Structural Comparison of Raw Sodium Bicarbonate and Hydrated Lime for Dry SO2 Removal †
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deng, Y.; Ansart, R.; Baeyens, J.; Zhang, H. Flue Gas Desulphurization in Circulating Fluidised Beds. Energies 2019, 12, 3908. [Google Scholar] [CrossRef]
- Carpenter, A.M. Low Water FGD Technologies; IEA Clean Coal Centre: London, UK, 2012; Vol December 2012. [Google Scholar]
- Walawska, B.; Szymanek, A.; Pajdak, A.; Nowak, M. Flue Gas Desulfurization by Mechanically and Thermally Activated Sodium Bicarbonate. Pol. J. Chem. Technol. 2014, 16, 56–62. [Google Scholar] [CrossRef]
- Omidi Bibalani, I.; Ale Ebrahim, H. Kinetic Study of Low-Temperature Sulfur Dioxide Removal Reaction by Sodium Carbonate Using Random Pore Model. Environ. Sci. Pollut. Res. 2022, 29, 6334–6346. [Google Scholar] [CrossRef] [PubMed]
- Makomere, R.S.; Rutto, H.L.; Koech, L. The Use of Cellulose Nanocrystals to Support Ca(OH)2 Nanoparticles with Diatomite Incorporation in Sulphur Capture at Low Temperatures: Optimisation and Modelling. Arab. J. Sci. Eng. 2023, 48, 8871–8885. [Google Scholar] [CrossRef]
