Non-Contact Blood Pressure Estimation Using Forehead and Palm Infrared Video
Abstract
1. Introduction
2. Dataset Protocol & Equipment
3. Proposed Methodology
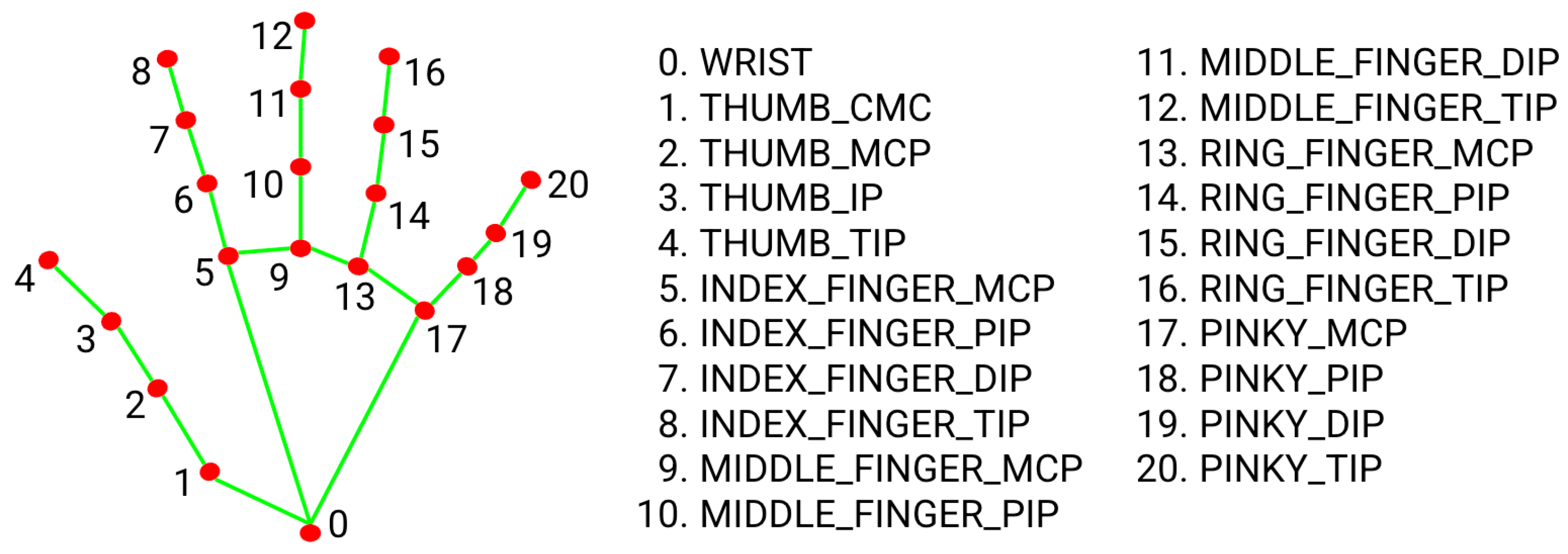
- Face & Hand Detection: Precisely locating facial and hand regions of interest using advanced computer vision techniques.
- ROI Extraction: Extracting critical facial landmarks and hand-knuckle coordinates from the detected areas.
- Motion Magnification: Amplifying subtle movements and physiological signals to reveal important dynamics.
- Filtering: Removing noise and artifacts to enhance data accuracy.
- Peak Detection: Identifying and characterizing relevant peaks in the signals.
