Microbial Exopolysaccharides: Structure, Diversity, Applications, and Future Frontiers in Sustainable Functional Materials
Abstract
:1. Introduction
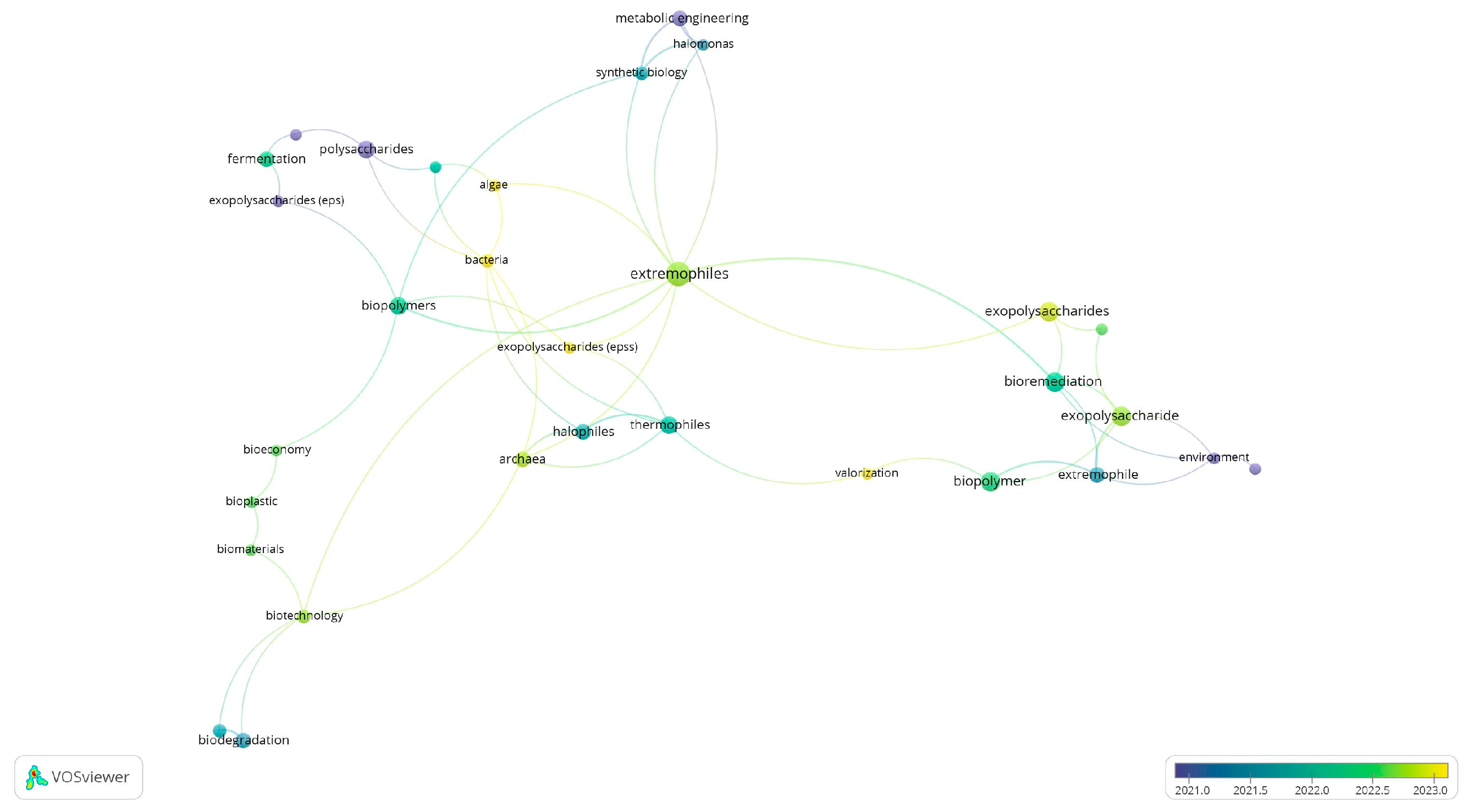
2. Bibliometric Analysis
2.1. Methodology
2.2. Data Collection
2.3. Search Results and Analysis
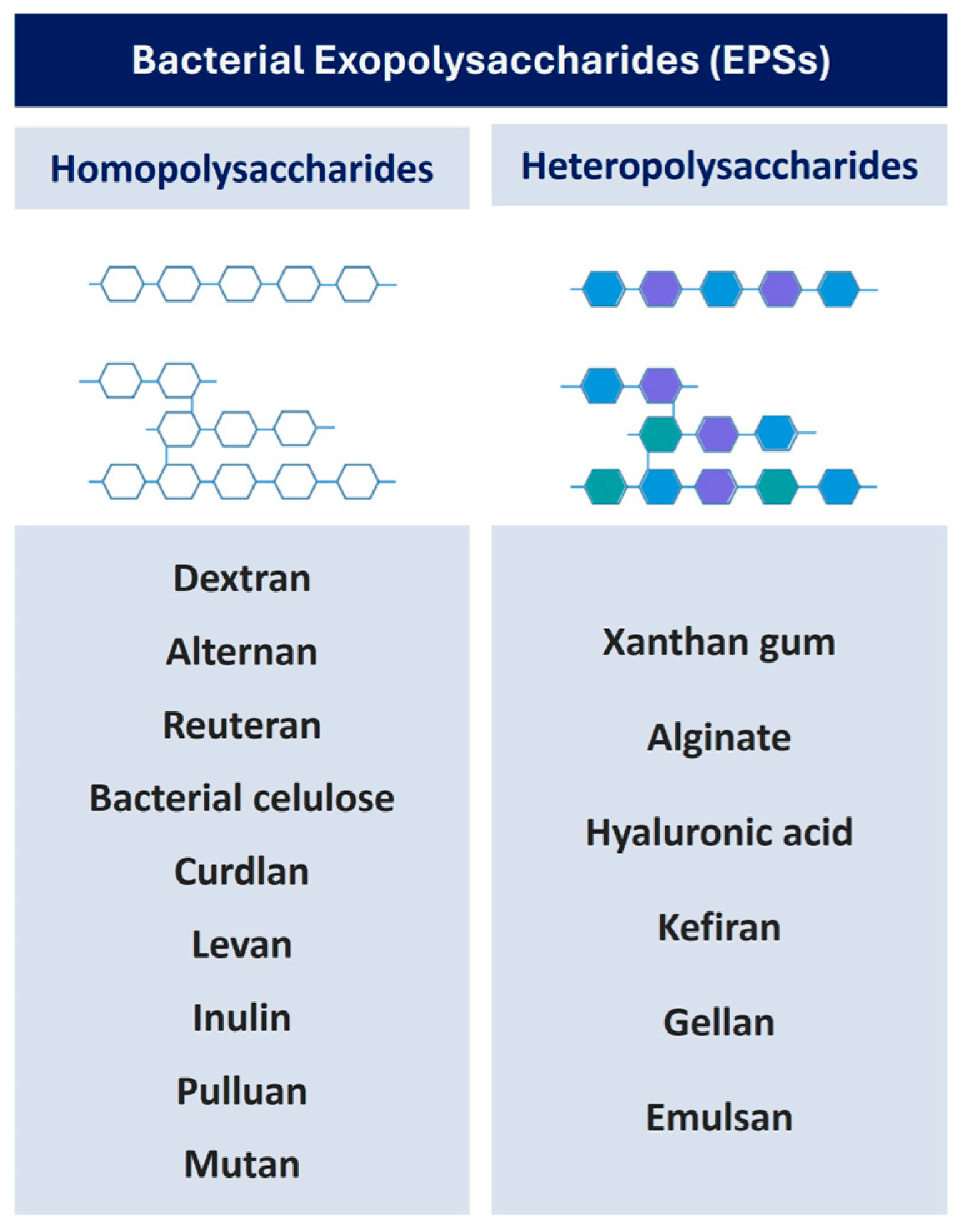
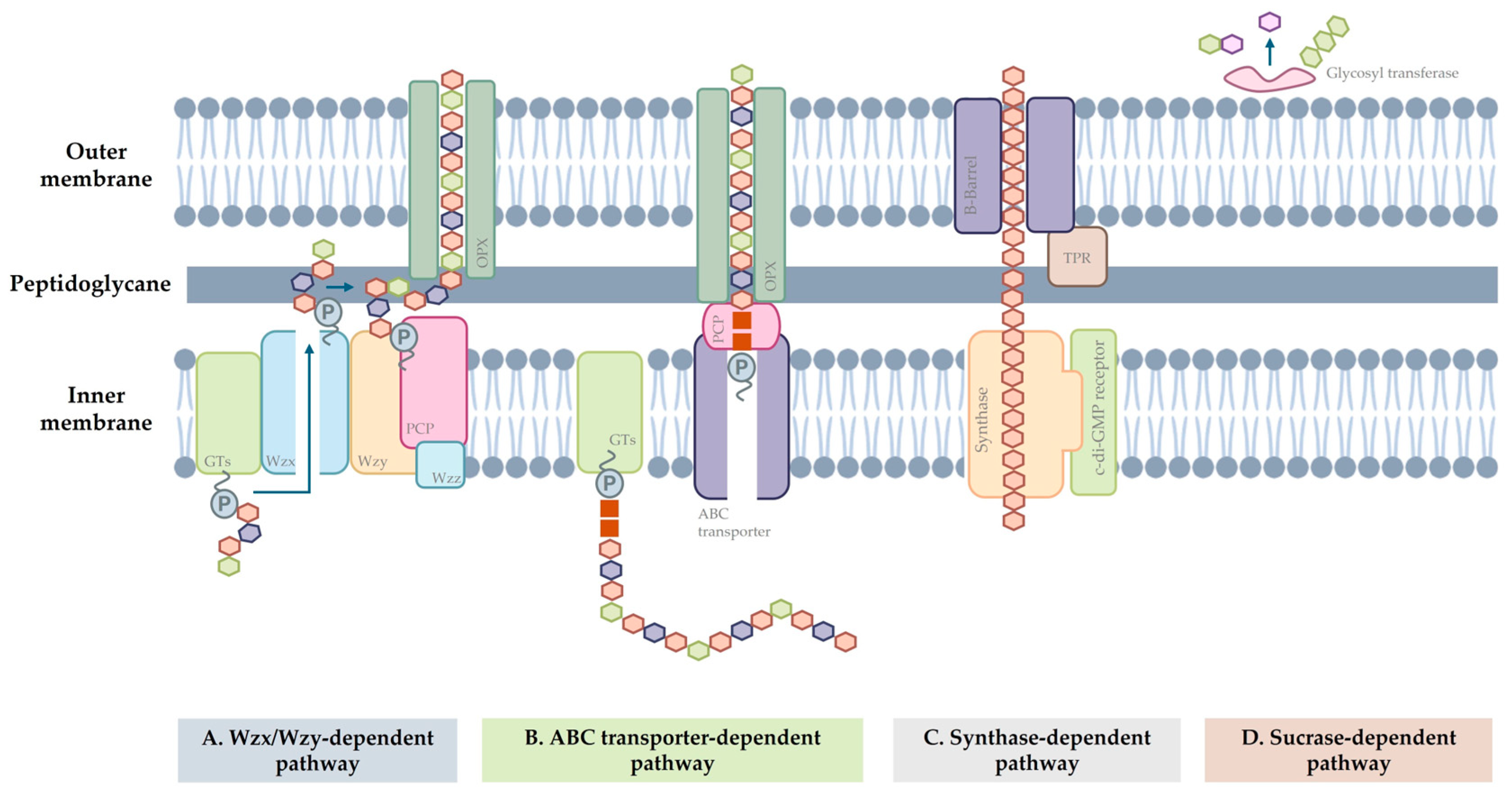
3. Exopolysaccharides (EPSs)
3.1. Homopolysaccharides EPSs
3.1.1. Dextran
3.1.2. Alternan
3.1.3. Reuteran
3.1.4. Bacterial Cellulose
3.1.5. Curdlan
3.1.6. Levan
3.1.7. Inulin
3.1.8. Pullulan
3.1.9. Mutan
3.2. Heteropolysaccharide EPSs
3.2.1. Xanthan Gum
3.2.2. Alginate
3.2.3. Hyaluronic Acid
3.2.4. Kefiran
3.2.5. Gellan
3.2.6. Emulsan
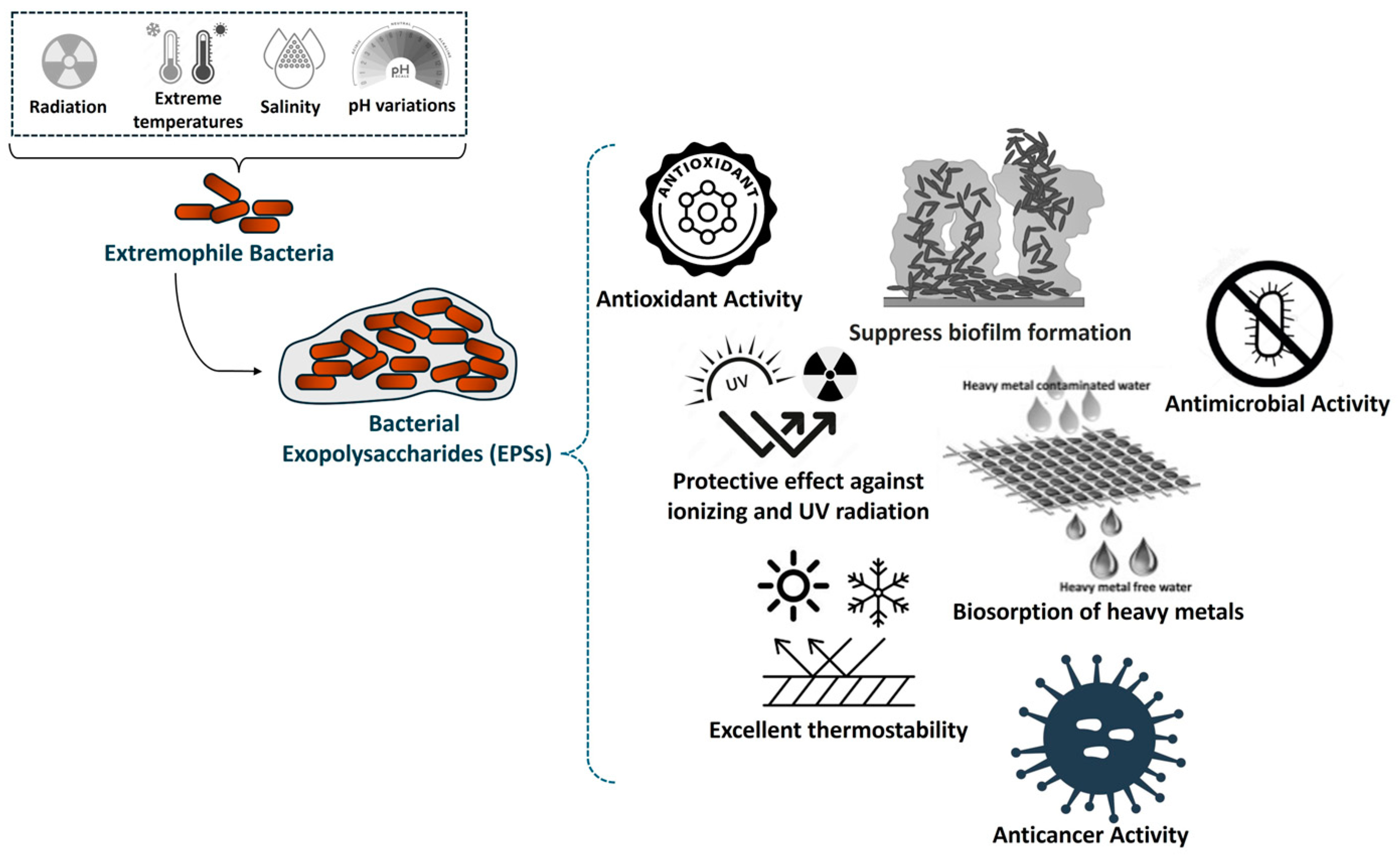
4. Emergent Functionalities and Applications of Exopolysaccharides (EPSs)
| Producer Organism | EPS | Sugar Carbon Source | Monosaccharide Composition | Molecular Weight (Da) | Functional Properties | Refs. |
|---|---|---|---|---|---|---|
| Deinococcus radiodurans | DeinoPol | Glucose, fructose, galactose, rhamnose, fucose, arabinose, mannose, and xylose | Xylose, galactose, fucose, glucose, arabinose, and fructose | 8.0 × 104–1.0 × 105 | Tolerance to ionizing radiation, UV radiation, desiccation, and oxidizing agents; robust antioxidant effects; ability to suppress Staphylococcus aureus biofilm formation; radioprotection. | [188] |
| Pantoea agglomerans KFS-9 | WSEPS | N.A. | Arabinose, glucose galactose, and gulcuronic acid | 7.6 × 105 | High antioxidant activity; protective effect against UV radiation. | [174] |
| Lactobacillus brevis-fermented Ecklonia cava | VLFEP | N.A. | Fucose, glucose, and mannose | >3.0 × 104 | Enhanced cell survival and proliferation in γ ray-irradiated cells; antioxidant activity; radioprotective effects. | [175] |
| Bacillus siamensis CV5 | CV5-EPS | Glucose | Rhamnose, fructose, mannose, and glucose | N.A. | Protective mechanism against radiation-induced damage; antioxidant activity. | [37] |
| Bacillus sp. QDR3-1 | EPS-M1 | Glucose | Mannose, glucose, galactose, and fucose | 3.38 × 104 | Biocompatible; protection against UV radiation; resistance to UV-induced stress; antioxidant activity. | [176] |
| Rhodococcus pyridinivorans ZZ47 | N.A. | Glucose | N.A. | N.A. | Antibiofilm activity; anti-angiogenic properties; antioxidant activity with no genotoxicity | [177] |
| Paenibacillus polymyxa PYQ1 | N.A. | Sucrose | N.A. | N.A. | Protection against short-wave UV radiation. | [178] |
| Geobacillus sp. TS3-9 | N.A. | Glucose, lactose, galactose, xylose, maltose, fructose, mannose, sucrose, and sorbose | D-mannose, D-glucose, and rhamnose | 3.2 × 106 | Radiation resistance; antioxidant activity. | [189] |
| Anoxybacillus sp. R4-33 | EPS-II | Glucose | D-mannose and D-glucose | 1.0 × 106 | Biosorption of heavy metals; antioxidant properties. | [37,190] |
| Geobacillus sp. WSUCF1 | EPS-1 EPS-2 | Glucose | EPS-1: mannose and glucose; EPS-2: mannan | 1.0 × 106 | Antioxidant activities; non-cytotoxicity; excellent thermostability. | [69,70] |
| Cold-adapted Pseudomonas sp. BGI-2 | N.A. | Glucose, galactose, mannose, mannitol, glycerol, and molasses | Glucose, galactose, and glucosamine | N.A. | Protect cell membranes; cryoprotective effect. | [179] |
| Chromohalobacter canadensis 28 | N.A. | Lactose | Glucosamine, glucose, rhamnose, xylose, and not identified sugar | >1.0 × 106 | High hydrophilicity; high swelling behavior; stabilizing properties; emulsifying properties; good foaming ability. | [181] |
| Halomonas smyrnensis K2 | EPS-K2 | Glucose | Mannose, glucose, and galactose | 3.96 × 105 | Remarkable thermostability; antioxidant activity; iron-chelating ability; DNA protection; antimicrobial activity; anti-biofilm activity. | [182] |
| Alkalibacillus sp. w3 | N.A. | Glucose, fructose, maltose, galactose, mannitol, sucrose, and lactose | N.A. | N.A. | Anticancer activity; antibacterial activity. | [184] |
| Bacillus xiamenensis RT6 | EPSt | Glucose | Glucose, mannose, and galactose | 2.71 × 104 | Antioxidant properties; metal chelation abilities. | [185] |
| Vibrio neocaledonicus sp. | N.A. | Glucose, sucrose, fructose, xylose, mannitol, and galactose | N.A. | 2.96 × 101 | Corrosion inhibition. | [186] |
| Rhodococcus erythropolis HX-2 | HPS | N.A. | Glucose, galactose, fucose, mannose, and glucuronic acid | 1.04 × 106 | Anticancer properties; moisture retention. | [187] |
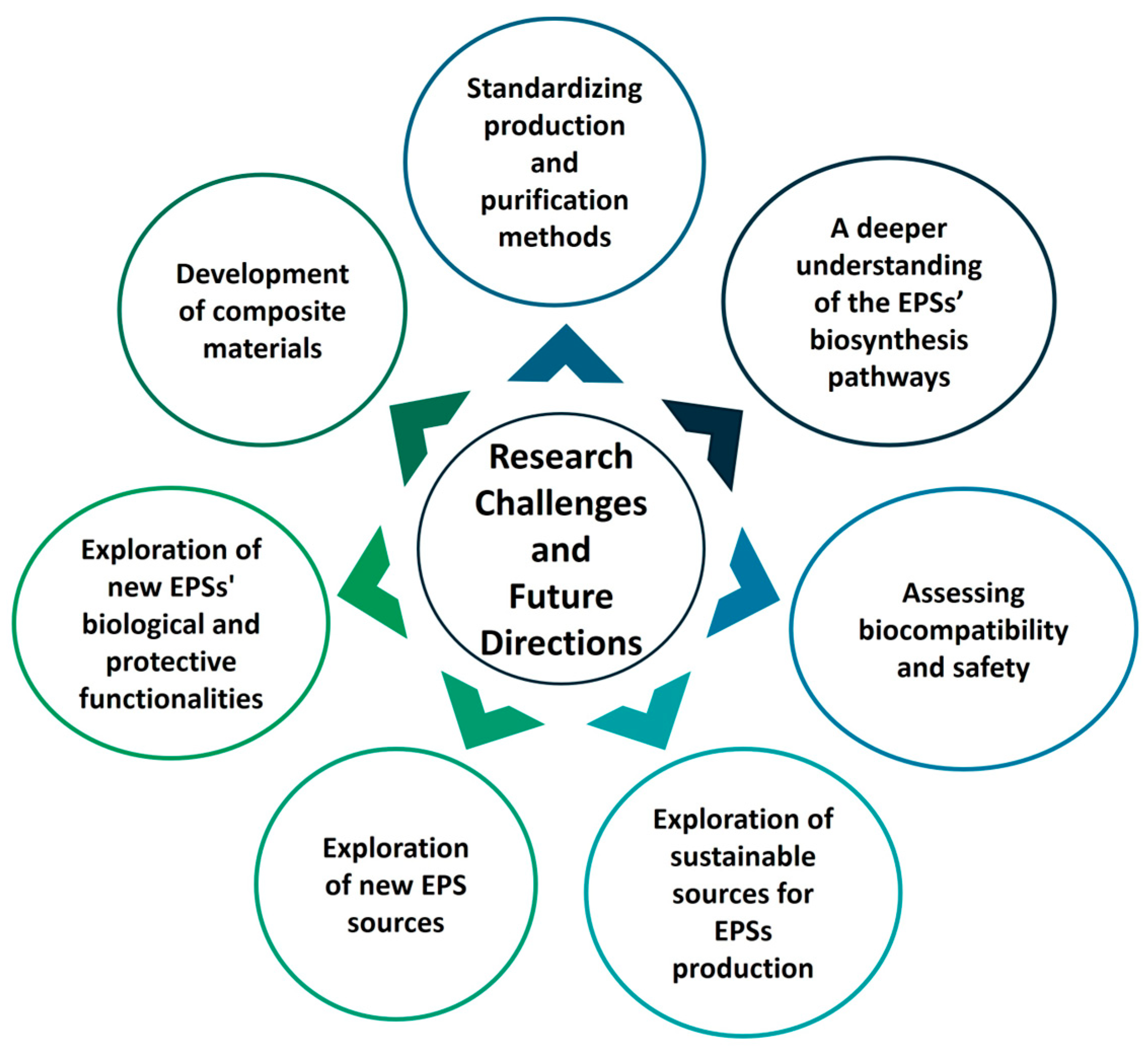
5. Challenges and Future Research Directions
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Álvarez, A.; Manjarres, J.J.; Ramírez, C.; Bolívar, G. Use of an Exopolysaccharide-Based Edible Coating and Lactic Acid Bacteria with Antifungal Activity to Preserve the Postharvest Quality of Cherry Tomato. LWT 2021, 151, 112225. [Google Scholar] [CrossRef]
- Guerrero, B.G.; Santos, K.d.L.; Kamimura, E.S.; de Oliveira, C.A.F. Application of Microbial Exopolysaccharides in Packaging Films for the Food Industry: A Review. Int. J. Food Sci. Technol. 2024, 59, 17–29. [Google Scholar] [CrossRef]
- Ahuja, V.; Bhatt, A.K.; Banu, J.R.; Kumar, V.; Kumar, G.; Yang, Y.H.; Bhatia, S.K. Microbial Exopolysaccharide Composites in Biomedicine and Healthcare: Trends and Advances. Polymers 2023, 15, 1801. [Google Scholar] [CrossRef] [PubMed]
- Salimi, F.; Farrokh, P. Recent Advances in the Biological Activities of Microbial Exopolysaccharides. World J. Microbiol. Biotechnol. 2023, 39, 213. [Google Scholar] [CrossRef] [PubMed]
- Netrusov, A.I.; Liyaskina, E.V.; Kurgaeva, I.V.; Liyaskina, A.U.; Yang, G.; Revin, V.V. Exopolysaccharides Producing Bacteria: A Review. Microorganisms 2023, 11, 1541. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. Chitin and Chitosan: Properties and Applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Kumari, R.; Kumar, M.; Vivekanand, V.; Pareek, N. Chitin Biorefinery: A Narrative and Prophecy of Crustacean Shell Waste Sustainable Transformation into Bioactives and Renewable Energy. Renew. Sustain. Energy Rev. 2023, 184, 113595. [Google Scholar] [CrossRef]
- Ravi Kumar, M.N.V. A Review of Chitin and Chitosan Applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M.; Harding, D.; Sashiwa, H. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef]
- Rawat, M.; Chauhan, M.; Pandey, A. Extremophiles and Their Expanding Biotechnological Applications. Arch. Microbiol. 2024, 206, 247. [Google Scholar] [CrossRef] [PubMed]
- Gahlawat, G.; Kumari, P.; Bhagat, N.R. Technological Advances in the Production of Polyhydroxyalkanoate Biopolymers. Curr. Sustain./Renew. Energy Rep. 2020, 7, 73–83. [Google Scholar] [CrossRef]
- Pei, C.; Lu, H.; Ma, J.; Eichler, J.; Guan, Z.; Gao, L.; Liu, L.; Zhou, H.; Yang, J.; Jin, C. AepG Is a Glucuronosyltransferase Involved in Acidic Exopolysaccharide Synthesis and Contributes to Environmental Adaptation of Haloarcula Hispanica. J. Biol. Chem. 2023, 299, 102911. [Google Scholar] [CrossRef] [PubMed]
- Villanova, V.; Galasso, C.; Fiorini, F.; Lima, S.; Brönstrup, M.; Sansone, C.; Brunet, C.; Brucato, A.; Scargiali, F. Biological and Chemical Characterization of New Isolated Halophilic Microorganisms from Saltern Ponds of Trapani, Sicily. Algal Res. 2021, 54, 102192. [Google Scholar] [CrossRef]
- Waoo, A.A.; Singh, S.; Pandey, A.; Kant, G.; Choure, K.; Amesho, K.T.T.; Srivastava, S. Microbial Exopolysaccharides in the Biomedical and Pharmaceutical Industries. Heliyon 2023, 9, e18613. [Google Scholar] [CrossRef] [PubMed]
- Tabernero, A.; Cardea, S. Microbial Exopolysaccharides as Drug Carriers. Polymers 2020, 12, 2142. [Google Scholar] [CrossRef] [PubMed]
- Nadzir, M.M.; Nurhayati, R.W.; Idris, F.N.; Nguyen, M.H. Biomedical Applications of Bacterial Exopolysaccharides: A Review. Polymers 2021, 13, 530. [Google Scholar] [CrossRef]
- Możejko-Ciesielska, J.; Ray, S.; Sankhyan, S. Recent Challenges and Trends of Polyhydroxyalkanoate Production by Extremophilic Bacteria Using Renewable Feedstocks. Polymers 2023, 15, 4385. [Google Scholar] [CrossRef] [PubMed]
- Angra, V.; Sehgal, R.; Gupta, R. Trends in PHA Production by Microbially Diverse and Functionally Distinct Communities. Microb. Ecol. 2023, 85, 572–585. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, K.; Umesh, M.; Kathirvel, P. Microbial Polyhydroxyalkanoates (PHAs): A Review on Biosynthesis, Properties, Fermentation Strategies and Its Prospective Applications for Sustainable Future. J. Polym. Environ. 2022, 30, 4903–4935. [Google Scholar] [CrossRef]
- Obulisamy, P.K.; Mehariya, S. Polyhydroxyalkanoates from Extremophiles: A Review. Bioresour. Technol. 2021, 325, 124653. [Google Scholar] [CrossRef]
- Mehariya, S.; Plöhn, M.; Jablonski, P.; Stagge, S.; Jönsson, L.J.; Funk, C. Biopolymer Production from Biomass Produced by Nordic Microalgae Grown in Wastewater. Bioresour. Technol. 2023, 376, 128901. [Google Scholar] [CrossRef] [PubMed]
- Pacholak, A.; Gao, Z.L.; Gong, X.Y.; Kaczorek, E.; Cui, Y.W. The Metabolic Pathways of Polyhydroxyalkanoates and Exopolysaccharides Synthesized by Haloferax Mediterranei in Response to Elevated Salinity. J. Proteom. 2021, 232, 104065. [Google Scholar] [CrossRef] [PubMed]
- Chacón, M.; Wongsirichot, P.; Winterburn, J.; Dixon, N. Genetic and Process Engineering for Polyhydroxyalkanoate Production from Pre- and Post-Consumer Food Waste. Curr. Opin. Biotechnol. 2024, 85, 103024. [Google Scholar] [CrossRef] [PubMed]
- Adak, S.; Kayalvizhi, R.; Bishai, M.; Jacob, S.; Kundu, D. Advancements in Microbial Production of Polyhydroxyalkanoates (PHA) from Wastes for Sustainable Active Food Packaging: An Eclectic Review. Biocatal. Agric. Biotechnol. 2024, 60, 103288. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, J.C.; Ma, Y.M.; Chen, G.Q. Engineering Biosynthesis of Polyhydroxyalkanoates (PHA) for Diversity and Cost Reduction. Metab. Eng. 2020, 58, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Rajesh Banu, J.; Ginni, G.; Kavitha, S.; Yukesh Kannah, R.; Kumar, V.; Adish Kumar, S.; Gunasekaran, M.; Tyagi, V.K.; Kumar, G. Polyhydroxyalkanoates Synthesis Using Acidogenic Fermentative Effluents. Int. J. Biol. Macromol. 2021, 193, 2079–2092. [Google Scholar] [CrossRef] [PubMed]
