Algae: Nature’s Renewable Resource for Fuels and Chemicals
Abstract
:1. Introduction
2. Selection of Research Articles
3. Growing Microalgae on Wastewater
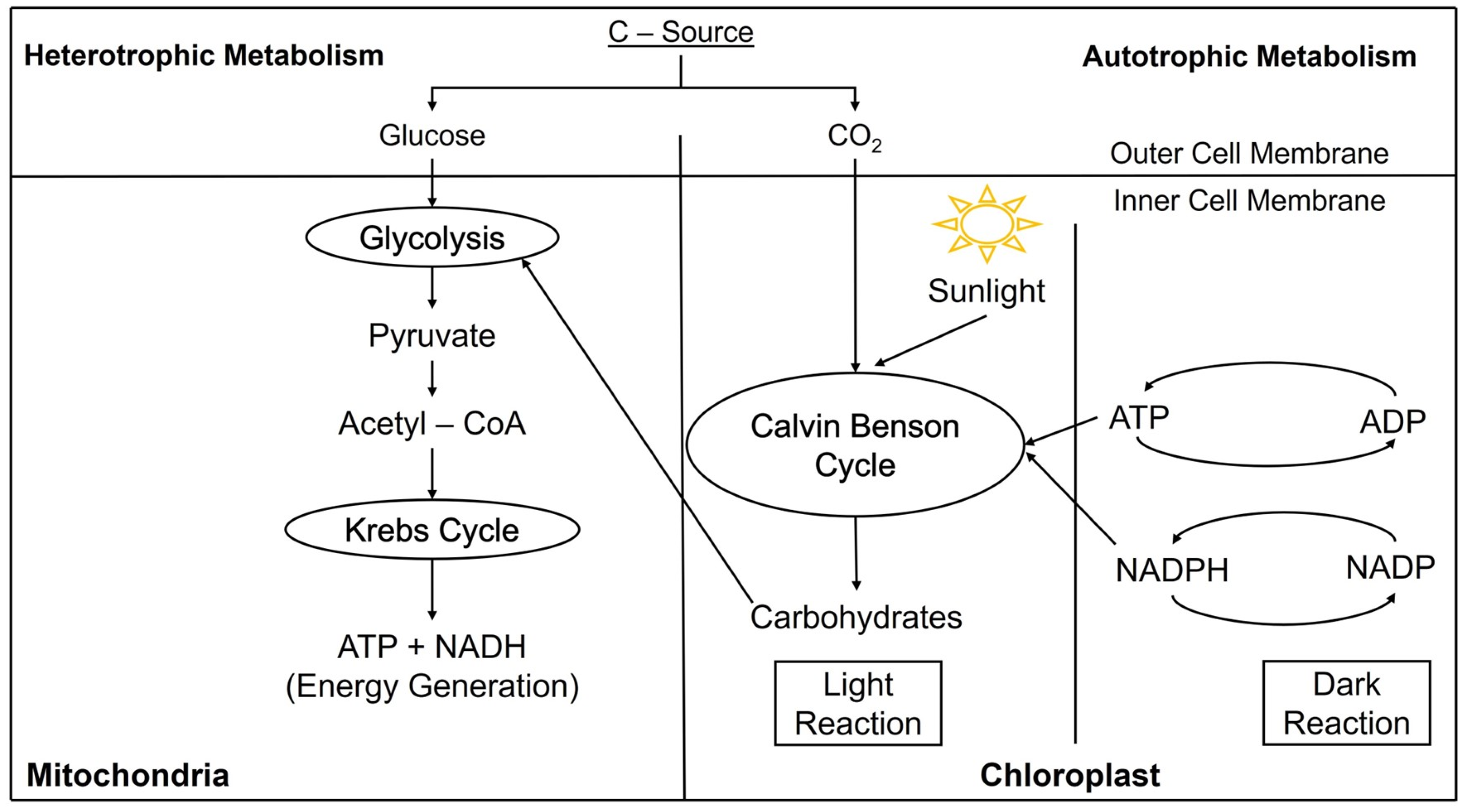
4. Algal Biomass Conversion Processes
4.1. Thermal Conversion Processes
Pyrolysis
4.2. Hydrothermal Processing
4.2.1. Hydrothermal Liquefaction
4.2.2. Hydrothermal Carbonization
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chakraborty, S.; Dunford, N.T.; Goad, C. A Kinetic Study of Microalgae, Municipal Sludge and Cedar Wood Co-Pyrolysis. Renew. Energy 2021, 165, 514–524. [Google Scholar] [CrossRef]
- Wolski, P.; Blankenship, B.W.; Umar, A.; Cabrera, M.; Simmons, B.A.; Sale, K.L.; Achinivu, E.C. Factors That Influence the Activity of Biomass-Degrading Enzymes in the Presence of Ionic Liquids—A Review. Front. Energy Res. 2023, 11, 1212719. [Google Scholar] [CrossRef]
- Jacinto, G.S.S.; Cruz, G.; Cabral, A.A.; Bezerra, G.V.P.; Peña Garcia, R.R.; Magalhães, U.N.; Gomes, W.C. Biotechnological Investigation of Pediastrum Boryanum and Desmodesmus Subspicatus Microalgae Species for a Potential Application in Bioenergy. Algal Res. 2023, 75, 103266. [Google Scholar] [CrossRef]
- Chakraborty, S.; Dunford, N.T. Evaluation of Microalgae, Sludge and Cedar Wood Blends for Aromatic Hydrocarbon-Enriched Bio-Oil Production. Energy Ecol. Environ. 2022, 7, 408–424. [Google Scholar] [CrossRef]
- Machado, L.; Carvalho, G.; Pereira, R.N. Effects of Innovative Processing Methods on Microalgae Cell Wall: Prospects towards Digestibility of Protein-Rich Biomass. Biomass 2022, 2, 80–102. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, K.; Tao, Y.; Yang, Q.; Xu, L.; Liu, C.; Ma, L.; Xiao, R. Biomass Directional Pyrolysis Based on Element Economy to Produce High-Quality Fuels, Chemicals, Carbon Materials—A Review. Biotechnol. Adv. 2023, 69, 108262. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gupta, R.; Zhang, Q.; You, S. Review of Biochar Production via Crop Residue Pyrolysis: Development and Perspectives. Bioresour. Technol. 2023, 369, 128423. [Google Scholar] [CrossRef] [PubMed]
- Hamzeh, Y.; Chirat, C.; Haarlemmer, G.; Lachenal, D.; Ashori, A.; Mortha, G.; Demey Cedeno, H. Extraction of Phenolic Compounds from Hydrothermal Processing of Black Liquor: Effect of Reactor Type and pH of Recovered Liquid Phase. Chem. Eng. J. 2023, 470, 144269. [Google Scholar] [CrossRef]
- Martins-Vieira, J.C.; Lachos-Perez, D.; Draszewski, C.P.; Celante, D.; Castilhos, F. Sugar, Hydrochar and Bio-Oil Production by Sequential Hydrothermal Processing of Corn Cob. J. Supercrit. Fluids 2023, 194, 105838. [Google Scholar] [CrossRef]
- Zhou, N.; Dunford, N.T. Thermal Degradation and Microwave-Assisted Pyrolysis of Green Algae and Cyanobacteria Isolated from the Great Salt Plains. Trans. ASABE 2017, 60, 561–569. [Google Scholar] [CrossRef]
- Clagnan, E.; D’Imporzano, G.; Dell’Orto, M.; Bani, A.; Dumbrell, A.J.; Parati, K.; Acién-Fernández, F.G.; Portillo-Hahnefeld, A.; Martel-Quintana, A.; Gómez-Pinchetti, J.L.; et al. Centrate as a Sustainable Growth Medium: Impact on Microalgal Inocula and Bacterial Communities in Tubular Photobioreactor Cultivation Systems. Bioresour. Technol. 2022, 363, 127979. [Google Scholar] [CrossRef]
- Soffian, M.S.; Halim, F.Z.A.; Aziz, F.; Rahman, M.A.; Amin, M.A.M.; Chee, D.N.A. Carbon-Based Material Derived from Biomass Waste for Wastewater Treatment. Environ. Adv. 2022, 9, 100259. [Google Scholar] [CrossRef]
- Wollmann, F.; Dietze, S.; Ackermann, J.-U.; Bley, T.; Walther, T.; Steingroewer, J.; Krujatz, F. Microalgae Wastewater Treatment: Biological and Technological Approaches. Eng. Life Sci. 2019, 19, 860–871. [Google Scholar] [CrossRef] [PubMed]
- Quijano, G.; Arcila, J.S.; Buitrón, G. Microalgal-Bacterial Aggregates: Applications and Perspectives for Wastewater Treatment. Biotechnol. Adv. 2017, 35, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.F.; Mofijur, M.; Parisa, T.A.; Islam, N.; Kusumo, F.; Inayat, A.; Le, V.G.; Badruddin, I.A.; Khan, T.M.Y.; Ong, H.C. Progress and Challenges of Contaminate Removal from Wastewater Using Microalgae Biomass. Chemosphere 2022, 286, 131656. [Google Scholar] [CrossRef]