3.1. Facial & Palmar Segmentation
3.2. Motion Magnification
3.3. Post-Processing & Peak Detection
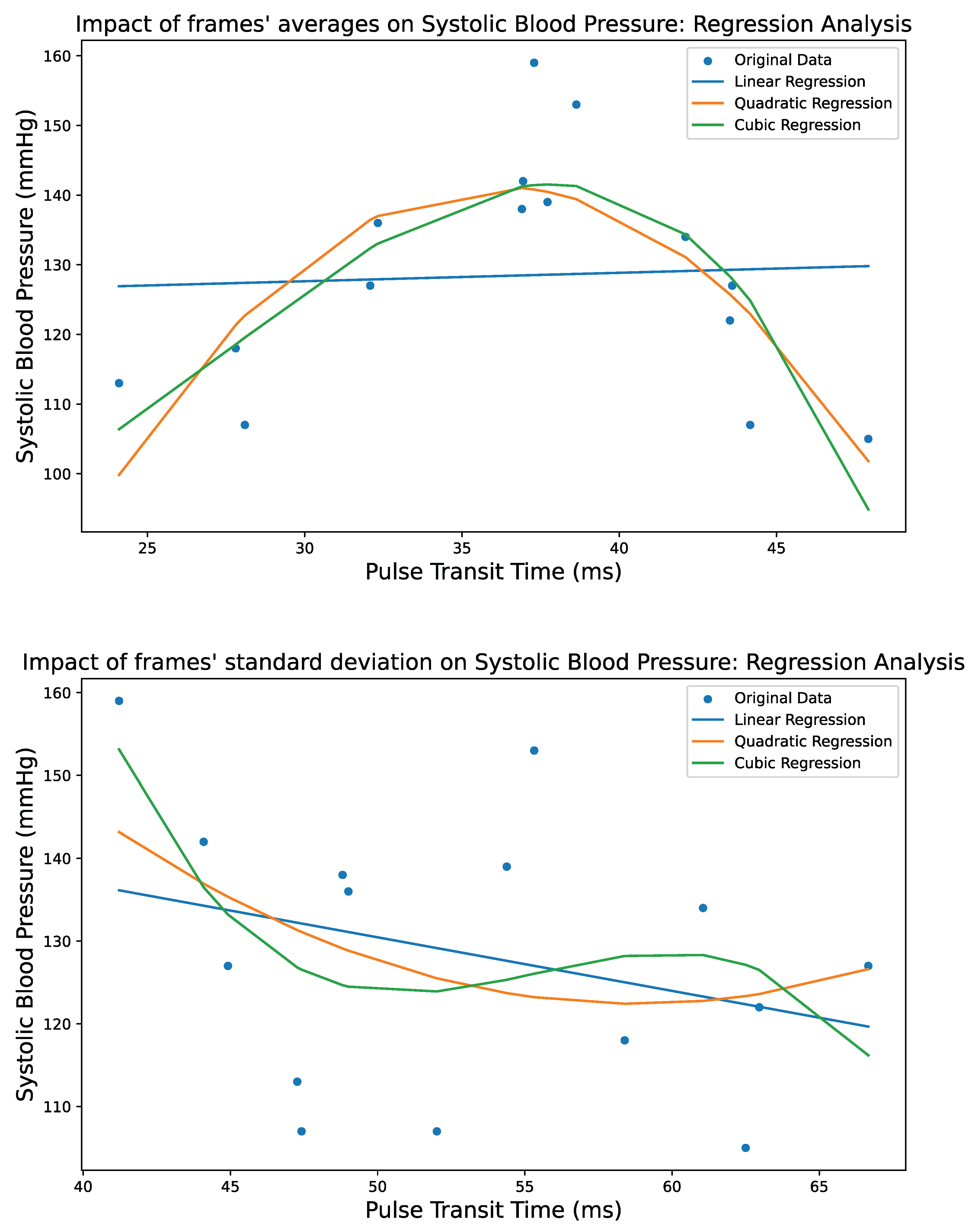
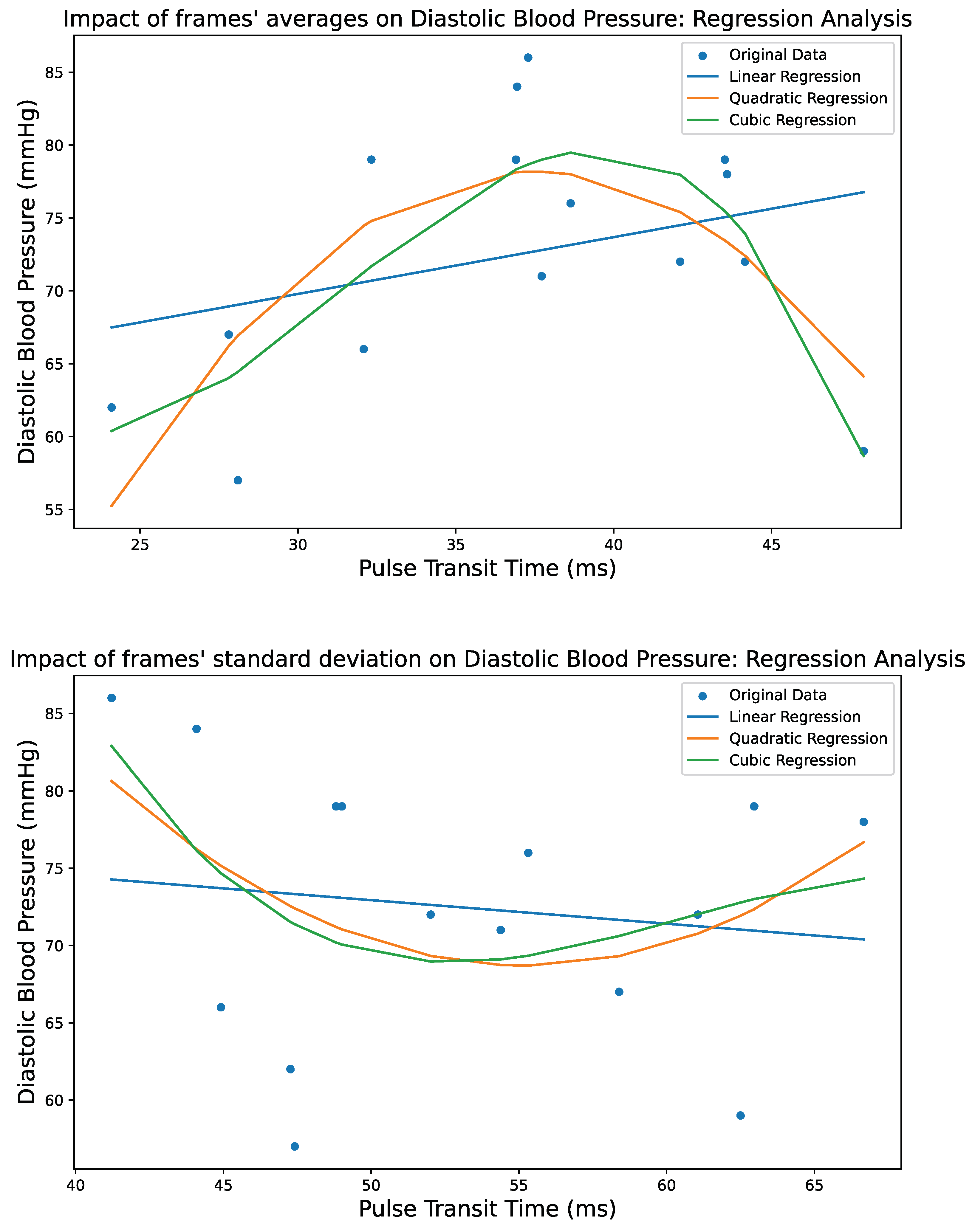
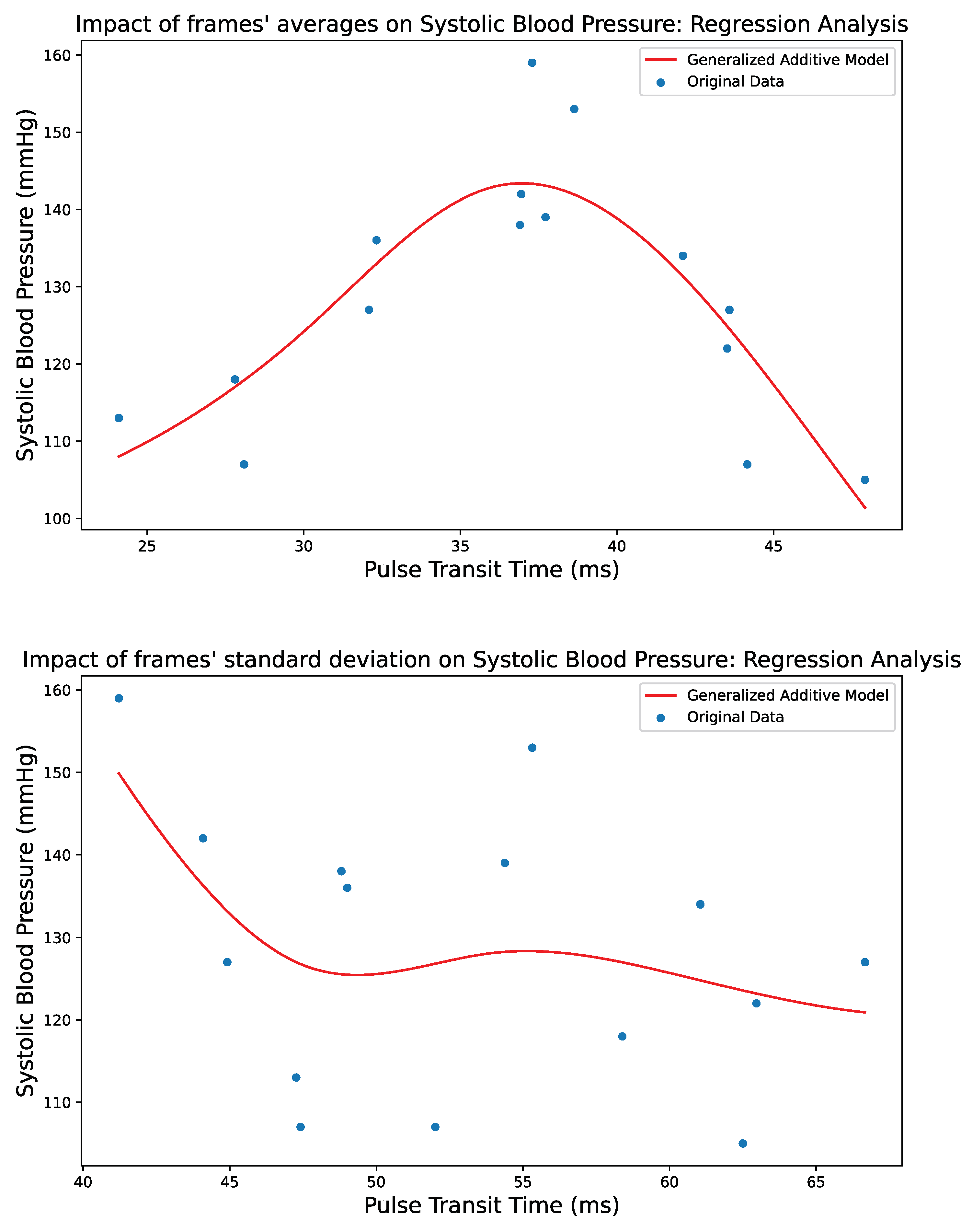
4. Regression Techniques
4.1. Generalized Additive Models
4.2. Polynomial Regression
4.2.1. Linear Regression
4.2.2. Quadratic Regression
4.2.3. Cubic Regression
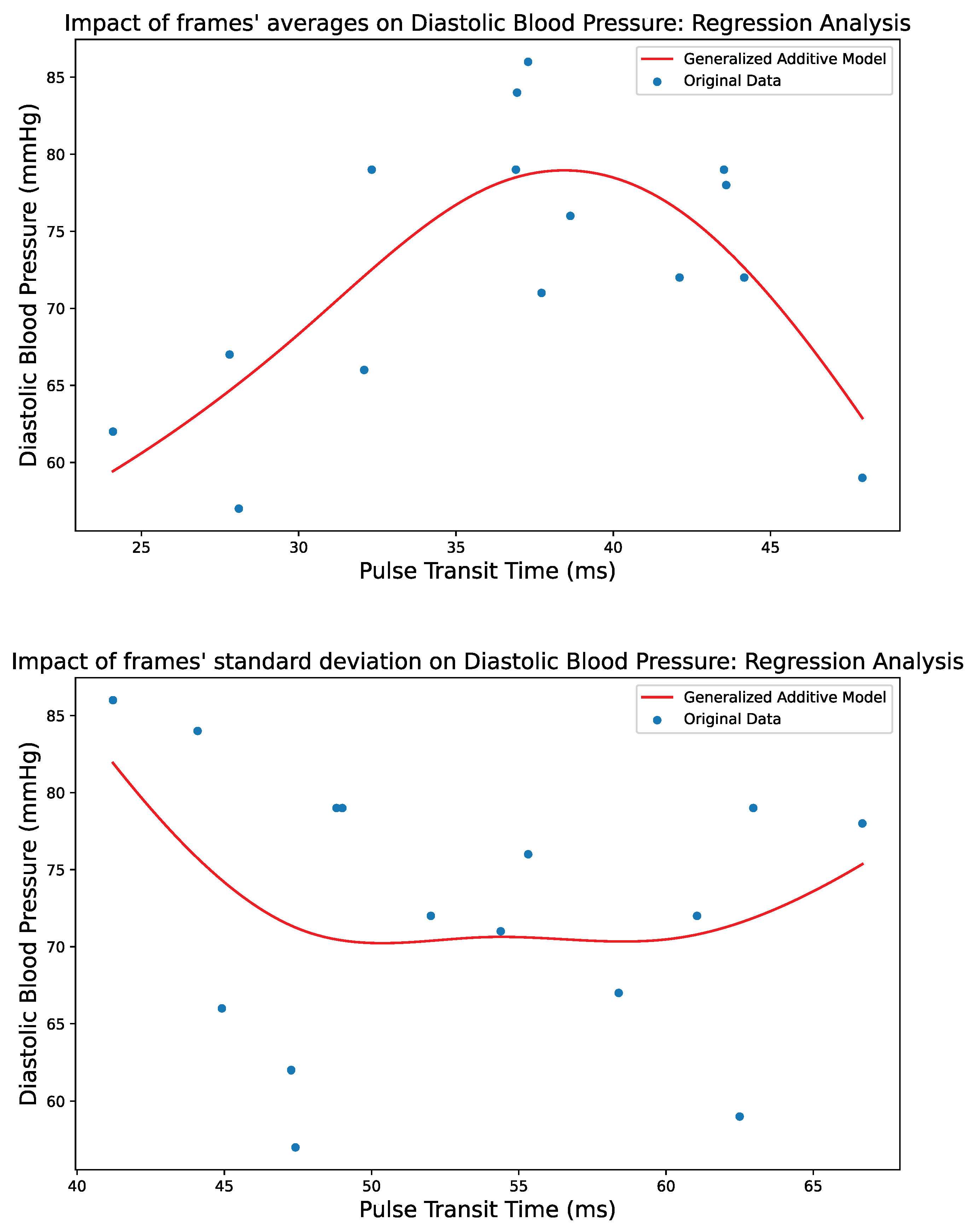
5. Results
5.1. Implementation
5.2. Validation Standards
5.3. Performance
5.4. Potential Biases
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Z.; Zhang, Z.; Ren, Y.; Wang, Y.; Fang, J.; Yue, H.; Ma, S.; Guan, F. Aging