- Maina, P.; Mbarawa, M. Enhancement of Lime Reactivity by Addition of Diatomite. Fuel Process. Technol. 2011, 92, 1910–1919. [Google Scholar] [CrossRef]
- Wang, K.-Q.; Gao, X.-M.; Lin, B.; Hua, D.-X.; Yan, Y.; Zhao, H.-Y.; Xiao, W.-D. An Efficient Calcium-Based Sorbent for Flue Gas Dry-Desulfurization: Promotion Roles of Nitrogen Oxide and Oxygen. RSC Adv. 2023, 13, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- Koech, L.; Everson, R.; Neomagus, H.; Rutto, H. Dissolution Kinetics of South African Coal Fly Ash and the Development of a Semi-Empirical Model to Predict Dissolution. Chem. Ind. Chem. Eng. Q. 2015, 21, 319–330. [Google Scholar] [CrossRef]
- Sudibyo, S.; Suharto, S.; Rarasati, S.A.A.; Wulandari, Y.R.; Shintawati, S.; Rohman, F.S. Optimization of Sodium Bicarbonate Production from Ammonium Hydroxide Using a Froth Flotation Column. Chem. Eng. Technol. 2022, 45, 1952–1959. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, M.; Xie, C. Effect of Reaction Conditions on Agglomeration of Aluminum Hydroxide in the Recovery of Waste Aluminum-Catalyst. Sep. Purif. Technol. 2020, 248, 116978. [Google Scholar] [CrossRef]
- Mchabe, D.; Hattingh, B.B.; Koech, L.; Rutto, H.; Neomagus, H.W.J.P. Sodium-Based Flue Gas Desulphurisation for the South African Coal-Fired Power Industry a Review. S. Afr. J. Chem. Eng. 2024, 48, 167–183. [Google Scholar] [CrossRef]
- Samanta, A.; Chanda, D.K.; Das, P.S.; Ghosh, J.; Mukhopadhyay, A.K.; Dey, A. Synthesis of Nano Calcium Hydroxide in Aqueous Medium. J. Am. Ceram. Soc. 2016, 99, 787–795. [Google Scholar] [CrossRef]
- Engelking, L.R. Chapter 93—Alkalinizing and Acidifying Solutions. In Textbook of Veterinary Physiological Chemistry, 3rd ed.; Engelking, L.R., Ed.; Academic Press: Boston, MA, USA, 2015; pp. 606–611. ISBN 978-0-12-391909-0. [Google Scholar]
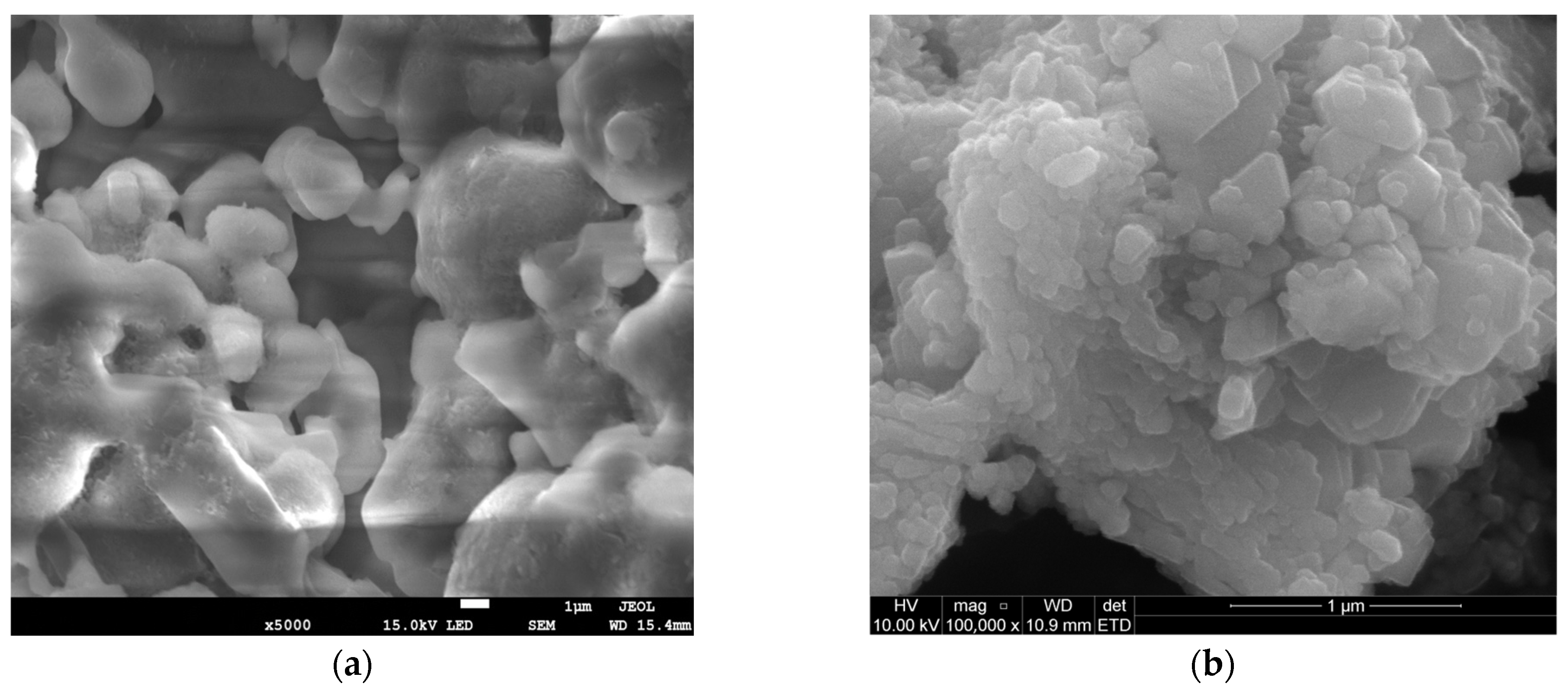
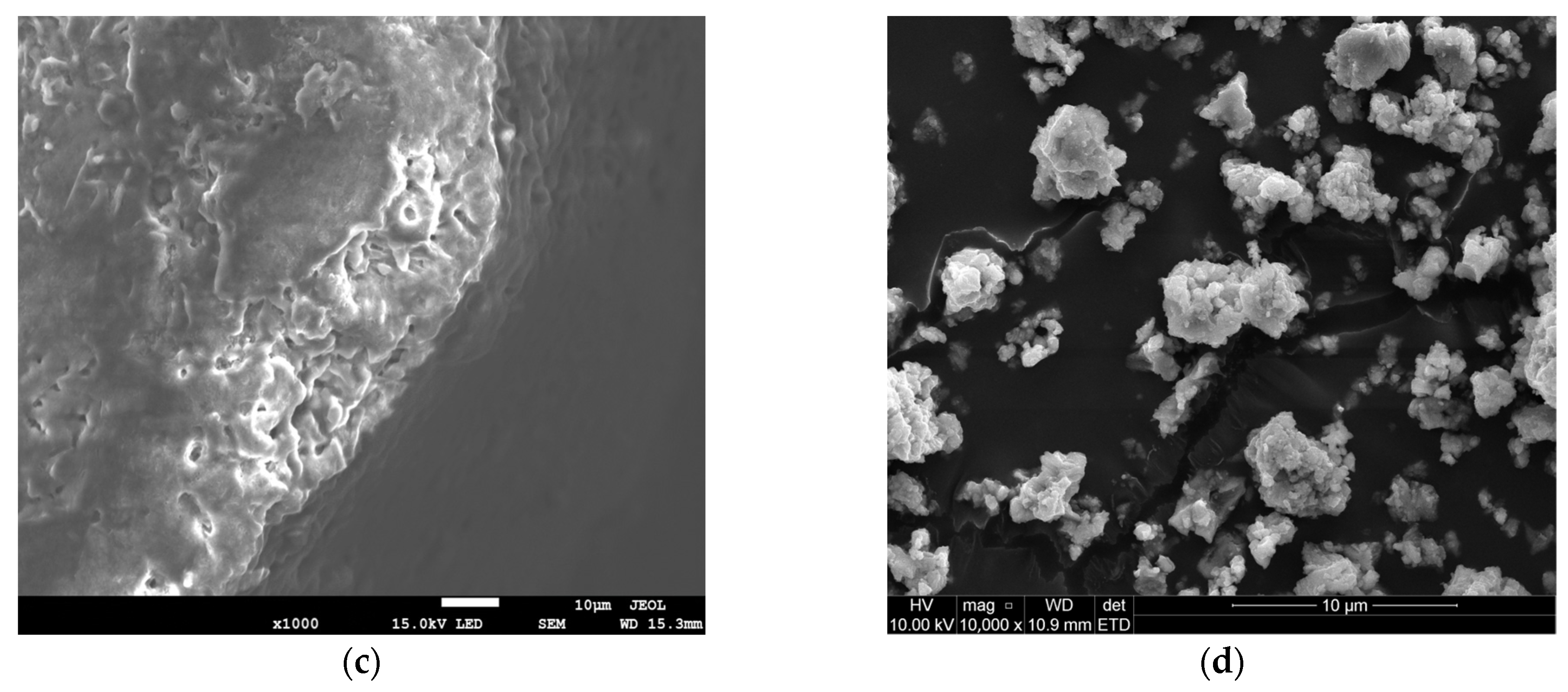
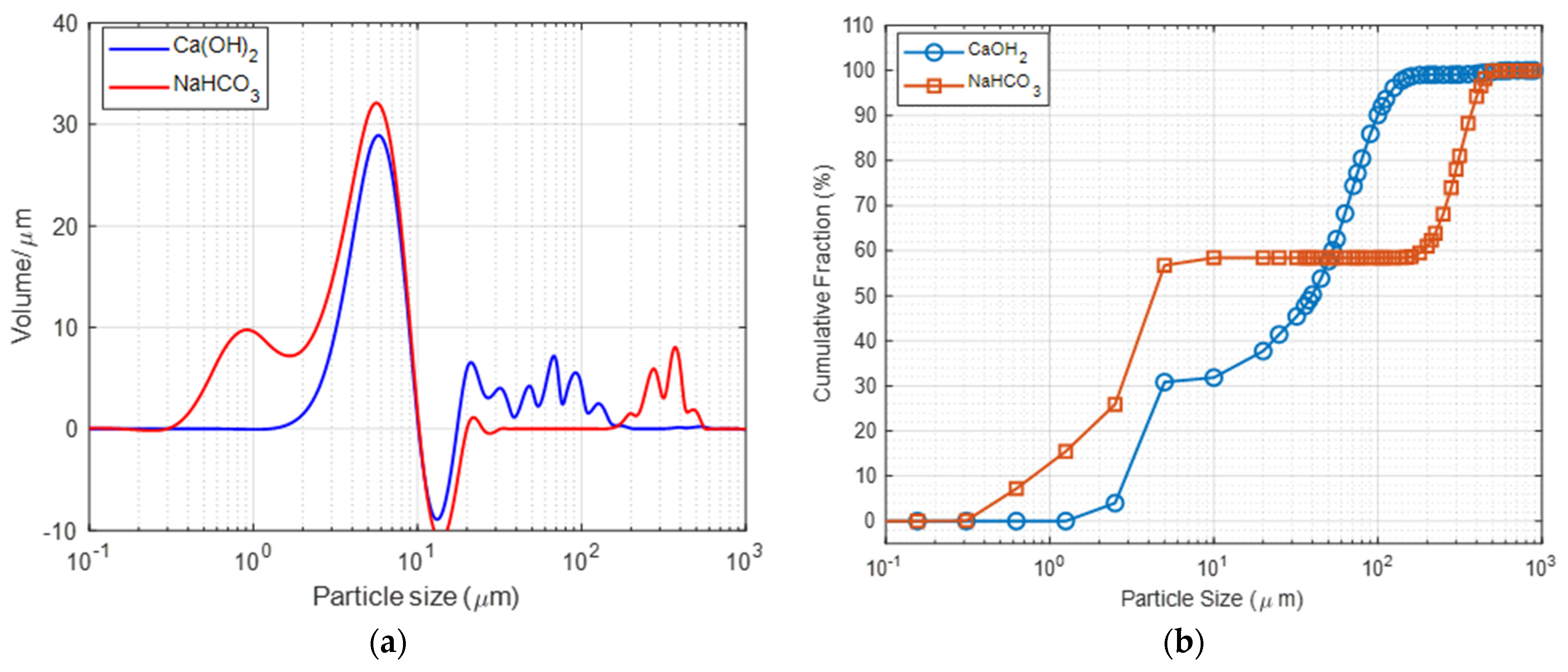
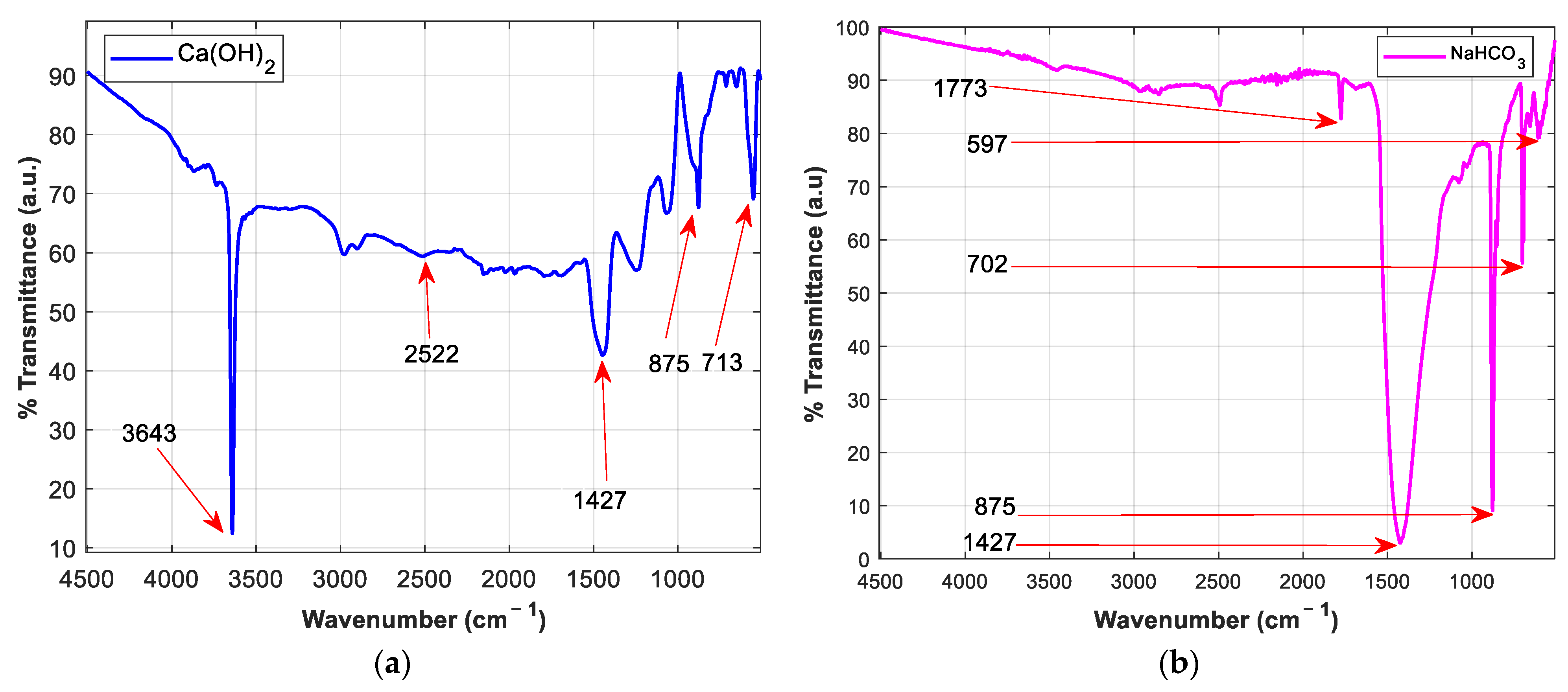