- Chawla, P.; Gola, D.; Dalvi, V.; Sreekrishnan, T.R.; Ariyadasa, T.U.; Malik, A. Design and Development of Mini-Photobioreactor System for Strategic High Throughput Selection of Optimum Microalgae-Wastewater Combination. Bioresour. Technol. Rep. 2022, 17, 100967. [Google Scholar] [CrossRef]
- Cheng, P.; Chen, D.; Liu, W.; Cobb, K.; Zhou, N.; Liu, Y.; Liu, H.; Wang, Q.; Chen, P.; Zhou, C.; et al. Auto-Flocculation Microalgae Species Tribonema Sp. and Synechocystis Sp. with T-IPL Pretreatment to Improve Swine Wastewater Nutrient Removal. Sci. Total Environ. 2020, 725, 138263. [Google Scholar] [CrossRef]
- Fan, H.; Wang, K.; Wang, C.; Yu, F.; He, X.; Ma, J.; Li, X. A Comparative Study on Growth Characters and Nutrients Removal from Wastewater by Two Microalgae under Optimized Light Regimes. Environ. Technol. Innov. 2020, 19, 100849. [Google Scholar] [CrossRef]
- Pham, T.-L.; Bui, M.H. Removal of Nutrients from Fertilizer Plant Wastewater Using Scenedesmus Sp.: Formation of Bioflocculation and Enhancement of Removal Efficiency. J. Chem. 2020, 2020, 8094272. [Google Scholar] [CrossRef]
- Satheesh, S.; Pugazhendi, A.; Al-Mur, B.A.; Balasubramani, R. Biohydrogen Production Coupled with Wastewater Treatment Using Selected Microalgae. Chemosphere 2023, 334, 138932. [Google Scholar] [CrossRef]
- Mostafaei, H.; Samimi, A.; Shokrollahzadeh, S.; Karamad, S.; Sheikhinejad, A. Nutrients Removal from Raw Municipal Wastewater Using Chlorella Vulgaris Microalgae. Adv. Environ. Technol. 2023, 9, 47–57. [Google Scholar] [CrossRef]
- Aketo, T.; Hoshikawa, Y.; Nojima, D.; Yabu, Y.; Maeda, Y.; Yoshino, T.; Takano, H.; Tanaka, T. Selection and Characterization of Microalgae with Potential for Nutrient Removal from Municipal Wastewater and Simultaneous Lipid Production. J. Biosci. Bioeng. 2020, 129, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Sisman-Aydin, G. Comparative Study on Phycoremediation Performance of Three Native Microalgae for Primary-Treated Municipal Wastewater. Environ. Technol. Innov. 2022, 28, 102932. [Google Scholar] [CrossRef]
- Ummalyma, S.B.; Chiang, A.; Herojit, N.; Arumugam, M. Sustainable Microalgal Cultivation in Poultry Slaughterhouse Wastewater for Biorefinery Products and Pollutant Removal. Bioresour. Technol. 2023, 374, 128790. [Google Scholar] [CrossRef] [PubMed]
- Concas, A.; Lutzu, G.A.; Turgut Dunford, N. Experiments and Modeling of Komvophoron Sp. Growth in Hydraulic Fracturing Wastewater. Chem. Eng. J. 2021, 426, 131299. [Google Scholar] [CrossRef]
- Lutzu, G.A.; Concas, A.; Dunford, N.T. Cultivation of Hydrocarbon-Tolerant Microalgae in Flowback Wastewaters Produced during Hydrofracking of Impermeable Rocks. J. Biol. Res.-Boll. Della Soc. Ital. Biol. Sper. 2022, 95. [Google Scholar] [CrossRef]
- Lutzu, G.A.; Concas, A.; Dunford, N.T. Microalgae Growth in Physically Pre-Treated Wastewater Generated During Hydraulic Fracturing. Chem. Eng. Trans. 2022, 92, 661–666. [Google Scholar] [CrossRef]
- Lutzu, G.A.; Dunford, N.T. Growing Algae in Produced Water Generated during Oil and Gas Production Using Hydraulic Fracturing Technique. CET J.-Chem. Eng. Trans. 2019, 74, 1261. [Google Scholar]
- Lutzu, G.A.; Dunford, N.T. Algal Treatment of Wastewater Generated during Oil and Gas Production Using Hydraulic Fracturing Technology. Environ. Technol. 2019, 40, 1027–1034. [Google Scholar] [CrossRef]
- Lutzu, G.A.; Marin, M.A.; Concas, A.; Dunford, N.T. Nutrient Enrichment of Wastewater Generated During Hydraulic Fracturing with Animal Wastewater to Enhance Microalgae Growth. Chem. Eng. Trans. 2021, 86, 115–120. [Google Scholar] [CrossRef]
- Lutzu, G.A.; Marin, M.A.; Concas, A.; Dunford, N.T. Growing Picochlorum Oklahomensis in Hydraulic Fracturing Wastewater Supplemented with Animal Wastewater. Water Air Soil Pollut. 2020, 231, 457. [Google Scholar] [CrossRef]
