Unveiling the Impact of LED Light on Growing Carrot Taproots: A Novel Hydroponic Cultivation System
Abstract
1. Introduction
2. Materials and Methods
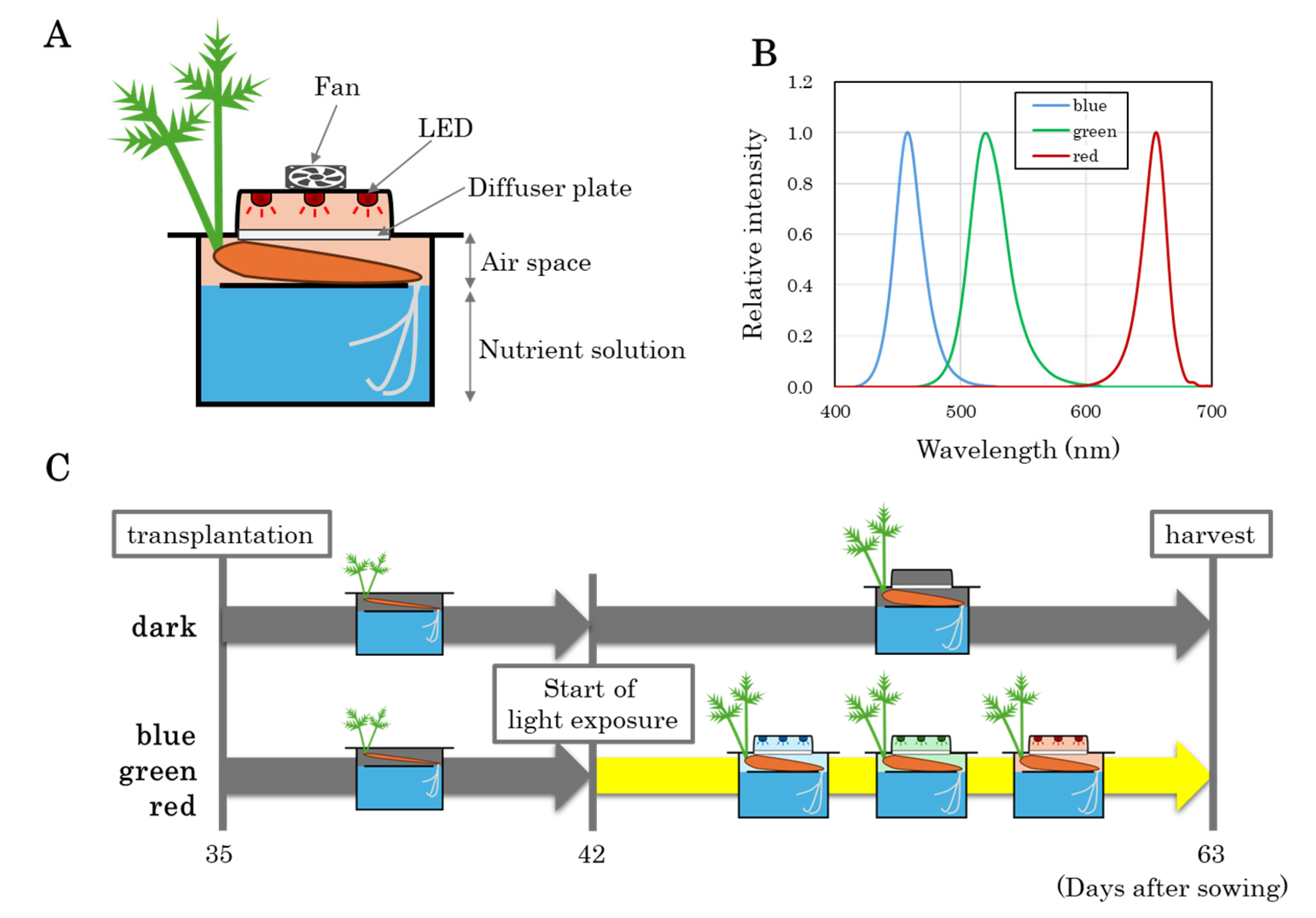
2.1. Experimental Conditions
2.2. Measurement of Taproot Color
2.3. Measurement of Anthocyanin Content
2.4. Measurement of Total Phenol Content
2.5. Measurement of Chlorophyll Content
2.6. Microscopic Observations
2.7. Data Analysis
3. Results
3.1. Morphological Changes in Carrot Taproots Under Different Light Conditions
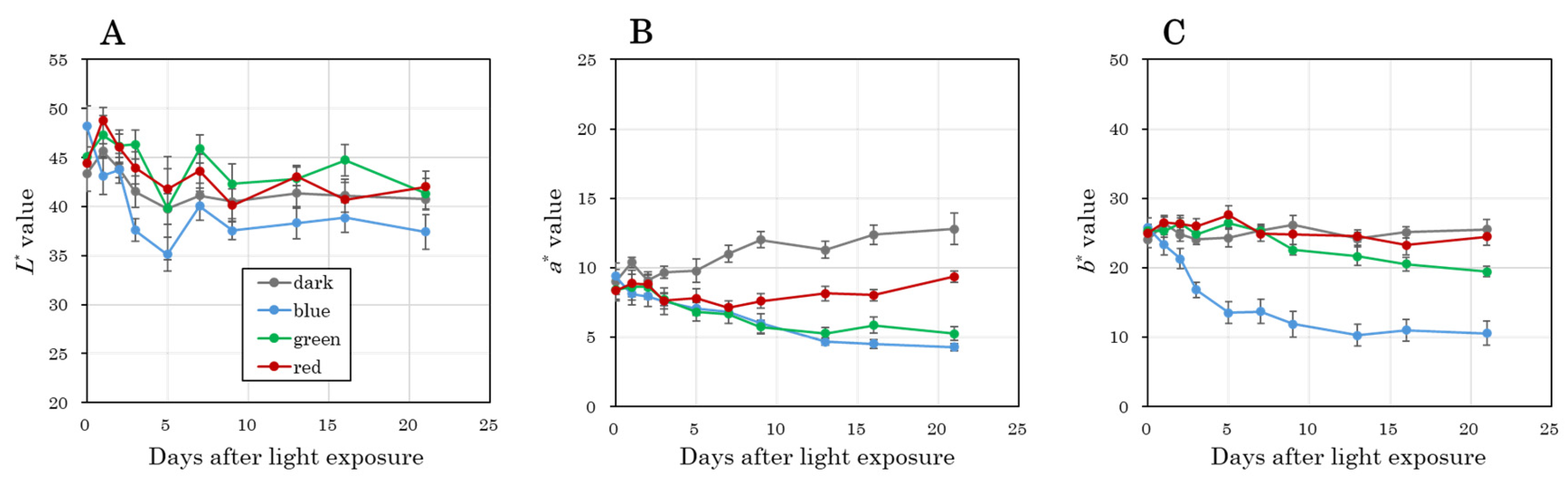
3.2. Time-Course Changes in Color Parameters of the Taproot Epidermis
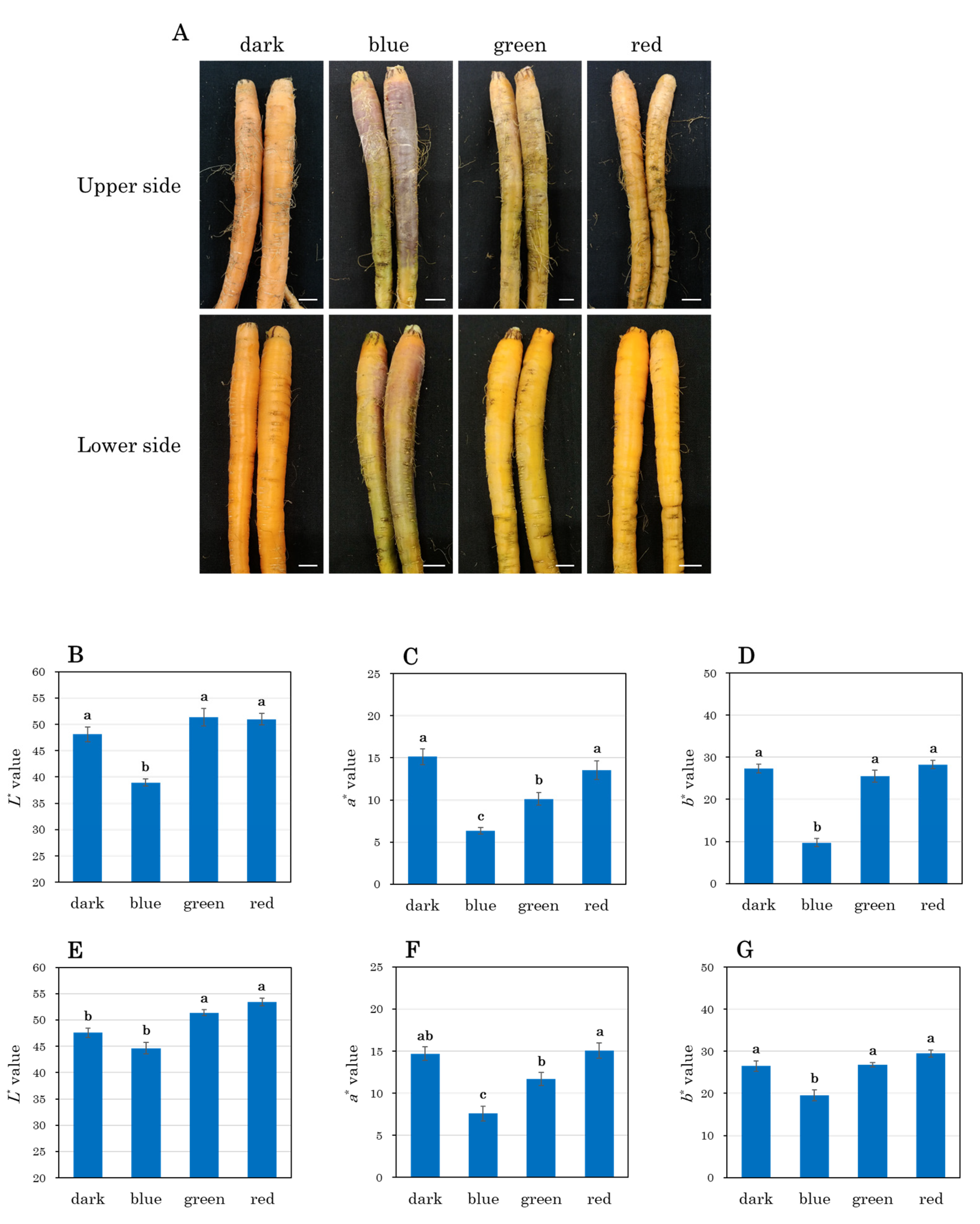
3.3. Visual and Quantitative Analysis of Taproots After 21 Days of Light Exposure
3.4. Cross-Sectional Analysis of Taproots
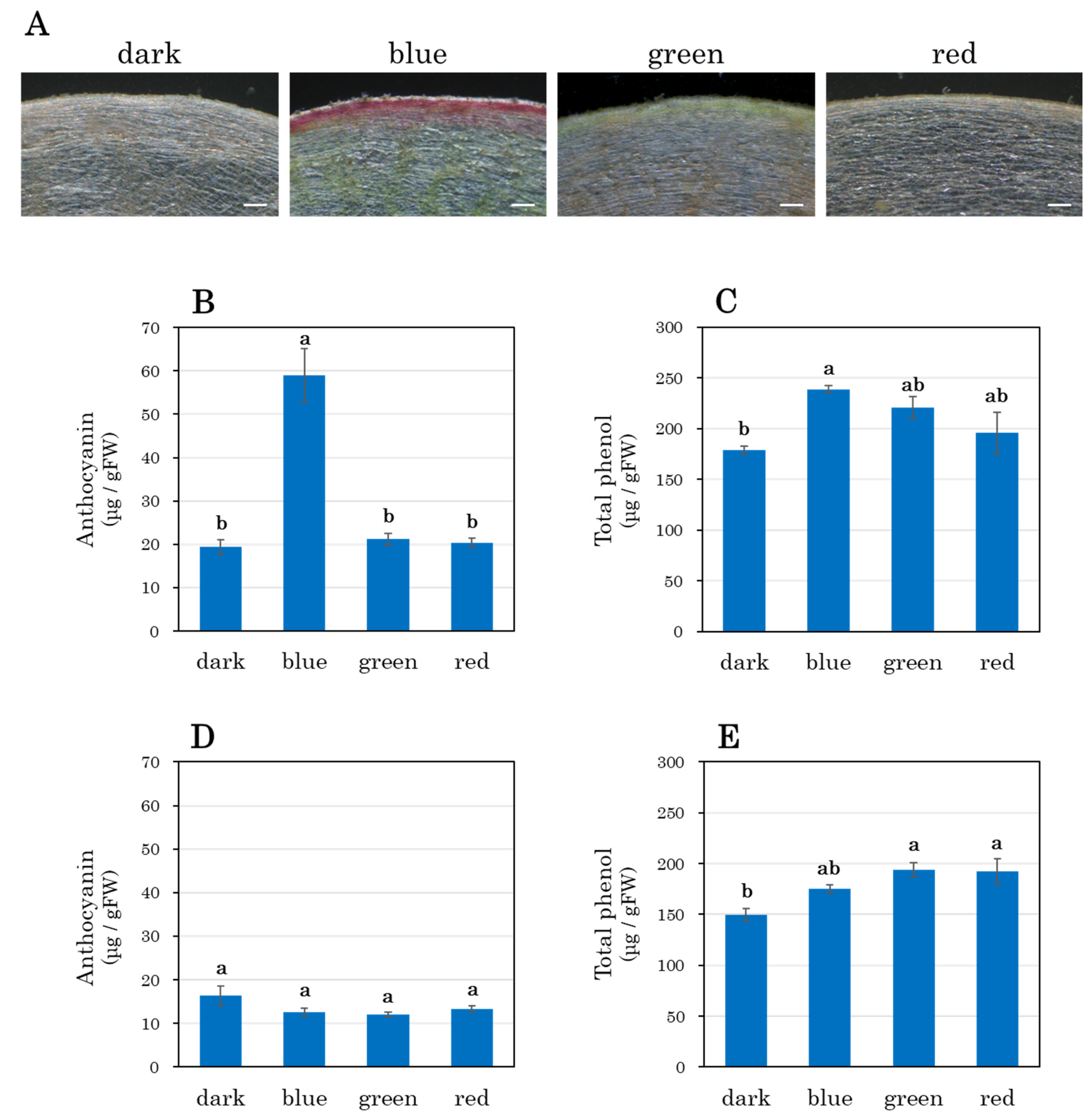
3.5. Anthocyanin and Total Phenol Accumulation
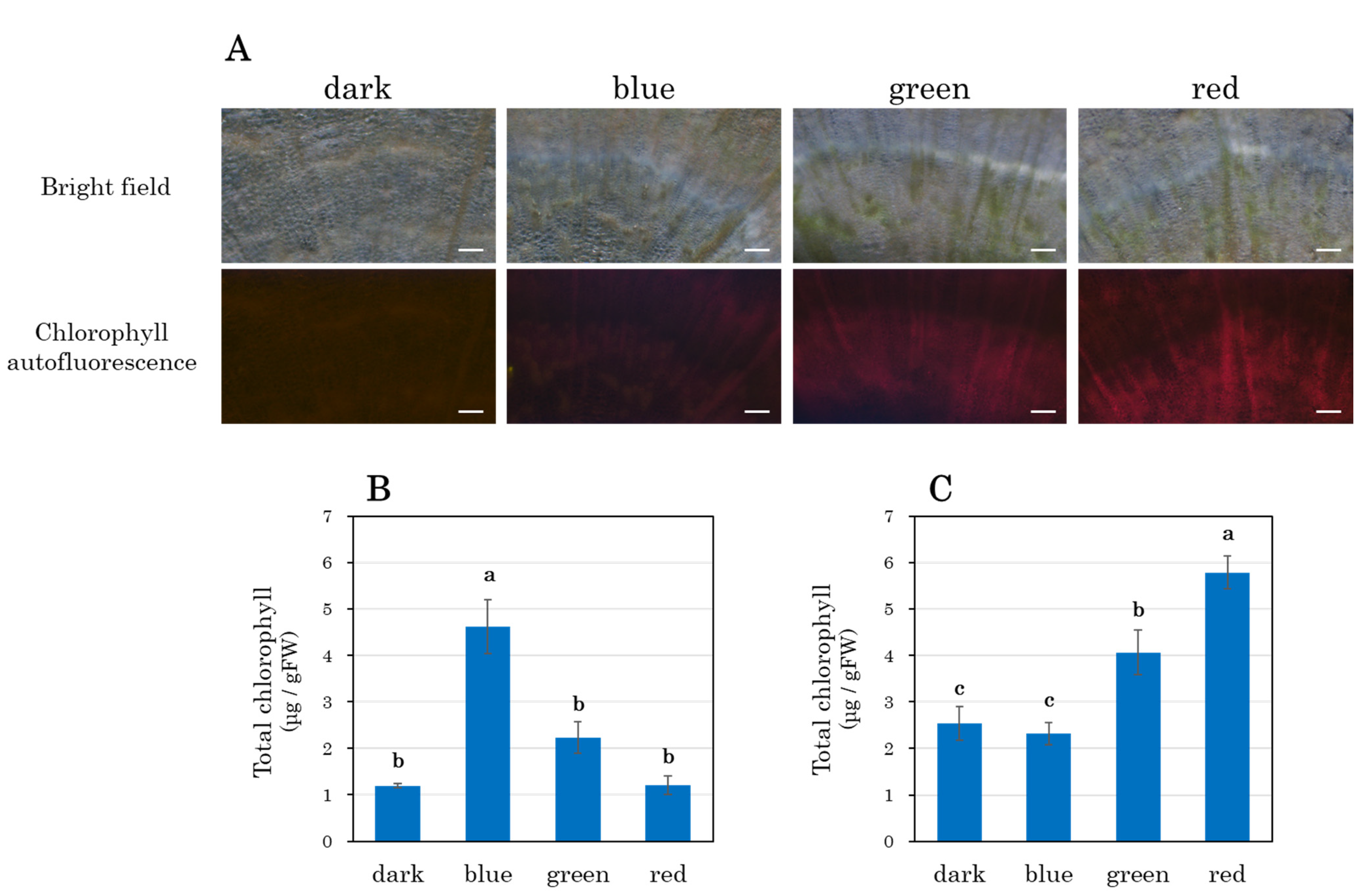
3.6. Chlorophyll Accumulation in the Stele
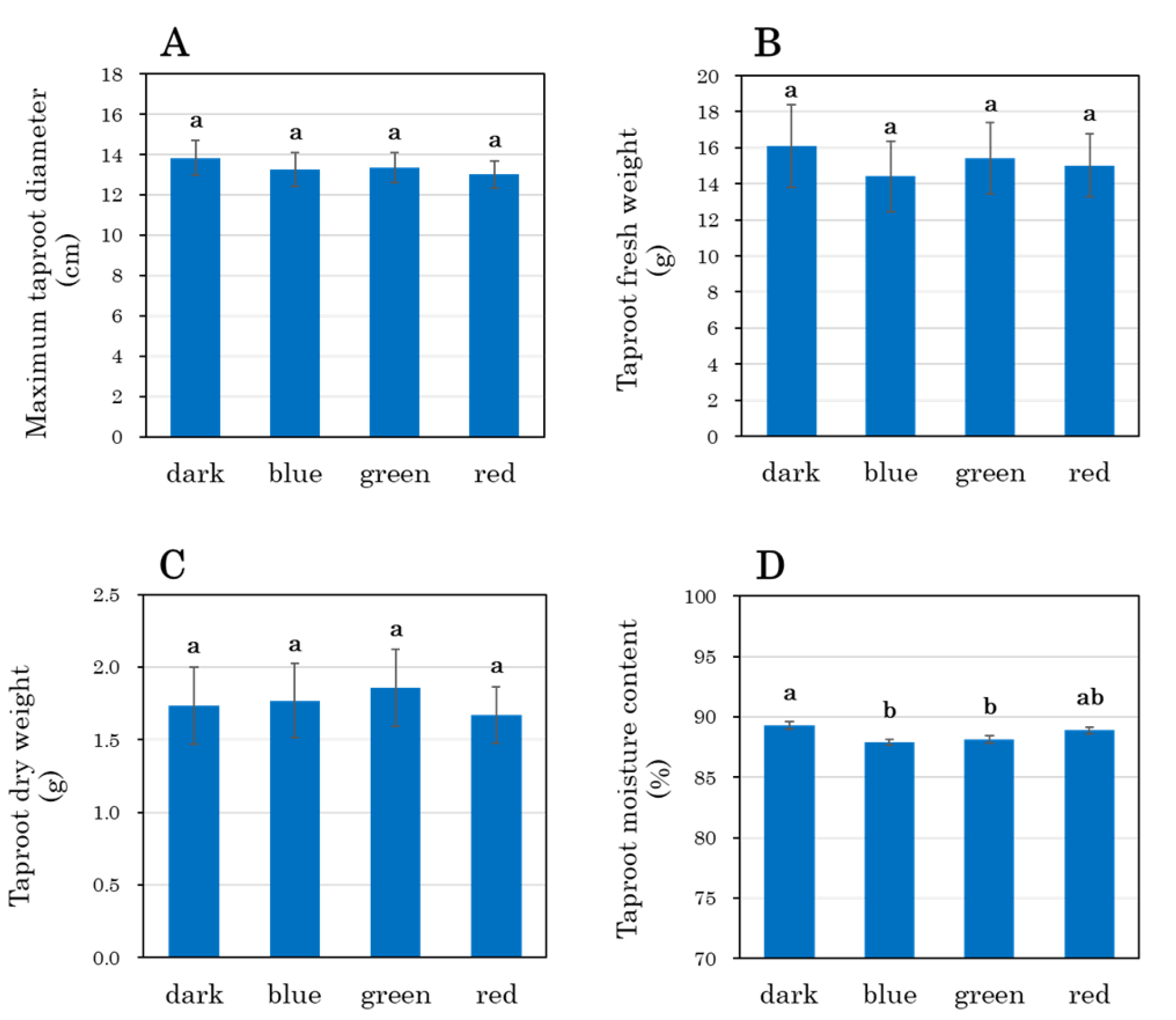
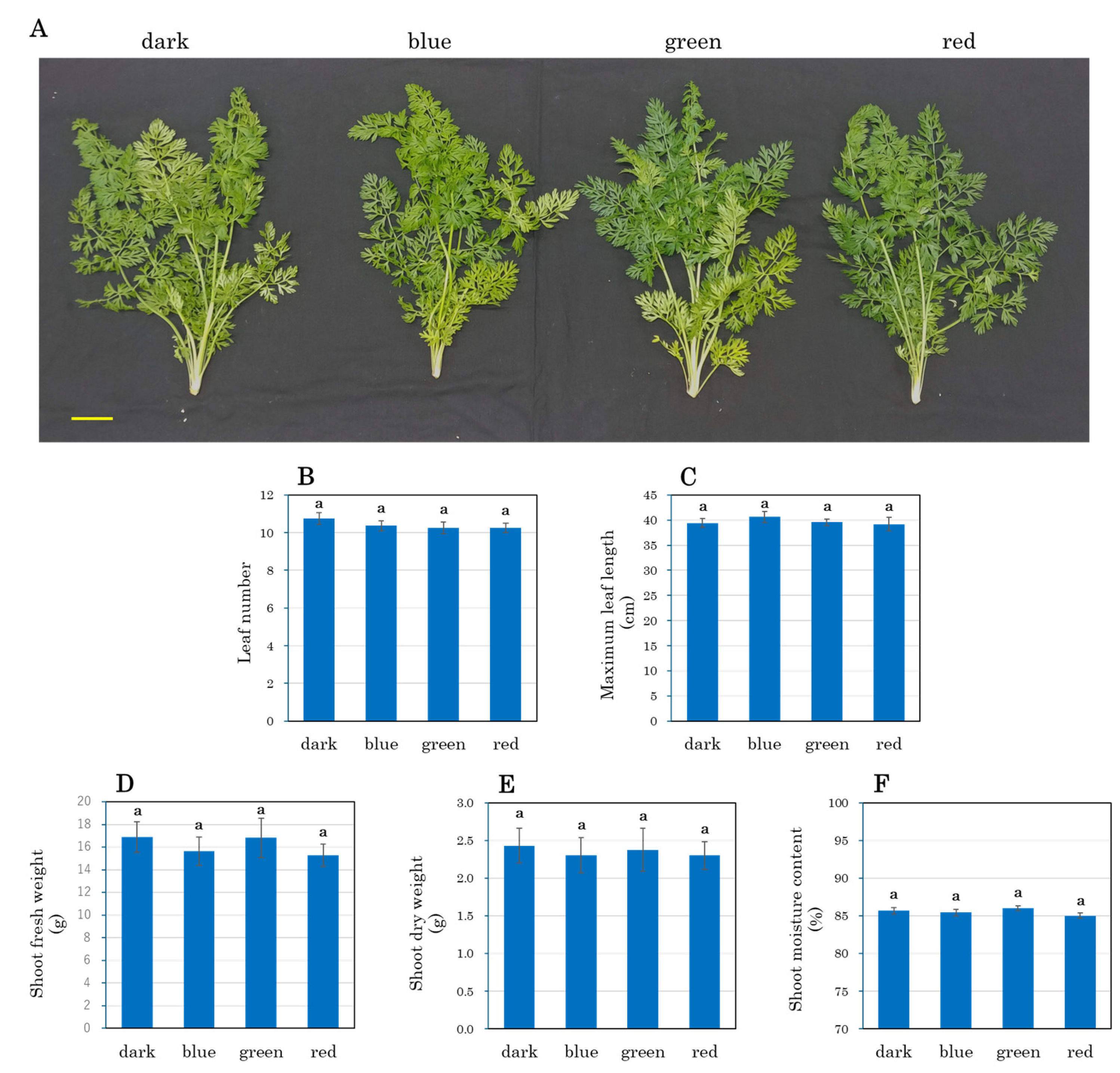
3.7. Growth Parameters of Carrot Taproots and Shoots
4. Discussion
4.1. Novel Hydroponic System and Root Development
4.2. Anthocyanin Accumulation
4.3. Chlorophyll Accumulation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Darko, E.; Heydarizadeh, P.; Schoefs, B.; Sabzalian, M.R. Photosynthesis under Artificial Light: The Shift in Primary and Secondary Metabolism. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130243. [Google Scholar] [CrossRef] [PubMed]
- Paradiso, R.; Proietti, S. Light-Quality Manipulation to Control Plant Growth and Photomorphogenesis in Greenhouse Horticulture: The State of the Art and the Opportunities of Modern LED Systems. J. Plant Growth Regul. 2022, 41, 742–780. [Google Scholar] [CrossRef]
- Kobayashi, K.; Baba, S.; Obayashi, T.; Sato, M.; Toyooka, K.; Keränen, M.; Aro, E.-M.; Fukaki, H.; Ohta, H.; Sugimoto, K.; et al. Regulation of Root Greening by Light and Auxin/Cytokinin Signaling in Arabidopsis. Plant Cell 2012, 24, 1081–1095. [Google Scholar] [CrossRef] [PubMed]
- Quian-Ulloa, R.; Stange, C. Carotenoid Biosynthesis and Plastid Development in Plants: The Role of Light. Int. J. Mol. Sci. 2021, 22, 1184. [Google Scholar] [CrossRef]
- Usami, T.; Mochizuki, N.; Kondo, M.; Nishimura, M.; Nagatani, A. Cryptochromes and Phytochromes Synergistically Regulate Arabidopsis Root Greening under Blue Light. Plant Cell Physiol. 2004, 45, 1798–1808. [Google Scholar] [CrossRef]
- Galen, C.; Rabenold, J.J.; Liscum, E. Functional Ecology of a Blue Light Photoreceptor: Effects of Phototropin-1 on Root Growth Enhance Drought Tolerance in Arabidopsis thaliana. New Phytol. 2007, 173, 91–99. [Google Scholar] [CrossRef]
