Biotransformation of Pollutants by Pycnoporus spp. in Submerged and Solid-State Fermentation: Mechanisms, Achievements, and Perspectives
Abstract
:1. Introduction
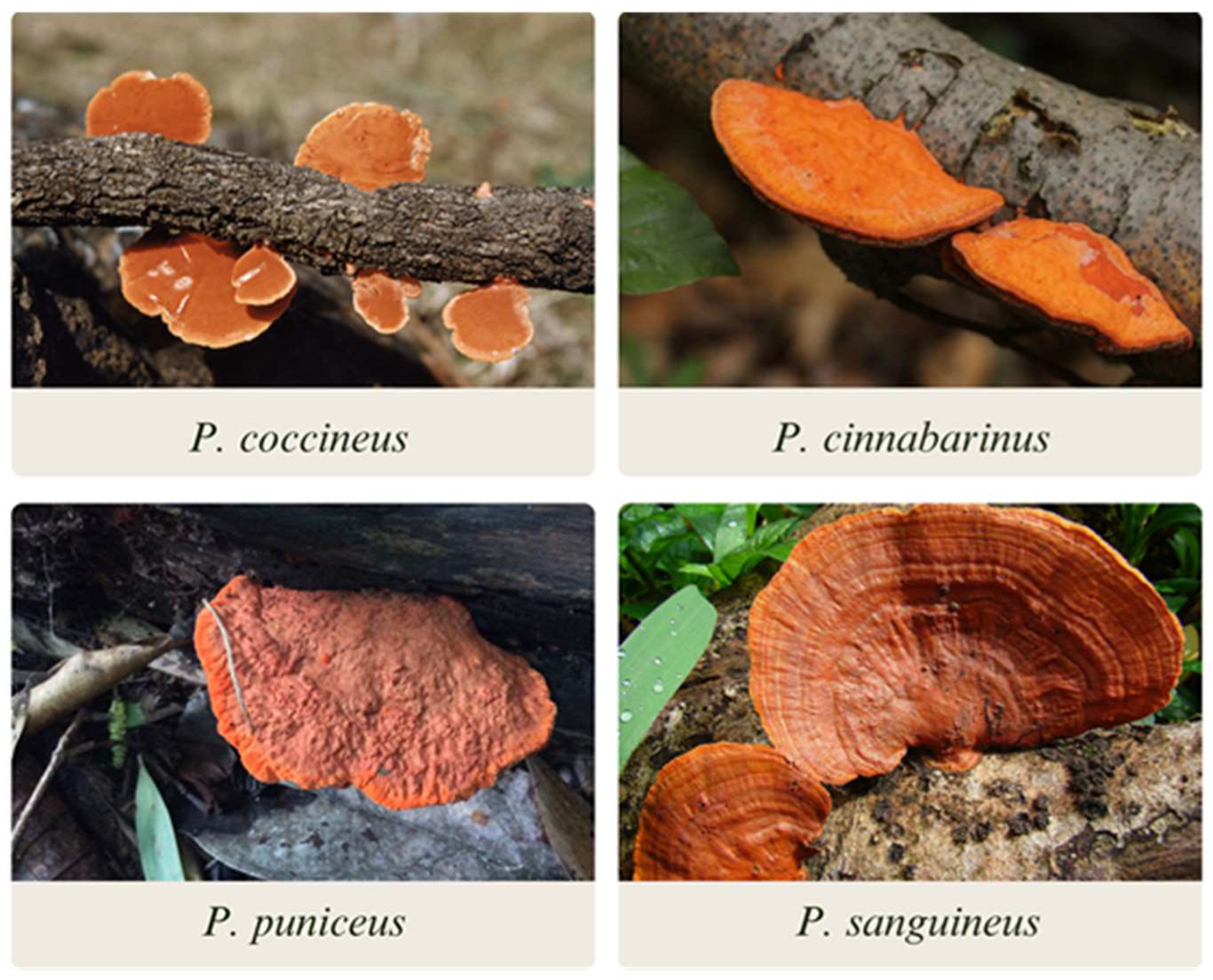
2. The genus Pycnoporus
3. General Mechanisms Used by Pycnoporus spp. for the Biodegradation of Pollutants
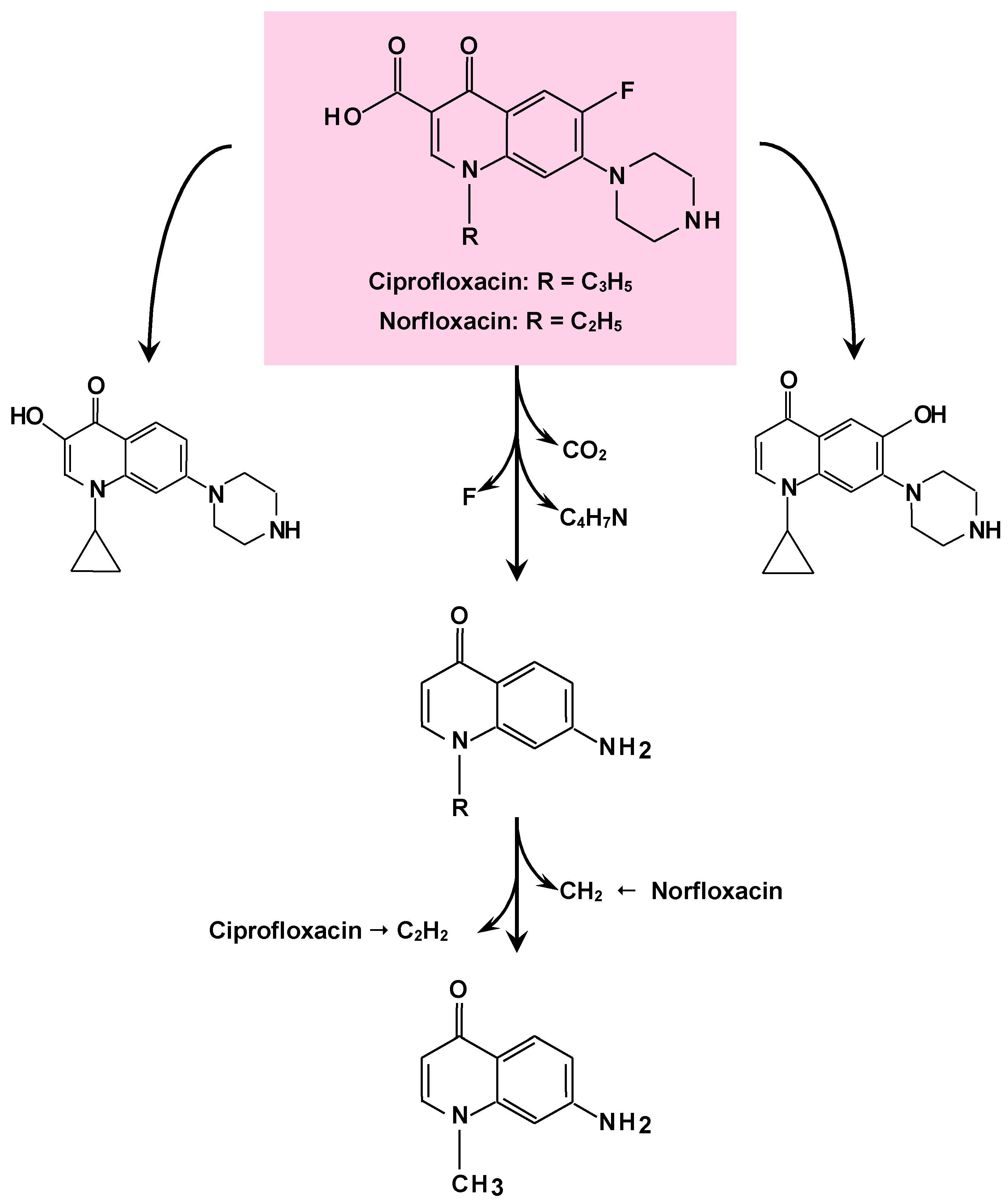
4. Degradation of Pharmaceuticals
5. Decolorization and Degradation of Synthetic Dyes by Pycnoporus spp. and Their Enzymes
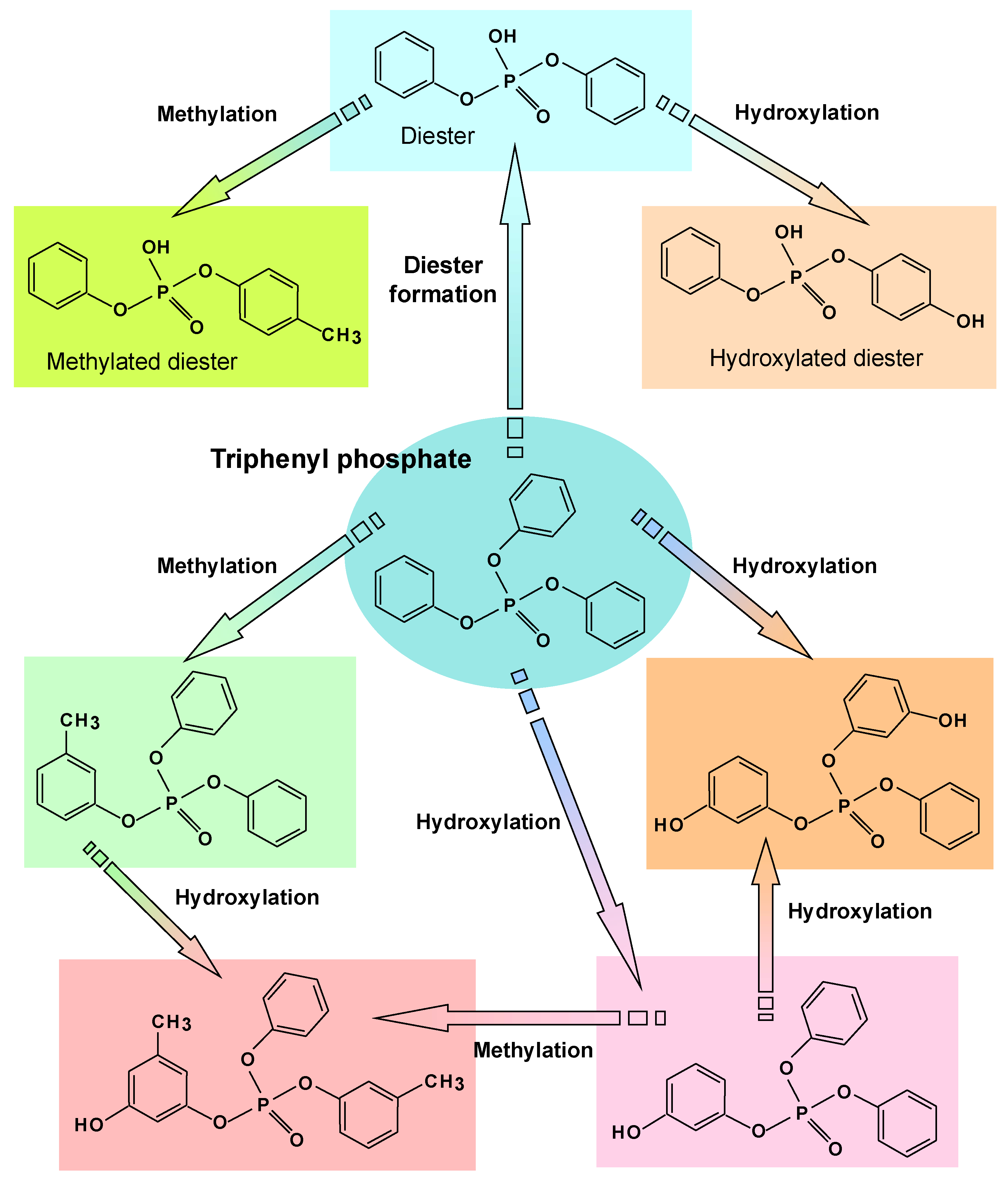
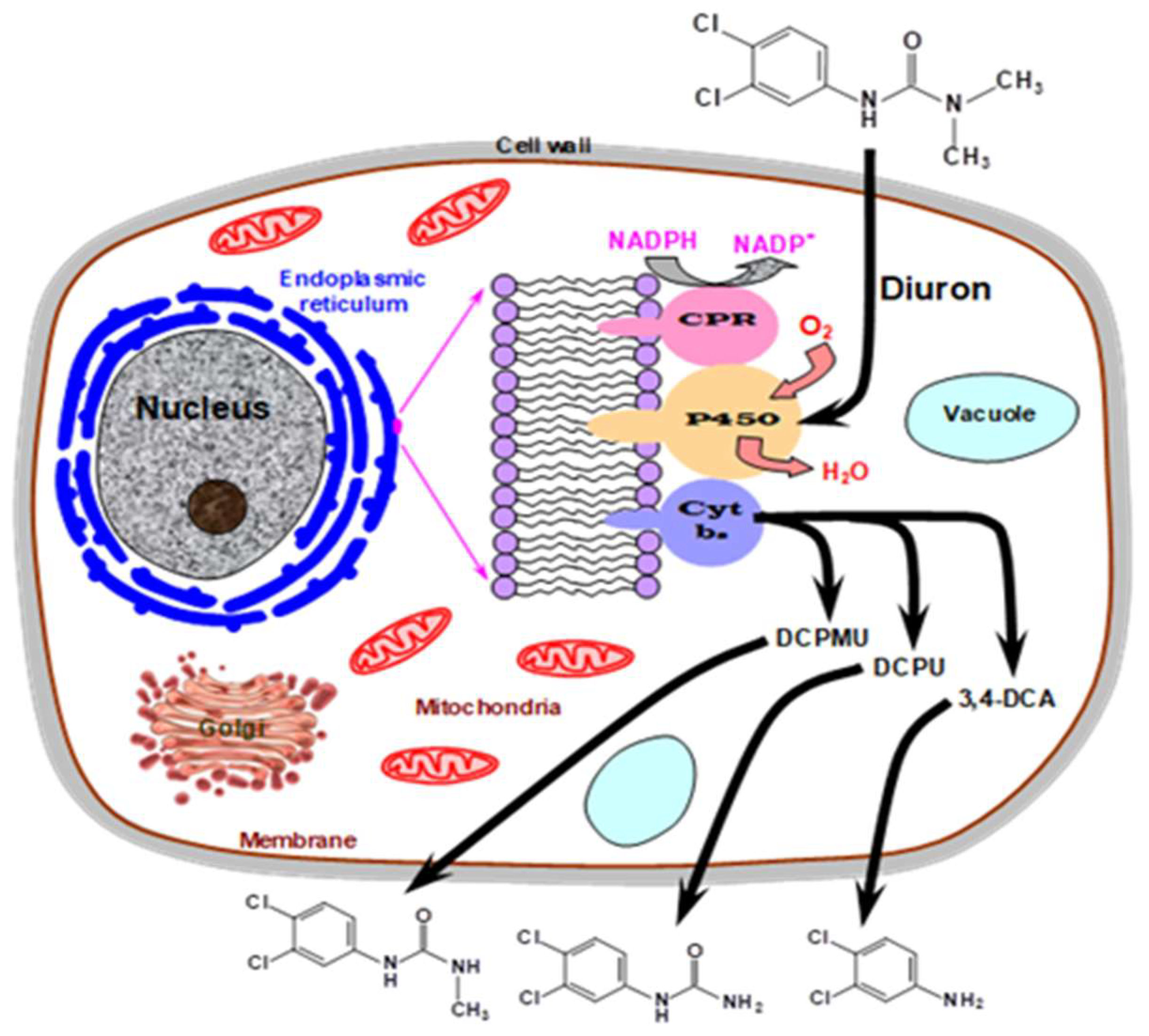
6. Degradation of Flame Retardants and Pesticides
7. Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Navina, B.K.; Velmurugan, N.K.; Kumar, P.S.; Rangasamy, G.; Palanivelu, J.; Thamarai, P.; Vickram, A.S.; Saravanan, A.; Shakoor, A. Fungal bioremediation approaches for the removal of toxic pollutants: Mechanistic understanding for biorefinery applications. Chemosphere 2024, 350, 141123. [Google Scholar] [CrossRef]
- Peralta, R.M.; da Silva, B.P.; Côrrea, R.C.G.; Kato, C.G.; Seixas, F.A.V.; Bracht, A. Enzymes from basidiomycetes—Peculiar and efficient tools for biotechnology. In Biotechnology of Microbial, Enzymes; Brahmachari, G., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 1, pp. 119–149. [Google Scholar] [CrossRef]
- Voběrková, S.; Solčány, V.; Vršanská, M.; Adam, V. Immobilization of ligninolytic enzymes from white-rot fungi in cross-linked aggregates. Chemosphere 2018, 202, 694–707. [Google Scholar] [CrossRef] [PubMed]
- Andlar, M.; Rezić, T.; Marđetko, N.; Kracher, D.; Ludwig, R.; Šantek, B. Lignocellulose degradation: An overview of fungi and fungal enzymes involved in lignocellulose degradation. Eng. Life Sci. 2018, 18, 768–778. [Google Scholar] [CrossRef] [PubMed]
- Bakratsas, G.; Antoniadis, K.; Athanasiou, P.E.; Katapodis, P.; Stamatis, H. Laccase and Biomass Production via Submerged Cultivation of Pleurotus ostreatus using wine lees. Biomass 2024, 4, 1–22. [Google Scholar] [CrossRef]
- Uber, T.M.; Backes, E.; Saute, V.M.S.; Silva, B.P.; Corrêa, R.C.G.; Kato, C.G.; Seixas, F.A.V.; Bracht, A.; Peralta, R.M. Enzymes from basidiomycetes—Peculiar and efficient tools for biotechnology. In Biotechnology of Microbial Enzymes—Production, Catalysis, and Industrial Applications; Brahmachari, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2023; Chapter 6; pp. 129–164. [Google Scholar] [CrossRef]
- Chio, C.; Sain, M.; Qin, W. Lignin utilization: A review of lignin depolymerization from various aspects. Renew. Sustain. Energy Rev. 2019, 107, 232–249. [Google Scholar] [CrossRef]
- Justo, A.; Hibbett, D.S. Phylogenetic classification of Trametes (Basidiomycota, Polyporales) based on a five–marker dataset. Taxon 2011, 60, 1567–1583. [Google Scholar] [CrossRef]
- Téllez-Téllez, M.; Villegas, E.; Rodríguez, A.; Acosta-Urdapilleta, M.L.; O’Donovan, A.; Díaz-Godínez, G. Fungi of Pycnoporus: Morphological and moleculer identification, worldwide distribution and biotechnological potential. Mycosphere 2016, 11, 1500. [Google Scholar] [CrossRef]
- Lomascolo, A.; Uzan-Boukhris, E.; Herpoel-Gimbert, I.; Sigoillot, J.C.; Lesage-Meessen, L. Peculiarities of Pycnoporus species for applications in biotechnology. Appl. Microbiol. Biotechnol. 2011, 92, 1129–1149. [Google Scholar] [CrossRef]
- Molinar, E.; Rios, N.; Spadafora, C.; Arnold, A.E.; Coley, P.D.; Kursar, T.A.; Gerwick, W.H.; Cubilla-Rios, L. Coibanoles, a new class of meroterpenoids produced by Pycnoporus sanguineus. Tetrahedron Lett. 2012, 53, 919–922. [Google Scholar] [CrossRef]