- Conrad, C.L.; Ben Yin, Y.; Hanna, T.; Atkinson, A.J.; Alvarez, P.J.J.; Tekavec, T.N.; Reynolds, M.A.; Wong, M.S. Fit-for-Purpose Treatment Goals for Produced Waters in Shale Oil and Gas Fields. Water Res. 2020, 173, 115467. [Google Scholar] [CrossRef] [PubMed]
- Soru, S.; Malavasi, V.; Concas, A.; Caboni, P.; Cao, G. A Novel Investigation of the Growth and Lipid Production of the Extremophile Microalga Coccomyxa Melkonianii SCCA 048 under the Effect of Different Cultivation Conditions: Experiments and Modeling. Chem. Eng. J. 2019, 377, 120589. [Google Scholar] [CrossRef]
- Chakraborty, S.; Mohanty, D.; Ghosh, S.; Das, D. Improvement of Lipid Content of Chlorella Minutissima MCC 5 for Biodiesel Production. J. Biosci. Bioeng. 2016, 122, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Maltsev, Y.; Kulikovskiy, M.; Maltseva, S. Nitrogen and Phosphorus Stress as a Tool to Induce Lipid Production in Microalgae. Microb. Cell Factories 2023, 22, 239. [Google Scholar] [CrossRef] [PubMed]
- Wan Mahari, W.A.; Wan Razali, W.A.; Manan, H.; Hersi, M.A.; Ishak, S.D.; Cheah, W.; Chan, D.J.C.; Sonne, C.; Show, P.L.; Lam, S.S. Recent Advances on Microalgae Cultivation for Simultaneous Biomass Production and Removal of Wastewater Pollutants to Achieve Circular Economy. Bioresour. Technol. 2022, 364, 128085. [Google Scholar] [CrossRef]
- Yang, L.; Li, H.; Wang, Q. A Novel One-Step Method for Oil-Rich Biomass Production and Harvesting by Co-Cultivating Microalgae with Filamentous Fungi in Molasses Wastewater. Bioresour. Technol. 2019, 275, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Iwama, R.; Okahashi, N.; Suzawa, T.; Yang, C.; Matsuda, F.; Horiuchi, H. Comprehensive Analysis of the Composition of the Major Phospholipids during the Asexual Life Cycle of the Filamentous Fungus Aspergillus Nidulans. Biochim. Biophys. Acta BBA-Mol. Cell Biol. Lipids 2023, 1868, 159379. [Google Scholar] [CrossRef]
- Kumar, N. Oxidative Stability of Biodiesel: Causes, Effects and Prevention. Fuel 2017, 190, 328–350. [Google Scholar] [CrossRef]
- Padri, M.; Boontian, N.; Teaumroong, N.; Piromyou, P.; Piasai, C. Co-Culture of Microalga Chlorella Sorokiniana with Syntrophic Streptomyces Thermocarboxydus in Cassava Wastewater for Wastewater Treatment and Biodiesel Production. Bioresour. Technol. 2022, 347, 126732. [Google Scholar] [CrossRef]
- Shen, X.-F.; Xu, Y.-P.; Tong, X.-Q.; Huang, Q.; Zhang, S.; Gong, J.; Chu, F.-F.; Zeng, R.J. The Mechanism of Carbon Source Utilization by Microalgae When Co-Cultivated with Photosynthetic Bacteria. Bioresour. Technol. 2022, 365, 128152. [Google Scholar] [CrossRef]
- Huo, S.; Kong, M.; Zhu, F.; Qian, J.; Huang, D.; Chen, P.; Ruan, R. Co-Culture of Chlorella and Wastewater-Borne Bacteria in Vinegar Production Wastewater: Enhancement of Nutrients Removal and Influence of Algal Biomass Generation. Algal Res. 2020, 45, 101744. [Google Scholar] [CrossRef]
- Japir, A.A.-W.; Salih, N.; Salimon, J. Synthesis and Characterization of Biodegradable Palm Palmitic Acid Based Bioplastic. Turk. J. Chem. 2021, 45, 585–599. [Google Scholar] [CrossRef]
- Muhamad, I.I.; Hassan, N.D.; Mamat, S.N.H.; Nawi, N.M.; Rashid, W.A.; Tan, N.A. Chapter 14—Extraction Technologies and Solvents of Phytocompounds From Plant Materials: Physicochemical Characterization and Identification of Ingredients and Bioactive Compounds From Plant Extract Using Various Instrumentations. In Ingredients Extraction by Physicochemical Methods in Food; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 523–560. ISBN 978-0-12-811521-3. [Google Scholar]
- Das, V.; Tripathi, A.M.; Borah, M.J.; Dunford, N.T.; Deka, D. Cobalt-Doped CaO Catalyst Synthesized and Applied for Algal Biodiesel Production. Renew. Energy 2020, 161, 1110–1119. [Google Scholar] [CrossRef]