- Rizzini, L.; Favory, J.-J.; Cloix, C.; Faggionato, D.; O’Hara, A.; Kaiserli, E.; Baumeister, R.; Schäfer, E.; Nagy, F.; Jenkins, G.I.; et al. Perception of UV-B by the Arabidopsis UVR8 Protein. Science 2011, 332, 103–106. [Google Scholar] [CrossRef]
- Wan, J.; Zhang, P.; Wang, R.; Sun, L.; Wang, W.; Zhou, H.; Xu, J. UV-B Radiation Induces Root Bending Through the Flavonoid-Mediated Auxin Pathway in Arabidopsis. Front. Plant Sci. 2018, 9, 618. [Google Scholar] [CrossRef]
- Kozai, T. Sustanable Plant Factory: Closed Plant Production System with Articicial Light for High Resource Use Efficiencies and Quality Produce. Acta Hortic. 2013, 1004, 27–40. [Google Scholar] [CrossRef]
- Hirawan, D.; Nurhadiansyah, E.; Hadiana, A. Comparison NFT and DFT Hydroponic Method Based on Internet of Things. AIP Conf. Proc. 2023, 2510, 030030. [Google Scholar] [CrossRef]
- Garzón, J.; Montes, L.; Garzón, J.; Lampropoulos, G. Systematic Review of Technology in Aeroponics: Introducing the Technology Adoption and Integration in Sustainable Agriculture Model. Agronomy 2023, 13, 2517. [Google Scholar] [CrossRef]
- Sakamoto, M.; Suzuki, T. Effect of Root-Zone Temperature on Growth and Quality of Hydroponically Grown Red Leaf Lettuce (Lactuca sativa L. Cv. Red Wave). Am. J. Plant Sci. 2015, 06, 2350. [Google Scholar] [CrossRef]
- Sakamoto, M.; Suzuki, T. Elevated Root-Zone Temperature Modulates Growth and Quality of Hydroponically Grown Carrots. Agric. Sci. 2015, 6, 749–757. [Google Scholar] [CrossRef]
- Sakamoto, M.; Uenishi, M.; Miyamoto, K.; Suzuki, T. Effect of Root-Zone Temperature on the Growth and Fruit Quality of Hydroponically Grown Strawberry Plants. J. Agric. Sci. 2016, 8, 122. [Google Scholar] [CrossRef]
- Levine, C.P.; Hayashi, S.; Ohmori, Y.; Kusano, M.; Kobayashi, M.; Nishizawa, T.; Kurimoto, I.; Kawabata, S.; Yamori, W. Controlling Root Zone Temperature Improves Plant Growth and Pigments in Hydroponic Lettuce. Ann. Bot. 2023, 132, 455–470. [Google Scholar] [CrossRef]
- Sakamoto, M.; Suzuki, T. N-Acetylcysteine Mitigates Oxidative Stress Induced by Transplanting Lettuce Seedlings into a DFT Hydroponic System. Agronomy 2024, 14, 2112. [Google Scholar] [CrossRef]
- Fujiuchi, N.; Fujiwara, K. LED Dim Light Irradiation of the Root Zone Influences Growth and Development of Leaf Lettuce (Lactuca sativa) Plants under Nutrient Film Technique Hydroponics. Environ. Control Biol. 2012, 50, 101–106. [Google Scholar] [CrossRef]