- Khatami, K.; Perez-Zabaleta, M.; Owusu-Agyeman, I.; Cetecioglu, Z. Waste to Bioplastics: How Close Are We to Sustainable Polyhydroxyalkanoates Production? Waste Manag. 2021, 119, 374–388. [Google Scholar] [CrossRef] [PubMed]
- Palmeiro-Sánchez, T.; O’Flaherty, V.; Lens, P.N.L. Polyhydroxyalkanoate Bio-Production and Its Rise as Biomaterial of the Future. J. Biotechnol. 2022, 348, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Tarrahi, R.; Fathi, Z.; Seydibeyoğlu, M.Ö.; Doustkhah, E.; Khataee, A. Polyhydroxyalkanoates (PHA): From Production to Nanoarchitecture. Int. J. Biol. Macromol. 2020, 146, 596–619. [Google Scholar] [CrossRef]
- Goswami, L.; Kushwaha, A.; Napathorn, S.C.; Kim, B.S. Valorization of Organic Wastes Using Bioreactors for Polyhydroxyalkanoate Production: Recent Advancement, Sustainable Approaches, Challenges, and Future Perspectives. Int. J. Biol. Macromol. 2023, 247, 125743. [Google Scholar] [CrossRef]
- Wang, J.; Huang, J.; Liu, S. The Production, Recovery, and Valorization of Polyhydroxybutyrate (PHB) Based on Circular Bioeconomy. Biotechnol. Adv. 2024, 72, 108340. [Google Scholar] [CrossRef]
- Paul, E.; Bessière, Y.; Dumas, C.; Girbal-Neuhauser, E. Biopolymers Production from Wastes and Wastewaters by Mixed Microbial Cultures: Strategies for Microbial Selection. Waste Biomass Valorization 2020, 12, 4213–4237. [Google Scholar] [CrossRef]
- Angelin, J.; Kavitha, M. Exopolysaccharides from Probiotic Bacteria and Their Health Potential. Int. J. Biol. Macromol. 2020, 162, 853. [Google Scholar] [CrossRef] [PubMed]
- Saha, I.; Datta, S. Bacterial Exopolysaccharides in Drug Delivery Applications. J. Drug Deliv. Sci. Technol. 2022, 74, 103557. [Google Scholar] [CrossRef]
- Yaşar Yıldız, S.; Radchenkova, N. Exploring Extremophiles from Bulgaria: Biodiversity, Biopolymer Synthesis, Functional Properties, Applications. Polymers 2023, 16, 69. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, H.; Karatas, N. Microbial Exopolysaccharides: Resources and Bioactive Properties. Process Biochem. 2018, 72, 41–46. [Google Scholar] [CrossRef]
- Guesmi, S.; Chouchane, H.; Neifar, M.; Hosni, F.; Cherif, A.; Sghaier, H. Radiation-Inducible Radioprotective Exopolysaccharides of Bacillus siamensis CV5 from Irradiated Roots of Cistanche Violacea to Decrease Free Radical Damage Produced by Ionizing Radiation. Int. J. Radiat. Biol. 2019, 95, 1552–1563. [Google Scholar] [CrossRef] [PubMed]
- López-Ortega, M.A.; Chavarría-Hernández, N.; del Rocío López-Cuellar, M.; Rodríguez-Hernández, A.I. A Review of Extracellular Polysaccharides from Extreme Niches: An Emerging Natural Source for the Biotechnology. From the Adverse to Diverse! Int. J. Biol. Macromol. 2021, 177, 559–577. [Google Scholar] [CrossRef]
- Wang, J.; Salem, D.R.; Sani, R.K. Extremophilic Exopolysaccharides: A Review and New Perspectives on Engineering Strategies and Applications. Carbohydr. Polym. 2019, 205, 8–26. [Google Scholar] [CrossRef]
- Radchenkova, N.; Rusinova-Videva, S. Extremophiles as a “Green Source” of New Exopolysaccharides with Ecological Importance and Multifunctional Applications. Polym.-Plast. Technol. Mater. 2024, 63, 247–269. [Google Scholar] [CrossRef]
- Laubach, J.; Joseph, M.; Brenza, T.; Gadhamshetty, V.; Sani, R.K. Exopolysaccharide and Biopolymer-Derived Films as Tools for Transdermal Drug Delivery. J. Control. Release 2021, 329, 971–987. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Zheng, C.; Wu, W.; Yu, G.; Wang, P. Exopolysaccharides from Marine Microbes: Source, Structure and Application. Mar. Drugs 2022, 20, 512. [Google Scholar] [CrossRef]
- Banerjee, A.; Sarkar, S.; Govil, T.; González-Faune, P.; Cabrera-Barjas, G.; Bandopadhyay, R.; Salem, D.R.; Sani, R.K. Extremophilic Exopolysaccharides: Biotechnologies and Wastewater Remediation. Front. Microbiol. 2021, 12, 721365. [Google Scholar] [CrossRef] [PubMed]
- Caccamo, M.T.; Zammuto, V.; Spanò, A.; Gugliandolo, C.; Magazù, S. Hydrating Capabilities of the Biopolymers Produced by the Marine Thermophilic Bacillus horneckiae SBP3 as Evaluated by ATR-FTIR Spectroscopy. Materials 2022, 15, 5988. [Google Scholar] [CrossRef]
- Parada-Pinilla, M.P.; Ferreira, M.A.; Roncallo, J.C.; Santos, S.N.; Melo, I.S.; Assef, A.N.B.; Wilke, D.V.; Silva, L.F.; Garrido, L.M.; Araújo, W.L.; et al. Biopolymer Production by Halotolerant Bacteria Isolated from Caatinga Biome. Braz. J. Microbiol. 2021, 52, 547–559. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, P.; Tang, X.; Xu, C. Characterisation and Bioactivities of an Exopolysaccharide from an Antarctic Bacterium Shewanella frigidimarina W32–2. Aquaculture 2021, 530, 735760. [Google Scholar] [CrossRef]
- Uma, G.; Babu, M.M.; Prakash, V.S.G.; Nisha, S.J.; Citarasu, T. Nature and Bioprospecting of Haloalkaliphilics: A Review. World J. Microbiol. Biotechnol. 2020, 36, 66. [Google Scholar] [CrossRef] [PubMed]
- Spanò, A.; Zammuto, V.; Macrì, A.; Agostino, E.; Nicolò, M.S.; Scala, A.; Trombetta, D.; Smeriglio, A.; Ingegneri, M.; Caccamo, M.T.; et al. Arsenic Adsorption and Toxicity Reduction of An Exopolysaccharide Produced by Bacillus licheniformis B3-15 of Shallow Hydrothermal Vent Origin. J. Mar. Sci. Eng. 2023, 11, 325. [Google Scholar] [CrossRef]
- Chambi, D.; Romero-Soto, L.; Villca, R.; Orozco-Gutiérrez, F.; Vega-Baudrit, J.; Quillaguamán, J.; Hatti-Kaul, R.; Martín, C.; Carrasco, C. Exopolysaccharides Production by Cultivating a Bacterial Isolate from the Hypersaline Environment of Salar de Uyuni (Bolivia) in Pretreatment Liquids of Steam-Exploded Quinoa Stalks and Enzymatic Hydrolysates of Curupaú Sawdust. Fermentation 2021, 7, 33. [Google Scholar] [CrossRef]
- Banerjee, S.; Cabrera-Barjas, G.; Tapia, J.; Fabi, J.P.; Delattre, C.; Banerjee, A. Characterization of Chilean Hot Spring-Origin Staphylococcus sp. BSP3 Produced Exopolysaccharide as Biological Additive. Nat. Prod. Bioprospect. 2024, 14, 15. [Google Scholar] [CrossRef]
- Chavan, S.; Yadav, B.; Tyagi, R.D.; Drogui, P. A Review on Production of Polyhydroxyalkanoate (PHA) Biopolyesters by Thermophilic Microbes Using Waste Feedstocks. Bioresour. Technol. 2021, 341, 125900. [Google Scholar] [CrossRef] [PubMed]
- Harirchi, S.; Sar, T.; Ramezani, M.; Aliyu, H.; Etemadifar, Z.; Nojoumi, S.A.; Yazdian, F.; Awasthi, M.K.; Taherzadeh, M.J. Bacillales: From Taxonomy to Biotechnological and Industrial Perspectives. Microorganisms 2022, 10, 2355. [Google Scholar] [CrossRef] [PubMed]
- Gurav, R.; Hwang, S.; Bhatia, S.K.; Kshirsagar, A.D.; Mandal, S.; Yang, Y.H. Exopolysaccharide Production Using Pinewood Hydrolysate as a Substrate for Psychrotrophic Bacterium Isolated from Svalbard Glacier Soil. Biomass Convers. Biorefin. 2023, 1, 1–11. [Google Scholar] [CrossRef]
- Możejko-Ciesielska, J.; Moraczewski, K.; Czaplicki, S. Halomonas alkaliantarctica as a Platform for Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Production from Biodiesel-Derived Glycerol. Environ. Microbiol. Rep. 2024, 16, e13225. [Google Scholar] [CrossRef] [PubMed]
- Erkorkmaz, B.A.; Kırtel, O.; Abaramak, G.; Nikerel, E.; Toksoy Öner, E. UV and Chemically Induced Halomonas smyrnensis Mutants for Enhanced Levan Productivity. J. Biotechnol. 2022, 356, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Mozejko-Ciesielska, J.; Marciniak, P.; Moraczewski, K.; Rytlewski, P.; Czaplicki, S.; Zadernowska, A. Cheese Whey Mother Liquor as Dairy Waste with Potential Value for Polyhydroxyalkanoate Production by Extremophilic Paracoccus Homiensis. Sustain. Mater. Technol. 2022, 33, e00449. [Google Scholar] [CrossRef]
- González, E.; Zuleta, C.; Zamora, G.; Maturana, N.; Ponce, B.; Rivero, M.V.; Rodríguez, A.; Soto, J.P.; Scott, F.; Díaz-Barrera, Á. Production of Poly (3-Hydroxybutyrate) and Extracellular Polymeric Substances from Glycerol by the Acidophile Acidiphilium cryptum. Extremophiles 2023, 27, 30. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Huang, X.; He, Z.; He, T. Exopolysaccharide Production by Salt-Tolerant Bacteria: Recent Advances, Current Challenges, and Future Prospects. Int. J. Biol. Macromol. 2024, 264, 130731. [Google Scholar] [CrossRef]
- Munir Ahamed, J.; Dahms, H.U.; Huang, Y.L. Heavy Metal Tolerance, and Metal Biosorption by Exopolysaccharides Produced by Bacterial Strains Isolated from Marine Hydrothermal Vents. Chemosphere 2024, 351, 141170. [Google Scholar] [CrossRef]
- Rodge, S.P.; Shende, K.S.; Patil, N.P. Polyhydroxyalkanoate Biosynthesis and Optimisation of Thermophilic Geobacillus stearothermophilus Strain K4E3_SPR_NPP. Extremophiles 2023, 27, 13. [Google Scholar] [CrossRef]
- Caccamo, M.T.; Gugliandolo, C.; Zammuto, V.; Magazù, S. Thermal Properties of an Exopolysaccharide Produced by a Marine Thermotolerant Bacillus licheniformis by ATR-FTIR Spectroscopy. Int. J. Biol. Macromol. 2020, 145, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Cornejo, S.; Otero, M.C.; Banerjee, A.; Gordillo-Fuenzalida, F. Biological Properties of Exopolysaccharides Produced by Bacillus spp. Microbiol. Res. 2023, 268, 127276. [Google Scholar] [CrossRef]
- Ibrahim, H.A.H.; Abou Elhassayeb, H.E.; El-Sayed, W.M.M. Potential Functions and Applications of Diverse Microbial Exopolysaccharides in Marine Environments. J. Genet. Eng. Biotechnol. 2022, 20, 151. [Google Scholar] [CrossRef]
- Ye, J.W.; Lin, Y.N.; Yi, X.Q.; Yu, Z.X.; Liu, X.; Chen, G.Q. Synthetic Biology of Extremophiles: A New Wave of Biomanufacturing. Trends Biotechnol. 2023, 41, 342–357. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Dey, P. Bacterial Exopolysaccharides as Emerging Bioactive Macromolecules: From Fundamentals to Applications. Res. Microbiol. 2023, 174, 104024. [Google Scholar] [CrossRef]
- López-Ortega, M.A.; Rodríguez-Hernández, A.I.; Camacho-Ruíz, R.M.; Córdova, J.; del Rocío López-Cuellar, M.; Chavarría-Hernández, N.; González-García, Y. Physicochemical Characterization and Emulsifying Properties of a Novel Exopolysaccharide Produced by Haloarchaeon Haloferax mucosum. Int. J. Biol. Macromol. 2020, 142, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Mukhia, S.; Kumar, R. Production, Characterisation, and Application of Exopolysaccharide Extracted from a Glacier Bacterium Mucilaginibacter sp. ERMR7:07. Process Biochem. 2022, 113, 27–36. [Google Scholar] [CrossRef]
