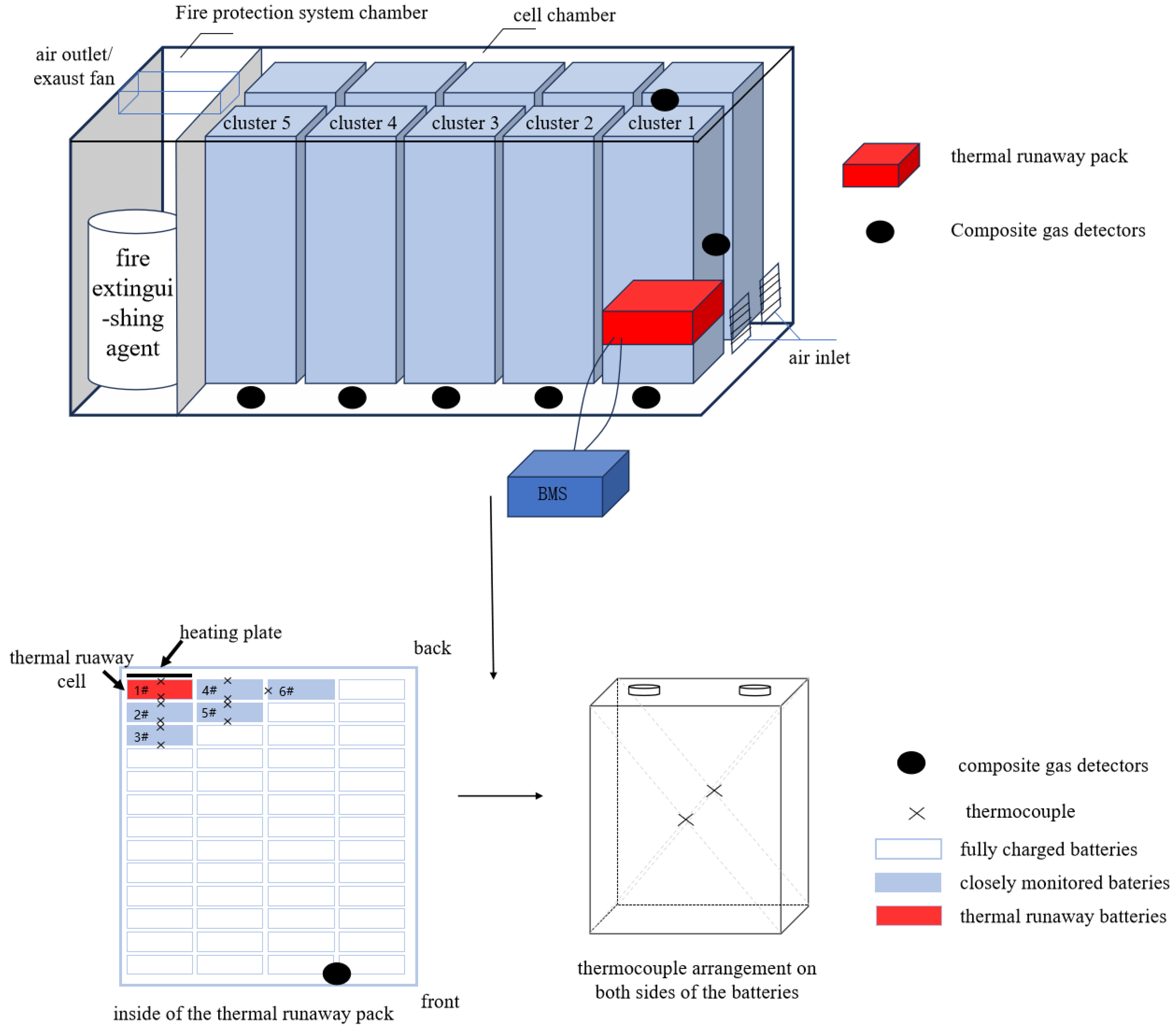
2.2. Experimental Setup
2.3. Test Method
3. Test Results and Analysis
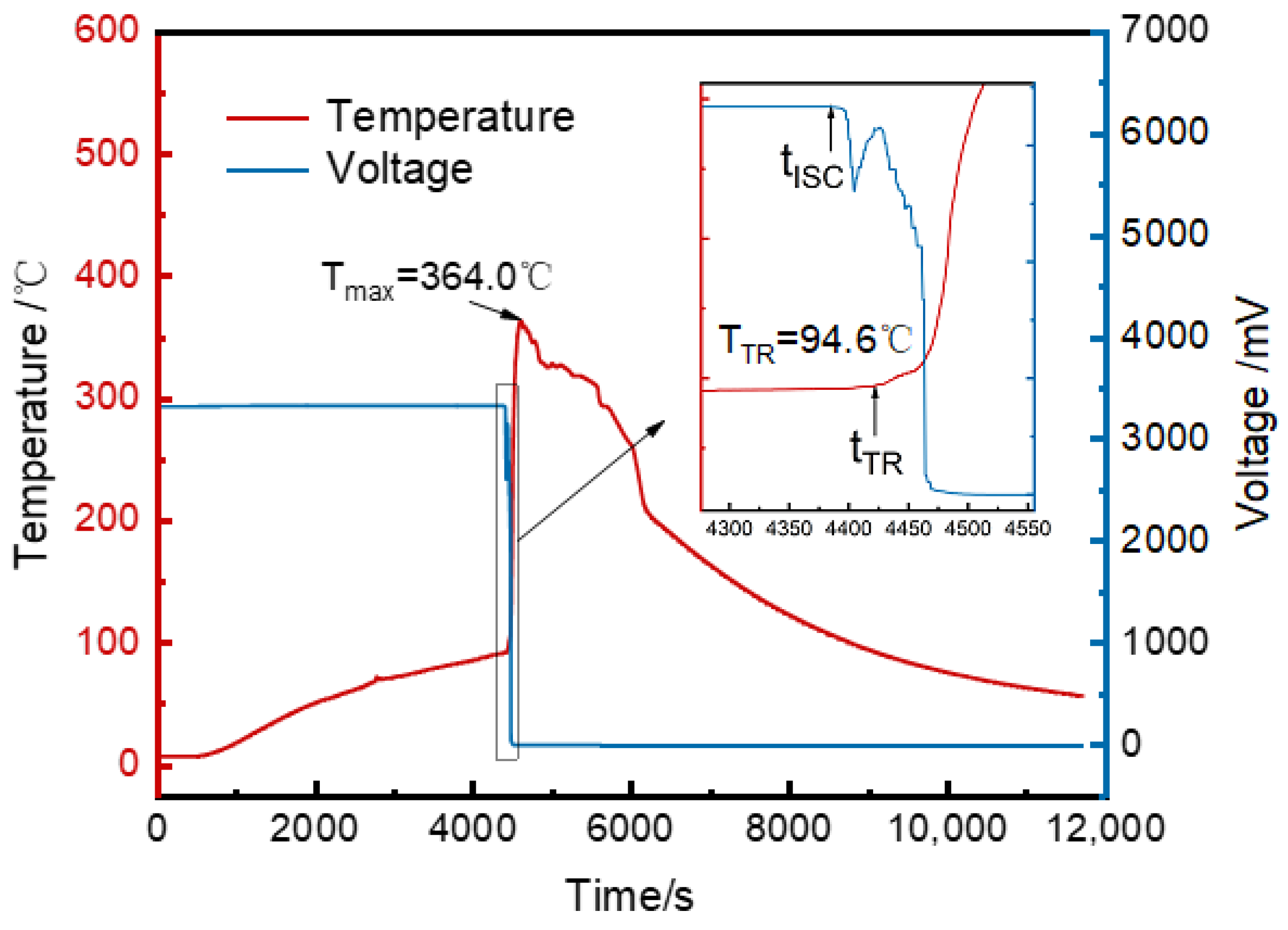
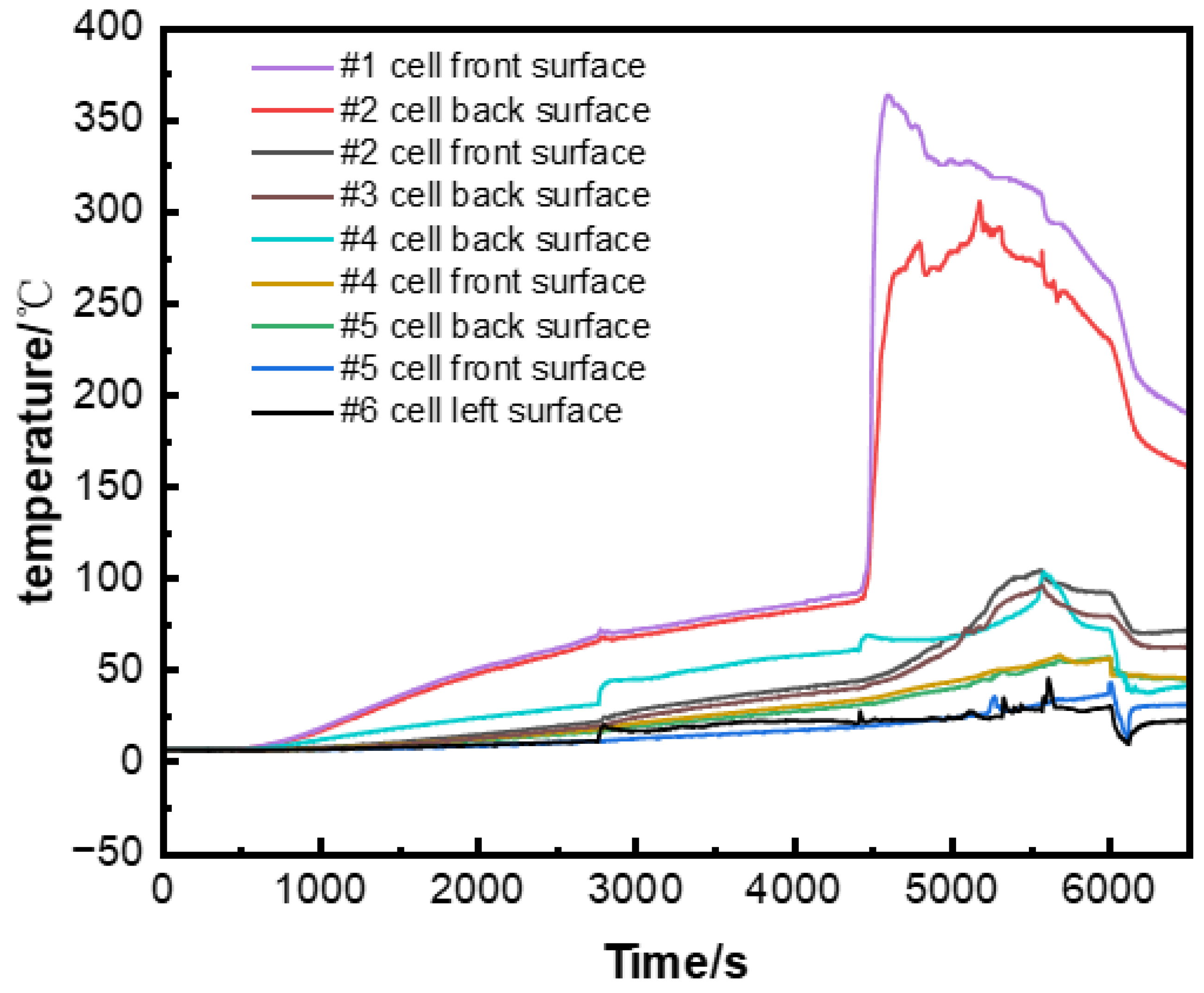
3.1. Temperature and Voltage Changes in the Runaway Cell and Temperature Analysis of Surrounding Cells
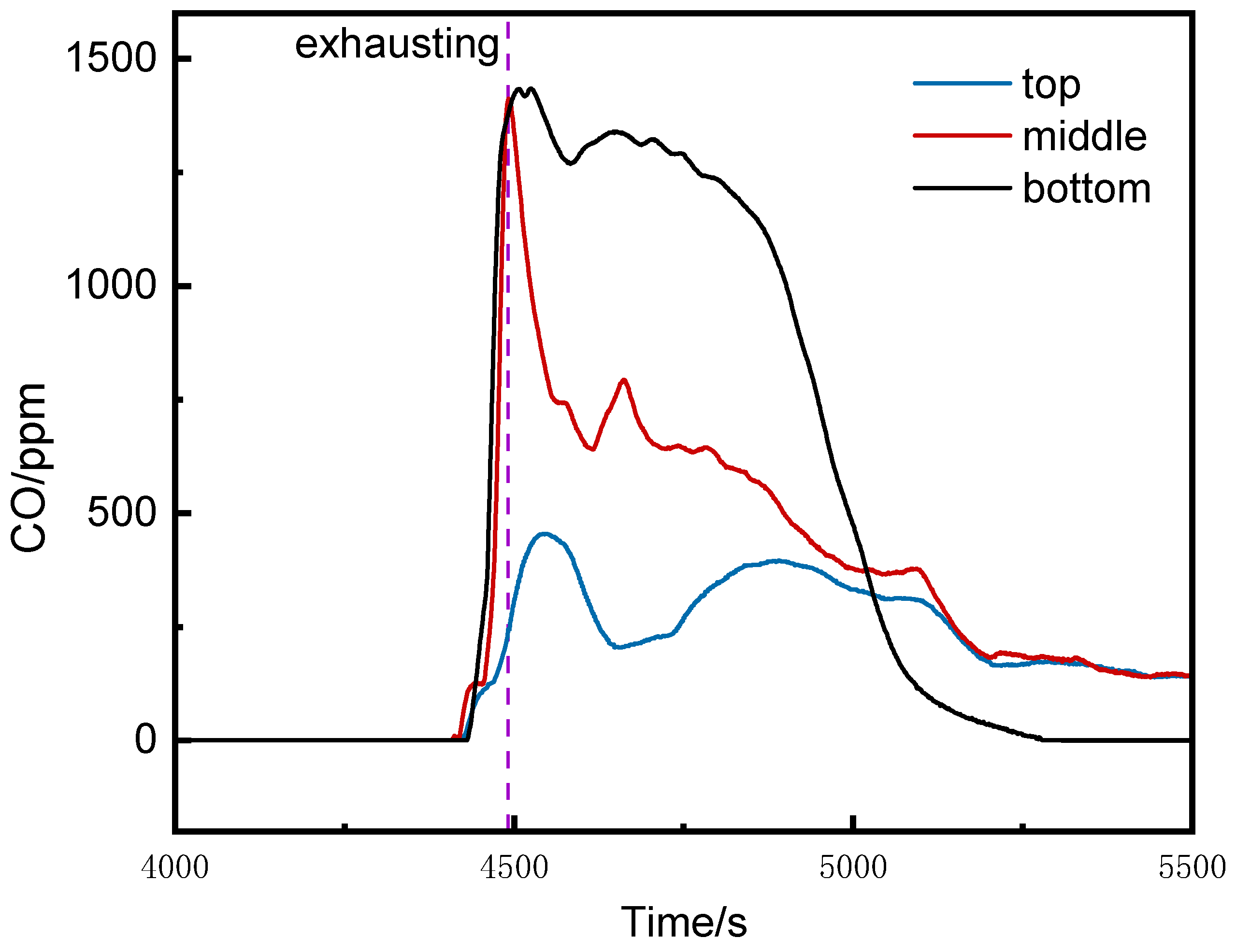
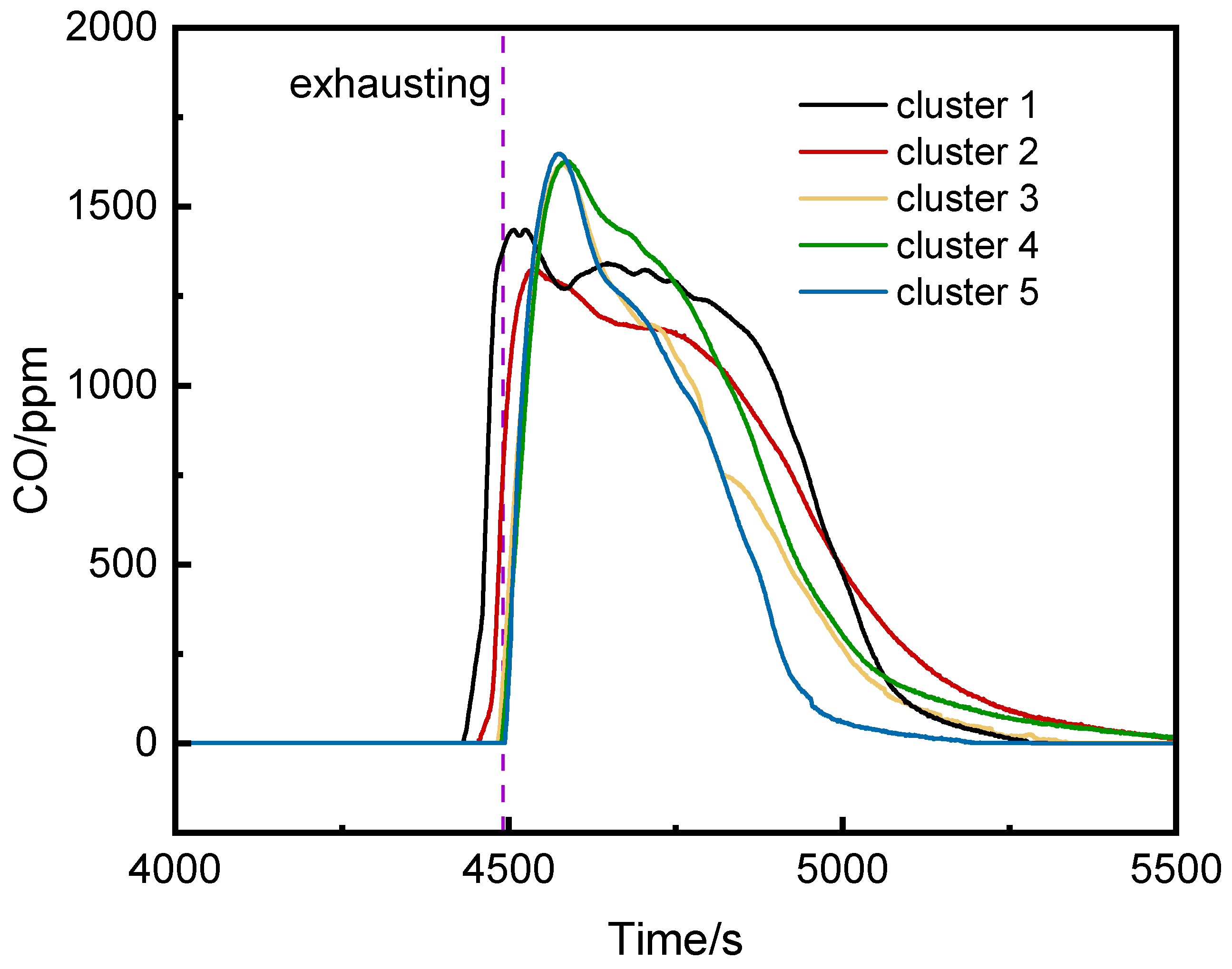
3.2. CO and VOC Concentration Analysis Inside the Pack and at Different Positions in the Cabin
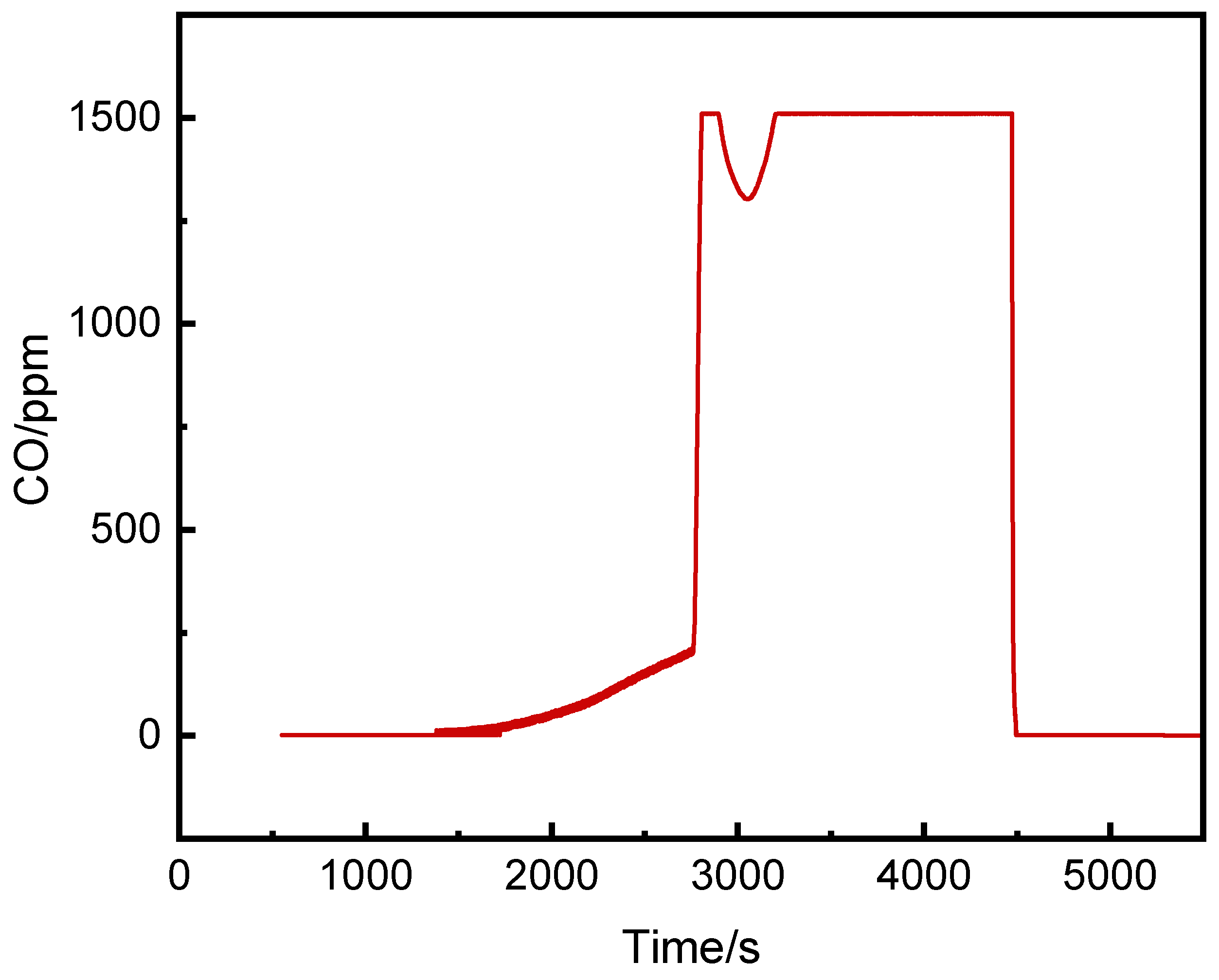
3.2.1. CO Concentration Analysis
- (1)
- CO Concentration Inside the Pack
- (2)
- CO Concentration at Different Positions in the Runaway Cluster
- (3)
- CO Concentration in the Runaway and Left Clusters
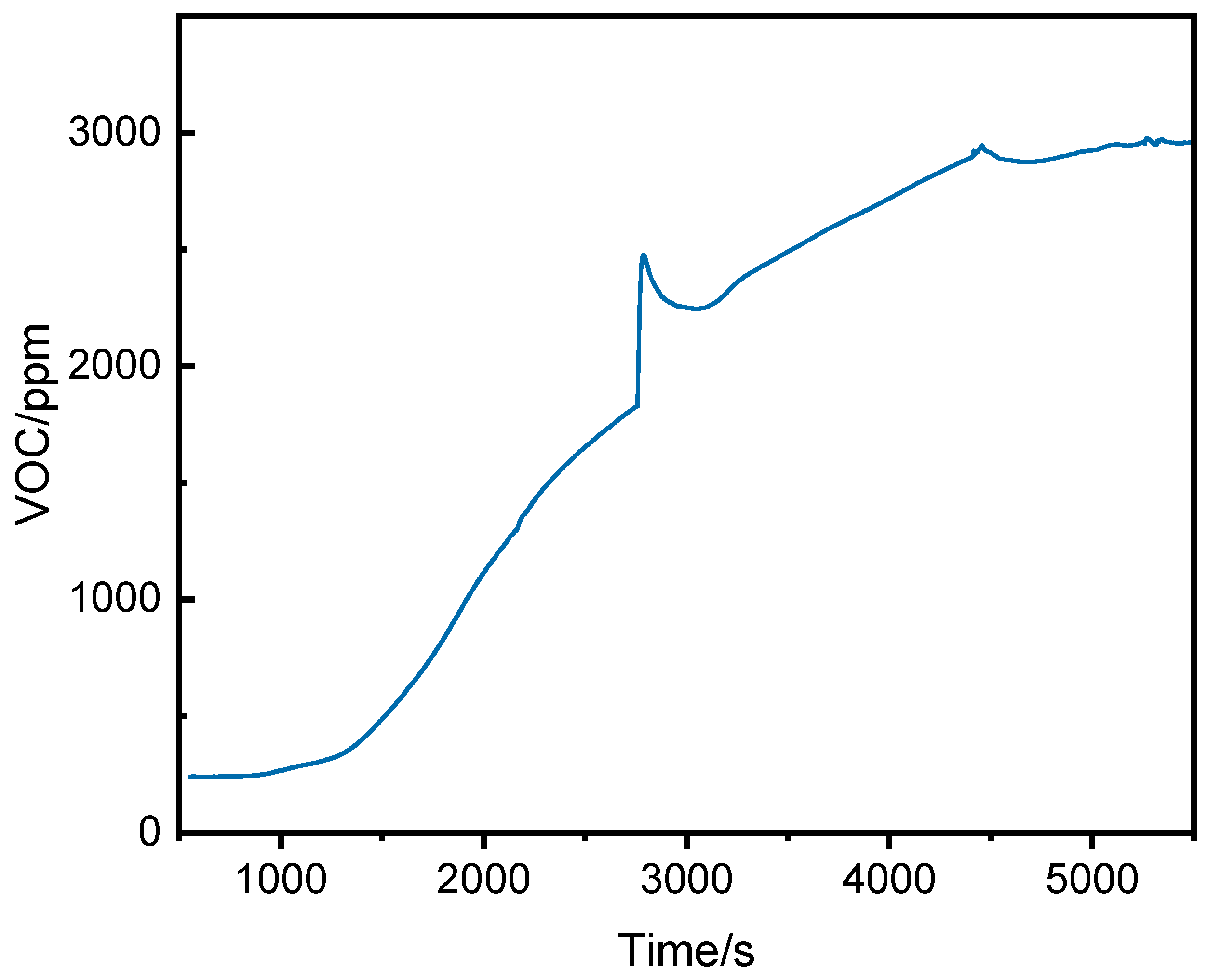
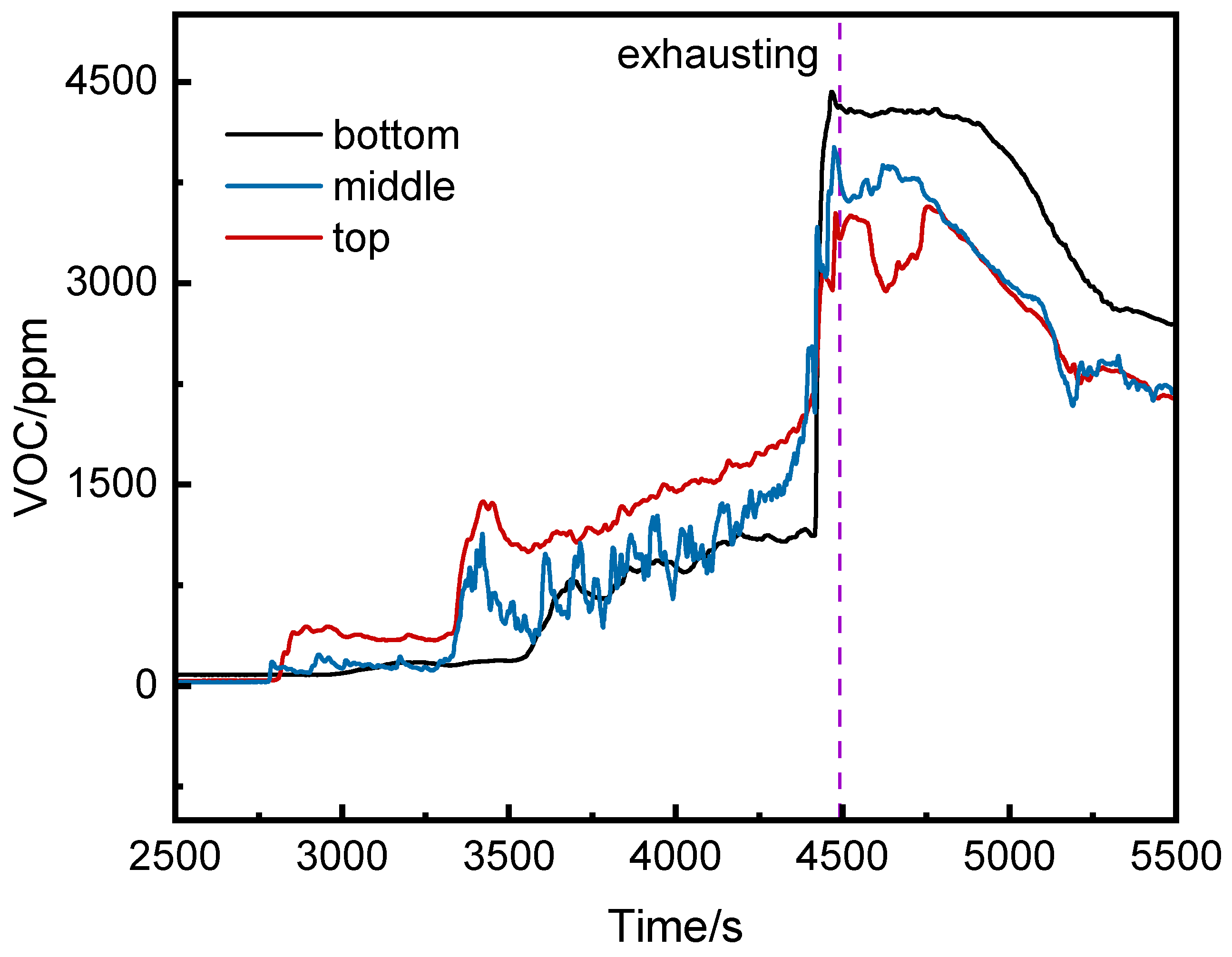
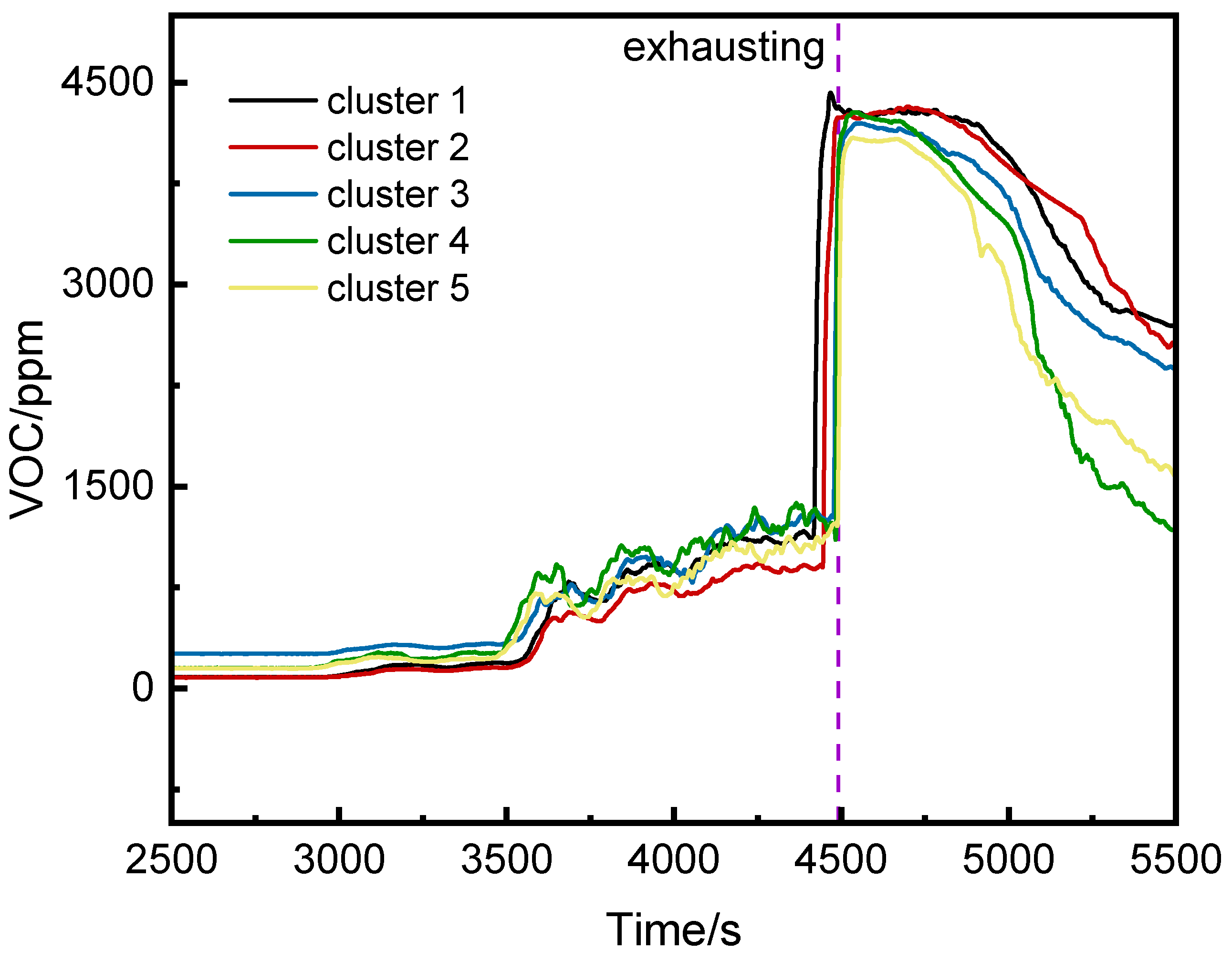
3.2.2. VOC Concentration Analysis
3.2.3. Comparison of VOC and CO Concentrations
4. Conclusions and Recommendations
- The thermal runaway trigger time was 37 s later than the voltage drop time and 1642 s earlier than vent opening.