- Paponov, M.; Ziegler, J.; Paponov, I.A. Light Exposure of Roots in Aeroponics Enhances the Accumulation of Phytochemicals in Aboveground Parts of the Medicinal Plants Artemisia annua and Hypericum perforatum. Front. Plant Sci. 2023, 14, 1079656. [Google Scholar] [CrossRef]
- Kon, S.; Toyofuku, K.; Muto, D.; Kimura, S.; Ogawa, A. Irradiating Roots of Komatsuna (Brassica napus) with Various Light Qualities Affects Growth and Nutrient Content in Leaves, Stems, and Roots. Sci. Hortic. 2024, 331, 113179. [Google Scholar] [CrossRef]
- Eguchi, T.; Yoshida, S. A Cultivation Method to Ensure Tuberous Root Formation in Sweetpotatoes (Ipomoea batatas (L.) Lam.). Environ. Control. Biol. 2004, 42, 259–266. [Google Scholar] [CrossRef]
- Kusakawa, T.; Inoue, M. Damage to Pot-cultured Carrot Growth due to a Temporarily Raised Groundwater Level and Flooding Period. Hort. Res. 2010, 9, 495–500. [Google Scholar] [CrossRef]
- Siqinbatu; Kitaya, Y.; Hirai, H.; Shibuya, T.; Endo, R. Effects of Soil Water Content on the Growth of Sweet Potato Grown in Sandy Soil. Eco-Eng 2014, 26, 75–80. [Google Scholar] [CrossRef]
- Sakamoto, M.; Suzuki, T. Impacts of Light Exposure and Soil Covering on Sweet Potato Storage Roots in a Novel Soilless Culture System. AgriEngineering 2024, 6, 3912–3930. [Google Scholar] [CrossRef]
- Terabayashi, S.; Harada, N.; Date, S.; Fujime, Y. Effects of Aeration and Root Immersion Level on the Development of Carrot Root in Hydroponics. Hortic. Res. 2008, 7, 439–444. [Google Scholar] [CrossRef]
- Que, F.; Wang, G.-L.; Feng, K.; Xu, Z.-S.; Wang, F.; Xiong, A.-S. Hypoxia Enhances Lignification and Affects the Anatomical Structure in Hydroponic Cultivation of Carrot Taproot. Plant Cell Rep. 2018, 37, 1021–1032. [Google Scholar] [CrossRef]
- Hayden, A. Aeroponic and Hydroponic Systems for Medicinal Herb, Rhizome, and Root Crops. HortScience 2006, 41, 536–538. [Google Scholar] [CrossRef]
- Selvaraj, M.G.; Montoya-P, M.E.; Atanbori, J.; French, A.P.; Pridmore, T. A Low-Cost Aeroponic Phenotyping System for Storage Root Development: Unravelling the below-Ground Secrets of Cassava (Manihot esculenta). Plant Methods 2019, 15, 131. [Google Scholar] [CrossRef]
- Tunio, M.H.; Gao, J.; Shaikh, S.A.; Lakhiar, I.A.; Qureshi, W.A.; Solangi, K.A.; Chandio, F.A. Potato Production in Aeroponics: An Emerging Food Growing System in Sustainable Agriculture for Food Security. Chil. J. Agric. Res. 2020, 80, 118–132. [Google Scholar] [CrossRef]
- Sakamoto, M.; Wada, M.; Suzuki, T. Effect of Partial Excision of Early Taproots on Growth and Components of Hydroponic Carrots. Horticulturae 2020, 6, 5. [Google Scholar] [CrossRef]