- Rana, S.; Upadhyay, L.S.B. Microbial Exopolysaccharides: Synthesis Pathways, Types and Their Commercial Applications. Int. J. Biol. Macromol. 2020, 157, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Salem, D.R.; Sani, R.K. Two New Exopolysaccharides from a Thermophilic Bacterium Geobacillus sp. WSUCF1: Characterization and Bioactivities. New Biotechnol. 2021, 61, 29–39. [Google Scholar] [CrossRef]
- Joulak, I.; Azabou, S.; Finore, I.; Poli, A.; Nicolaus, B.; Donato, P.D.I.; Bkhairia, I.; Dumas, E.; Gharsallaoui, A.; Immirzi, B.; et al. Structural Characterization and Functional Properties of Novel Exopolysaccharide from the Extremely Halotolerant Halomonas elongata S6. Int. J. Biol. Macromol. 2020, 164, 95–104. [Google Scholar] [CrossRef]
- Ayyash, M.; Stathopoulos, C.; Abu-Jdayil, B.; Esposito, G.; Baig, M.; Turner, M.S.; Baba, A.S.; Apostolopoulos, V.; Al-Nabulsi, A.; Osaili, T. Exopolysaccharide Produced by Potential Probiotic Enterococcus faecium MS79: Characterization, Bioactivities and Rheological Properties Influenced by Salt and pH. LWT 2020, 131, 109741. [Google Scholar] [CrossRef]
- Samalens, F.; Thomas, M.; Claverie, M.; Castejon, N.; Zhang, Y.; Pigot, T.; Blanc, S.; Fernandes, S.C.M. Progresses and Future Prospects in Biodegradation of Marine Biopolymers and Emerging Biopolymer-Based Materials for Sustainable Marine Ecosystems. Green Chem. 2022, 24, 1762–1779. [Google Scholar] [CrossRef]
- Kochhar, N.; Kavya, I.K.; Shrivastava, S.; Ghosh, A.; Rawat, V.S.; Sodhi, K.K.; Kumar, M. Perspectives on the Microorganism of Extreme Environments and Their Applications. Curr. Res. Microb. Sci. 2022, 3, 100134. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Kumar, V.; Yadav, V.; Sarsaiya, S.; Awasthi, S.K.; Sindhu, R.; Binod, P.; Kumar, V.; Pandey, A.; Zhang, Z. Current State of the Art Biotechnological Strategies for Conversion of Watermelon Wastes Residues to Biopolymers Production: A Review. Chemosphere 2022, 290, 133310. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Kumar, V.; Jia, L.; Sarsaiya, S.; Kumar, D.; Juneja, A.; Zhang, Z.; Sindhu, R.; Binod, P.; Bhatia, S.K.; et al. Biopolymer Poly-Hydroxyalkanoates (PHA) Production from Apple Industrial Waste Residues: A Review. Chemosphere 2021, 284, 131427. [Google Scholar] [CrossRef]
- Tiwari, O.N.; Bobby, M.N.; Kondi, V.; Halder, G.; Kargarzadeh, H.; Ikbal, A.M.A.; Bhunia, B.; Thomas, S.; Efferth, T.; Chattopadhyay, D.; et al. Comprehensive Review on Recent Trends and Perspectives of Natural Exo-Polysaccharides: Pioneering Nano-Biotechnological Tools. Int. J. Biol. Macromol. 2024, 265, 130747. [Google Scholar] [CrossRef]
- Alvarado, R.; Fuentes, A.; Ortiz, J.; Herrera, H.; Arriagada, C. Metal(Loid)-Resistant Bacterial Consortia with Antimycotic Properties Increase Tolerance of Chenopodium Quinoa Wild. to Metal(Loid) Stress. Rhizosphere 2022, 23, 100569. [Google Scholar] [CrossRef]
- Obruča, S.; Dvořák, P.; Sedláček, P.; Koller, M.; Sedlář, K.; Pernicová, I.; Šafránek, D. Polyhydroxyalkanoates Synthesis by Halophiles and Thermophiles: Towards Sustainable Production of Microbial Bioplastics. Biotechnol. Adv. 2022, 58, 107906. [Google Scholar] [CrossRef]
- Wang, Q.C.; Wei, M.; Zhang, J.; Yue, Y.; Wu, N.; Geng, L.; Sun, C.; Zhang, Q.; Wang, J. Structural Characteristics and Immune-Enhancing Activity of an Extracellular Polysaccharide Produced by Marine Halomonas sp. 2E1. Int. J. Biol. Macromol. 2021, 183, 1660–1668. [Google Scholar] [CrossRef]
- Basu, B. The Radiophiles of Deinococcaceae Family: Resourceful Microbes for Innovative Biotechnological Applications. Curr. Res. Microb. Sci. 2022, 3, 100153. [Google Scholar] [CrossRef]
- Aparici-Carratalá, D.; Esclapez, J.; Bautista, V.; Bonete, M.J.; Camacho, M. Archaea: Current and Potential Biotechnological Applications. Res. Microbiol. 2023, 174, 104080. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.; Wysokowski, M.; Jesionowski, T. The Philosophy of Extreme Biomimetics. Sustain. Mater. Technol. 2022, 32, e00447. [Google Scholar] [CrossRef]
- Chen, F.; Ji, H.J.; Choi, J.I.; Han, S.H.; Lim, S.; Seo, H.S.; Ahn, K.B. Anti-Allergic Function of the Cell Wall (DeinoWall) from Deinococcus radiodurans. Mol. Immunol. 2022, 151, 103–113. [Google Scholar] [CrossRef]
- Fenibo, E.O.; Selvarajan, R.; Wang, H.; Wang, Y.; Abia, A.L.K. Untapped Talents: Insight into the Ecological Significance of Methanotrophs and Its Prospects. Sci. Total Environ. 2023, 903, 166145. [Google Scholar] [CrossRef] [PubMed]
- Gaur, S.; Kaur, M.; Kalra, R.; Rene, E.R.; Goel, M. Application of Microbial Resources in Biorefineries: Current Trend and Future Prospects. Heliyon 2024, 10, e28615. [Google Scholar] [CrossRef]
- Mapstone, L.J.; Leite, M.N.; Purton, S.; Crawford, I.A.; Dartnell, L. Cyanobacteria and Microalgae in Supporting Human Habitation on Mars. Biotechnol. Adv. 2022, 59, 107946. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Kumar, V.; Harirchi, S.; Vigneswaran, V.S.; Rajendran, K.; Sharma, P.; Wah Tong, Y.; Binod, P.; Sindhu, R.; Sarsaiya, S.; et al. Recovery of Value-Added Products from Biowaste: A Review. Bioresour. Technol. 2022, 360, 127565. [Google Scholar] [CrossRef]
- Hosseini Nasab, M.; Noaparast, M.; Abdollahi, H.; Amoozegar, M.A. Kinetics of Two-Step Bioleaching of Ni and Co from Iron Rich-Laterite Using Supernatant Metabolites Produced by Salinivibrio kushneri as Halophilic Bacterium. Hydrometallurgy 2020, 195, 105387. [Google Scholar] [CrossRef]
- Aragón-León, A.; Moreno-Vilet, L.; González-Ávila, M.; Mondragón-Cortez, P.M.; Sassaki, G.L.; Martínez-Pérez, R.B.; Camacho-Ruíz, R.M. Inulin from Halophilic Archaeon Haloarcula: Production, Chemical Characterization, Biological, and Technological Properties. Carbohydr. Polym. 2023, 321, 121333. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, G.; Wang, F.; Zhao, H.; Wei, Y.; Liu, L.; Zhang, H. Extraction, Characterization, Antioxidant Activity and Rheological Behavior of a Polysaccharide Produced by the Extremely Salt Tolerant Bacillus subtilis LR-1. LWT 2022, 162, 113413. [Google Scholar] [CrossRef]
- Senko, H.; Kajić, S.; Huđ, A.; Palijan, G.; Petek, M.; Rajnović, I.; Šamec, D.; Udiković-Kolić, N.; Mešić, A.; Brkljačić, L.; et al. Will the Beneficial Properties of Plant-Growth Promoting Bacteria Be Affected by Waterlogging Predicted in the Wake of Climate Change: A Model Study. Appl. Soil Ecol. 2024, 198, 105379. [Google Scholar] [CrossRef]
- Kumar, P.; Sharma, A.; Bamrah, G.K.; Choi, K.Y. Novel Fungal Diversity: A New Prospect for the Commercial Production of Future Anti-Cancer Compounds. Fungal Biol. Rev. 2024, 48, 100355. [Google Scholar] [CrossRef]
- Anand, U.; Vaishnav, A.; Sharma, S.K.; Sahu, J.; Ahmad, S.; Sunita, K.; Suresh, S.; Dey, A.; Bontempi, E.; Singh, A.K.; et al. Current Advances and Research Prospects for Agricultural and Industrial Uses of Microbial Strains Available in World Collections. Sci. Total Environ. 2022, 842, 156641. [Google Scholar] [CrossRef] [PubMed]
- Najar, I.N.; Sharma, P.; Das, R.; Tamang, S.; Mondal, K.; Thakur, N.; Gandhi, S.G.; Kumar, V. From Waste Management to Circular Economy: Leveraging Thermophiles for Sustainable Growth and Global Resource Optimization. J. Environ. Manag. 2024, 360, 121136. [Google Scholar] [CrossRef] [PubMed]
- Ameen, F.; AlNadhari, S.; Al-Homaidan, A.A. Marine Microorganisms as an Untapped Source of Bioactive Compounds. Saudi J. Biol. Sci. 2021, 28, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Martínez, G.M.; Pire, C.; Martínez-Espinosa, R.M. Hypersaline Environments as Natural Sources of Microbes with Potential Applications in Biotechnology: The Case of Solar Evaporation Systems to Produce Salt in Alicante County (Spain). Curr. Res. Microb. Sci. 2022, 3, 100136. [Google Scholar] [CrossRef] [PubMed]
- Zampieri, R.M.; Adessi, A.; Caldara, F.; De Philippis, R.; Dalla Valle, L.; La Rocca, N. In Vivo Anti-Inflammatory and Antioxidant Effects of Microbial Polysaccharides Extracted from Euganean Therapeutic Muds. Int. J. Biol. Macromol. 2022, 209, 1710–1719. [Google Scholar] [CrossRef] [PubMed]
- Erginer, M.; Gökalsin, B.; Tornaci, S.; Sesal, C.; Toksoy Öner, E. Exploring the Potential of Halomonas Levan and Its Derivatives as Active Ingredients in Cosmeceutical and Skin Regenerating Formulations. Int. J. Biol. Macromol. 2023, 240, 124418. [Google Scholar] [CrossRef]
- Urbanek, A.K.; Strzelecki, M.C.; Mirończuk, A.M. The Potential of Cold-Adapted Microorganisms for Biodegradation of Bioplastics. Waste Manag. 2021, 119, 72–81. [Google Scholar] [CrossRef]
- Aytar Celik, P.; Barut, D.; Enuh, B.M.; Erdogan Gover, K.; Nural Yaman, B.; Burcin Mutlu, M.; Cabuk, A. A Novel Higher Polyhydroxybutyrate Producer Halomonas halmophila 18H with Unique Cell Factory Attributes. Bioresour. Technol. 2023, 372, 128669. [Google Scholar] [CrossRef]
- Parrilli, E.; Tedesco, P.; Fondi, M.; Tutino, M.L.; Lo Giudice, A.; de Pascale, D.; Fani, R. The Art of Adapting to Extreme Environments: The Model System Pseudoalteromonas. Phys. Life Rev. 2021, 36, 137–161. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, M.; Wang, J.; Xu, D.; Zhong, C. Engineering Microbial Systems for the Production and Functionalization of Biomaterials. Curr. Opin. Microbiol. 2022, 68, 102154. [Google Scholar] [CrossRef] [PubMed]
- Kourilova, X.; Pernicova, I.; Sedlar, K.; Musilova, J.; Sedlacek, P.; Kalina, M.; Koller, M.; Obruca, S. Production of Polyhydroxyalkanoates (PHA) by a Thermophilic Strain of Schlegelella thermodepolymerans from Xylose Rich Substrates. Bioresour. Technol. 2020, 315, 123885. [Google Scholar] [CrossRef] [PubMed]
- Mukti, I.J.; Sardari, R.R.R.; Kristjansdottir, T.; Hreggvidsson, G.O.; Karlsson, E.N. Medium Development and Production of Carotenoids and Exopolysaccharides by the Extremophile Rhodothermus marinus DSM16675 in Glucose-Based Defined Media. Microb. Cell Fact. 2022, 21, 220. [Google Scholar] [CrossRef]