- Welti, S.; Moreau, P.; Favel, A.; Courtecuisse, R.; Haon, M.; Navarro, D.; Taussac, S.; Lesage-Meessen, L. Molecular phylogeny of Trametes and related genera, and description of a new genus Leiotrametes. Fungal Divers. 2012, 55, 47–64. [Google Scholar] [CrossRef]
- Schinagl, C.W.; Siewert, B.; Hammerle, F.; Spes, G.; Peintner, U.; Schlierenzauer, M.; Vrabl, P. Growth, morphology, and formation of cinnabarin in Pycnoporus cinnabarinus in relation to different irradiation spectra. Photochem. Photobiol. Sci. 2023, 22, 2861–2875. [Google Scholar] [CrossRef]
- Lesage-Meessen, L.; Haon, M.; Uzan, E.; Levasseur, A.; Piumi, F.; Navarro, D.; Taussac, S.; Favel, A.; Lomascolo, A. Phylogeographic relationships in the polypore fungus Pycnoporus inferred from molecular data. FEMS Microbiol. Lett. 2011, 325, 37–48. [Google Scholar] [CrossRef]
- Rohr, C.O.; Levin, L.; Mentaberry, A.; Wirth, S. A First Insight into Pycnoporus sanguineus BAFC 2126 Transcriptome. PLoS ONE 2013, 8, e81033. [Google Scholar] [CrossRef]
- Levasseur, A.; Lomascolo, A.; Chabrol, O.; Ruiz-Dueñas, F.J.; Boukhris-Uzan, E.; Piumi, F.; Kües, U.; Ram, A.F.J.; Murat, C.; Haon, M.; et al. The genome of the white-rot fungus Pycnoporus cinnabarinus: A basidiomycete model with a versatile arsenal for lignocellulosic biomass breakdown. BMC Genom. 2014, 15, 486. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Jia, G.; Sun, H.; Sun, T.; Hou, D. Genome sequence of the fungus Pycnoporus sanguineus, which produces cinnabarinic acid and pH-and thermo-stable laccases. Gene 2020, 742, 144586. [Google Scholar] [CrossRef]
- Miyauchi, S.; Hage, H.; Drula, É.; Lesage-Meessen, L.; Berrin, J.; Navarro, D.; Favel, A.; Chaduli, D.; Grisel, S.; Haon, M.; et al. Conserved white-rot enzymatic mechanism for wood decay in the Basidiomycota genus Pycnoporus. DNA Res. 2020, 27, dsaa011. [Google Scholar] [CrossRef] [PubMed]
- Gil, R.G.; Brambila, O.M.G.; Bedrán, H.V.; Martínez, J.C.G.; Luna, J.A.C.; Brambila, M.M.G. Depolymerization of lignin by extracellular activity of Pycnoporus cinnabarinus, to obtain cellulose. Int. J. Chem. React. Eng. 2023, 21, 445–460. [Google Scholar] [CrossRef]
- Asther, M.; Lomascolo, A.; Mi, A.; Moukha, S.; Lesage-Meessen, L. Metabolic pathways of biotransformation and biosynthesis of aromatic compounds for the flavour industry by the basidiomycete Pycnoporus cinnabarinus. Micol. Neotrop. Apl. 1998, 11, 69–76. [Google Scholar]
- Smânia, A.; Marques, C.J.S.; Smânia, E.F.A.; Zanetti, C.R.; Carobrez, S.G.; Tramonte, R.; Loguercio-Leite, C. Toxicity and antiviral activity of cinnabarin obtained from Pycnoporus sanguineus (Fr.) Murr. Phytother. Res. 2003, 17, 1069–1072. [Google Scholar] [CrossRef]
- Meng, D.; Shao, X.; Luo, S.P.; Tian, Q.; Liao, X. Pigment production by a newly isolated strain Pycnoporus sanguineus SYBC-L7 in solid-state fermentation. Front. Microbiol. 2022, 13, 1015913. [Google Scholar] [CrossRef]
- Bentil, J.A.; Thygesen, A.; Mensah, M.; Lange, L.; Meyer, A.S. Cellulase production by white rot basidiomycetous fungi: Solid state versus submerged cultivation. Appl. Microbiol. Biotechnol. 2018, 102, 5827–5839. [Google Scholar] [CrossRef] [PubMed]
- Wattanakitjanukul, N.; Sukkasem, C.; Chiersilp, B.; Boonsawang, P. Use of palm empty fruit bunches for the production of ligninolytic enzymes by Xylaria sp. in solid state fermentation. Waste Biomass Valori. 2020, 11, 3953–3964. [Google Scholar] [CrossRef]
- Yazid, A.N.; Barrena, R.; Komilis, D.; Sánchez, A. Solid-state fermentation as a novel paradigm for organic waste valorization: A review. Sustainability 2017, 9, 224. [Google Scholar] [CrossRef]