- Das, V.; Dunford, N.; Deka, D. Waste Utilization and Biodiesel Production from Desmodesmus Maximus Grown in Swine Wastewater Using Waste Eggshells as a Catalyst. Aquac. Eng. 2022, 99, 102293. [Google Scholar] [CrossRef]
- Xu, H.; Ou, L.; Li, Y.; Hawkins, T.R.; Wang, M. Life Cycle Greenhouse Gas Emissions of Biodiesel and Renewable Diesel Production in the United States. Environ. Sci. Technol. 2022, 56, 7512–7521. [Google Scholar] [CrossRef]
- Ha, G.-S.; El-Dalatony, M.M.; Kim, D.-H.; Salama, E.-S.; Kurade, M.B.; Roh, H.-S.; El-Fatah Abomohra, A.; Jeon, B.-H. Biocomponent-Based Microalgal Transformations into Biofuels during the Pretreatment and Fermentation Process. Bioresour. Technol. 2020, 302, 122809. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Zhang, Y.; Wu, X.; Gong, X.; Wang, Q. Hydrolysis of Chlorella Biomass for Fermentable Sugars in the Presence of HCl and MgCl2. Bioresour. Technol. 2011, 102, 10158–10161. [Google Scholar] [CrossRef]
- Calijuri, M.L.; Silva, T.A.; Magalhães, I.B.; de Paula Pereira, A.S.A.; Marangon, B.B.; de Assis, L.R.; Lorentz, J.F. Bioproducts from Microalgae Biomass: Technology, Sustainability, Challenges and Opportunities. Chemosphere 2022, 305, 135508. [Google Scholar] [CrossRef]
- Beckstrom, B.D.; Wilson, M.H.; Crocker, M.; Quinn, J.C. Bioplastic Feedstock Production from Microalgae with Fuel Co-Products: A Techno-Economic and Life Cycle Impact Assessment. Algal Res. 2020, 46, 101769. [Google Scholar] [CrossRef]
- Jahirul, M.I.; Rasul, M.G.; Schaller, D.; Khan, M.M.K.; Hasan, M.M.; Hazrat, M.A. Transport Fuel from Waste Plastics Pyrolysis—A Review on Technologies, Challenges and Opportunities. Energy Convers. Manag. 2022, 258, 115451. [Google Scholar] [CrossRef]
- Babatabar, M.A.; Yousefian, F.; Mousavi, M.V.; Hosseini, M.; Tavasoli, A. Pyrolysis of Lignocellulosic and Algal Biomasses in a Fixed-Bed Reactor: A Comparative Study on the Composition and Application Potential of Bioproducts. Int. J. Energy Res. 2022, 46, 9836–9850. [Google Scholar] [CrossRef]
- Sun, J.; Norouzi, O.; Mašek, O. A State-of-the-Art Review on Algae Pyrolysis for Bioenergy and Biochar Production. Bioresour. Technol. 2022, 346, 126258. [Google Scholar] [CrossRef] [PubMed]
- Su, G.; Ong, H.C.; Gan, Y.Y.; Chen, W.-H.; Chong, C.T.; Ok, Y.S. Co-Pyrolysis of Microalgae and Other Biomass Wastes for the Production of High-Quality Bio-Oil: Progress and Prospective. Bioresour. Technol. 2022, 344, 126096. [Google Scholar] [CrossRef] [PubMed]
- Crujeira, A.T.; Trancoso, M.A.; Eusébio, A.; Oliveira, A.C.; Passarinho, P.C.; Abreu, M.; Marques, I.P.; Marques, P.A.S.S.; Marques, S.; Albergaria, H.; et al. Admissibility Grid to Support the Decision for the Preferential Routing of Portuguese Endogenous Waste Biomass for the Production of Biogas, Advanced Biofuels, Electricity and Heat. Biomass 2023, 3, 336–366. [Google Scholar] [CrossRef]
- Vuppaladadiyam, A.K.; Varsha Vuppaladadiyam, S.S.; Sikarwar, V.S.; Ahmad, E.; Pant, K.K.; Murugavelh, S.; Pandey, A.; Bhattacharya, S.; Sarmah, A.; Leu, S.-Y. A Critical Review on Biomass Pyrolysis: Reaction Mechanisms, Process Modeling and Potential Challenges. J. Energy Inst. 2023, 108, 101236. [Google Scholar] [CrossRef]
- Yang, C.; Li, R.; Zhang, B.; Qiu, Q.; Wang, B.; Yang, H.; Ding, Y.; Wang, C. Pyrolysis of Microalgae: A Critical Review. Fuel Process. Technol. 2019, 186, 53–72. [Google Scholar] [CrossRef]
- Hong, Y.; Xie, C.; Chen, W.; Luo, X.; Shi, K.; Wu, T. Kinetic Study of the Pyrolysis of Microalgae under Nitrogen and CO2 Atmosphere. Renew. Energy 2020, 145, 2159–2168. [Google Scholar] [CrossRef]
- Azizi, K.; Keshavarz Moraveji, M.; Arregi, A.; Amutio, M.; Lopez, G.; Olazar, M. On the Pyrolysis of Different Microalgae Species in a Conical Spouted Bed Reactor: Bio-Fuel Yields and Characterization. Bioresour. Technol. 2020, 311, 123561. [Google Scholar] [CrossRef]
- Bordoloi, N.; Narzari, R.; Sut, D.; Saikia, R.; Chutia, R.S.; Kataki, R. Characterization of Bio-Oil and Its Sub-Fractions from Pyrolysis of Scenedesmus Dimorphus. Spec. Issue New Horiz. Biofuels Prod. Technol. 2016, 98, 245–253. [Google Scholar] [CrossRef]