- Michael, H.S.R.; Subiramanian, S.R.; Thyagarajan, D.; Mohammed, N.B.; Saravanakumar, V.K.; Govindaraj, M.; Maheswari, K.M.; Karthikeyan, N.; Ramesh Kumar, C. Melanin Biopolymers from Microbial World with Future Perspectives—A Review. Arch. Microbiol. 2023, 205, 306. [Google Scholar] [CrossRef]
- Mozejko-Ciesielska, J.; Moraczewski, K.; Czaplicki, S.; Singh, V. Production and Characterization of Polyhydroxyalkanoates by Halomonas alkaliantarctica Utilizing Dairy Waste as Feedstock. Sci. Rep. 2023, 13, 22289. [Google Scholar] [CrossRef] [PubMed]
- Dhagat, S.; Jujjavarapu, S.E. Biorefinery System for Production of Thermostable Exopolysaccharide by a Novel Thermophile Brevibacillus borstelensis MK878423 and Its Study on Impact of Glucose Utilization. Biomass Convers. Biorefin. 2023, 13, 7521–7531. [Google Scholar] [CrossRef]
- Genc, B.; Tunç, M.T.; Adiguzel, A. Characterization of Water-Soluble Extracellular Polysaccharide from Aeribacillus pallidus IM17. Indian J. Microbiol. 2023, 1, 1–10. [Google Scholar] [CrossRef]
- Khaswal, A.; Chaturvedi, N.; Mishra, S.K.; Kumar, P.R.; Paul, P.K. Current Status and Applications of Genus Geobacillus in the Production of Industrially Important Products—A Review. Folia Microbiol. 2022, 67, 389–404. [Google Scholar] [CrossRef]
- Nair, P.G.; Joseph, E.; Yadav, R.; Rajput, V.; Nisal, A.; Dharne, M.S. Production of Poly-Gamma-Glutamic Acid (γ-PGA) from Sucrose by an Osmotolerant Bacillus paralicheniformis NCIM 5769 and Genome-Based Predictive Biosynthetic Pathway. Biomass Convers Biorefin 2023, 1, 1–11. [Google Scholar] [CrossRef]
- Nampoothiri, M. Exopolysaccharides For Probiotic Fortification And Food Processing. In Technology Match Maker, Proceedings of SIMM3, Online, 26 October 2023; TechEx.in: Pune, India, 2023. [Google Scholar]
- Narayan, R.; Arcovito, A.; Dahiya, D.; Singh Nigam, P. Dextran of Diverse Molecular-Configurations Used as a Blood-Plasma Substitute, Drug-Delivery Vehicle and Food Additive Biosynthesized by Leuconostoc, Lactobacillus and Weissella. Appl. Sci. 2023, 13, 12526. [Google Scholar] [CrossRef]
- Basiri, S. Applications of Microbial Exopolysaccharides in the Food Industry. Avicenna J. Med. Biochem. 2021, 9, 107–120. [Google Scholar] [CrossRef]
- Gangoiti, J.; Corwin, S.F.; Lamothe, L.M.; Vafiadi, C.; Hamaker, B.R.; Dijkhuizen, L. Synthesis of Novel α-Glucans with Potential Health Benefits through Controlled Glucose Release in the Human Gastrointestinal Tract. Crit. Rev. Food Sci. Nutr. 2020, 60, 123–146. [Google Scholar] [CrossRef] [PubMed]
- Zikmanis, P.; Brants, K.; Kolesovs, S.; Semjonovs, P. Extracellular Polysaccharides Produced by Bacteria of the Leuconostoc Genus. World J. Microbiol. Biotechnol. 2020, 36, 161. [Google Scholar] [CrossRef]
- Chen, Z.; Ni, D.; Zhang, W.; Stressler, T.; Mu, W. Lactic Acid Bacteria-Derived α-Glucans: From Enzymatic Synthesis to Miscellaneous Applications. Biotechnol. Adv. 2021, 47, 107708. [Google Scholar] [CrossRef]
- Tabibloghmany, F.S.; Ehsandoost, E. An Overview of Healthy and Functionality of Exopolysaccharides Produced by Lactic Acid Bacteria in the Dairy Industry. Eur. J. Nutr. Food Saf. 2014, 4, 63–86. [Google Scholar] [CrossRef]
- Bianchet, R.T.; Vieira Cubas, A.L.; Machado, M.M.; Siegel Moecke, E.H. Applicability of Bacterial Cellulose in Cosmetics—Bibliometric Review. Biotechnol. Rep. 2020, 27, e00502. [Google Scholar] [CrossRef]
- Choi, S.M.; Rao, K.M.; Zo, S.M.; Shin, E.J.; Han, S.S. Bacterial Cellulose and Its Applications. Polymers 2022, 14, 1080. [Google Scholar] [CrossRef]
- Lahiri, D.; Nag, M.; Dutta, B.; Dey, A.; Sarkar, T.; Pati, S.; Edinur, H.A.; Kari, Z.A.; Noor, N.H.M.; Ray, R.R. Bacterial Cellulose: Production, Characterization, and Application as Antimicrobial Agent. Int. J. Mol. Sci. 2021, 22, 12984. [Google Scholar] [CrossRef]
- Anane, R.F.; Sun, H.; Zhao, L.; Wang, L.; Lin, C.; Mao, Z. Improved Curdlan Production with Discarded Bottom Parts of Asparagus Spear. Microb. Cell Fact. 2017, 16, 59. [Google Scholar] [CrossRef]
- Chaudhari, V.; Buttar, H.S.; Bagwe-Parab, S.; Tuli, H.S.; Vora, A.; Kaur, G. Therapeutic and Industrial Applications of Curdlan With Overview on Its Recent Patents. Front. Nutr. 2021, 8, 646988. [Google Scholar] [CrossRef] [PubMed]
- Jadaun, J.S.; Narnoliya, L.K.; Agarwal, N.; Singh, S.P. Catalytic Biosynthesis of Levan and Short-Chain Fructooligosaccharides from Sucrose-Containing Feedstocks by Employing the Levansucrase from Leuconostoc mesenteroides MTCC10508. Int. J. Biol. Macromol. 2019, 127, 486–495. [Google Scholar] [CrossRef]
- Gupta, N.; Jangid, A.K.; Pooja, D.; Kulhari, H. Inulin: A Novel and Stretchy Polysaccharide Tool for Biomedical and Nutritional Applications. Int. J. Biol. Macromol. 2019, 132, 852–863. [Google Scholar] [CrossRef] [PubMed]
- Teferra, T.F. Possible Actions of Inulin as Prebiotic Polysaccharide: A Review. Food Front. 2021, 2, 407–416. [Google Scholar] [CrossRef]
- Prajapati, V.D.; Jani, G.K.; Khanda, S.M. Pullulan: An Exopolysaccharide and Its Various Applications. Carbohydr. Polym. 2013, 95, 540–549. [Google Scholar] [CrossRef]
- Dailin, D.J.; Low, L.Z.M.I.; Malek, R.A.; Izyan, N.; Azelee, W.; Abd Manas, N.H.; Chin keat, H.; Sukmawati, D.; El Enshasy, H.; Arab, A. Pullulan, a Biopolymer with Potential Applications in Pharmaceutical and Cosmeceutical: A Review. Biosci. Res. 2019, 16, 2604–2616. [Google Scholar]
- Cruz-Santos, M.M.; Antunes, F.A.F.; Arruda, G.L.; Shibukawa, V.P.; Prado, C.A.; Ortiz-Silos, N.; Castro-Alonso, M.J.; Marcelino, P.R.F.; Santos, J.C. Production and Applications of Pullulan from Lignocellulosic Biomass: Challenges and Perspectives. Bioresour. Technol. 2023, 385, 129460. [Google Scholar] [CrossRef] [PubMed]
- Wani, S.M.; Mir, S.A.; Khanday, F.A.; Masoodi, F.A. Advances in Pullulan Production from Agro-Based Wastes by Aureobasidium Pullulans and Its Applications. Innov. Food Sci. Emerg. Technol. 2021, 74, 102846. [Google Scholar] [CrossRef]
- Dailin, D.; Low, L.Z.M.I.; Kumar, K.; Malek, R.; Natasya, K.; Keat, H.; Sukmawati, D.; Enshasy, H.E. Agro-Industrial Waste: A Potential Feedstock for Pullulan Production. Biosci. Biotechnol. Res. Asia 2019, 16, 229–250. [Google Scholar]
- de Souza, C.K.; Ghosh, T.; Lukhmana, N.; Tahiliani, S.; Priyadarshi, R.; Hoffmann, T.G.; Purohit, S.D.; Han, S.S. Pullulan as a Sustainable Biopolymer for Versatile Applications: A Review. Mater. Today Commun. 2023, 36, 106477. [Google Scholar] [CrossRef]
- Sheng, L.; Tong, Q.; Ma, M. Why Sucrose Is the Most Suitable Substrate for Pullulan Fermentation by Aureobasidium pullulans CGMCC1234? Enzyme Microb. Technol. 2016, 92, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Boddapati, S.; Rai, R.; Gummadi, S.N. Structural Analysis and Antioxidative Properties of Mutan (Water-Insoluble Glucan) and Carboxymethyl Mutan from Streptococcus Mutans. Process Biochem. 2020, 97, 130–139. [Google Scholar] [CrossRef]
- Costa Oliveira, B.E.; Ricomini Filho, A.P.; Burne, R.A.; Zeng, L. The Route of Sucrose Utilization by Streptococcus mutans Affects Intracellular Polysaccharide Metabolism. Front. Microbiol. 2021, 12, 636684. [Google Scholar] [CrossRef]
- Yu, S.H.; Kwak, S.H.; Nguyen, T.T.H.; Seo, Y.S.; Song, C.; Mok, I.K.; Kim, D. Decrease of insoluble glucan formation in Streptococcus mutans by co-cultivation with Enterococcus faecium T7 and glucanase addition. Biotechnol. Lett. 2018, 40, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.F.; Fornari, R.C.G.; Mazutti, M.A.; de Oliveira, D.; Padilha, F.F.; Cichoski, A.J.; Cansian, R.L.; Di Luccio, M.; Treichel, H. Production and Characterization of Xantham Gum by Xanthomonas campestris Using Cheese Whey as Sole Carbon Source. J. Food Eng. 2009, 90, 119–123. [Google Scholar] [CrossRef]
- Don, M.M.; Shoparwe, N.F. Kinetics of Hyaluronic Acid Production by Streptococcus zooepidemicus Considering the Effect of Glucose. Biochem. Eng. J. 2010, 49, 95–103. [Google Scholar] [CrossRef]
- Serra, M.; Casas, A.; Toubarro, D.; Barros, A.N.; Teixeira, J.A. Microbial Hyaluronic Acid Production: A Review. Molecules 2023, 28, 2084. [Google Scholar] [CrossRef] [PubMed]
- Gentry, B.; Cazón, P.; O’Brien, K. A Comprehensive Review of the Production, Beneficial Properties, and Applications of Kefiran, the Kefir Grain Exopolysaccharide. Int. Dairy J. 2023, 144, 105691. [Google Scholar] [CrossRef]
- Tan, K.X.; Chamundeswari, V.N.; Loo, S.C.J. Prospects of Kefiran as a Food-Derived Biopolymer for Agri-Food and Biomedical Applications. RSC Adv. 2020, 10, 25339–25351. [Google Scholar] [CrossRef]
- Viswanathan, H.S.; Rahman, S.S.A.; Venkatachalam, P.; Karuppiah, S. Production of Gellan Gum Using Milk Skin Residue (MSR)—A Tea Shop Waste: Statistical Optimization and Downstream Processing. Biomass Convers. Biorefin. 2023, 13, 189–203. [Google Scholar] [CrossRef]
- Yi, G.; Son, J.; Yoo, J.; Park, C.; Koo, H. Emulsan-Based Nanoparticles for in Vivo Drug Delivery to Tumors. Biochem. Biophys. Res. Commun. 2019, 508, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Amani, H.; Kariminezhad, H. Study on Emulsification of Crude Oil in Water Using Emulsan Biosurfactant for Pipeline Transportation. Pet. Sci. Technol. 2016, 34, 216–222. [Google Scholar] [CrossRef]
- Castro, G.R.; Panilaitis, B.; Kaplan, D.L. Emulsan, a Tailorable Biopolymer for Controlled Release. Bioresour. Technol. 2008, 99, 4566–4571. [Google Scholar] [CrossRef] [PubMed]
- Panilaitis, B.; Castro, G.R.; Solaiman, D.; Kaplan, D.L. Biosynthesis of Emulsan Biopolymers from Agro-Based Feedstocks. J. Appl. Microbiol. 2007, 102, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.M.; Singh, T.; Majhi, B.; Misra, S.; Chauhan, P.S. Biosurfactant Producing Plant Growth–Promoting Bacteria: Eco-Friendly Approaches for Charcoal Rot Management. In Macrophomina Phaseolina Ecobiology, Pathology and Management; Academic Press: Cambridge, MA, USA, 2023; pp. 313–321. [Google Scholar] [CrossRef]