- Ahmed, P.M.; Álvarez, A.; De Figueroa, L.I.C.; Pajot, H.F. Boldly going green: Utilizing Pycnoporus sp. for laccase production and sustainable vinasse treatment. Int. J. Environ. Sci. Technol. 2023, in press. [Google Scholar] [CrossRef]
- Bai, Y.; Liang, H.; Wang, L.; Li, Y.; Cheng, L.; Gao, D. Bioremediation of Diesel-Contaminated Soil by Fungal Solid-State Fermentation. Bull. Environ. Contam. Toxicol. 2024, 112, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Rawal, R.S.; Mehant, A.; Suman, S.K. Deciphering ligninolytic enzymes in the secretome of Pycnoporus sp. and their potential in degradation of 2-chlorophenol. Environ. Sci. Pollut. R. 2023, 30, 92830–92841. [Google Scholar] [CrossRef] [PubMed]
- Falkoski, D.L.; Guimarães, V.M.; de Almeida, M.N.; Alfenas, A.C.; Colodette, J.L.; de Rezende, S.T. Characterization of cellulolytic extract from Pycnoporus sanguineus PF-2 and its application in biomass saccharification. Appl. Biochem. Biotechnol. 2012, 166, 1586–1603. [Google Scholar] [CrossRef] [PubMed]
- Azmi, M.A.A.M.; Jalil, R.; Kalil, M.S. Production of Cellulase from Pycnoporus sanguineus. Ind. J. Sci. Technol. 2016, 9, 21. [Google Scholar] [CrossRef]
- Maphatsoe, M.M.; Hashem, C.; Ling, J.G.; Horvat, M.; Rumbold, K.; Bakar, F.D.A.; Winkler, M. Characterization and immobilization of Pycnoporus cinnabarinus carboxylic acid reductase, PcCAR2. J. Biotechnol. 2022, 345, 47–54. [Google Scholar] [CrossRef]
- Akhtar, M.K.; Turner, N.J.; Jones, P.R. Carboxylic acid reductase is a versatile enzyme for the conversion of fatty acids into fuels and chemical commodities. Proc. Natl. Acad. Sci. USA 2012, 110, 87–92. [Google Scholar] [CrossRef]
- Ferreira, A.N.; Silva, A.T.; Nascimento, J.S.D.; De Souza, C.B.; Da Costa Silva, M.; Grillo, L.A.M.; Da Luz, J.M.R.; Pereira, H.J.V. Production, characterization, and application of a new chymotrypsin-like protease from Pycnoporus sanguineus. Biocatal. Biotransfor. 2023, in press. [Google Scholar] [CrossRef]
- Bilal, M.; Rasheed, T.; Nabeel, F.; Iqbal, H.M.N.; Zhao, Y. Hazardous contaminants in the environment and their laccase-assisted degradation—A review. J. Environ. Manag. 2019, 234, 253–264. [Google Scholar] [CrossRef]
- Scarpa, J.C.P.; Marques, N.P.; Monteiro, D.A.; Martins, G.M.; de Paula, A.V.; Silva, R.; Gomes, E.; Cocchini, D.A. Saccharification of pretreated sugarcane bagasse using enzymes solution from Pycnoporus sanguineus MCA 16 and cellulosic ethanol production. Ind. Crops Prod. 2019, 141, 111795. [Google Scholar] [CrossRef]
- Wang, F.; Xu, L.; Zhao, L.; Ding, Z.; Ma, H.; Terry, N. Fungal laccase production from lignocellulosic agricultural wastes by solid-state fermentation: A review. Microorganisms 2019, 7, 665. [Google Scholar] [CrossRef]
- Barber-Zucker, S.; Mateljak, I.; Goldsmith, M.; Kupervaser, M.; Alcalde, M.; Fleishman, S.J. Designed High-Redox Potential Laccases exhibit high functional diversity. ACS Catal. 2022, 12, 13164–13173. [Google Scholar] [CrossRef]
- Uzan, E.; Nousiainen, P.; Balland, V.; Sipila, J.; Piumi, F.; Navarro, D.; Asther, M.; Record, E.; Lomascolo, E. High redox potential laccases from the ligninolytic fungi Pycnoporus coccineus and Pycnoporus sanguineus suitable for white biotechnology: From gene cloning to enzyme characterization and applications. J. Appl. Microbiol. 2010, 108, 2199–2213. [Google Scholar] [CrossRef]