- The CO concentrations at the middle and bottom of the runaway cluster reached alarm thresholds 25 s and 39 s earlier than at the top, with peak concentrations over three times higher. Ventilation caused higher CO concentrations on the left side of the runaway cluster.
- Before thermal runaway, VOC concentrations at the middle and top of the runaway cluster were generally higher than at the bottom. After thermal runaway, the bottom VOC concentration exceeded other positions.
- VOC provided earlier warnings than CO (2296 s, 1681 s, and 1464 s earlier at the top, middle, and bottom, respectively) but its initial detection values were more volatile.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, G.; Ping, P.; Peng, R.; Lv, H.; Zhao, H.; Gao, W.; Kong, D. A semi reduced-order model for multi-scale simulation of fire propagation of lithium-ion batteries in energy storage system. Renew. Sustain. Energy Rev. 2023, 186, 113672. [Google Scholar] [CrossRef]
- Lai, X.; Yao, J.; Jin, C.; Feng, X.; Wang, H.; Xu, C.; Zheng, Y. A review of lithium-ion battery failure hazards: Test standards, accident analysis, and safety suggestions. Batteries 2022, 8, 248. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, F.; Feng, J.; Jin, C.; Wang, H.; Xu, C.; Ouyang, M. Propagation dynamics of the thermal runaway front in large-scale lithium-ion batteries: Theoretical and experiment validation. Int. J. Heat Mass Transf. 2024, 225, 125393. [Google Scholar] [CrossRef]
- Zhang, X.; Yao, J.; Zhu, L.; Wu, J.; Wei, D.; Wang, Q.; Chen, H.; Li, K.; Gao, Z.; Xu, C.; et al. Experimental and simulation investigation of thermal runaway propagation in lithium-ion battery pack systems. J. Energy Storage 2024, 77, 109868. [Google Scholar] [CrossRef]
- Jin, Y.; Zheng, Z.K.; Wei, D.H.; Jiang, X.; Lu, H.F.; Sun, L.; Tao, F.B.; Guo, D.L.; Liu, Y.; Gao, J.F.; et al. Detection of micro-scale Li dendrite via H2 gas capture for early safety warning. Joule 2020, 4, 1714–1729. [Google Scholar] [CrossRef]
- Koch, S.; Birke, K.P.; Kuhn, R. Fast thermal runaway detection for lithium-ion cells in large scale traction batteries. Batteries 2018, 4, 16. [Google Scholar] [CrossRef]