and age-related diseases: From mechanisms to therapeutic strategies. Biogerontology 2021, 22, 165–187. [Google Scholar] [CrossRef]
- Mancia, G.; Kreutz, R.; Brunström, M.; Burnier, M.; Grassi, G.; Januszewicz, A.; Muiesan, M.L.; Tsioufis, K.; Agabiti-Rosei, E.; Algharably, E.A.E.; et al. 2023 ESH Guidelines for the Management of Arterial Hypertension the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension Endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA). J. Hypertens. 2023, 41, 1874–2071. [Google Scholar] [PubMed]
- Cheng, S.; Xanthakis, V.; Sullivan, L.M.; Vasan, R.S. Blood Pressure Tracking over the Adult Life Course. Hypertension 2012, 60, 1393–1399. [Google Scholar] [CrossRef] [PubMed]
- Muntner, P.; Shimbo, D.; Carey, R.M.; Charleston, J.B.; Gaillard, T.; Misra, S.; Myers, M.G.; Ogedegbe, G.; Schwartz, J.E.; Townsend, R.R.; et al. Measurement of Blood Pressure in Humans: A Scientific Statement from the American Heart Association. Hypertension 2019, 73, e35–e66. [Google Scholar] [CrossRef]
- Buford, T.W. Hypertension and Aging. Ageing Res. Rev. 2016, 26, 96–111. [Google Scholar] [CrossRef]
- Oliveros, E.; Patel, H.; Kyung, S.; Fugar, S.; Goldberg, A.; Madan, N.; Williams, K.A. Hypertension in Older Adults: Assessment, Management, and Challenges. Clin. Cardiol. 2019, 43, 99–107. [Google Scholar] [CrossRef]
- Magny, E.; Donadio, C.; Maronnat, F.; Nghiem, D.; Berthelot, E.; Belmin, J.; Lafuente-Lafuente, C. Hypotensions in the Elderly: Clinical and Therapeutic Features. Presse Medicale 2019, 48, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Ermasova, S.A.; Shvarts, Y.G. Transient Hypotension in Elderly Hypertensive Patients: What and How to Manage? e-J. Cardiol. Pract. 2022, 22. Available online: https://www.escardio.org/Journals/E-Journal-of-Cardiology-Practice/Volume-22/transient-hypotension-in-elderly-hypertensive-patients-what-and-how-to-manage (accessed on 4 February 2024).