- Zimbardi, A.L.R.L.; Camargo, P.F.; Carli, S.; Neto, S.A.; Meleiro, L.P.; Rosa, J.C.; De Andrade, A.R.; Jorge, J.A.; Furriel, R.P.M. A high redox potential laccase from Pycnoporus sanguineus RP15: Potential application for dye decolorization. Int. J. Mol. Sci. 2016, 17, 672. [Google Scholar] [CrossRef]
- Tian, Q.; Dou, X.; Huang, L.; Wang, L.; Meng, D.; Zhai, L.; Shen, Y.; You, C.; Zhang, G.; Liao, X. Characterization of a robust cold-adapted and thermostable laccase from Pycnoporus sp. SYBC-L10 with a strong ability for the degradation of tetracycline and oxytetracycline by laccase-mediated oxidation. J. Hazard. Mat. 2020, 382, 121084. [Google Scholar] [CrossRef]
- Guardado, A.L.P.; Belleville, M.P.; Alanis, M.J.R.; Sanches-Marcan, J. Effect of redox mediators in pharmaceuticals degradation by laccase: A comparative study. Process Biochem. 2019, 78, 123–131. [Google Scholar] [CrossRef]
- Malci, K.; Kurt-Gur, G.; Tamerler, C.; Yazgan-Karatas, A. Combinatorial decolorization performance of Pycnoporus sanguineus MUCL 38531 sourced recombinant laccase/mediator systems on toxic textile dyes. Int. J. Environ. Sci.Technol. 2023, 20, 951–996. [Google Scholar] [CrossRef]
- Ramirez-Cavazos, L.I.; Junghanns, C.; Nair, R.; Cardenas-Chavez, D.L.; Hernandez-Luna, C.; Agathos, S.N.; Parra, R. Enhanced production of thermostable laccases from a native strain of Pycnoporus sanguineus using central composite design. J. Zhejiang Univ. Sci. B 2014, 15, 343–352. [Google Scholar] [CrossRef]
- Golveia, J.C.S.; Santiago, M.F.; Sales, P.T.F.; Sartoratto, A.; Ponezi, A.N.; Thomaz, D.V.; Gil, E.S.; Bara, M.T.F. Cupuaçu (Theobroma grandiflorum) residue and its potential application in the bioremediation of 17-A-ethinylestradiol as a Pycnoporus sanguineus laccase inducer. Prep. Biochem. Biotechnol. 2018, 48, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Awasthi, M.K.; Zhang, Z.; Wong, J.W.C. Sustainable composting and its environmental implications. In Sustainable Resource Recovery and Zero Waste Approaches; Mohammad, J.T., Bolton, K., Wong, J., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 115–132. [Google Scholar] [CrossRef]
- Cheute, V.M.S.; Backes, E.; Corrêa, R.C.G.; Corrêa, V.G.; Bracht, A.; Peralta, R.M. The global market for mushrooms, their uses as dietary supplements and associated safety issues. In Food Chemistry, Function and Analysis; Stojkovic, D., Barros, L., Eds.; Royal Society of Chemistry: London, UK, 2022; Chapter 11; pp. 383–397. [Google Scholar] [CrossRef]
- Gutiérrez Soto, G.; Medina González, G.E.; García Zambrano, E.A.; Treviño Ramírez, J.E.; Hernández Luna, C.E. Selection and characterization of a native Pycnoporus sanguineus strain as a lignocellulolytic extract producer from submerged cultures of various agroindustrial wastes. Bioresources 2015, 10, 3564–3576. [Google Scholar] [CrossRef]
- Rodríguez, M.D.; Paiva, I.M.A.; Castrillo, M.L.; Zapata, P.D.; Villalba, L.L. KH2PO4 improves cellulase production of Irpex lacteus and Pycnoporus sanguineus. J. King Saud Univ. Sci. 2019, 31, 434–444. [Google Scholar] [CrossRef]
- Saat, M.N.; Annuar, M.S.M.; Alias, Z.; Chuan, L.T.; Chisti, Y. Modeling of growth and laccase production by Pycnoporus sanguineus. Bioproc. Biosyst. Eng. 2014, 37, 765–775. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yin, H.; Peng, H.; Feng, M.; Lu, G.; Dang, Z. Degradation of 2, 2′, 4, 4′-tetrabromodiphenyl ether by Pycnoporus sanguineus in the presence of copper ions. J. Environ. Sci. 2019, 83, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Daou, M.; Piumi, F.; Cullen, D.; Record, E.; Faulds, C.B. Heterologous production and characterization of two glyoxal oxidases from Pycnoporus cinnabarinus. Appl. Environ. Microbiol. 2016, 82, 4867–4875. [Google Scholar] [CrossRef]