- Aboulkas, A.; Hammani, H.; El Achaby, M.; Bilal, E.; Barakat, A.; El Harfi, K. Valorization of Algal Waste via Pyrolysis in a Fixed-Bed Reactor: Production and Characterization of Bio-Oil and Bio-Char. Bioresour. Technol. 2017, 243, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Jamro, I.A.; Wang, J.; Ullah, A.; Kumari, L.; Cui, B.; Tao, J.; Guo, D.; Yan, B.; Aborisade, M.A.; et al. Co-Pyrolysis of Microalgae Residue and Sewage Sludge: An in-Depth Characterization of Kinetics, Drivers, and Gas-Oil-Char Behaviors. J. Anal. Appl. Pyrolysis 2024, 179, 106438. [Google Scholar] [CrossRef]
- de Morais, E.G.; da Silveira, J.T.; Schüler, L.M.; de Freitas, B.C.B.; Costa, J.A.V.; de Morais, M.G.; Ferrer, I.; Barreira, L. Biomass Valorization via Pyrolysis in Microalgae-Based Wastewater Treatment: Challenges and Opportunities for a Circular Bioeconomy. J. Appl. Phycol. 2023, 35, 2689–2708. [Google Scholar] [CrossRef]
- Chen, W.; Yang, H.; Chen, Y.; Xia, M.; Yang, Z.; Wang, X.; Chen, H. Algae Pyrolytic Poly-Generation: Influence of Component Difference and Temperature on Products Characteristics. Energy 2017, 131, 1–12. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, J.; Pan, M.; Hao, Y.; Hu, R.; Xiao, W.; Li, G.; Lyu, T. Valorisation of Microalgae Residues after Lipid Extraction: Pyrolysis Characteristics for Biofuel Production. Biochem. Eng. J. 2022, 179, 108330. [Google Scholar] [CrossRef]
- Zhou, H.; Wu, C.; Onwudili, J.A.; Meng, A.; Zhang, Y.; Williams, P.T. Polycyclic Aromatic Hydrocarbon Formation from the Pyrolysis/Gasification of Lignin at Different Reaction Conditions. Energy Fuels 2014, 28, 6371–6379. [Google Scholar] [CrossRef]
- Li, G.; Hu, R.; Wang, N.; Yang, T.; Xu, F.; Li, J.; Wu, J.; Huang, Z.; Pan, M.; Lyu, T. Cultivation of Microalgae in Adjusted Wastewater to Enhance Biofuel Production and Reduce Environmental Impact: Pyrolysis Performances and Life Cycle Assessment. J. Clean. Prod. 2022, 355, 131768. [Google Scholar] [CrossRef]
- Wei, D.; Chen, C.; Huang, X.; Zhao, J.; Fan, D.; Zeng, T.; Bi, Y. Products and Pathway Analysis of Rice Straw and Chlorella Vulgaris by Microwave-Assisted Co-Pyrolysis. J. Energy Inst. 2023, 107, 101182. [Google Scholar] [CrossRef]
- Hua, M.-Y.; Li, B.-X. Co-Pyrolysis Characteristics of the Sugarcane Bagasse and Enteromorpha Prolifera. Energy Convers. Manag. 2016, 120, 238–246. [Google Scholar] [CrossRef]
- Rangel, M.D.; Mayer, F.M.; Carvalho, M.D.; Saboia, G.; de Andrade, A.M. Selecting Catalysts for Pyrolysis of Lignocellulosic Biomass. Biomass 2023, 3, 31–63. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, B.; Adhikari, B. Maillard Reaction between Pea Protein Isolate and Maltodextrin via Wet-Heating Route for Emulsion Stabilisation. Future Foods 2022, 6, 100193. [Google Scholar] [CrossRef]
- Chen, X.; Peng, X.; Ma, X.; Wang, J. Investigation of Mannich Reaction during Co-Liquefaction of Microalgae and Sweet Potato Waste. Bioresour. Technol. 2019, 284, 286–292. [Google Scholar] [CrossRef] [PubMed]
- González Martínez, M.; Ohra-aho, T.; Tamminen, T.; da Silva Perez, D.; Campargue, M.; Dupont, C. Detailed Structural Elucidation of Different Lignocellulosic Biomass Types Using Optimized Temperature and Time Profiles in Fractionated Py-GC/MS. J. Anal. Appl. Pyrolysis 2019, 140, 112–124. [Google Scholar] [CrossRef]
- Kazemi Targhi, N.; Tavakoli, O.; Nazemi, A.H. Co-Pyrolysis of Lentil Husk Wastes and Chlorella Vulgaris: Bio-Oil and Biochar Yields Optimization. J. Anal. Appl. Pyrolysis 2022, 165, 105548. [Google Scholar] [CrossRef]
- Gong, X.; Zhang, B.; Zhang, Y.; Huang, Y.; Xu, M. Investigation on Pyrolysis of Low Lipid Microalgae Chlorella Vulgaris and Dunaliella Salina. Energy Fuels 2014, 28, 95–103. [Google Scholar] [CrossRef]