- Wang, H.; Xu, H.; Zhao, Z.; Wang, Q.; Jin, C.; Li, Y.; Sheng, J.; Li, K.; Du, Z.; Xu, C.; et al. An experimental analysis on thermal runaway and its propagation in Cell-to-Pack lithium-ion batteries. Appl. Therm. Eng. 2022, 211, 118418. [Google Scholar] [CrossRef]
- Kurzawski, A.; Gray, L.; Torres-Castro, L.; Hewson, J. An investigation into the effects of state of charge and heating rate on propagating thermal runaway in Li-ion batteries with experiments and simulations. Fire Saf. J. 2023, 140, 103885. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, P.; Shang, R. Effects of heating position on the thermal runaway propagation of a lithium-ion battery module in a battery enclosure. Appl. Therm. Eng. 2023, 222, 119830. [Google Scholar] [CrossRef]
- Li, Z.; Guo, Y.; Zhang, P. Effects of the battery enclosure on the thermal behaviors of lithium-ion battery module during thermal runaway propagation by external-heating. J. Energy Storage 2022, 48, 104002. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, J.; Zhai, H.; Wang, Q. Experimental investigation on the characteristics of thermal runaway and its propagation of large-format lithium ion batteries under overcharging and overheating conditions. Energy 2021, 233, 121103. [Google Scholar] [CrossRef]
- Kang, S.; Kwon, M.; Choi, J.Y.; Choi, S. Full-scale fire testing of battery electric vehicles. Appl. Energy 2022, 332, 120497. [Google Scholar] [CrossRef]
- Svetlík, J.; Tancos, Z.; Tancos, P.; Markova, I.; Slastan, K. Large-Scale Fire Tests of Battery Electric Vehicle (BEV): Slovak Case Study. Appl. Sci. 2024, 14, 4013. [Google Scholar] [CrossRef]
- Sun, L.; Wei, C.; Guo, D.; Liu, J.; Zhao, Z.; Zheng, Z.; Jin, Y. Comparative study on thermal runaway characteristics of lithium iron phosphate battery modules under different overcharge conditions. Fire Technol. 2020, 56, 1555–1574. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, K.; Zhang, Q.; Chen, H.; Fan, M.; Geng, M.; Wei, B.; Xie, B. Simulation of Dispersion and Explosion Characteristics of LiFePO4 Lithium-Ion Battery Thermal Runaway Gases. ACS Omega 2024, 9, 17036–17044. [Google Scholar] [CrossRef]
