Fluidic Properties of Diamond and SplitP Structures with Varying Porosity Levels in Tissue Engineering Applications: A Computational Fluid Dynamics Analysis †
Abstract
:1. Introduction
2. Material and Methods
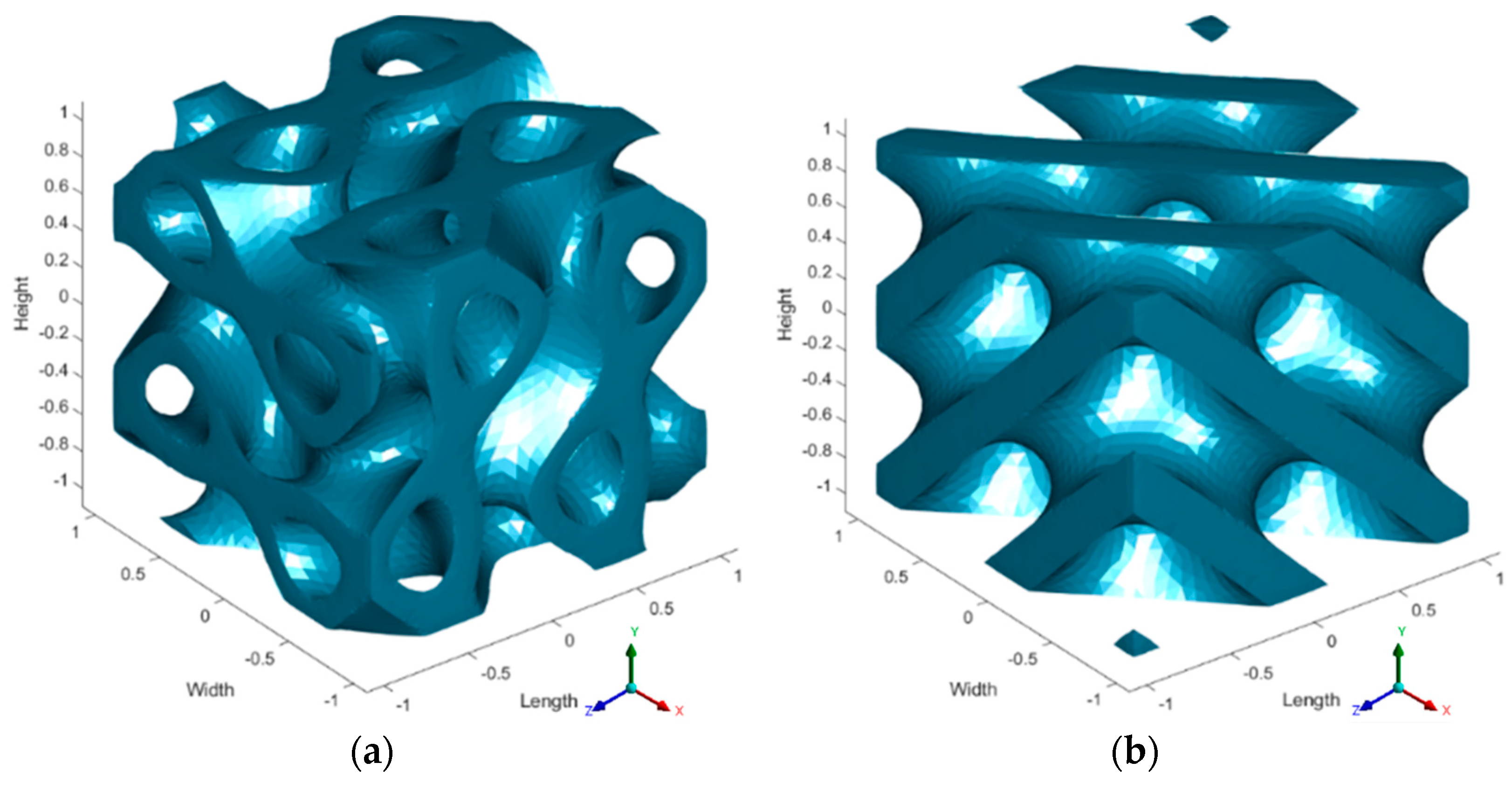