- Kumar, A.; Yan, B.; Tao, J.; Li, J.; Kumari, L.; Tafa Oba, B.; Akintayo Aborisade, M.; Ali Jamro, I.; Chen, G. Co-Pyrolysis of de-Oiled Microalgal Biomass Residue and Waste Tires: Deeper Insights from Thermal Kinetics, Behaviors, Drivers, Bio-Oils, Bio-Chars, and in-Situ Evolved Gases Analyses. Chem. Eng. J. 2022, 446, 137160. [Google Scholar] [CrossRef]
- Dai, M.; Yu, Z.; Fang, S.; Ma, X. Behaviors, Product Characteristics and Kinetics of Catalytic Co-Pyrolysis Spirulina and Oil Shale. Energy Convers. Manag. 2019, 192, 1–10. [Google Scholar] [CrossRef]
- Kumar, A.; Yan, B.; Cheng, Z.; Tao, J.; Hassan, M.; Li, J.; Kumari, L.; Oba, B.T.; Aborisade, M.A.; Jamro, I.A.; et al. Co-Pyrolysis of Hydrothermally Pre-Treated Microalgae Residue and Polymeric Waste (Plastic/Tires): Comparative and Dynamic Analyses of Pyrolytic Behaviors, Kinetics, Chars, Oils, and in-Situ Gas Emissions. Fuel 2023, 331, 125814. [Google Scholar] [CrossRef]
- Tang, F.; Yu, Z.; Li, Y.; Chen, L.; Ma, X. Catalytic Co-Pyrolysis Behaviors, Product Characteristics and Kinetics of Rural Solid Waste and Chlorella Vulgaris. Bioresour. Technol. 2020, 299, 122636. [Google Scholar] [CrossRef]
- Saiyud, N.; Deethayat, T.; Asanakham, A.; Duongbia, N.; Kamopas, W.; Kiatsiriroat, T. Biochar Production from Co-Pyrolysis of Coffee Ground and Native Microalgae Consortium. Biomass Convers. Biorefin. 2022, 14, 6855–6863. [Google Scholar] [CrossRef]
- Chen, W.; Chen, Y.; Yang, H.; Li, K.; Chen, X.; Chen, H. Investigation on Biomass Nitrogen-Enriched Pyrolysis: Influence of Temperature. Bioresour. Technol. 2018, 249, 247–253. [Google Scholar] [CrossRef]
- Tangmankongworakoon, N. An Approach to Produce Biochar from Coffee Residue for Fuel and Soil Amendment Purpose. Int. J. Recycl. Org. Waste Agric. 2019, 8, 37–44. [Google Scholar] [CrossRef]
- Yadav, A.; Ansari, K.B.; Simha, P.; Gaikar, V.G.; Pandit, A.B. Vacuum Pyrolysed Biochar for Soil Amendment. Resour.-Effic. Technol. 2016, 2, S177–S185. [Google Scholar] [CrossRef]
- Mishra, R.K.; Mohanty, K. A Review of the Next-Generation Biochar Production from Waste Biomass for Material Applications. Sci. Total Environ. 2023, 904, 167171. [Google Scholar] [CrossRef]
- Chakraborty, I.; Sathe, S.M.; Dubey, B.K.; Ghangrekar, M.M. Waste-Derived Biochar: Applications and Future Perspective in Microbial Fuel Cells. Bioresour. Technol. 2020, 312, 123587. [Google Scholar] [CrossRef]
- Roy, S.; Marzorati, S.; Schievano, A.; Pant, D. Microbial Fuel Cells. In Encyclopedia of Sustainable Technologies; Abraham, M.A., Ed.; Elsevier: Oxford, UK, 2017; pp. 245–259. ISBN 978-0-12-804792-7. [Google Scholar]
- Xu, C.; Poon, K.; Choi, M.M.F.; Wang, R. Using Live Algae at the Anode of a Microbial Fuel Cell to Generate Electricity. Environ. Sci. Pollut. Res. 2015, 22, 15621–15635. [Google Scholar] [CrossRef]
- Zhou, M.; He, H.; Jin, T.; Wang, H. Power Generation Enhancement in Novel Microbial Carbon Capture Cells with Immobilized Chlorella Vulgaris. J. Power Sources 2012, 214, 216–219. [Google Scholar] [CrossRef]
- Wang, H.; Liu, D.; Lu, L.; Zhao, Z.; Xu, Y.; Cui, F. Degradation of Algal Organic Matter Using Microbial Fuel Cells and Its Association with Trihalomethane Precursor Removal. Bioresour. Technol. 2012, 116, 80–85. [Google Scholar] [CrossRef]
- Liu, T.; Rao, L.; Yuan, Y.; Zhuang, L. Bioelectricity Generation in a Microbial Fuel Cell with a Self-Sustainable Photocathode. Sci. World J. 2015, 2015, 864568. [Google Scholar] [CrossRef]
- Bi, Z.; He, B.B. 26—Biochar from Microalgae. In 3rd Generation Biofuels; Jacob-Lopes, E., Zepka, L.Q., Severo, I.A., Maroneze, M.M., Eds.; Woodhead Publishing: Sawston, UK, 2022; pp. 613–637. ISBN 978-0-323-90971-6. [Google Scholar]
- Behera, B.; Mari Selvam, S.; Balasubramanian, P. Hydrothermal Processing of Microalgal Biomass: Circular Bio-Economy Perspectives for Addressing Food-Water-Energy Nexus. Bioresour. Technol. 2022, 359, 127443. [Google Scholar] [CrossRef]
- Kumar, A.; Saini, K.; Bhaskar, T. Hydochar and Biochar: Production, Physicochemical Properties and Techno-Economic Analysis. Bioresour. Technol. 2020, 310, 123442. [Google Scholar] [CrossRef]