- Díaz-Godínez, G.; Téllez-Téllez, M.; Rodríguez, A.; Obregón-Barbosa, V.; Acosta-Urdapilleta, M.D.L.; Villegas, E. Enzymatic, antioxidant, antimicrobial, and insecticidal activities of Pleurotus pulmonarius and Pycnoporus cinnabarinus grown separately in an airlift reactor. Bioresources 2016, 11, 4186–4200. [Google Scholar] [CrossRef]
- Niderhaus, C.; Garrido, M.; Insani, M.; Campos, E.; Wirth, S. Heterologous production and characterization of a thermostable GH10 family endo-xylanase from Pycnoporus sanguineus BAFC 2126. Proc. Biochem. 2018, 67, 92–98. [Google Scholar] [CrossRef]
- Liu, J.; Zhonghua, Y.; Liao, X.; Liu, J.; Mao, F.; Huang, Q. Scalable production, fast purification, and spray drying of native Pycnoporus laccase and circular dichroism characterization. J. Clean. Prod. 2016, 127, 600–609. [Google Scholar] [CrossRef]
- Echezonachi, S.O. The role of white rot fungi in bioremediation. In Microbes and Microbial Biotechnology for Green Remediation Elsevier eBooks; Malik, J.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 305–322. [Google Scholar] [CrossRef]
- Zhuo, R.; Fan, F. A comprehensive insight into the application of white rot fungi and their lignocellulolytic enzymes in the removal of organic pollutants. Sci. Total Environ. 2021, 778, 146132. [Google Scholar] [CrossRef] [PubMed]
- Ellouze, M.; Sayadi, S. White-rot fungi and their enzymes as a biotechnological tool for xenobiotic bioremediation. In Management of Hazardous Wastes; El-Din, H., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 103–120. [Google Scholar] [CrossRef]
- Morsi, R.; Bilal, M.; Iqbal, H.M.N.; Ashraf, S.S. Laccases and peroxidases: The smart, greener and futuristic biocatalytic tools to mitigate recalcitrant emerging pollutants. Sci. Total Environ. 2020, 714, 136572. [Google Scholar] [CrossRef]
- Mattila, H.K.; Österman-Udd, J.; Mali, T.; Lundell, T. Basidiomycota Fungi and ROS: Genomic Perspective on Key Enzymes Involved in Generation and Mitigation of Reactive Oxygen Species. Front. Fungal Biol. 2022, 3, 837605. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. ROS are good. Trends Plants Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Maciel, G.M.; Inácio, F.D.; Sá-Nakanishi, A.B.; Haminiuk, C.W.I.; Castoldi, R.; Comar, J.F.; Bracht, A.; Peralta, R.M. Response of Ganoderma lucidum and Trametes sp. to the herbicide picloram: Tolerance, antioxidants and production of ligninolytic enzymes. Pest. Biochem. Physiol. 2013, 105, 84–92. [Google Scholar] [CrossRef]
- Backes, E.; Kato, C.G.; De Oliveira, V.A.; Uber, T.M.; Santos, L.F.O.D.; Corrêa, R.C.G.; Bracht, A.; Peralta, R.M. Overproduction of Laccase by Trametes versicolor and Pycnoporus sanguineus in Farnesol-Pineapple Waste Solid Fermentation. Fermentation 2023, 9, 188. [Google Scholar] [CrossRef]
- Cresnar, B.; Petric, S. Cytochrome P450 enzymes in the fungal kingdom. Biochim. Biophys. Acta Proteins Proteom. 2011, 1814, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Wei, J.; Yang, B.; Zhang, M.; Zhuo, R. Bioremediation of organic pollutants by white rot fungal cytochrome P450: The role and mechanism of CYP450 in biodegradation. Chemosphere 2022, 301, 134776. [Google Scholar] [CrossRef]
- Łebkowska, M.; Załęska-Radziwiłł, M. Application of white-rot fungi for biodegradation of refractory organic compounds—A review. Desalin. Water Treat. 2014, 52, 3708–3713. [Google Scholar] [CrossRef]