- Lee, J.-H.; Goto, E. Ozone Control as a Novel Method to Improve Health-Promoting Bioactive Compounds in Red Leaf Lettuce (Lactuca sativa L.). Front. Plant Sci. 2022, 13, 1045239. [Google Scholar] [CrossRef]
- Sakamoto, M.; Suzuki, T. Methyl Jasmonate and Salinity Increase Anthocyanin Accumulation in Radish Sprouts. Horticulturae 2019, 5, 62. [Google Scholar] [CrossRef]
- Chazaux, M.; Schiphorst, C.; Lazzari, G.; Caffarri, S. Precise Estimation of Chlorophyll a, b and Carotenoid Content by Deconvolution of the Absorption Spectrum and New Simultaneous Equations for Chl Determination. Plant J. 2022, 109, 1630–1648. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Wang, L.; Wu, Y.; Xi, Z. Roles of Non-Visible Light and Temperature in the Regulation of Anthocyanin Synthesis in Fruits and Vegetables. Food Front. 2024, 5, 1968–1983. [Google Scholar] [CrossRef]
- White, J.M. Carrot Yield When Grown under Three Soil Water Concentrations. HortScience 1992, 27, 105–106. [Google Scholar] [CrossRef]
- Terabayashi, S.; Yomo, T.; Namiki, T. Root Development of Root Crops Grown in Deep Flow and Ebb & Flood Culture. Environ. Control Biol. 1997, 35, 99–105. [Google Scholar] [CrossRef]
- Uewada, T. The solution culture of sweet potatoes. Environ. Control. Biol. 1990, 28, 135–140. [Google Scholar] [CrossRef]
- Zhang, H.; Schonhof, I.; Krumbein, A.; Gutezeit, B.; Li, L.; Stützel, H.; Schreiner, M. Water Supply and Growing Season Influence Glucosinolate Concentration and Composition in Turnip Root (Brassica rapa ssp. Rapifera L.). J. Plant Nutr. Soil Sci. 2008, 171, 255–265. [Google Scholar] [CrossRef]
- Eguchi, T.; Kitano, M.; Eguchi, H. Growth of Tuberous Root as Affected by the Ambient Humidity in Sweetpotato (Ipomoea batatas Lam.). Environ. Control. Biol. 1999, 37, 197–201. [Google Scholar] [CrossRef]
- Hozyo, Y.; Kato, S. Thickening Growth and Re-thickening Growth of Tuberous Roots of Sweet Potato Pants (Ipomoea batatas Poiret). Jpn. J. Crop Sci. 1976, 45, 131–138. [Google Scholar] [CrossRef]
- Fuentes, P.; Pizarro, L.; Moreno, J.C.; Handford, M.; Rodriguez-Concepcion, M.; Stange, C. Light-Dependent Changes in Plastid Differentiation Influence Carotenoid Gene Expression and Accumulation in Carrot Roots. Plant Mol. Biol. 2012, 79, 47–59. [Google Scholar] [CrossRef]
- Mbinda, W.; Dixelius, C.; Oduor, R. Induced Expression of Xerophyta viscosa XvSap1 Gene Enhances Drought Tolerance in Transgenic Sweet Potato. Front. Plant Sci. 2019, 10, 1119. [Google Scholar] [CrossRef] [PubMed]
- Boguszewska-Mańkowska, D.; Zarzyńska, K.; Nosalewicz, A. Drought Differentially Affects Root System Size and Architecture of Potato Cultivars with Differing Drought Tolerance. Am. J. Potato Res. 2020, 97, 54–62. [Google Scholar] [CrossRef]