- Ağbulut, Ü.; Sirohi, R.; Lichtfouse, E.; Chen, W.-H.; Len, C.; Show, P.L.; Le, A.T.; Nguyen, X.P.; Hoang, A.T. Microalgae Bio-Oil Production by Pyrolysis and Hydrothermal Liquefaction: Mechanism and Characteristics. Bioresour. Technol. 2023, 376, 128860. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; Fu, P.; Song, C.; Fan, Y. Valorization of Waste Biomass through Hydrothermal Liquefaction: A Review with Focus on Linking Hydrothermal Factors to Products Characteristics. Ind. Crops Prod. 2023, 191, 116017. [Google Scholar] [CrossRef]
- Obeid, R.; Smith, N.; Lewis, D.M.; Hall, T.; van Eyk, P. A Kinetic Model for the Hydrothermal Liquefaction of Microalgae, Sewage Sludge and Pine Wood with Product Characterisation of Renewable Crude. Chem. Eng. J. 2022, 428, 131228. [Google Scholar] [CrossRef]
- Obeid, R.; Lewis, D.M.; Smith, N.; Hall, T.; van Eyk, P. Reaction Kinetics and Characterization of Species in Renewable Crude from Hydrothermal Liquefaction of Mixtures of Polymer Compounds to Represent Organic Fractions of Biomass Feedstocks. Energy Fuels 2020, 34, 419–429. [Google Scholar] [CrossRef]
- Obeid, R.; Lewis, D.M.; Smith, N.; Hall, T.; van Eyk, P. Reaction Kinetics and Characterisation of Species in Renewable Crude from Hydrothermal Liquefaction of Monomers to Represent Organic Fractions of Biomass Feedstocks. Chem. Eng. J. 2020, 389, 124397. [Google Scholar] [CrossRef]
- Yuan, T.; Tahmasebi, A.; Yu, J. Comparative Study on Pyrolysis of Lignocellulosic and Algal Biomass Using a Thermogravimetric and a Fixed-Bed Reactor. Bioresour. Technol. 2015, 175, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Mustapha, S.I.; Mohammed, U.A.; Bux, F.; Isa, Y.M. Catalytic Hydrothermal Liquefaction of Nutrient-Stressed Microalgae for Production of High-Quality Bio-Oil over Zr-Doped HZSM-5 Catalyst. Biomass Bioenergy 2022, 163, 106497. [Google Scholar] [CrossRef]
- Shakya, R.; Whelen, J.; Adhikari, S.; Mahadevan, R.; Neupane, S. Effect of Temperature and Na2CO3 Catalyst on Hydrothermal Liquefaction of Algae. Algal Res. 2015, 12, 80–90. [Google Scholar] [CrossRef]
- Abu El-Rub, Z.; Bramer, E.A.; Brem, G. Review of Catalysts for Tar Elimination in Biomass Gasification Processes. Ind. Eng. Chem. Res. 2004, 43, 6911–6919. [Google Scholar] [CrossRef]
- Hossain, M.R.; Khalekuzzaman, M.; Kabir, S.B.; Islam, M.B.; Bari, Q.H. Production of Light Oil-Prone Biocrude through Co-Hydrothermal Liquefaction of Wastewater-Grown Microalgae and Peat. J. Anal. Appl. Pyrolysis 2022, 161, 105423. [Google Scholar] [CrossRef]
- Dandamudi, K.P.R.; Muhammed Luboowa, K.; Laideson, M.; Murdock, T.; Seger, M.; McGowen, J.; Lammers, P.J.; Deng, S. Hydrothermal Liquefaction of Cyanidioschyzon Merolae and Salicornia Bigelovii Torr.: The Interaction Effect on Product Distribution and Chemistry. Fuel 2020, 277, 118146. [Google Scholar] [CrossRef]
- Kasmiarno, L.D.; Panannangan, J.K.; Steven, S.; Rizkiana, J.; Hernowo, P.; Achmad, F.; Muraza, O.; Prakoso, T.; Istyami, A.N.; Pratiwi, M.; et al. Exploration of Bio-Hydrocarbon Gases Production via Pyrolysis of Fresh Natural Rubber: Experimental and Volatile State Kinetic Modeling Studies. J. Anal. Appl. Pyrolysis 2024, 177, 106275. [Google Scholar] [CrossRef]
- KGK, D.; Kumari, S.; Shailender, G.; Malla, R.R. Marine Natural Compound Cyclo(L-Leucyl-L-Prolyl) Peptide Inhibits Migration of Triple Negative Breast Cancer Cells by Disrupting Interaction of CD151 and EGFR Signaling. Chem. Biol. Interact. 2020, 315, 108872. [Google Scholar] [CrossRef] [PubMed]
- Lauritano, C.; Montuori, E.; De Falco, G.; Carrella, S. In Silico Methodologies to Improve Antioxidants’ Characterization from Marine Organisms. Antioxidants 2023, 12, 710. [Google Scholar] [CrossRef] [PubMed]
- Atef, B.; Ishak, R.A.H.; Badawy, S.S.; Osman, R. Exploring the Potential of Oleic Acid in Nanotechnology-Mediated Dermal Drug Delivery: An up-to-Date Review. J. Drug Deliv. Sci. Technol. 2022, 67, 103032. [Google Scholar] [CrossRef]