- Ijoma, G.N.; Tekere, M. Potential microbial applications of co-cultures involving ligninolytic fungi in the bioremediation of recalcitrant xenobiotic compounds. Int. J. Environ. Sci. Technol. 2017, 14, 1787–1806. [Google Scholar] [CrossRef]
- da Silva, V.E.; Tadayozzi, Y.S.; Putti, F.F.; Santos, F.A.; Forti, J.C. Degradation of commercial glyphosate-based herbicide via advanced oxidative processes in aqueous media and phytotoxicity evaluation using maize seeds. Sci. Total Environ. 2022, 840, 156656. [Google Scholar] [CrossRef]
- Forti, J.C.; Loretti, G.H.; Tadayozzi, Y.S.; de Andrade, A.R. A phytotoxicity assessment of the efficiency 2,4-D degradation by different oxidative processes. J. Environ. Manag. 2020, 266, 110588. [Google Scholar] [CrossRef]
- Gao, N.; Liu, C.X.; Xu, Q.M.; Cheng, J.S.; Yuan, Y.J. Simultaneous removal of ciprofloxacin, norfloxacin, sulfamethoxazole by co-producing oxidative enzymes system of Phanerochaete chrysosporium and Pycnoporus sanguineus. Chemosphere 2018, 195, 146–155. [Google Scholar] [CrossRef]
- Garcia, L.F.; Lacerda, M.F.A.R.; Thomaz, D.V.; de Souza-Golveia, J.C.; Pereira, M.D.G.C.; de Souza-Gil, E.; Schimidt, F.; Santiago, M.F. Optimization of laccase–alginate–chitosan-based matrix toward 17 α-ethinylestradiol removal. Prep. Biochem. Biotechnol. 2019, 49, 375–383. [Google Scholar] [CrossRef] [PubMed]
- González-Coronel, L.A.; Cobas, M.; Rostro-Alanis, M.J.; Parra-Saldívar, R.; Hernandez-Luna, C.; Pazos, M.; Sanromán, M.Á. Immobilization of laccase of Pycnoporus sanguineus CS43. New Biotechnol. 2017, 39, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Long, S.Y.; Huang, J.; Xiao, H.; Ju-Ying, Z. Immobilization of Pycnoporus sanguineus laccase on magnetic chitosan microspheres. Biochem. Eng. J. 2005, 25, 15–23. [Google Scholar] [CrossRef]
- García-Morales, R.; García-García, A.; Orana-Navar, C.; Osma, J.F.; Nigam, K.D.P.; Ornelas-Soto, N. Biotransformation of emerging pollutants in groundwater by laccase from P. sanguineus CS43 immobilized onto titania nanoparticles. J. Environ. Chem. Eng. 2018, 6, 710–717. [Google Scholar] [CrossRef]
- Rodriguez-Delgado, M.; Ornelas-Soto, N.; Martínez-Lorán, E.; Hernandez-Luna, C.; García-García, A.; Contreras-Torres, F.F. Enhanced enzymatic activity of laccase (from Pycnoporus sanguineus CS43) immobilized on sputtered nanostructured gold thin films. J. Nanosci. Nanotechnol. 2017, 17, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.S.; Don, M.M. Optimization of process variables for the synthesis of silver nanoparticles by Pycnoporus sanguineus using statistical experimental design. J. Korean Soc. Appl. Biol. Chem. 2013, 56, 11–20. [Google Scholar] [CrossRef]
- Barrios-Estrada, C.; Rostro-Alanis, M.J.; Parra, A.L.; Belleville, M.P.; Sanchez-Marcano, J.; Iqbal, H.M.N.; Parra-Saldivar, T. Potentialities of active membranes with immobilized laccase for Bisphenol A degradation. Int. J. Biol. Macromol. 2018, 108, 837–844. [Google Scholar] [CrossRef]
- Kyomuhimbo, H.D.; Brink, H.G. Applications and immobilization strategies of the copper-centred laccase enzyme: A review. Heliyon 2023, 9, e13156. [Google Scholar] [CrossRef]
- Uber, T.M.; Buzzo, A.J.D.R.; Scaratti, G.; De Amorim, S.M.; Helm, C.V.; Maciel, G.M.; Peralta, R.A.; Moreira, R.F.P.M.; Bracht, A. Comparative detoxification of Remazol Brilliant Blue R by free and immobilized laccase of Oudemansiella canarii. Biocatal. Biotransform. 2022, 40, 17–28. [Google Scholar] [CrossRef]