- Frezza, E.E.; Mezghebe, H.M. Indications and complications of arterial catheter use in surgical or medical intensive care units: Analysis of 4932 patients. Am. Surg. 1998, 64, 127–131. [Google Scholar]
- Paik, J.J.; Hirpara, R.; Heller, J.A.; Hummers, L.K.; Wigley, F.M.; Shah, A.A. Thrombotic Complications after Radial Arterial Line Placement in Systemic Sclerosis: A Case Series. Semin. Arthritis Rheum. 2016, 46, 196–199. [Google Scholar] [CrossRef][Green Version]
- Scheer, B.; Perel, A.; Pfeiffer, U.J. Clinical Review: Complications and Risk Factors of Peripheral Arterial Catheters Used for Haemodynamic Monitoring in Anaesthesia and Intensive Care Medicine. Crit. Care 2002, 6, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Shimbo, D.; Artinian, N.T.; Basile, J.N.; Krakoff, L.R.; Margolis, K.L.; Rakotz, M.K.; Wozniak, G. Self-Measured Blood Pressure Monitoring at Home: A Joint Policy Statement from the American Heart Association and American Medical Association. Circulation 2020, 142, e42–e63. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Ye, Q.; Yang, X.; Choudhury, S.D. Robust Blood Pressure Estimation Using an RGB Camera. J. Ambient. Intell. Humaniz. Comput. 2018, 11, 4329–4336. [Google Scholar] [CrossRef]
- Rong, M.; Li, K. A Blood Pressure Prediction Method Based on Imaging Photoplethysmography in Combination with Machine Learning. Biomed. Signal Process. Control 2021, 64, 102328. [Google Scholar] [CrossRef]
- Yoshizawa, M.; Sugita, N.; Abe, M.; Tanaka, A.; Homma, N.; Yambe, T. Non-Contact Blood Pressure Estimation Using Video Pulse Waves for Ubiquitous Health Monitoring. In Proceedings of the 2017 IEEE 6th Global Conference on Consumer Electronics (GCCE), Nagoya, Japan, 24–27 October 2017. [Google Scholar]
- Takahashi, R.; Ogawa-Ochiai, K.; Tsumura, N. Non-Contact Method of Blood Pressure Estimation Using Only Facial Video. Artif. Life Robot. 2020, 25, 343–350. [Google Scholar] [CrossRef]
- Goudarzi, R.H.; Mousavi, S.S.; Charmi, M. Using Imaging Photoplethysmography (IPPG) Signal for Blood Pressure Estimation. In Proceedings of the 2020 International Conference on Machine Vision and Image Processing (MVIP), Qom, Iran, 18–20 February 2020. [Google Scholar]
- Topouchian, J.A.; Agnoletti, D.; Blacher, J.; Youssef, A.E.; Ibanez, I.; Khabouth, J.; Khawaja, S.; Beaino, L.; Asmar, R. Validation of four automatic devices for self-measurement of blood pressure according to the international protocol of the European Society of Hypertension. Vasc. Health Risk Manag. 2011, 7, 709–717. [Google Scholar]
- Stride BP. BP Monitors. Available online: https://www.stridebp.org/bp-monitors (accessed on 11 October 2023).