- Rahman, S.S.A.; Pasupathi, S.; Karuppiah, S. Conventional Optimization and Characterization of Microbial Dextran Using Treated Sugarcane Molasses. Int. J. Biol. Macromol. 2022, 220, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Koirala, P.; Maina, N.H.; Nihtilä, H.; Katina, K.; Coda, R. Brewers’ Spent Grain as Substrate for Dextran Biosynthesis by Leuconostoc pseudomesenteroides DSM20193 and Weissella confusa A16. Microb. Cell Fact. 2021, 20, 23. [Google Scholar] [CrossRef]
- Tripathy, A.; Patel, M.K.; Chakraborty, S. Microbial Production of Dextran Using Pineapple Waste Extract: A Two-Step Statistical Optimization of Submerged Fermentation Conditions and Structural Characterization. Biotechnol. Bioprocess. Eng. 2024, 29, 387–403. [Google Scholar] [CrossRef]
- Rahman, S.S.A.; Venkatachalam, P.; Karuppiah, S. Cost-Effective Production of Dextran Using Saccharum Officinarum Juice (SOJ) as a Potential Feedstock: Downstream Processing and Characterization. Biomass Convers. Biorefin. 2022, 12, 4863–4875. [Google Scholar] [CrossRef]
- İspirli, H.; Özmen, D.; Yılmaz, M.T.; Sağdıç, O.; Dertli, E. Impact of Glucan Type Exopolysaccharide (EPS) Production on Technological Characteristics of Sourdough Bread. Food Control 2020, 107, 106812. [Google Scholar] [CrossRef]
- Meng, X.; Dobruchowska, J.M.; Gerwig, G.J.; Kamerling, J.P.; Dijkhuizen, L. Synthesis of Oligo- and Polysaccharides by Lactobacillus reuteri 121 Reuteransucrase at High Concentrations of Sucrose. Carbohydr. Res. 2015, 414, 85–92. [Google Scholar] [CrossRef]
- Amorim, L.F.A.; Li, L.; Gomes, A.P.; Fangueiro, R.; Gouveia, I.C. Sustainable Bacterial Cellulose Production by Low Cost Feedstock: Evaluation of Apple and Tea by-Products as Alternative Sources of Nutrients. Cellulose 2023, 30, 5589–5606. [Google Scholar] [CrossRef]
- Ghozali, M.; Meliana, Y.; Chalid, M. Synthesis and Characterization of Bacterial Cellulose by Acetobacter Xylinum Using Liquid Tapioca Waste. Mater. Today Proc. 2021, 44, 2131–2134. [Google Scholar] [CrossRef]
- Ramírez Tapias, Y.A.; Di Monte, M.V.; Peltzer, M.A.; Salvay, A.G. Bacterial Cellulose Films Production by Kombucha Symbiotic Community Cultured on Different Herbal Infusions. Food Chem. 2022, 372, 131346. [Google Scholar] [CrossRef] [PubMed]
- El-Bestawy, E.; Eltaweil, A.S.; Khallaf, N.S. Effective Production of Bacterial Cellulose Using Acidic Dairy Industry By-Products and Agro Wastes. Sustain. Chem. Pharm. 2023, 33, 101064. [Google Scholar] [CrossRef]
- Hasanin, M.S.; Abdelraof, M.; Hashem, A.H.; El Saied, H. Sustainable Bacterial Cellulose Production by Achromobacter Using Mango Peel Waste. Microb. Cell Fact. 2023, 22, 24. [Google Scholar] [CrossRef] [PubMed]
- Rofeal, M.; Abdelmalek, F.; Pietrasik, J.; Steinbüchel, A. Sustainable Curdlan Biosynthesis by Rahnella Variigena ICRI91 via Alkaline Hydrolysis of Musa Sapientum Peels and Its Edible, Active and Modified Hydrogel for Quercetin Controlled Release. Int. J. Biol. Macromol. 2023, 225, 416–429. [Google Scholar] [CrossRef]
- Mohsin, A.; Sun, J.; Khan, I.M.; Hang, H.; Tariq, M.; Tian, X.; Ahmed, W.; Niazi, S.; Zhuang, Y.; Chu, J.; et al. Sustainable Biosynthesis of Curdlan from Orange Waste by Using Alcaligenes Faecalis: A Systematically Modeled Approach. Carbohydr. Polym. 2019, 205, 626–635. [Google Scholar] [CrossRef]
- West, T.P. Production of the Polysaccharide Curdlan by Agrobacterium Species on Processing Coproducts and Plant Lignocellulosic Hydrolysates. Fermentation 2020, 6, 16. [Google Scholar] [CrossRef]
- de Siqueira, E.C.; Toksoy Öner, E. Co-Production of Levan with Other High-Value Bioproducts: A Review. Int. J. Biol. Macromol. 2023, 235, 123800. [Google Scholar] [CrossRef]
- Veerapandian, B.; Shanmugam, S.R.; Sivaraman, S.; Sriariyanun, M.; Karuppiah, S.; Venkatachalam, P. Production and Characterization of Microbial Levan Using Sugarcane (Saccharum spp.) Juice and Chicken Feather Peptone as a Low-Cost Alternate Medium. Heliyon 2023, 9, e17424. [Google Scholar] [CrossRef]
- Bhat, I.M.; Wani, S.M.; Mir, S.A.; Masoodi, F.A. Advances in Xanthan Gum Production, Modifications and Its Applications. Biocatal. Agric. Biotechnol. 2022, 42, 102328. [Google Scholar] [CrossRef]
- Hu, J.; Chen, Z.; Huang, X.; Yan, Z.; Li, Y.; Zhu, Y.; Zheng, Z.; Zhou, P. Hyaluronic Acid Applied as a Natural Flavor Enhancer and Its Mechanism Exploration. Food Biosci. 2023, 55, 102969. [Google Scholar] [CrossRef]
- Shukla, P.; Anand, S.; Srivastava, P.; Mishra, A. Hyaluronic Acid Production by Utilizing Agro-Industrial Waste Cane Molasses. 3 Biotech 2022, 12, 208. [Google Scholar] [CrossRef] [PubMed]
- Amado, I.R.; Vázquez, J.A.; Pastrana, L.; Teixeira, J.A. Microbial Production of Hyaluronic Acid from Agro-Industrial by-Products: Molasses and Corn Steep Liquor. Biochem. Eng. J. 2017, 117, 181–187. [Google Scholar] [CrossRef]
- Davidović, S.; Miljković, M.; Gordic, M.; Cabrera-Barjas, G.; Nesic, A.; Dimitrijević-Branković, S. Dextran-Based Edible Coatings to Prolong the Shelf Life of Blueberries. Polymers 2021, 13, 4252. [Google Scholar] [CrossRef]
- Cheirsilp, B.; Suksawang, S.; Yeesang, J.; Boonsawang, P. Co-Production of Functional Exopolysaccharides and Lactic Acid by Lactobacillus kefiranofaciens Originated from Fermented Milk, Kefir. J. Food Sci. Technol. 2018, 55, 331–340. [Google Scholar] [CrossRef]
- Raghunandan, K.; Kumar, A.; Kumar, S.; Permaul, K.; Singh, S. Production of Gellan Gum, an Exopolysaccharide, from Biodiesel-Derived Waste Glycerol by Sphingomonas spp. 3 Biotech 2018, 8, 71. [Google Scholar] [CrossRef]
- Wang, D.; Kim, H.; Lee, S.; Kim, D.H.; Joe, M.H. Improved Gellan Gum Production by a Newly-Isolated Sphingomonas Azotifigens GL-1 in a Cheese Whey and Molasses Based Medium. Process Biochem. 2020, 95, 269–278. [Google Scholar] [CrossRef]
- D’Almeida, A.P.; de Albuquerque, T.L.; Rocha, M.V.P. Recent Advances in Emulsan Production, Purification, and Application: Exploring Bioemulsifiers Unique Potentials. Int. J. Biol. Macromol. 2024, 133672. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, J.; Ji, H.J.; Kim, M.K.; Kim, K.W.; Choi, J.I.; Han, S.H.; Lim, S.; Seo, H.S.; Ahn, K.B. Deinococcus radiodurans Exopolysaccharide Inhibits Staphylococcus aureus Biofilm Formation. Front. Microbiol. 2021, 12, 712086. [Google Scholar] [CrossRef]
- Park, H.R.; Lee, J.H.; Ji, H.J.; Lim, S.; Ahn, K.B.; Seo, H.S. Radioprotection of Deinococcal Exopolysaccharide BRD125 by Regenerating Hematopoietic Stem Cells. Front. Oncol. 2022, 12, 898185. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jiang, X.; Mu, H.; Liang, X.; Guan, H. Structure and Protective Effect of Exopolysaccharide from P. agglomerans Strain KFS-9 against UV Radiation. Microbiol. Res. 2007, 162, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.W.; Ahn, G.; Lee, B.J.; Wijesinghe, W.A.J.P.; Kim, D.; Yang, H.; Kim, Y.M.; Park, S.J.; Jee, Y.; Jeon, Y.J. Radio-Protective Effect of Polysaccharides Isolated from Lactobacillus brevis-Fermented Ecklonia Cava. Int. J. Biol. Macromol. 2013, 52, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Hu, X.; Qin, L.; Li, H.; Yang, Y.; Zhang, X.; Lu, J.; Li, Y.; Bao, M. Characterization and Protective Effect against Ultraviolet Radiation of a Novel Exopolysaccharide from Bacillus marcorestinctum QDR3-1. Int. J. Biol. Macromol. 2022, 221, 1373–1383. [Google Scholar] [CrossRef] [PubMed]
- Taşkaya, A.; Güvensen, N.C.; Güler, C.; Şancı, E.; Yavaşoğlu, N.Ü.K. Exopolysaccharide from Rhodococcus pyridinivorans ZZ47 Strain: Evaluation of Biological Activity and Toxicity. J. Agric. Prod. 2023, 4, 63–71. [Google Scholar] [CrossRef]
- Li, Z.Q.; Huang, Y.L.; Zhang, J.; Mi, D.; Zhou, W.W. Ultrasound Stimulated Production of Exopolysaccharide with Anti-UV Radiation Activity by Increasing Cell Permeability of Paenibacillus polymyxa. Process Biochem. 2023, 126, 252–259. [Google Scholar] [CrossRef]
- Ali, P.; Shah, A.A.; Hasan, F.; Hertkorn, N.; Gonsior, M.; Sajjad, W.; Chen, F. A Glacier Bacterium Produces High Yield of Cryoprotective Exopolysaccharide. Front. Microbiol. 2020, 10, 466434. [Google Scholar] [CrossRef] [PubMed]
- Biswas, J. Diversity and Production of Extracellular Polysaccharide by Halophilic Microorganisms. Biodiversity Int. J. 2017, 1, 32–39. [Google Scholar] [CrossRef]
- Radchenkova, N.; Boyadzhieva, I.; Atanasova, N.; Poli, A.; Finore, I.; Di Donato, P.; Nicolaus, B.; Panchev, I.; Kuncheva, M.; Kambourova, M. Extracellular Polymer Substance Synthesized by a Halophilic Bacterium Chromohalobacter Canadensis 28. Appl. Microbiol. Biotechnol. 2018, 102, 4937–4949. [Google Scholar] [CrossRef]
- Joulak, I.; Finore, I.; Poli, A.; Abid, Y.; Bkhairia, I.; Nicolaus, B.; Di Donato, P.; Dal Poggetto, G.; Gharsallaoui, A.; Attia, H.; et al. Hetero-Exopolysaccharide from the Extremely Halophilic Halomonas smyrnensis K2: Production, Characterization and Functional Properties in Vitro. 3 Biotech 2020, 10, 395. [Google Scholar] [CrossRef]
- Jain, R.M.; Mody, K.; Mishra, A.; Jha, B. Isolation and Structural Characterization of Biosurfactant Produced by an Alkaliphilic Bacterium Cronobacter Sakazakii Isolated from Oil Contaminated Wastewater. Carbohydr. Polym. 2012, 87, 2320–2326. [Google Scholar] [CrossRef]
- Arayes, M.A.; Mabrouk, M.E.M.; Sabry, S.A.; Abdella, B. Exopolysaccharide Production from Alkalibacillus sp. W3: Statistical Optimization and Biological Activity. Biologia 2023, 78, 229–240. [Google Scholar] [CrossRef]
- Huang-Lin, E.; Sánchez-León, E.; Amils, R.; Abrusci, C. Potential Applications of an Exopolysaccharide Produced by Bacillus xiamenensis RT6 Isolated from an Acidic Environment. Polymers 2022, 14, 3918. [Google Scholar] [CrossRef] [PubMed]
- Moradi, M.; Song, Z.; Xiao, T. Exopolysaccharide Produced by Vibrio neocaledonicus sp. as a Green Corrosion Inhibitor: Production and Structural Characterization. J. Mater. Sci. Technol. 2018, 34, 2447–2457. [Google Scholar] [CrossRef]
- Hu, X.; Li, D.; Qiao, Y.; Wang, X.; Zhang, Q.; Zhao, W.; Huang, L. Purification, Characterization and Anticancer Activities of Exopolysaccharide Produced by Rhodococcus erythropolis HX-2. Int. J. Biol. Macromol. 2020, 145, 646–654. [Google Scholar] [CrossRef]
- Lin, S.M.; Baek, C.Y.; Jung, J.H.; Kim, W.S.; Song, H.Y.; Lee, J.H.; Ji, H.J.; Zhi, Y.; Kang, B.S.; Bahn, Y.S.; et al. Antioxidant Activities of an Exopolysaccharide (DeinoPol) Produced by the Extreme Radiation-Resistant Bacterium Deinococcus radiodurans. Sci. Rep. 2020, 10, 55. [Google Scholar] [CrossRef]