- Zhang, R.-R.; Wang, Y.-H.; Li, T.; Tan, G.-F.; Tao, J.-P.; Su, X.-J.; Xu, Z.-S.; Tian, Y.-S.; Xiong, A.-S. Effects of Simulated Drought Stress on Carotenoid Contents and Expression of Related Genes in Carrot Taproots. Protoplasma 2021, 258, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Asaduzzaman, M.; Kobayashi, Y.; Mondal, M.F.; Ban, T.; Matsubara, H.; Adachi, F.; Asao, T. Growing Carrots Hydroponically Using Perlite Substrates. Sci. Hortic. 2013, 159, 113–121. [Google Scholar] [CrossRef]
- Tao, R.; Bai, S.; Ni, J.; Yang, Q.; Zhao, Y.; Teng, Y. The Blue Light Signal Transduction Pathway Is Involved in Anthocyanin Accumulation in ‘Red Zaosu’ Pear. Planta 2018, 248, 37–48. [Google Scholar] [CrossRef]
- Hong, G.-J.; Hu, W.-L.; Li, J.-X.; Chen, X.-Y.; Wang, L.-J. Increased Accumulation of Artemisinin and Anthocyanins in Artemisia annua Expressing the Arabidopsis Blue Light Receptor CRY1. Plant Mol. Biol. Rep. 2009, 27, 334–341. [Google Scholar] [CrossRef]
- Ma, Y.; Ma, X.; Gao, X.; Wu, W.; Zhou, B. Light Induced Regulation Pathway of Anthocyanin Biosynthesis in Plants. Int. J. Mol. Sci. 2021, 22, 11116. [Google Scholar] [CrossRef]
- Araguirang, G.E.; Richter, A.S. Activation of Anthocyanin Biosynthesis in High Light—What Is the Initial Signal? New Phytol. 2022, 236, 2037–2043. [Google Scholar] [CrossRef]
- Xu, Z.; Mahmood, K.; Rothstein, S.J. ROS Induces Anthocyanin Production Via Late Biosynthetic Genes and Anthocyanin Deficiency Confers the Hypersensitivity to ROS-Generating Stresses in Arabidopsis. Plant Cell Physiol. 2017, 58, 1364–1377. [Google Scholar] [CrossRef]
- Zheng, X.-T.; Yu, Z.-C.; Tang, J.-W.; Cai, M.-L.; Chen, Y.-L.; Yang, C.-W.; Chow, W.S.; Peng, C.-L. The Major Photoprotective Role of Anthocyanins in Leaves of Arabidopsis thaliana under Long-Term High Light Treatment: Antioxidant or Light Attenuator? Photosynth. Res. 2021, 149, 25–40. [Google Scholar] [CrossRef]
- Lv, Q.; Zhao, Q.; Zhu, C.; Ding, M.; Chu, F.; Li, X.; Cheng, K.; Zhao, X. Hydrogen Peroxide Mediates High-Intensity Blue Light-Induced Hypocotyl Phototropism of Cotton Seedlings. Stress Biol. 2023, 3, 27. [Google Scholar] [CrossRef] [PubMed]
- Sawatdee, S.; Jarunglumlert, T.; Pavasant, P.; Sakihama, Y.; Flood, A.E.; Prommuak, C. Effect of Mixed Light Emitting Diode Spectrum on Antioxidants Content and Antioxidant Activity of Red Lettuce Grown in a Closed Soilless System. BMC Plant Biol. 2023, 23, 351. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.-Y.; Chang, M.-Y.; Wu, C.-C.; Fang, W. Quantitative Evaluation of Electric Light Recipes for Red Leaf Lettuce Cultivation in Plant Factories. HortTechnology 2018, 28, 755–763. [Google Scholar] [CrossRef]