- Parati, G.; Ochoa, J.E. Blood Pressure Variability. In Updates in Hypertension and Cardiovascular Protection; Springer: Cham, Switzerland, 2018; pp. 395–417. [Google Scholar]
- McLean, K.M.; Sacks, J.M.; Kuo, Y.R.; Wollstein, R.; Rubin, J.P.; Lee, W.P.A. Anatomical Landmarks to the Superficial and Deep Palmar Arches. Plast. Reconstr. Surg. 2008, 121, 181–185. [Google Scholar] [CrossRef]
- Milutinovic, J.; Zelic, K.; Nedeljkovic, N. Evaluation of Facial Beauty Using Anthropometric Proportions. Sci. World J. 2014, 2014, 428250. [Google Scholar] [CrossRef]
- Kaya, K.S.; Türk, B.; Cankaya, M.; Seyhun, N.; Coşkun, B.U. Assessment of Facial Analysis Measurements by Golden Proportion. Braz. J. Otorhinolaryngol. 2019, 85, 494–501. [Google Scholar] [CrossRef]
- Fernandes, J.W. The Legacy of Art in Plastic Surgery. Plast. Reconstr.-Surg.-Glob. Open 2021, 9, e3519. [Google Scholar] [CrossRef] [PubMed]
- Stogiannopoulos, T.; Cheimariotis, G.A.; Mitianoudis, N. A Study of Machine Learning Regression Techniques for Non-Contact SPO2 Estimation from Infrared Motion-Magnified Facial Video. Information 2023, 14, 301. [Google Scholar] [CrossRef]
- Fernandez, J. Mediapipe Hands. GitHub. Available online: https://github.com/google/mediapipe/blob/master/docs/solutions/hands.md (accessed on 4 April 2023).
- Wu, H.Y.; Rubinstein, M.; Shih, E.; Guttag, J.; Durand, F.; Freeman, W. Eulerian Video Magnification for Revealing Subtle Changes in the World. ACM Trans. Graph. 2012, 31, 1–8. [Google Scholar] [CrossRef]
- Garratt, C.; Ward, D.; Antoniou, A.; Camm, A.J. Misuse of Verapamil in Pre-Excited Atrial Fibrillation. Lancet 1989, 333, 367–369. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Zhao, Y.; Dong, L.; Jian, Y.; Jin, X.; Li, B.; Feng, Y.; Liu, M.; Liu, X.; Wu, H. Non-Contact Detection of Oxygen Saturation Based on Visible Light Imaging Device Using Ambient Light. Opt. Express 2013, 21, 17464–17471. [Google Scholar] [CrossRef] [PubMed]
- Brieva, J.; Moya-Albor, E.; Ponce, H. A Non-Contact SpO2 Estimation Using a Video Magnification Technique. In Proceedings of the 17th International Symposium on Medical Information Processing and Analysis, Campinas, Brazil, 17–19 November 2021. [Google Scholar]
- de Fátima Galvão Rosa, A.; Betini, R.C. Noncontact SPO2 Measurement Using Eulerian Video Magnification. IEEE Trans. Instrum. Meas. 2019, 69, 2120–2130. [Google Scholar] [CrossRef]
- Lauridsen, H.; Hedwig, S.D.; Perrin, K.L.; Williams, C.J.; Wrege, P.H.; Bertelsen, M.F.; Pedersen, M.; Butcher, J.T. Extracting Physiological Information in Experimental Biology via Eulerian Video Magnification. BMC Biol. 2019, 17, 103. [Google Scholar] [CrossRef] [PubMed]
- Hastie, T.; Tibshirani, R. Generalized Additive Models. In Statistical Science; Routledge: Oxfordshire, UK, 1986; Volume 1. [Google Scholar]
- MacCullagh, P.; Nelder, J.A. Generalized Linear Models; Chapman and Hall: London, UK, 1989. [Google Scholar]
- Video Magnification. Available online: https://people.csail.mit.edu/mrub/vidmag/#code (accessed on 14 October 2023).