- Lou, Z.; Huang, J.; Wang, M.; Zhang, Y.; Lv, K.; Yao, H. Inhibition performances of lithium-ion battery pack fires by fine water mist in an energy-storage cabin: A simulation study. Phys. Fluids 2024, 36, 045141. [Google Scholar] [CrossRef]
- GB 15322.1-2019; Combustible Gas Detectors-Part 1: Point-Type Combustible Gas Detectors for Industrial and Commercial Use. Standardization Administration of China: Beijing, China, 2019.
- GB 50116-2013; Code for Design of Automatic Fire Alarm System. Standardization Administration of China: Beijing, China, 2013.
- Parhizi, M.; Ahmed, M.; Jain, A. Determination of the core temperature of a Li-ion cell during thermal runaway. J. Power Sources 2017, 370, 27–35. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, L.; Tian, J.; Mei, W.; Jiang, L.; Sun, J.; Wang, Q. Modeling the propagation of internal thermal runaway in lithium-ion battery. Appl. Energy 2024, 362, 123004. [Google Scholar] [CrossRef]
- Liu, H.; Wei, Z.; He, W.; Zhao, J. Thermal issues about Li-ion batteries and recent progress in battery thermal management systems: A review. Energy Convers. Manag. 2017, 150, 304–330. [Google Scholar] [CrossRef]
- Mao, B.; Fear, C.; Chen, H.; Zhou, H.; Zhao, C.; Mukherjee, P.P.; Sun, J.; Wang, Q. Experimental and modeling investigation on the gas generation dynamics of lithium-ion batteries during thermal runaway. eTransportation 2022, 15, 100212. [Google Scholar] [CrossRef]
- Jia, Z.; Qin, P.; Li, Z.; Wei, Z.; Jin, K.; Jiang, L.; Wang, Q. Analysis of gas release during the process of thermal runaway of lithium-ion batteries with three different cathode materials. J. Energy Storage 2022, 50, 104302. [Google Scholar] [CrossRef]
- Hildebrand, S.; Ferrario, F.; Lebedeva, N. Comparative Overview of Methods for the Detection of Airborne Electrolyte Components Released from Lithium-Ion Batteries. Energy Technol. 2023, 12, 2300647. [Google Scholar] [CrossRef]
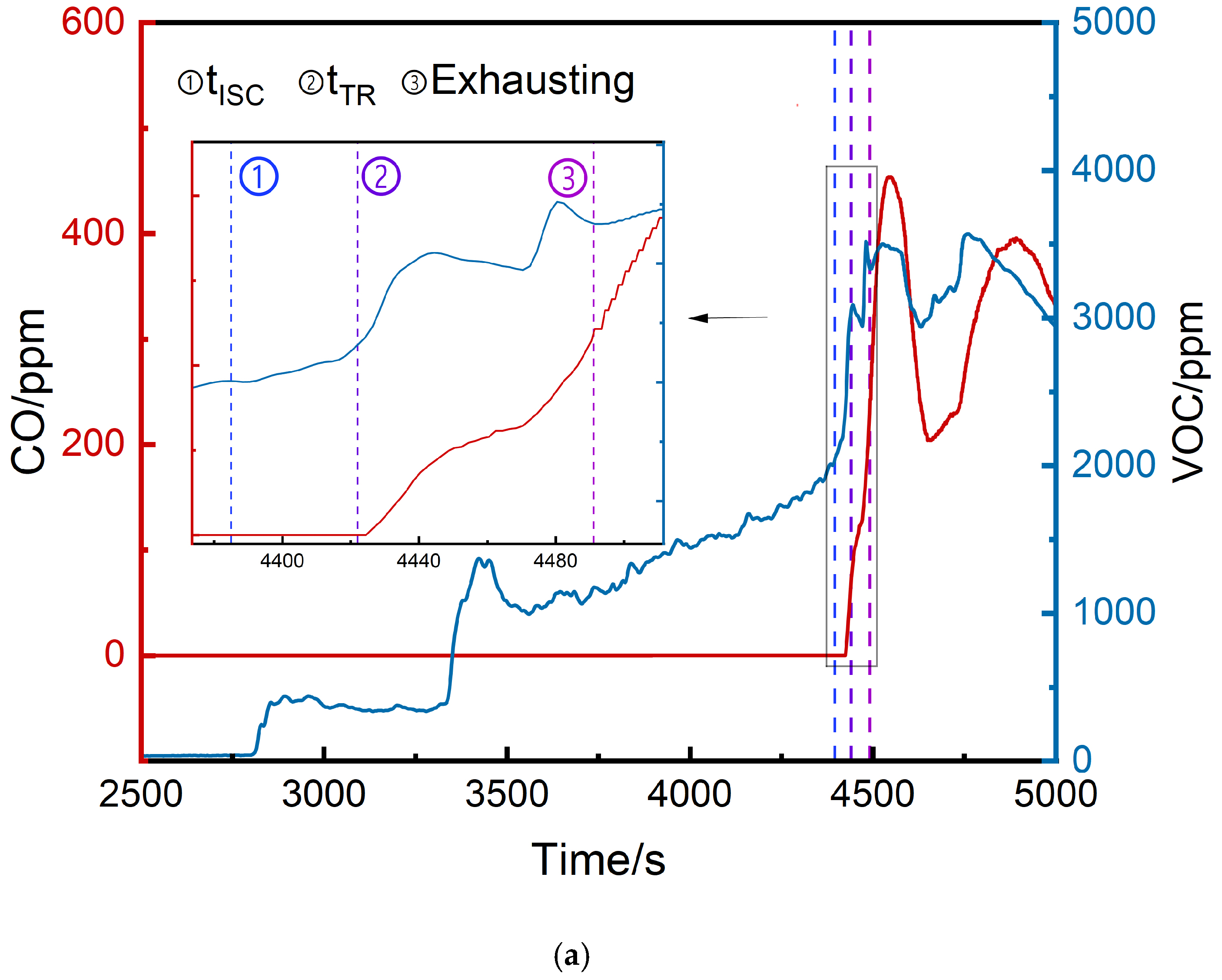
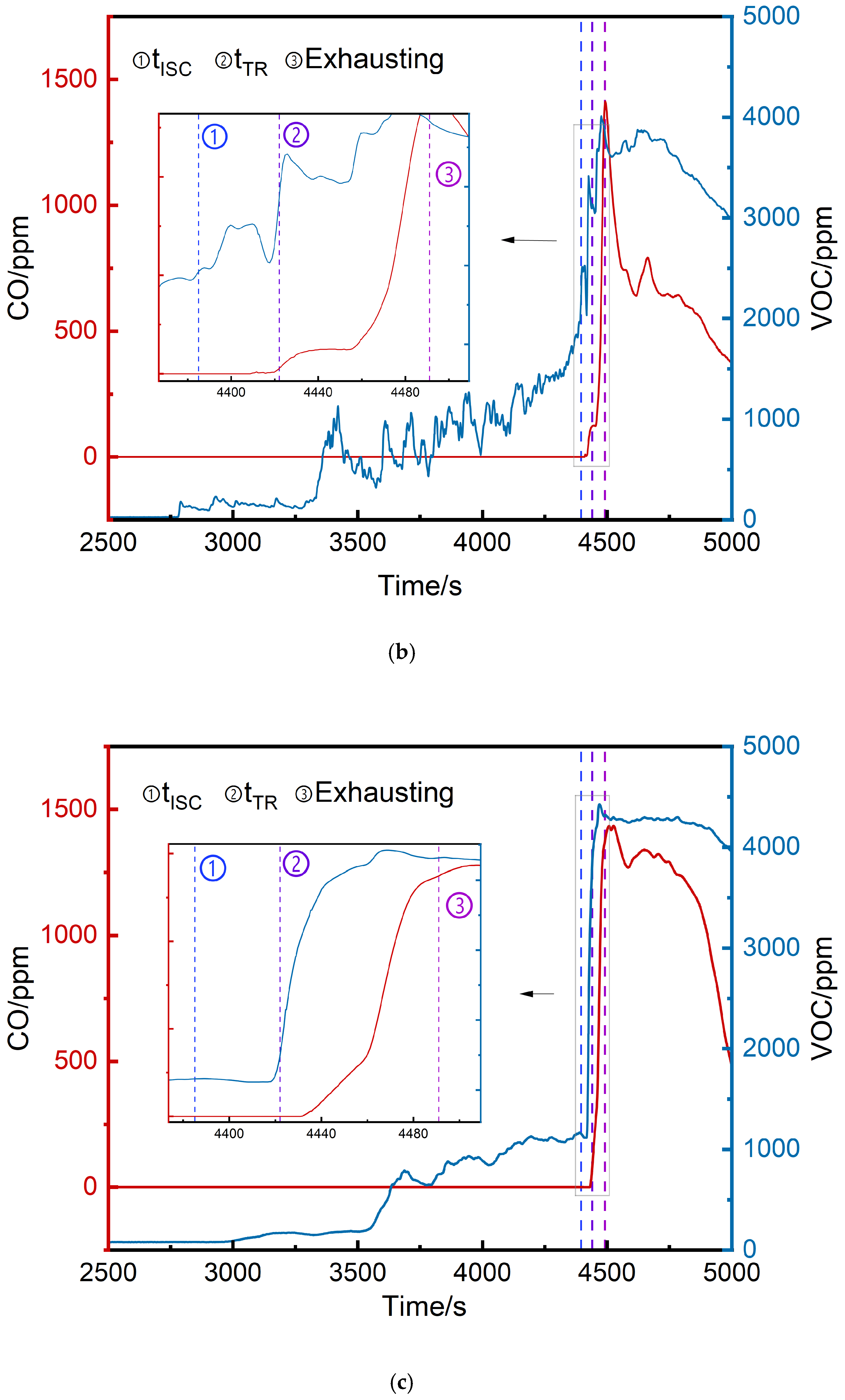
| Position | Initial VOC Detection Values (ppm) | Initial CO Detection Values (ppm) | tVOC (s) | tCO (s) |
|---|---|---|---|---|