- Liang, X. Structural Characterization and Bioactivity of Exopolysaccharide Synthesized by Geobacillus sp. TS3-9 Isolated from Radioactive Radon Hot Spring. Adv. Biotechnol. Microbiol. 2017, 4, 555634. [Google Scholar] [CrossRef]
- Zhao, S.; Cao, F.; Zhang, H.; Zhang, L.; Zhang, F.; Liang, X. Structural Characterization and Biosorption of Exopolysaccharides from Anoxybacillus sp. R4-33 Isolated from Radioactive Radon Hot Spring. Appl. Biochem. Biotechnol. 2014, 172, 2732–2746. [Google Scholar] [CrossRef]
- Bibi, A.; Xiong, Y.; Rajoka, M.S.R.; Mehwish, H.M.; Radicetti, E.; Umair, M.; Shoukat, M.; Khan, M.K.I.; Aadil, R.M. Recent Advances in the Production of Exopolysaccharide (EPS) from Lactobacillus spp. and Its Application in the Food Industry: A Review. Sustainability 2021, 13, 12429. [Google Scholar] [CrossRef]
- Xiong, J.; Liu, D.M.; Huang, Y.Y. Exopolysaccharides from Lactiplantibacillus plantarum: Isolation, Purification, Structure–Function Relationship, and Application. Eur. Food Res. Technol. 2023, 249, 1431–1448. [Google Scholar] [CrossRef]




| No. | Article Title | Source Title | Publication Year | Impact Factor | Reference |
|---|---|---|---|---|---|
| 1 | Exremophiles as a green source of new exopolysaccharides with ecological importance and multifunctional applications | Polymer-Plastics Technology and Materials | 2024 | 2.6 | [40] |
| 2 | Exploring extremophiles from Bulgaria: Biodiversity, biopolymer synthesis, functional properties, applications | Polymers | 2024 | 4.7 | [35] |
| 3 | Extremophiles and their expanding biotechnological applications | Archives of Microbiology | 2024 | 2.3 | [10] |
| 4 | Exopolysaccharides from marine microbes: Source, structure and application | Marine Drugs | 2022 | 4.9 | [42] |
| 5 | Extremophilic exopolysaccharides: Biotechnologies and wastewater remediation | Frontiers in Microbiology | 2021 | 4.0 | [43] |
| 6 | Hydrating capabilities of the biopolymers produced by the marine thermophilic Bacillus horneckiae sBP3 as evaluated by aTR-fTIR spectroscopy | Materials | 2022 | 3.1 | [44] |
| 7 | Recent challenges and trends of polyhydroxyalkanoate production by extremophilic bacteria using renewable feedstocks | Polymers | 2023 | 4.7 | [17] |
| 8 | Biopolymer production by halotolerant bacteria isolated from Caatinga biome | Brazilian Journal of Microbiology | 2021 | 2.1 | [45] |
| 9 | Characterisation and bioactivities of an exopolysaccharide from an Antarctic bacterium Shewanella frigidimarina W32-2 | Aquaculture | 2021 | 3.9 | [46] |
| 10 | Nature and bioprospecting of haloalkaliphilics: A review | World Journal of Microbiology and Biotechnology | 2020 | 4.0 | [47] |
| 11 | Arsenic adsorption and toxicity reduction of an exopolysaccharide produced by Bacillus licheniformis B3-15 of shallow hydrothermal vent origin | Journal of Marine Science and Engineering | 2023 | 2.7 | [48] |
| 12 | Exopolysaccharides production by cultivating a bacterial isolate from the hypersaline environment of Salar de Uyuni (Bolivia) in pretreatment liquids of steam-exploded quinoa stalks and enzymatic hydrolysates of Curupau sawdust | Fermentation-Basel | 2021 | 3.3 | [49] |
| 13 | Exopolysaccharide and biopolymer-derived films as tools for transdermal drug delivery | Journal of Controlled Release | 2021 | 10.5 | [41] |
| 14 | Characterization of Chilean hot spring-origin Staphylococcus sp. BSP3 produced exopolysaccharide as biological additive | Natural Products and Bioprospecting | 2024 | 4.8 | [50] |
| 15 | A review on production of polyhydroxyalkanoate (PHA) biopolyesters by thermophilic microbes using waste feedstocks | Bioresource Technology | 2021 | 9.7 | [51] |
| 16 | Bacillales: From taxonomy to biotechnological and industrial perspectives | Microorganisms | 2022 | 4.1 | [52] |
| 17 | Exopolysaccharide production using pinewood hydrolysate as a substrate for psychrotrophic bacterium isolated from Svalbard glacier soil | Biomass Conversion and Biorefinery | 2023 | 3.5 | [53] |
| 18 | Halomonas alkaliantarctica as a platform for poly(3-hydroxybutyrate-co-3-hydroxyvalerate) production from biodiesel-derived glycerol | Environmental Microbiology Reports | 2024 | 3.6 | [54] |
| 19 | UV and chemically induced Halomonas smyrnensis mutants for enhanced levan productivity | Journal of Biotechnology | 2022 | 4.1 | [55] |
| 20 | Cheese whey mother liquor as dairy waste with potential value for polyhydroxyalkanoate production by extremophilic Paracoccus homiensis | Sustainable Materials and Technologies | 2022 | 8.6 | [56] |
| 21 | Production of poly (3-hydroxybutyrate) and extracellular polymeric substances from glycerol by the acidophile Acidiphilium cryptum | Extremophiles | 2023 | 2.6 | [57] |
| 22 | Exopolysaccharide production by salt-tolerant bacteria: Recent advances, current challenges, and future prospects | International Journal of Biological Macromolecules | 2024 | 7.7 | [58] |
| 23 | Heavy metal tolerance, and metal biosorption by exopolysaccharides produced by bacterial strains isolated from marine hydrothermal vents | Chemosphere | 2024 | 8.1 | [59] |
| 24 | Polyhydroxyalkanoate biosynthesis and optimisation of thermophilic Geobacillus stearothermophilus strain K4E3_SPR_NPP | Extremophiles | 2023 | 2.6 | [60] |
| 25 | Trends in PHA production by microbially diverse and functionally distinct communities | Microbial Ecology | 2023 | 3.3 | [18] |
| 26 | Microbial polyhydroxyalkanoates (PHAs): A review on biosynthesis, properties, fermentation strategies and its prospective applications for sustainable future | Journal of Polymers and the Environment | 2022 | 4.7 | [19] |
| 27 | Technological advances in the production of polyhydroxyalkanoate biopolymers | Current Sustainable/Renewable Energy Reports | 2020 | 3.1 | [11] |
| 28 | Thermal properties of an exopolysaccharide produced by a marine thermotolerant Bacillus licheniformis by ATR-FTIR spectroscopy | International Journal of Biological Macromolecules | 2020 | 7.7 | [61] |
| 29 | Biological properties of exopolysaccharides produced by Bacillus spp. | Microbiological Research | 2023 | 6.1 | [62] |
| 30 | Potential functions and applications of diverse microbial exopolysaccharides in marine environments | Journal of Genetic Engineering and Biotechnology | 2022 | 3.6 | [63] |
| 31 | Synthetic biology of extremophiles: a new wave of biomanufacturing | Trends in Biotechnology | 2023 | 14.3 | [64] |
| 32 | Bacterial exopolysaccharides as emerging bioactive macromolecules: from fundamentals to applications | Research in Microbiology | 2023 | 2.5 | [65] |
| 33 | Polyhydroxyalkanoates from extremophiles: A review | Bioresource Technology | 2021 | 9.7 | [20] |
| 34 | Physicochemical characterization and emulsifying properties of a novel exopolysaccharide produced by haloarchaeon Haloferax mucosum | International Journal of Biological Macromolecules | 2020 | 7.7 | [66] |
| 35 | Production, characterisation, and application of exopolysaccharide extracted from a glacier bacterium Mucilaginibacter sp. ERMR7:07 | Process Biochemistry | 2022 | 3.7 | [67] |
| 36 | Microbial exopolysaccharides: Synthesis pathways, types and their commercial applications | International Journal of Biological Macromolecules | 2020 | 7.7 | [68] |
| 37 | Two new exopolysaccharides from a thermophilic bacterium Geobacillus sp. WSUCF1: Characterization and bioactivities | New Biotechnology | 2021 | 4.5 | [69] |
| 38 | Structural characterization and functional properties of novel exopolysaccharide from the extremely halotolerant Halomonas elongata S6 | International Journal of Biological Macromolecules | 2020 | 7.7 | [70] |
| 39 | AepG is a glucuronosyltransferase involved in acidic exopolysaccharide synthesis and contributes to environmental adaptation of Haloarcula hispanica | Journal of Biological Chemistry | 2023 | 4.0 | [12] |
| 40 | Exopolysaccharide produced by potential probiotic Enterococcus faecium MS79: Characterization, bioactivities and rheological properties influenced by salt and pH | LWT | 2020 | 6.0 | [71] |
| 41 | Biopolymer production from biomass produced by Nordic microalgae grown in wastewater | Bioresource Technology | 2023 | 9.7 | [21] |
| 42 | Progresses and future prospects in biodegradation of marine biopolymers and emerging biopolymer-based materials for sustainable marine ecosystems | Green Chemistry | 2022 | 9.3 | [72] |
| 43 | A review of extracellular polysaccharides from extreme niches: An emerging natural source for the biotechnology. From the adverse to diverse! | International Journal of Biological Macromolecules | 2021 | 7.7 | [38] |
| 44 | Perspectives on the microorganism of extreme environments and their applications | Current Research in Microbial Sciences | 2022 | 4.8 | [73] |
| 45 | Current state of the art biotechnological strategies for conversion of watermelon wastes residues to biopolymers production: A review | Chemosphere | 2022 | 8.1 | [74] |
| 46 | Biopolymer poly-hydroxyalkanoate (PHA) production from apple industrial waste residues: A review | Chemosphere | 2021 | 8.1 | [75] |
| 47 | Comprehensive review on recent trends and perspectives of natural exo-polysaccharides: Pioneering nano-biotechnological tools | International Journal of Biological Macromolecules | 2024 | 7.7 | [76] |
| 48 | The metabolic pathways of polyhydroxyalkanoates and exopolysaccharides synthesized by Haloferax mediterranei in response to elevated salinity | Journal of Proteomics | 2021 | 2.8 | [22] |
| 49 | Metal(loid)-resistant bacterial consortia with antimycotic properties increase tolerance of Chenopodium quinoa Wild. to metal(loid) stress | Rhizosphere | 2022 | 3.4 | [77] |
| 50 | Polyhydroxyalkanoates synthesis by halophiles and thermophiles: towards sustainable production of microbial bioplastics | Biotechnology Advances | 2022 | 12.1 | [78] |
| 51 | Structural characteristics and immune-enhancing activity of an extracellular polysaccharide produced by marine Halomonas sp. 2E1 | International Journal of Biological Macromolecules | 2021 | 7.7 | [79] |