| Physical Property | Ca(OH)2 | NaHCO3 |
|---|---|---|
| BET surface area, m2/g | 4.2360 | 0.2303 |
| BET pore size, Å | 601.753 | 117.312 |
| BJH pore volume, cm3/g | 0.089822 | 0.000639 |
| * HK maximum pore volume, cm3/g | 0.003861 | 0.000705 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makomere, R.; Koech, L.; Rutto, H.; Alugongo, A.; Kiambi, S.; Kibambe, N. Structural Comparison of Raw Sodium Bicarbonate and Hydrated Lime for Dry SO2 Removal. Eng. Proc. 2024, 67, 63. https://doi.org/10.3390/engproc2024067063
Makomere R, Koech L, Rutto H, Alugongo A, Kiambi S, Kibambe N. Structural Comparison of Raw Sodium Bicarbonate and Hydrated Lime for Dry SO2 Removal. Engineering Proceedings. 2024; 67(1):63. https://doi.org/10.3390/engproc2024067063
Chicago/Turabian StyleMakomere, Robert, Lawrence Koech, Hilary Rutto, Alfayo Alugongo, Sammy Kiambi, and Ngeleshi Kibambe. 2024. "Structural Comparison of Raw Sodium Bicarbonate and Hydrated Lime for Dry SO2 Removal" Engineering Proceedings 67, no. 1: 63. https://doi.org/10.3390/engproc2024067063