- Chen, X.; Fan, Y.; Guo, Y.; Li, S.; Zhang, B.; Li, H.; Liu, L.-J. Blue Light Photoreceptor Cryptochrome 1 Promotes Wood Formation and Anthocyanin Biosynthesis in Populus. Plant Cell Environ. 2024, 47, 2044–2057. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, L.; Gao, L.; Chen, Z.; Bie, Y.; Zhao, Q.; Zhang, S.; Hu, X.; Liu, Q.; Wang, X.; et al. Differential Photoregulation of the Nuclear and Cytoplasmic CRY1 in Arabidopsis. New Phytol. 2022, 234, 1332–1346. [Google Scholar] [CrossRef]
- Salisbury, F.J.; Hall, A.; Grierson, C.S.; Halliday, K.J. Phytochrome Coordinates Arabidopsis Shoot and Root Development. Plant J. 2007, 50, 429–438. [Google Scholar] [CrossRef]
- Binder, B.Y.K.; Peebles, C.A.M.; Shanks, J.V.; San, K.-Y. The effects of UV-B stress on the production of terpenoid indole alkaloids in Catharanthus roseus hairy roots. Biotechnol. Prog. 2009, 25, 861–865. [Google Scholar] [CrossRef]
- Chen, I.-G.J.; Lee, M.-S.; Lin, M.-K.; Ko, C.-Y.; Chang, W.-T. Blue Light Decreases Tanshinone IIA Content in Salvia miltiorrhiza Hairy Roots via Genes Regulation. J. Photochem. Photobiol. B Biol. 2018, 183, 164–171. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Zhao, B.; Yuan, X. Improved Growth of Artemisia annua L Hairy Roots and Artemisinin Production under Red Light Conditions. Biotechnol. Lett. 2001, 23, 1971–1973. [Google Scholar] [CrossRef]
- Abbasi, B.H.; Tian, C.-L.; Murch, S.J.; Saxena, P.K.; Liu, C.-Z. Light-Enhanced Caffeic Acid Derivatives Biosynthesis in Hairy Root Cultures of Echinacea purpurea. Plant Cell Rep. 2007, 26, 1367–1372. [Google Scholar] [CrossRef]
- Yokawa, K.; Kagenishi, T.; Kawano, T.; Mancuso, S.; Baluška, F. Illumination of Arabidopsis Roots Induces Immediate Burst of ROS Production. Plant Signal Behav. 2011, 6, 1460–1464. [Google Scholar] [CrossRef] [PubMed]
- Ashenafi, E.L.; Nyman, M.C.; Shelley, J.T.; Mattson, N.S. Spectral Properties and Stability of Selected Carotenoid and Chlorophyll Compounds in Different Solvent Systems. Food Chem. Adv. 2023, 2, 100178. [Google Scholar] [CrossRef]
- Yokawa, K.; Fasano, R.; Kagenishi, T.; Baluška, F. Light as Stress Factor to Plant Roots—Case of Root Halotropism. Front. Plant Sci. 2014, 5, 718. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, C.; Sircar, D.; Chatterjee, M.; Das, S.; Mitra, A. Combating Photooxidative Stress in Green Hairy Roots of Daucus Carota Cultivated under Light Irradiation. J. Plant Physiol. 2014, 171, 179–187. [Google Scholar] [CrossRef]