| 52 | The radiophiles of Deinococcaceae family: Resourceful microbes for innovative biotechnological applications | Current Research in Microbial Sciences | 2022 | 4.8 | [80] |
| 53 | Archaea: current and potential biotechnological applications | Research in Microbiology | 2023 | 2.5 | [81] |
| 54 | The philosophy of extreme biomimetics | Sustainable Materials and Technologies | 2022 | 8.6 | [82] |
| 55 | Anti-allergic function of the cell wall (DeinoWall) from Deinococcus radiodurans | Molecular Immunology | 2022 | 3.2 | [83] |
| 56 | Untapped talents: insight into the ecological significance of methanotrophs and its prospects | Science of The Total Environment | 2023 | 8.2 | [84] |
| 57 | Genetic and process engineering for polyhydroxyalkanoate production from pre- and post-consumer food waste | Current Opinion in Biotechnology | 2024 | 7.1 | [23] |
| 58 | Application of microbial resources in biorefineries: Current trend and future prospects | Heliyon | 2024 | 3.4 | [85] |
| 59 | Cyanobacteria and microalgae in supporting human habitation on Mars | Biotechnology Advances | 2022 | 12.1 | [86] |
| 60 | Advancements in microbial production of polyhydroxyalkanoates (PHAs) from wastes for sustainable active food packaging: An eclectic review | Biocatalysis and Agricultural Biotechnology | 2024 | 3.4 | [24] |
| 61 | Recovery of value-added products from biowaste: A review | Bioresource Technology | 2022 | 9.7 | [87] |
| 62 | Engineering biosynthesis of polyhydroxyalkanoates (PHAs) for diversity and cost reduction | Metabolic Engineering | 2020 | 6.8 | [25] |
| 63 | Kinetics of two-step bioleaching of Ni and Co from iron rich-laterite using supernatant metabolites produced by Salinivibrio kushneri as halophilic bacterium | Hydrometallurgy | 2020 | 4.8 | [88] |
| 64 | Polyhydroxyalkanoates synthesis using acidogenic fermentative effluents | International Journal of Biological Macromolecules | 2021 | 7.7 | [26] |
| 65 | Waste to bioplastics: How close are we to sustainable polyhydroxyalkanoates production? | Waste Management | 2021 | 7.1 | [27] |
| 66 | Inulin from halophilic archaeon Haloarcula: Production, chemical characterization, biological, and technological properties | Carbohydrate Polymers | 2023 | 10.7 | [89] |
| 67 | Extraction, characterization, antioxidant activity and rheological behavior of a polysaccharide produced by the extremely salt tolerant Bacillus subtilis LR-1 | LWT | 2022 | 6.0 | [90] |
| 68 | Polyhydroxyalkanoate bio-production and its rise as biomaterial of the future | Journal of Biotechnology | 2022 | 4.1 | [28] |
| 69 | Will the beneficial properties of plant-growth promoting bacteria be affected by waterlogging predicted in the wake of climate change: A model study | Applied Soil Ecology | 2024 | 4.8 | [91] |
| 70 | Novel fungal diversity: A new prospect for the commercial production of future anti-cancer compounds | Fungal Biology Reviews | 2024 | 5.7 | [92] |
| 71 | Polyhydroxyalkanoates (PHAs): From production to nanoarchitecture | International Journal of Biological Macromolecules | 2020 | 7.7 | [29] |
| 72 | Current advances and research prospects for agricultural and industrial uses of microbial strains available in world collections | Science of The Total Environment | 2022 | 8.2 | [93] |
| 73 | From waste management to circular economy: Leveraging thermophiles for sustainable growth and global resource optimization | Journal of Environmental Management | 2024 | 8.0 | [94] |
| 74 | Valorization of organic wastes using bioreactors for polyhydroxyalkanoate production: Recent advancement, sustainable approaches, challenges, and future perspectives | International Journal of Biological Macromolecules | 2023 | 7.7 | [30] |
| 75 | Marine microorganisms as an untapped source of bioactive compounds | Saudi Journal of Biological Sciences | 2021 | 3.1 | [95] |
| 76 | Hypersaline environments as natural sources of microbes with potential applications in biotechnology: The case of solar evaporation systems to produce salt in Alicante County (Spain). | Current Research in Microbial Sciences | 2022 | 4.8 | [96] |
| 77 | The production, recovery, and valorization of polyhydroxybutyrate (PHB) based on circular bioeconomy | Biotechnology Advances | 2024 | 12.1 | [31] |
| 78 | In vivo anti-inflammatory and antioxidant effects of microbial polysaccharides extracted from Euganean therapeutic muds | International Journal of Biological Macromolecules | 2022 | 7.7 | [97] |
| 79 | Biological and chemical characterization of new isolated halophilic microorganisms from saltern ponds of Trapani, Sicily | Algal Research | 2021 | 4.6 | [13] |
| 80 | Exploring the potential of Halomonas levan and its derivatives as active ingredients in cosmeceutical and skin regenerating formulations | International Journal of Biological Macromolecules | 2023 | 7.7 | [98] |
| 81 | The potential of cold-adapted microorganisms for biodegradation of bioplastics | Waste Management | 2021 | 7.1 | [99] |
| 82 | A novel higher polyhydroxybutyrate producer Halomonas halmophila 18H with unique cell factory attributes | Bioresource Technology | 2023 | 9.7 | [100] |
| 83 | The art of adapting to environments: The model system Pseudoalteromonas | Physics of Life Reviews | 2021 | 13.7 | [101] |
| 84 | Engineering microbial systems for the production and functionalization of biomaterials | Current Opinion in Microbiology | 2022 | 5.9 | [102] |
| 85 | Production of polyhydroxyalkanoates (PHAs) by a thermophilic strain of Schlegelella thermodepolymerans from xylose rich substrates | Bioresource Technology | 2020 | 9.7 | [103] |
| 86 | Biopolymers production from wastes and wastewaters by mixed microbial cultures: Strategies for microbial selection | Waste and Biomass Valorization | 2020 | 2.6 | [32] |
| 87 | Medium development and production of carotenoids and exopolysaccharides by the extremophile Rhodothermus marinus DSM16675 in glucose-based defined media | Microbial Cell Factories | 2022 | 4.3 | [104] |
| 88 | Melanin biopolymers from microbial world with future perspectives—a review | Archives of Microbiology | 2022 | 2.3 | [105] |
| 89 | Production and characterization of polyhydroxyalkanoates by Halomonas alkaliantarctica utilizing dairy waste as feedstock | Scientific Reports | 2023 | 3.8 | [106] |
| 90 | Biorefinery system for production of thermostable exopolysaccharide by a novel thermophile Brevibacillus borstelensis MK878423 and its study on impact of glucose utilization | Biomass Conversion and Biorefinery | 2023 | 3.5 | [107] |
| 91 | Characterization of water-soluble extracellular polysaccharide from Aeribacillus pallidus IM17 | Indian Journal of Microbiology | 2023 | 2.1 | [108] |
| 92 | Current status and applications of genus Geobacillus in the production of industrially important products—a review | Folia Microbiologica | 2022 | 2.4 | [109] |
| 93 | Production of poly-gamma-glutamic acid (γ-PGA) from sucrose by an osmotolerant Bacillus paralicheniformis NCIM 5769 and genome-based predictive biosynthetic pathway | Biomass Conversion and Biorefinery | 2023 | 3.5 | [110] |
| EPS | Producer Organisms | Substrates | Monomer Composition | Molecular Weight (Da) | Functional Properties | Applications | Refs. |
|---|---|---|---|---|---|---|---|
| Dextran | Leuconostoc mesenteriodes, Leuconostoc dextranicum, Lactobacillus hilgardii, Streptococcus mutans | Sucrose | Glucose | 3.0 × 103–3.0 × 106 | Thickening, viscosifying, emulsifying, and stabilizing properties; non-toxic, biodegradable, and biocompatible; anticancer, antibacterial, and antifungal capabilities; high aqueous solubility. | It is used to improve the softness, moisture retention, and texture of bakery products and confectionery, prevents crystallization, and stabilizes and thickens ice creams and jams. It has potential for biodegradable edible coatings and films for perishable food. It is used as a carrier for drug delivery, blood plasma volume expander, and surgical sealan. It enhances skin health and appearance by acting as moisturizers and thickeners in cosmetics. It is also used as a chromatographic media. | [2,3,14,15,16,36,112] |
| Alternan | Leuconostoc mesenteroides, Leuconostoc citreum, and Streptococcus salivarius | Sucrose | Glucose | l06–l07 | Solubility in water, low viscosity, and strong resistance to enzymatic hydrolysis. | It is used as a prebiotic ingredient, low caloric bulking agent, and binder for food. Lipid-substitute texturizer in cosmetic preparations. It also promote the growth, migration, and differentiation of human mesenchymal stem cells (MSCs). It enhances the strength of dry paper when added during the papermaking process. It can be also used as inks and glues. | [4,113,114,115,116] |
| Reuteran | Limosilactobacillus reuteri | Sucrose | Glucose | 2.8 × 107 | Water-soluble and non Newtonian behavior in aqueous solution. | It is used as a dietary fiber and prebiotic additive to enhance satiety and gut health. It enhances the quality and texture of wheat bread and gluten-free sourdough. It induces or enhances satiety in humans and animals. | [113,116,117] |
| Bacterial Cellulose | Gluconacetobacter, Agrobacterium, Rhizobium, Salmonella, and Sarcina | Sucrose, fructose, molasses, arabitol, and mannitol | Glucose | 3.0 × 105– 2.0 × 106 | High purity, surface area, yield, polymerization degree, crystallinity, tensile strength, water-holding capacity, lightweight nature, transparency, flexibility, biocompatibility, biodegradability, renewability, non-toxicity, and non-immunogenicity. | It is used as a promising biomaterial for wound dressings, tissue engineering, and drug delivery. It is applied in artificial blood vessels and implants. It is used in cosmeceutical face masks to act as a carrier for bioactive agents and enhance skin hydration. It acts as an emulsion stabilizer in cosmetic formulations. It is used as a thickener, gelling agent, and a natural non-digestible fiber. It is used in food packaging. It is used for pollutant detection and waste decomposition. It is also applied in nanoelectronics, textile fashion, biocatalytic technologies, and paper production. | [5,16,36,113,118,119,120] |