- Li, X.; Xu, Q.M.; Cheng, J.S.; Yuan, Y.J. Improving the bioremoval of sulfamethoxazole and alleviating cytotoxicity of its biotransformation by laccase producing system under coculture of Pycnoporus sanguineus and Alcaligenes faecalis. Bioresour. Technol. 2016, 220, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Cavazos, L.I.; Junghanns, C.; Ornelas-Soto, N.; Cardenas-Chávez, D.L.; Hernandez-Luna, C.; Demarche, P.; Enaud, E.; Garcia-Morales, R.; Agathos, S.N.; Parra, R. Purification and characterization of two thermostable laccases from Pycnoporus sanguineus and potential role in degradation of endocrine disrupting chemicals. J. Mol. Catal. B Enzym. 2014, 108, 32–42. [Google Scholar] [CrossRef]
- Rodriguez-Delgado, M.; Orona-Navar, C.; García-Morales, R.; Hernandez-Luna, C.; Parra, R.; Mahlknecht, J.; Ornelas-Soto, N. Biotransformation kinetics of pharmaceutical and industrial micropollutants in groundwaters by a laccase cocktail from Pycnoporus sanguineus CS43 fungi. Int. Biodeterior. Biodegrad. 2016, 108, 34–41. [Google Scholar] [CrossRef]
- Herath, I.S.; Udayanga, D.; Jayasanka, D.J.; Hewawasam, C. Textile dye decolorization by white rot fungi–A review. Bioresour. Technol. Rep. 2024, 25, 101687. [Google Scholar] [CrossRef]
- Aouni, A.; Fersi, C.; Cuartas-Uribe, B.; Bes-Piá, A.; Alcaina–Miranda, M.I.; Dhahbim, M. Reactive dyes rejection and textile effluent treatment study using ultrafiltration and nanofiltration processes. Desalination 2012, 297, 87–96. [Google Scholar] [CrossRef]
- Jeyabalan, J.; Veluchamy, A.; Priyan, V.V.; Kumar, A.; Chandrasekar, R.; Narayanasamy, S. A review on the laccase assisted decolourization of dyes: Recent trends and research progress. J. Taiwan Inst. Chem. Eng. 2023, 151, 105081. [Google Scholar] [CrossRef]
- Yang, C.H.; Shih, M.C.; Chiu, H.C.; Huang, K.S. Magnetic Pycnoporus sanguineus-loaded alginate composite beads for removing dye from aqueous solutions. Molecules 2014, 19, 8276–8288. [Google Scholar] [CrossRef]
- Iracheta-Cárdenas, M.M.; Rocha-Peña, M.A.; Galán-Wong, L.J.; Arévalo-Niño, K.; Tovar-Herrera, O.E. A Pycnoporus sanguineus laccase for denim bleaching and its comparison with an enzymatic commercial formulation. J. Environ. Manag. 2016, 77, 93–100. [Google Scholar] [CrossRef]
- Gioia, L.; Manta, C.; Ovsejevi, K.; Burgueño, J.; Menéndez, P.; Rodriguez-Couto, S. Enhancing laccase production by a newly-isolated strain of Pycnoporus sanguineus with high potential for dye decolouration. RSC Adv. 2014, 4, 34096–34103. [Google Scholar] [CrossRef]
- Feng, M.; Li, H.; You, S.; Zhang, J.; Lin, H.; Wang, M.; Zhou, J. Effect of hexavalent chromium on the biodegradation of tetrabromobisphenol A (TBBPA) by Pycnoporus sanguineus. Chemosphere 2019, 235, 995–1006. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Yin, H.; Peng, H.; Liu, X.; Yang, P.; Lu, G.; Dang, Z. Influence of co-existed tetrabromobisphenol A (TBBPA) and hexavalent chromium on the cellular characteristics of Pycnoporus sanguineus during their removal and reduction. Ecotoxicol. Environ. Saf. 2017, 142, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Zhou, J.; Yu, X.; Wang, H.; Guo, Y.; Mao, W. Bioremediation of triphenyl phosphate by Pycnoporus sanguineus: Metabolic pathway, proteomic mechanism and biotoxicity assessment. J. Hazard. Mater. 2021, 417, 125983. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Wang, X.; Thai, P.K.; Baduel, C.; Gallen, C.; Banks, A.P.W.; Bainton, P.; English, K.; Mueller, J.F. Organophosphate and brominated flame retardants in Australian indoor environments: Levels, sources, and preliminary assessment of human exposure. Environ. Pollut. 2018, 235, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Li, D.Q.; Mu-Ning, Z.; Liao, Y.; Xie, Z.; Guo, T.; Li, J.; Zhang, S.; Liang, Z. Organophosphorus flame retardants and plasticizers: Sources, occurrence, toxicity and human exposure. Environ. Pollut. 2015, 196, 29–46. [Google Scholar] [CrossRef]