2.1. Scaffold Design
2.2. Governing Equations
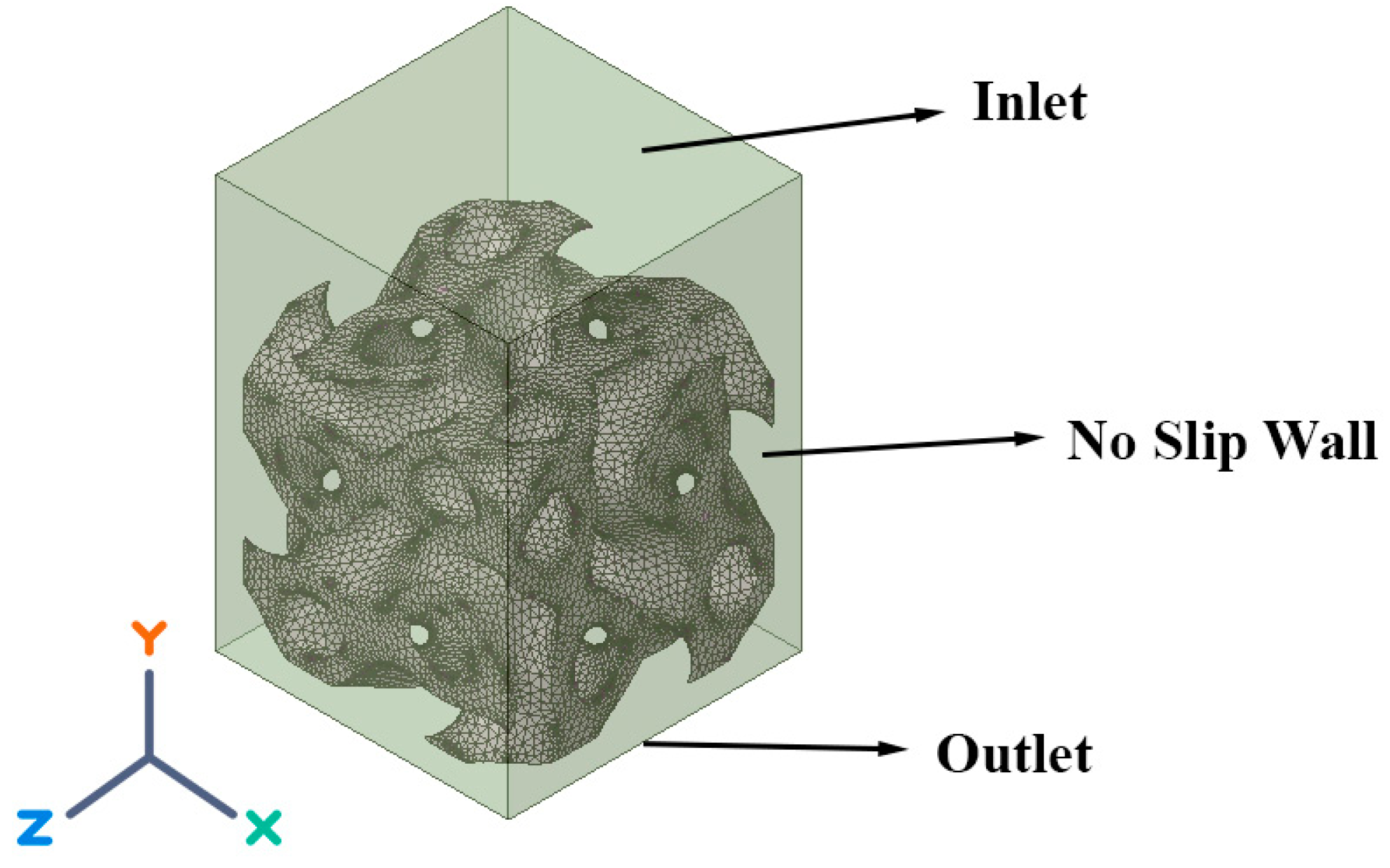
2.3. Discretization and Boundary Conditions
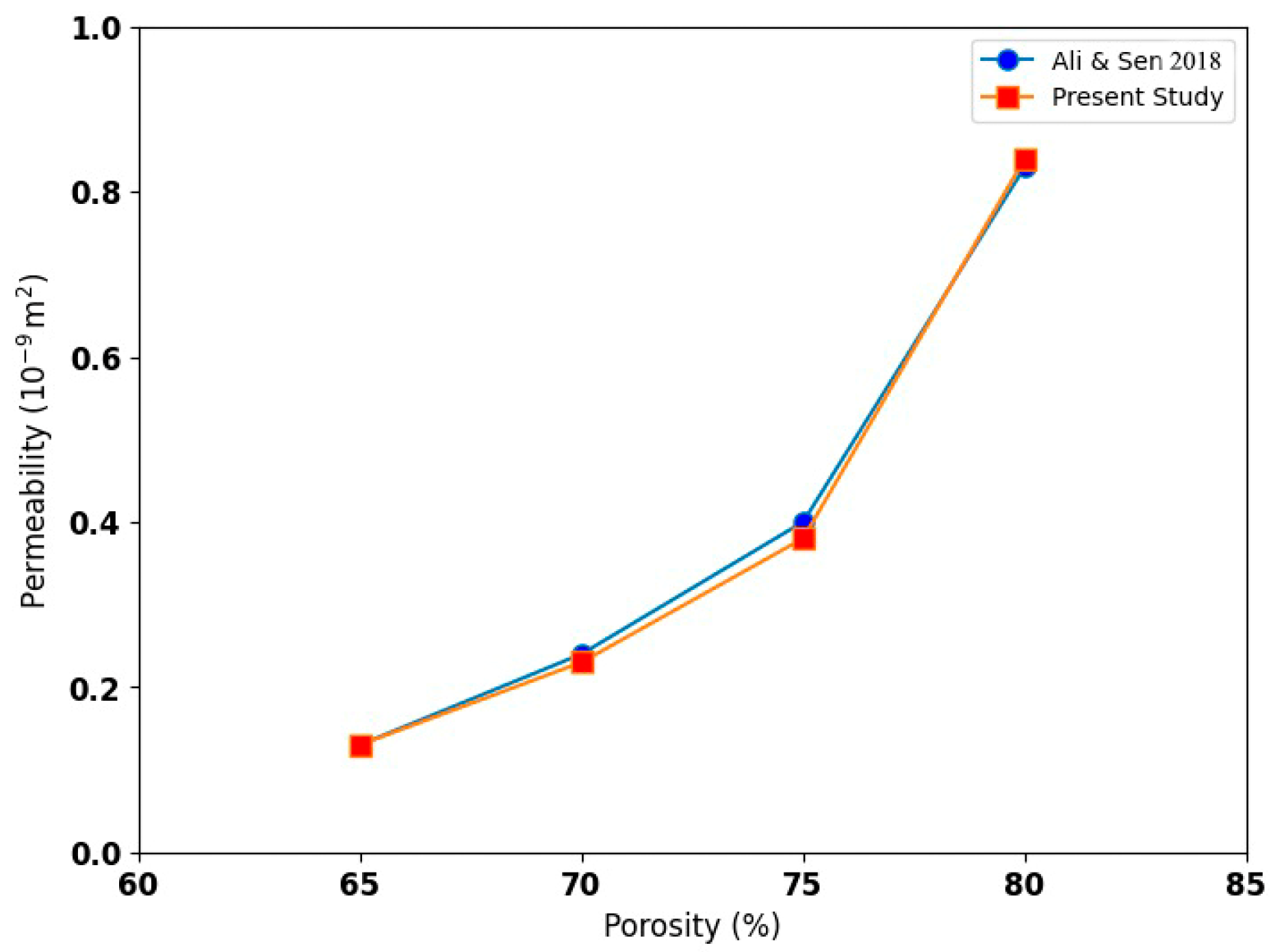
3. Validation Study
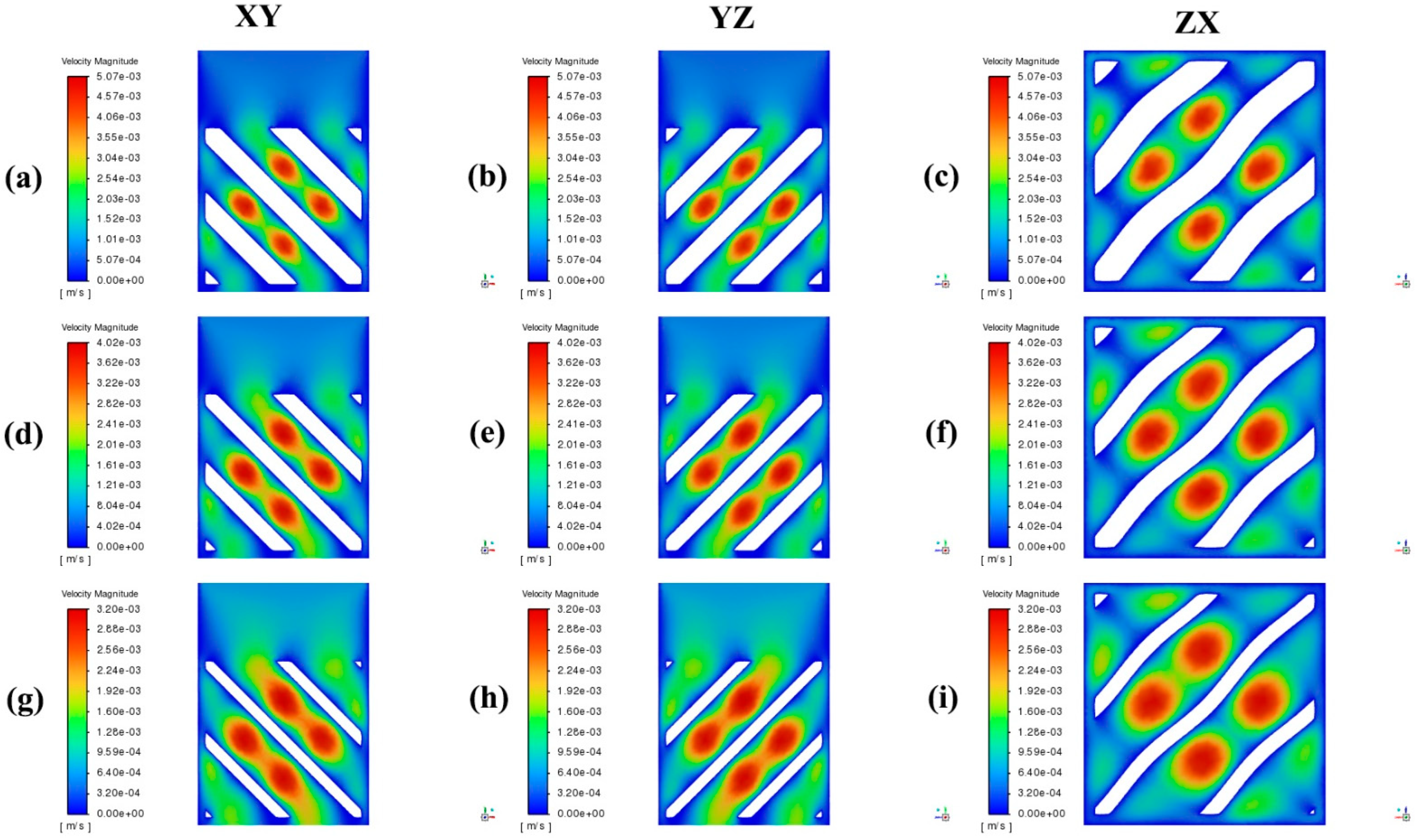