| Curdlan | Agrobacterium sp., Rhizobium sp., Bacillus sp., and Cellulomonas sp. | Glucose and sucrose | Glucose | 5.0 × 104–2.0 × 106 | Soluble in alkaline solutions but insoluble in water (can be solubilized in water with salt), has antitumoral properties immunomodulatory capability, thermostability, thermogelation properties, water-holding capacity, and potential antioxidant capability. | It is used for drug encapsulation, modulation of immune responses, and scaffold or wound dressing production. It promotes mesenchymal cell adhesion and enhances bone growth. It is used as drug delivery vehicles for sustained release. It is used as a thickener, stabilizer, texturizer, binder, and dietary fiber. It enhances the creaminess, stability, and texture of food products. It is used as an edible and biodegradable food packaging film. It is also used to remove heavy metals when combined with activated carbon adsorbents. | [3,4,14,15,36,113,121,122] |
| Levan | Acetobacter, Bacillus, Brenneria, Geobacillus, Halomonas, Lactobacillus, Zymomonas, and Saccharomyces | Sucrose | Fructose | 104–108 | Soluble in both water and oil, while remaining insoluble in most organic solvents; Antitumor, antioxidant, antibacterial, anti-inflammatory, anti-hyperlipidemic, radioprotective, immunomodulatory, and prebiotic activities; heat stability, high adhesive strength, and film-forming ability. | It serves as a thickener, emulsifier, stabilizer, gelling agent, film-former, encapsulant, cryoprotector, osmoregulator, and flavor carrier. It is used as a prebiotic food supplement and for food packaging. It is used as a plasma volume expander, agent for combating obesity, antitumor agent, and hyperglycemic inhibitor. It is used as an adhesive for wood bonding and a biological binder for producing wood biocomposite materials. It is suitable for cosmetic formulations. It is also used as adsorbent for heavy metals. | [3,5,15,36,113,123] |
| Inulin | Streptococcus mutans, Limosilactobacillus reuteri, Leuconostoc citreum, and Lactobacillus johnsonii | Fructose and glucose | Fructose | 5.0 × 102– 1.3 × 104 | Soluble in water; enhances water viscosity; enhances calcium absorption; Inhibits biofilm formation; antioxidant activity. | It is used to reduce food calories and replace fats and sugars. It has a prebiotic function. It acts as a stabilizing agent and cryoprotectant foodstuffs. It is used to reduce cancer risk (especially colon cancer). It is used as a carrier for drug delivery in colon diseases. It reduces irritable bowel disease risk and provides constipation relief. | [4,89,113,124,125] |
| Pullulan | Aureobasidium pullulans, Cytaria spp., Teloschistes flavicans, Rhodotorula bacarum, and Cryphonectria parasitica | Glucose, sucrose, mannose, galactose, and fructose | Glucose | 5.0 × 103–9.0 × 106 | Highly soluble in water; thermal stable; adhesive properties; robust mechanical strength; resistance to pH changes; non-mutagenic, non-carcinogenic, and non-immunogenic; ability to form strong, flexible films and fibers; biodegradable; biocompatible; odorless and tasteless; act as a thickening and stabilizing; prebiotic and antioxidant properties. | It is used as a carrier for gene and drug delivery, and in tissue engineering, biomedical imaging, plasma expanders, nasal vaccine adjuvants, vaccine formulations, skincare products and cosmetic formulations. It is used as a dietary fiber in low-calorie foods and in packaging films, edible films, food coating, carrier for flavors and antimicrobial compounds, viscosity stabilizer and thickening agent, and prebiotics. It is used in waste remediation, analytical techniques, and energy and electronics sectors. | [3,15,36,113,126,127,128,129,130,131,132] |
| Mutan | Streptococcus mutans and Streptococcus sobrinus | Sucrose | Glucose | 5.65 × 103 | Sticky, colorless, and water-insoluble. | It is used to inhibit dental plaque and dental caries. It is also used to adsorb heavy metals. | [4,118,133,134,135] |
| Xanthan gum | Xanthomonas campestris | Glucose and sucrose | Glucose, glucuronic acid, and pyruvic acid | 2.0 × 106–2.0 × 107 | Soluble in water; high viscosity; non-toxic; resistant to environmental factors; pseudoplastic; biodegradable; cost-effective; antioxidant effects; antimicrobial and antitumoral properties; viscosifying and stabilizing properties. | It is used as a carrier and scaffold for drug delivery. It is used in tissue engineering, in wound dressings, in intra-articular injections, and as an adjuvant in the immune system. It is used in the production of cosmetics, detergents, insecticides, and eco-friendly absorbents. It acts as a thickener, stabilizer, emulsifier, foam stabilizing agent, and crystal formation inhibitor in various food products. It is also used in oil recovery and viscosity control in drilling, and in 3D printing technology. | [3,5,15,16,36,113,136] |
| Alginate | Pseudomonas aeruginosa and Azotobacter vinelandii | Glucose and sucrose | Guluronic acid and mannuronic acid | 0.5 × 106–1.5 × 106 | Soluble in water; biocompatible and biodegradable; ability to form hydrogels; water-holding capacity; viscosity regulator and stabilizing properties. | It is used for encapsulating drugs, growth factors, and cells. It is used to produce scaffolds for tissue engineering. It is used as fillers and carriers for osteoinductive factors in bone engineering. It is used as a thickening agent, gelling agent, and excipient in skin and cosmetic formulations. It serves as a regulator of viscosity, stabilizer, and material for packaging. | [4,14,15,16,113] |
| Hyaluronic Acid | Streptococcus equi, Streptococcus equisimilis, Streptococcus pyogenes, and Streptococcus thermophilus | Glucose and sucrose | Glucuronic acid and N-acetylglucosamine | 1.0 × 106–2.0 × 106 | High water retention capacity; biocompatible, biodegradable, and non-immunogenic; moisture-absorption properties; viscoelastic; enhance cell adhesion and proliferation; ability to form a non-Newtonian solutions with gel-like properties; anti-inflammatory properties; Compatible with biological systems; Angiogenesis modulation. | It is used as a moisturizing agent in skincare and cosmetic products. It is used in the formulation of pharmaceuticals and artificial tear solutions. It is used in cancer treatment. It triggers angiogenesis, wound healing, and cell motility. It is used in tissue engineering scaffolds. It is used in the treatment of osteoarthritis. It is used as a replacement for natural eye fluid during ophthalmic surgeries and joint lubricant to replace synovial fluid. It is used to prevent adhesions in abdominal surgeries. It is used as a surface coating in medical applications. It is used as a food ingredient. | [3,15,16,36,137,138] |
| Kefiran | Lactobacillus kefiri, Lactobacillus parakefir, Lactobacillus kefiranofaciens, Lactobacillus kefirgranum, and Lactobacillus delbrueckii subsp. bulgaricus | Lactose, molasses, and whey lactose | Glucose and galactose | 5.0 × 104–1.5 × 107 | Water soluble; semi-crystalline nature; resistance to hydrolysis; gels and films formation ability; water vapor barrier properties; antibacterial and antioxidant properties; antitumor and mucosal adjuvant properties; biodegradable and biocompatible. | It is used for tissue engineering, controlled drug and probiotic delivery, and wound dressings. It is also used as edible biofilms and biodegradable food packaging. It is used for food stabilization and in thickening agents in fermented dairy products. | [2,4,16,113,139,140] |
| Gellan | Sphingomonas paucimobilis | Fructose, sucrose, glucose, and lactose | Glucose and rhamnose | 5.0 × 103–2.0 × 106 | Insoluble in cold water; resistance to enzymatic degradation; thermo-reversible gel formation; reduced susceptibility to pH changes; low mechanical resistance and high polyelectrolyte content. | It is used as a stabilizer, binder, thickener, and gelling agent in food products, and enhances texture and flavor release in desserts, jams, and ice creams. It acts as a carrier for vitamin C and encapsulates heat-sensitive ingredients. It is used for controlled drug release and tablet disintegration. It supports cell adhesion, proliferation, and differentiation. It is used in paper coatings, and serves as a water flocculent. It is helpful in the bioremediation of polluted soils and aquifers. | [14,15,16,36,141] |
| Emulsan | Acinetobacter venetianus and Acinetobacter calcoaceticus | Sucrose | Glucose, α- and β-hydroxydodecanoic acid | 1.0 × 106 | Amphiphilic properties; chemical and biological versatility; bioemulsifying properties. | It is used as stable hydrocarbon-in-water emulsions for industrial and environmental purposes. It is used for drug delivery. It is used as a bioemulsifier in food processing. It is also used in personal care products like cleansing creams, lotions, shampoo, soap, and toothpaste. It acts as a vaccine adjuvant. | [14,36,142,143,144,145,146] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mouro, C.; Gomes, A.P.; Gouveia, I.C. Microbial Exopolysaccharides: Structure, Diversity, Applications, and Future Frontiers in Sustainable Functional Materials. Polysaccharides 2024, 5, 241-287. https://doi.org/10.3390/polysaccharides5030018
Mouro C, Gomes AP, Gouveia IC. Microbial Exopolysaccharides: Structure, Diversity, Applications, and Future Frontiers in Sustainable Functional Materials. Polysaccharides. 2024; 5(3):241-287. https://doi.org/10.3390/polysaccharides5030018
Chicago/Turabian StyleMouro, Cláudia, Ana P. Gomes, and Isabel C. Gouveia. 2024. "Microbial Exopolysaccharides: Structure, Diversity, Applications, and Future Frontiers in Sustainable Functional Materials" Polysaccharides 5, no. 3: 241-287. https://doi.org/10.3390/polysaccharides5030018
APA StyleMouro, C., Gomes, A. P., & Gouveia, I. C. (2024). Microbial Exopolysaccharides: Structure, Diversity, Applications, and Future Frontiers in Sustainable Functional Materials. Polysaccharides, 5(3), 241-287. https://doi.org/10.3390/polysaccharides5030018