| top | 29 | 0 | 2130 | 4426 |
| middle | 23 | 0 | 2728 | 4409 |
| bottom | 80 | 0 | 2967 | 4431 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Fan, R.; Wang, M.; Sun, X.; Wang, X. Research of Characteristics of the Thermal Runaway Process of Full-Size Prefabricated Cabin Energy Storage System. Fire 2025, 8, 164. https://doi.org/10.3390/fire8050164
Zhao Y, Fan R, Wang M, Sun X, Wang X. Research of Characteristics of the Thermal Runaway Process of Full-Size Prefabricated Cabin Energy Storage System. Fire. 2025; 8(5):164. https://doi.org/10.3390/fire8050164
Chicago/Turabian StyleZhao, Yufei, Rong Fan, Maohai Wang, Xuan Sun, and Xuefeng Wang. 2025. "Research of Characteristics of the Thermal Runaway Process of Full-Size Prefabricated Cabin Energy Storage System" Fire 8, no. 5: 164. https://doi.org/10.3390/fire8050164
APA StyleZhao, Y., Fan, R., Wang, M., Sun, X., & Wang, X. (2025). Research of Characteristics of the Thermal Runaway Process of Full-Size Prefabricated Cabin Energy Storage System. Fire, 8(5), 164. https://doi.org/10.3390/fire8050164