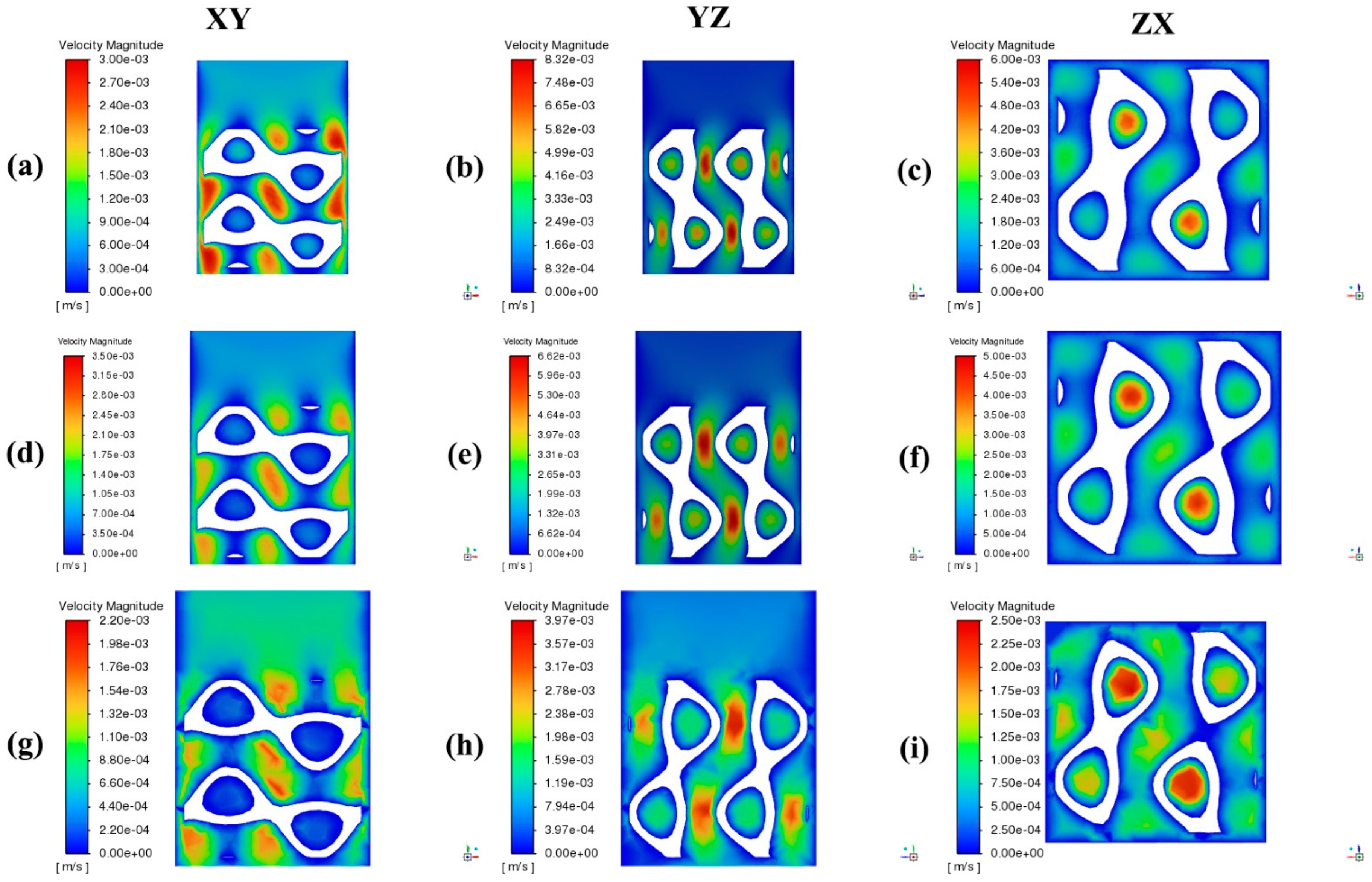
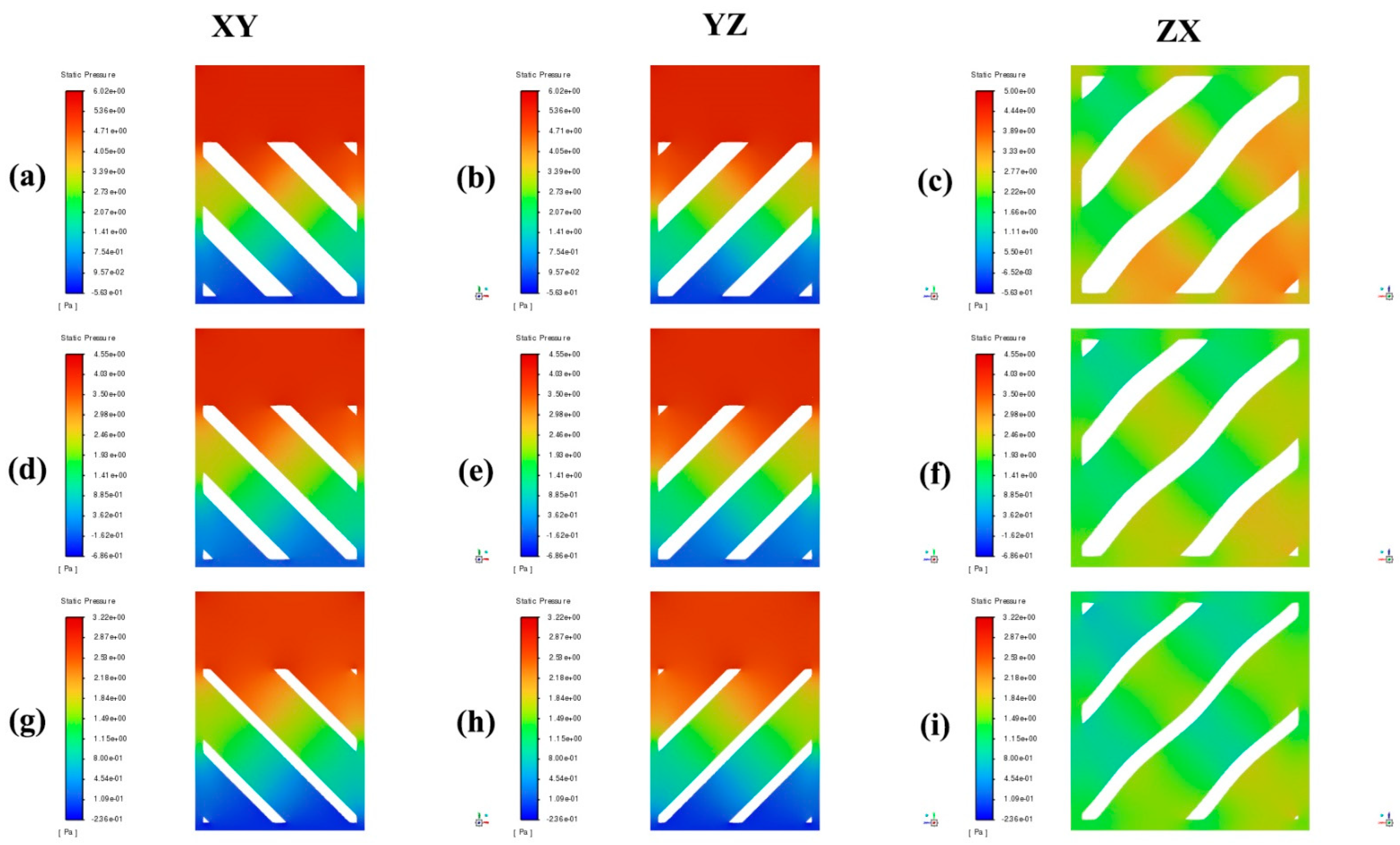
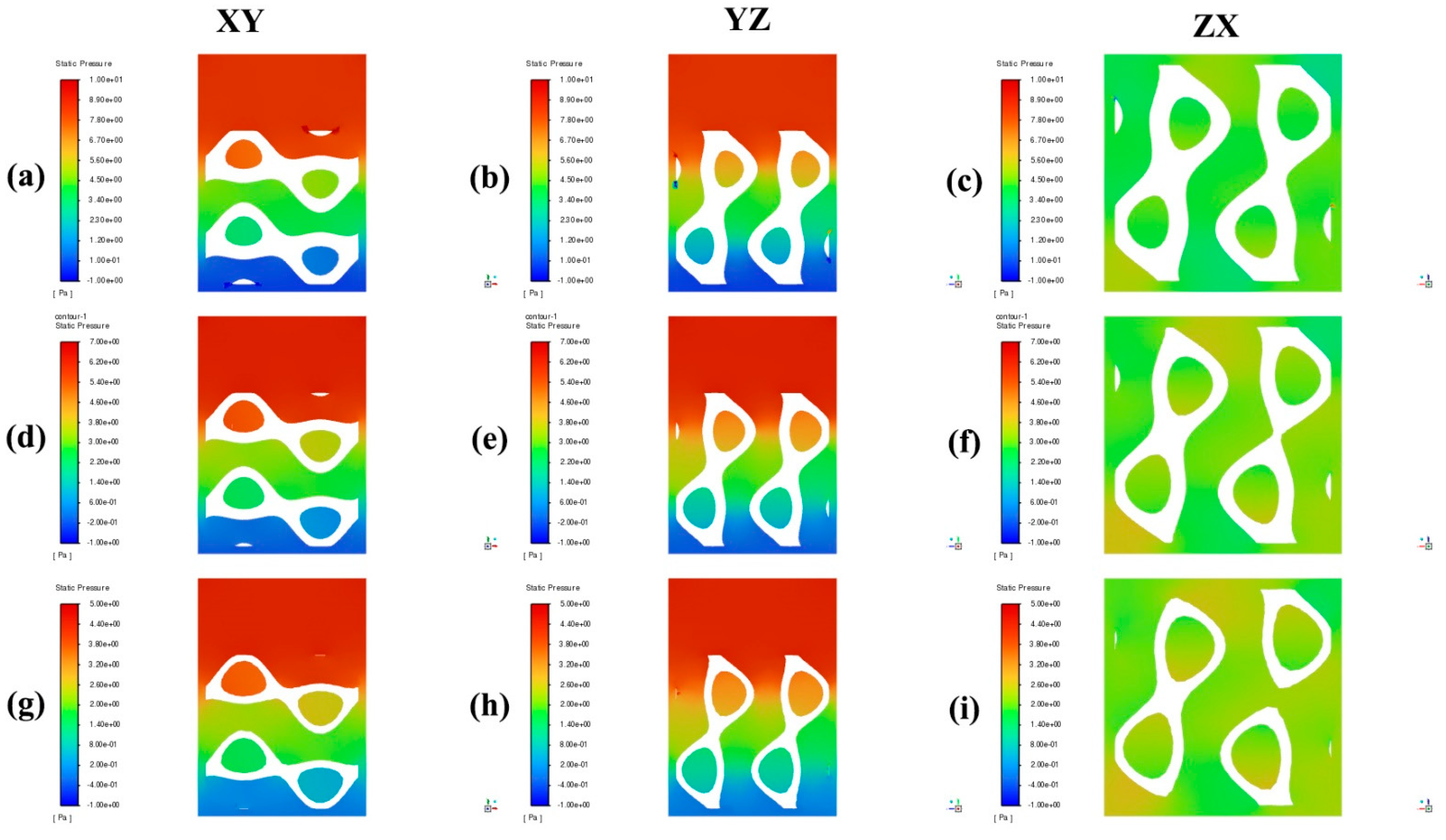
4. Results and Discussion
5. Conclusions
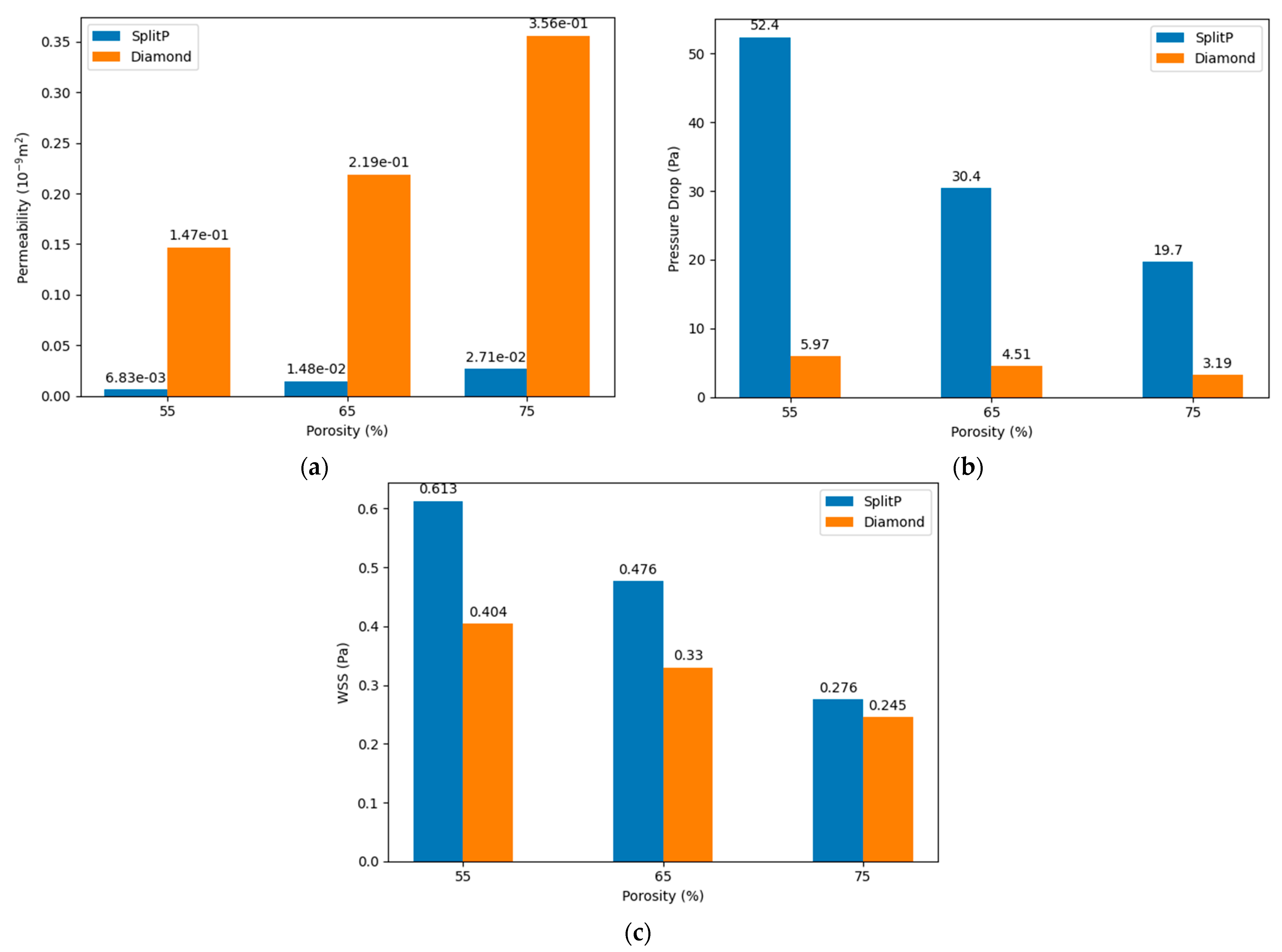
- Increasing the porosity significantly enhances the permeability within TPMS scaffolds. Scaffolds with 75% porosity demonstrated the highest permeability, facilitating superior fluid transport, which is essential for efficient nutrient delivery and waste removal. Among the designs analyzed, the Diamond structure generally exhibited slightly higher permeability compared to the SplitP structure, suggesting that it is more effective for applications requiring optimal fluid flow.
- Higher porosity in TPMS scaffolds significantly reduces the pressure drop across the structure. Both the Diamond and SplitP scaffolds exhibited the lowest pressure drop at 75% porosity, which is essential for effective nutrient delivery and waste removal in tissue engineering applications. Among the two designs, the Diamond structure consistently demonstrated a lower pressure drop than the SplitP structure, making it more efficient for applications that demand minimal resistance to fluid flow.