- Reis, A.; Kleinowski, A.M.; Klein, F.R.S.; Telles, R.T.; do Amarante, L.; Braga, E.J.B. Light Quality on the in Vitro Growth and Production of Pigments in the Genus alternanthera. J. Crop Sci. Biotechnol. 2015, 18, 349–357. [Google Scholar] [CrossRef]
- Ye, S.; Shao, Q.; Xu, M.; Li, S.; Wu, M.; Tan, X.; Su, L. Effects of Light Quality on Morphology, Enzyme Activities, and Bioactive Compound Contents in Anoectochilus roxburghii. Front. Plant Sci. 2017, 8, 857. [Google Scholar] [CrossRef]
- Mukherjee, C.; Samanta, T.; Mitra, A. Redirection of Metabolite Biosynthesis from Hydroxybenzoates to Volatile Terpenoids in Green Hairy Roots of Daucus carota. Planta 2016, 243, 305–320. [Google Scholar] [CrossRef]
- Kobayashi, K.; Ohnishi, A.; Sasaki, D.; Fujii, S.; Iwase, A.; Sugimoto, K.; Masuda, T.; Wada, H. Shoot Removal Induces Chloroplast Development in Roots via Cytokinin Signaling. Plant Physiol. 2017, 173, 2340–2355. [Google Scholar] [CrossRef]
- Bielach, A.; Podlesáková, K.; Marhavy, P.; Duclercq, J.; Cuesta, C.; Müller, B.; Grunewald, W.; Tarkowski, P.; Benková, E. Spatiotemporal Regulation of Lateral Root Organogenesis in Arabidopsis by Cytokinin. Plant Cell 2012, 24, 3967–3981. [Google Scholar] [CrossRef]
- Moore, S.; Jervis, G.; Topping, J.F.; Chen, C.; Liu, J.; Lindsey, K. A Predictive Model for Ethylene-Mediated Auxin and Cytokinin Patterning in the Arabidopsis Root. Plant Commun. 2024, 5, 100886. [Google Scholar] [CrossRef]
- Tanios, S.; Eyles, A.; Tegg, R.; Wilson, C. Potato Tuber Greening: A Review of Predisposing Factors, Management and Future Challenges. Am. J. Potato Res. 2018, 95, 248–257. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Chen, W.; Yang, Z.; Li, X.; Wang, L.; Cao, S.; Shi, L. The Changes in Chlorophyll, Solanine, and Phytohormones during Light-Induced Greening in Postharvest Potatoes. Postharvest Biol. Technol. 2025, 219, 113291. [Google Scholar] [CrossRef]
- Petermann, J.B.; Morris, S.C. The Spectral Responses of Chlorophyll and Glycoalkaloid Synthesis in Potato Tubers (Solanum tuberosum). Plant Sci. 1985, 39, 105–110. [Google Scholar] [CrossRef]
- Buschmann, C.; Meier, D.; Kleudgen, H.K.; Lichtenthaler, H.K. Regulation of Chloroplast Development by Red and Blue Light. In Annual European Symposium on Photomorphogenesis; Song, P.-S., Ed.; Pergamon: Oxford, UK, 1978; pp. 195–198. ISBN 978-0-08-022677-4. [Google Scholar]