- O’Brien, E.; Petrie, J.; Littler, W.; De Swiet, M.; Padfield, P.L.; Altman, D.; Bland, M.; Coats, A.; Atkins, N. The British Hypertension Society protocol for the evaluation of blood pressure measuring devices. J. Hypertens. 1993, 11 (Suppl. S2), S43–S62. [Google Scholar]
- White, W.B.; Berson, A.S.; Robbins, C.; Jamieson, M.J.; Prisant, L.M.; Roccella, E.; Sheps, S.G. National Standard for Measurement of Resting and Ambulatory Blood Pressures with Automated Sphygmomanometers. Hypertension 1993, 21, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Rong, M.; Li, K. A Multi-Type Features Fusion Neural Network for Blood Pressure Prediction Based on Photoplethysmography. Biomed. Signal Process. Control. 2021, 68, 102772. [Google Scholar] [CrossRef]



| Grade | ≤5 mm Hg | ≤10 mm Hg | ≤15 mm Hg |
|---|---|---|---|
| A | 60 | 85 | 95 |
| B | 50 | 75 | 90 |
| C | 40 | 65 | 85 |
| D | ≤40 | ≤65 | ≤85 |
| Linear Regression | Quadratic Regression | Cubic Regression | GAM | |
|---|---|---|---|---|
| Systolic Blood Pressure-CP5 1 | 20 (D) | 60 (A) | 80 (A) | 58 (B) |
| Systolic Blood Pressure-CP10 2 | 53.33 (D) | 60 (D) | 93.33 (A) | 92.53 (A) |
| Systolic Blood Pressure-CP15 3 | 66.67 (D) | 86.67 (C) | 100 (A) | 100 (A) |
| Diastolic Blood Pressure-CP5 1 | 40 (C) | 66.67 (A) | 80 (A) | 68.4 (A) |
| Diastolic Blood Pressure-CP10 2 | 86.67 (A) | 100 (A) | 100 (A) | 100 (A) |
| Diastolic Blood Pressure-CP15 3 | 93.33 (B) | 100 (A) | 100 (A) | 100 (A) |
| Systolic Blood Pressure-MAE 4 | 12.01 | 7.04 | 3.8 | 4.99 |
| Systolic Blood Pressure-SD 5 | 8.43 | 5.41 | 3.11 | 3.37 |
| Diastolic Blood Pressure-MAE 4 | 6.38 | 3.86 | 2.08 | 4.03 |
| Diastolic Blood Pressure-SD 5 | 3.91 | 2.71 | 2.33 | 2.48 |
| Systolic Blood Pressure- Score | 0.1664 | 0.6948 | 0.9068 | 0.8598 |
| Diastolic Blood Pressure- Score | 0.2311 | 0.6944 | 0.8658 | 0.6924 |
| BHS-SBP | BHS-DBP | AAMI-SBP | AAMI-DBP | |
|---|---|---|---|---|
| Fan [13] | - | - | ||
| Rong [14] | Passed | Passed | ||
| Goudarzi [17] | - | - | ||
| Rong [38] | Passed | Passed | ||
| Proposed Method | Passed | Passed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stogiannopoulos, T.; Mitianoudis, N. Non-Contact Blood Pressure Estimation Using Forehead and Palm Infrared Video. BioMedInformatics 2024, 4, 437-453. https://doi.org/10.3390/biomedinformatics4010025
Stogiannopoulos T, Mitianoudis N. Non-Contact Blood Pressure Estimation Using Forehead and Palm Infrared Video. BioMedInformatics. 2024; 4(1):437-453. https://doi.org/10.3390/biomedinformatics4010025
Chicago/Turabian StyleStogiannopoulos, Thomas, and Nikolaos Mitianoudis. 2024. "Non-Contact Blood Pressure Estimation Using Forehead and Palm Infrared Video" BioMedInformatics 4, no. 1: 437-453. https://doi.org/10.3390/biomedinformatics4010025
APA StyleStogiannopoulos, T., & Mitianoudis, N. (2024). Non-Contact Blood Pressure Estimation Using Forehead and Palm Infrared Video. BioMedInformatics, 4(1), 437-453. https://doi.org/10.3390/biomedinformatics4010025