- The WSS decreases as the porosity increases, with the lowest values observed at 75% porosity. A lower WSS is favorable for initial cell adhesion, although optimal differentiation requires adjustments to the porosity.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shahid, M.N.; Shahid, M.U.; Rasheed, S.; Irfan, M.; Obeidi, M.A. Computational Investigation of the Fluidic Properties of Triply Periodic Minimal Surface (TPMS) Structures in Tissue Engineering. Designs 2024, 8, 69. [Google Scholar] [CrossRef]
- Sidambe, A.T. Biocompatibility of Advanced Manufactured Titanium Implants-A Review. Materials 2014, 7, 8168–8188. [Google Scholar] [CrossRef] [PubMed]
- Zuniga, J.; Katsavelis, D.; Peck, J.; Stollberg, J.; Petrykowski, M.; Carson, A.; Fernandez, C. Cyborg Beast: A Low-Cost 3d-Printed Prosthetic Hand for Children with Upper-Limb Differences. BMC Res. Notes 2015, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Hutmacher, D.W.; Schantz, J.T.; Lam, C.X.F.; Tan, K.C.; Lim, T.C. State of the Art and Future Directions of Scaffold-Based Bone Engineering from a Biomaterials Perspective. J. Tissue Eng. Regen. Med. 2007, 1, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.R.; Fernandes, P.R.; Guedes, J.M.; Hollister, S.J. Permeability Analysis of Scaffolds for Bone Tissue Engineering. J. Biomech. 2012, 45, 938–944. [Google Scholar] [CrossRef] [PubMed]
- Berger, S.A.; Jou, L.D. Flows in Stenotic Vessels. Annu. Rev. Fluid. Mech. 2000, 32, 347–382. [Google Scholar] [CrossRef]
- Marin, A.C.; Lacroix, D. The Inter-Sample Structural Variability of Regular Tissue-Engineered Scaffolds Significantly Affects the Micromechanical Local Cell Environment. Interface Focus 2015, 5, 20140097. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhang, J.; Zhao, W. Effects of the Inlet Conditions and Blood Models on Accurate Prediction of Hemodynamics in the Stented Coronary Arteries. AIP Adv. 2015, 5, 57109. [Google Scholar] [CrossRef]
- Nadeem, S.; Akbar, N.S.; Hendi, A.A.; Hayat, T. Power Law Fluid Model for Blood Flow through a Tapered Artery with a Stenosis. Appl. Math. Comput. 2011, 217, 7108–7116. [Google Scholar] [CrossRef]
- Ali, D.; Ozalp, M.; Blanquer, S.B.G.; Onel, S. Permeability and Fluid Flow-Induced Wall Shear Stress in Bone Scaffolds with TPMS and Lattice Architectures: A CFD Analysis. Eur. J. Mech. B Fluids 2020, 79, 376–385. [Google Scholar] [CrossRef]
- Bobbert, F.S.L.; Zadpoor, A.A. Effects of Bone Substitute Architecture and Surface Properties on Cell Response, Angiogenesis, and Structure of New Bone. J. Mater. Chem. B 2017, 5, 6175–6192. [Google Scholar] [CrossRef]
- Vossenberg, P.; Higuera, G.A.; Van Straten, G.; Van Blitterswijk, C.A.; Van Boxtel, A.J.B. Darcian Permeability Constant as Indicator for Shear Stresses in Regular Scaffold Systems for Tissue Engineering. Biomech. Model. Mechanobiol. 2009, 8, 499–507. [Google Scholar] [CrossRef]
- Johnston, B.M.; Johnston, P.R.; Corney, S.; Kilpatrick, D. Non-Newtonian Blood Flow in Human Right Coronary Arteries: Steady State Simulations. J. Biomech. 2004, 37, 709–720. [Google Scholar] [CrossRef]
- Ali, D.; Sen, S. Permeability and Fluid Flow-Induced Wall Shear Stress of Bone Tissue Scaffolds: Computational Fluid Dynamic Analysis Using Newtonian and Non-Newtonian Blood Flow Models. Comput. Biol. Med. 2018, 99, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Tang, Q.; Han, X.; Feng, Q.; Song, J.; Setchi, R.; Liu, Y.; Liu, Y.; Goulas, A.; Engstrøm, D.S.; et al. Manufacturability, Mechanical Properties, Mass-Transport Properties and Biocompatibility of Triply Periodic Minimal Surface (TPMS) Porous Scaffolds Fabricated by Selective Laser Melting. Mater. Des. 2020, 195, 109034. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahid, M.N.; Khan, M.M.; Shahid, M.U.; Rasheed, S. Fluidic Properties of Diamond and SplitP Structures with Varying Porosity Levels in Tissue Engineering Applications: A Computational Fluid Dynamics Analysis. Eng. Proc. 2024, 75, 39. https://doi.org/10.3390/engproc2024075039
Shahid MN, Khan MM, Shahid MU, Rasheed S. Fluidic Properties of Diamond and SplitP Structures with Varying Porosity Levels in Tissue Engineering Applications: A Computational Fluid Dynamics Analysis. Engineering Proceedings. 2024; 75(1):39. https://doi.org/10.3390/engproc2024075039
Chicago/Turabian StyleShahid, Muhammad Noman, Muhammad Mahabat Khan, Muhammad Usman Shahid, and Shummaila Rasheed. 2024. "Fluidic Properties of Diamond and SplitP Structures with Varying Porosity Levels in Tissue Engineering Applications: A Computational Fluid Dynamics Analysis" Engineering Proceedings 75, no. 1: 39. https://doi.org/10.3390/engproc2024075039