- Miao, Y.; Chen, Q.; Qu, M.; Gao, L.; Hou, L. Blue Light Alleviates ‘Red Light Syndrome’ by Regulating Chloroplast Ultrastructure, Photosynthetic Traits and Nutrient Accumulation in Cucumber Plants. Sci. Hortic. 2019, 257, 108680. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Q.; Xin, Y.; Mei, Z.; Gao, A.; Liu, W.; Yu, L.; Chen, X.; Chen, Z.; Wang, N. Analyses of the Photosynthetic Characteristics, Chloroplast Ultrastructure, and Transcriptome of Apple (Malus domestica) Grown under Red and Blue Lights. BMC Plant Biol. 2021, 21, 483. [Google Scholar] [CrossRef]
- Garrido, A.; Conde, A.; Serôdio, J.; De Vos, R.C.H.; Cunha, A. Fruit Photosynthesis: More to Know about Where, How and Why. Plants 2023, 12, 2393. [Google Scholar] [CrossRef]
- Jiao, J.; Fu, J.-X.; Yao, L.; Gai, Q.-Y.; He, X.-J.; Feng, X.; Fu, Y.-J. The Growth, Adventitious Bud Formation, Bioactive Flavonoid Production, Antioxidant Response, and Cryptochrome-Mediated Light Signal Transduction in Isatis tinctoria L. Hairy Root Cultures Exposed to LED Lights. Ind. Crops Prod. 2023, 195, 116496. [Google Scholar] [CrossRef]
- Berkovich, Y.A.; Smolyanina, S.O.; Krivobok, N.M.; Erokhin, A.N.; Agureev, A.N.; Shanturin, N.A. Vegetable Production Facility as a Part of a Closed Life Support System in a Russian Martian Space Flight Scenario. Adv. Space Res. 2009, 44, 170–176. [Google Scholar] [CrossRef]
- Finetto, C.; Lobascio, C.; Rapisarda, A. Concept of a Lunar FARM: Food and Revitalization Module. Acta Astronaut. 2010, 66, 1329–1340. [Google Scholar] [CrossRef]
- Kamran, M.; Auroux, L.; Johnson, K.; Lewsey, M.G. Optimising Plant Form and Function for Controlled Environment Agriculture in Space and on Earth. Mod. Agric. 2023, 1, 86–97. [Google Scholar] [CrossRef]
- Ellery, A. Supplementing Closed Ecological Life Support Systems with In-Situ Resources on the Moon. Life 2021, 11, 770. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakamoto, M.; Funaki, A.; Sakagami, F.; Kaida, T.; Suzuki, T. Unveiling the Impact of LED Light on Growing Carrot Taproots: A Novel Hydroponic Cultivation System. Eng 2025, 6, 87. https://doi.org/10.3390/eng6050087
Sakamoto M, Funaki A, Sakagami F, Kaida T, Suzuki T. Unveiling the Impact of LED Light on Growing Carrot Taproots: A Novel Hydroponic Cultivation System. Eng. 2025; 6(5):87. https://doi.org/10.3390/eng6050087
Chicago/Turabian StyleSakamoto, Masaru, Ayuhiko Funaki, Fumiya Sakagami, Taichi Kaida, and Takahiro Suzuki. 2025. "Unveiling the Impact of LED Light on Growing Carrot Taproots: A Novel Hydroponic Cultivation System" Eng 6, no. 5: 87. https://doi.org/10.3390/eng6050087
APA StyleSakamoto, M., Funaki, A., Sakagami, F., Kaida, T., & Suzuki, T. (2025). Unveiling the Impact of LED Light on Growing Carrot Taproots: A Novel Hydroponic Cultivation System. Eng, 6(5), 87. https://doi.org/10.3390/eng6050087