- Lee, S.; Cho, H.J.; Choi, W.; Moon, H. Organophosphate flame retardants (OPFRs) in water and sediment: Occurrence, distribution, and hotspots of contamination of Lake Shihwa, Korea. Mar. Pollut. Bull. 2018, 130, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, R.; He, J.; Ma, H.; Zhao, F.; Tao, S.; Liu, J.; Hu, J. Triphenyl phosphate at environmental levels retarded ovary development and reduced egg production in Japanese medaka (Oryzias latipes). Environ. Sci. Technol. 2019, 53, 14709–14715. [Google Scholar] [CrossRef]
- Henn, C.; Arakaki, R.M.; Monteiro, D.A.; Boscolo, M.; Da Silva, R.; Gomes, E. Degradation of the organochlorinated herbicide diuron by rainforest basidiomycetes. BioMed Res. Int. 2020, 2020, 5324391. [Google Scholar] [CrossRef]
- Guengerich, F.P.; Waterman, M.R.; Egli, M. Recent structural insights into cytochrome P450 function. Trends Pharmacol. Sci. 2016, 37, 625–640. [Google Scholar] [CrossRef]
- Coelho-Moreira, J.S.; Brugnari, T.; Sá-Nakanishi, A.B.; Castoldi, R.; de Souza, C.G.M.; Bracht, A.; Peralta, R.M. Evaluation of diuron tolerance and biotransformation by the white-rot fungus Ganoderma Lucidum. Fungal Biol. 2018, 122, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ning, Y.N.; Zhang, X.; Zhao, Y.; Yang, X.; Wu, K.; Yang, S.; La, G.; Sun, X.; Li, X. Contrasting characteristics of anthracene and pyrene degradation by wood rot fungus Pycnoporus sanguineus H1. Int. Biodeterior. Biodegrad. 2015, 105, 228–232. [Google Scholar] [CrossRef]
- Zerva, A.; Simić, S.; Topakas, E.; Nikodinovic-Runic, J. Applications of Microbial laccases: Patent Review of the past decade (2009–2019). Catalysts 2019, 9, 1023. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheute, V.M.S.; Uber, T.M.; dos Santos, L.F.O.; Backes, E.; Dantas, M.P.; Contato, A.G.; Castoldi, R.; de Souza, C.G.M.; Corrêa, R.C.G.; Bracht, A.; et al. Biotransformation of Pollutants by Pycnoporus spp. in Submerged and Solid-State Fermentation: Mechanisms, Achievements, and Perspectives. Biomass 2024, 4, 313-328. https://doi.org/10.3390/biomass4020015
Cheute VMS, Uber TM, dos Santos LFO, Backes E, Dantas MP, Contato AG, Castoldi R, de Souza CGM, Corrêa RCG, Bracht A, et al. Biotransformation of Pollutants by Pycnoporus spp. in Submerged and Solid-State Fermentation: Mechanisms, Achievements, and Perspectives. Biomass. 2024; 4(2):313-328. https://doi.org/10.3390/biomass4020015
Chicago/Turabian StyleCheute, Vinícius Mateus Salvatori, Thaís Marques Uber, Luís Felipe Oliva dos Santos, Emanueli Backes, Marina Proença Dantas, Alex Graça Contato, Rafael Castoldi, Cristina Giatti Marques de Souza, Rúbia Carvalho Gomes Corrêa, Adelar Bracht, and et al. 2024. "Biotransformation of Pollutants by Pycnoporus spp. in Submerged and Solid-State Fermentation: Mechanisms, Achievements, and Perspectives" Biomass 4, no. 2: 313-328. https://doi.org/10.3390/biomass4020015
APA StyleCheute, V. M. S., Uber, T. M., dos Santos, L. F. O., Backes, E., Dantas, M. P., Contato, A. G., Castoldi, R., de Souza, C. G. M., Corrêa, R. C. G., Bracht, A., & Peralta, R. M. (2024). Biotransformation of Pollutants by Pycnoporus spp. in Submerged and Solid-State Fermentation: Mechanisms, Achievements, and Perspectives. Biomass, 4(2), 313-328. https://doi.org/10.3390/biomass4020015