- Xia, J.; Han, L.; Zhang, C.; Guo, H.; Rong, N.; Baloch, H.A.; Wu, P.; Xu, G.; Ma, K. Hydrothermal Co-Liquefaction of Rice Straw and Nannochloropsis: The Interaction Effect on Mechanism, Product Distribution and Composition. J. Anal. Appl. Pyrolysis 2022, 161, 105368. [Google Scholar] [CrossRef]
- Ellersdorfer, M. Hydrothermal Co-Liquefaction of Chlorella Vulgaris with Food Processing Residues, Green Waste and Sewage Sludge. Biomass Bioenergy 2020, 142, 105796. [Google Scholar] [CrossRef]
- Obeid, F.; Van, T.C.; Guo, B.; Surawski, N.C.; Hornung, U.; Brown, R.J.; Ramirez, J.A.; Thomas-Hall, S.R.; Stephens, E.; Hankamer, B.; et al. The Fate of Nitrogen and Sulphur during Co-Liquefaction of Algae and Bagasse: Experimental and Multi-Criterion Decision Analysis. Biomass Bioenergy 2021, 151, 106119. [Google Scholar] [CrossRef]
- Pagano, M.; Hernando, H.; Cueto, J.; Cruz, P.L.; Dufour, J.; Moreno, I.; Serrano, D.P. Insights on the Acetic Acid Pretreatment of Wheat Straw: Changes Induced in the Biomass Properties and Benefits for the Bio-Oil Production by Pyrolysis. Chem. Eng. J. 2023, 454, 140206. [Google Scholar] [CrossRef]
- Chen, H.; Xu, H.; Zhu, H.; Yan, S.; Zhang, S.; Zhang, H.; Guo, X.; Hu, X.; Gao, W. A Review on Bioslurry Fuels Derived from Bio-Oil and Biochar: Preparation, Fuel Properties and Application. Fuel 2024, 355, 129283. [Google Scholar] [CrossRef]
- Fakayode, O.A.; Wahia, H.; Zhang, L.; Zhou, C.; Ma, H. State-of-the-Art Co-Pyrolysis of Lignocellulosic and Macroalgae Biomass Feedstocks for Improved Bio-Oil Production—A Review. Fuel 2023, 332, 126071. [Google Scholar] [CrossRef]
- Aliyu, A.; Lee, J.G.M.; Harvey, A.P. Microalgae for Biofuels: A Review of Thermochemical Conversion Processes and Associated Opportunities and Challenges. Bioresour. Technol. Rep. 2021, 15, 100694. [Google Scholar] [CrossRef]
- Zhi, Y.; Xu, D.; Jiang, G.; Yang, W.; Chen, Z.; Duan, P.; Zhang, J. A Review of Hydrothermal Carbonization of Municipal Sludge: Process Conditions, Physicochemical Properties, Methods Coupling, Energy Balances and Life Cycle Analyses. Fuel Process. Technol. 2024, 254, 107943. [Google Scholar] [CrossRef]
- Mathimani, T.; Mallick, N. A Review on the Hydrothermal Processing of Microalgal Biomass to Bio-Oil—Knowledge Gaps and Recent Advances. J. Clean. Prod. 2019, 217, 69–84. [Google Scholar] [CrossRef]
- de Siqueira Castro, J.; Assemany, P.P.; de Oliveira Carneiro, A.C.; Ferreira, J.; de Jesus Júnior, M.M.; de Ávila Rodrigues, F.; Calijuri, M.L. Hydrothermal Carbonization of Microalgae Biomass Produced in Agro-Industrial Effluent: Products, Characterization and Applications. Sci. Total Environ. 2021, 768, 144480. [Google Scholar] [CrossRef]
- Sagar, V.; Lynam, J.G.; Parrenin, A.G. Sugar Extraction from Secondary Agricultural Waste Biomass Using Hydrothermal Carbonization and Direct Contact Membrane Distillation. Biomass 2023, 3, 323–335. [Google Scholar] [CrossRef]
- Liu, H.; Chen, Y.; Yang, H.; Gentili, F.G.; Söderlind, U.; Wang, X.; Zhang, W.; Chen, H. Hydrothermal Carbonization of Natural Microalgae Containing a High Ash Content. Fuel 2019, 249, 441–448. [Google Scholar] [CrossRef]
- Cantero-Tubilla, B.; Cantero, D.A.; Martinez, C.M.; Tester, J.W.; Walker, L.P.; Posmanik, R. Characterization of the Solid Products from Hydrothermal Liquefaction of Waste Feedstocks from Food and Agricultural Industries. J. Supercrit. Fluids 2018, 133, 665–673. [Google Scholar] [CrossRef]
- Guo, D.; Wang, Y.; Gao, Y.; Lyu, Y.; Lin, Y.; Pan, Y.; Zhu, L.; Zhu, Y. Nitrogen Migration in Products during the Microwave-Assisted Hydrothermal Carbonization of Spirulina Platensis. Bioresour. Technol. 2022, 351, 126968. [Google Scholar] [CrossRef]
- Khoo, C.G.; Hirose, Y.; Matsumura, Y.; Lam, M.K.; Tan, I.S.; Lee, K.T. Valorisation of Chlorella Vulgaris Biomass for Multi-Products Synthesis via Hydrothermal Processing. Energy Convers. Manag. X 2023, 20, 100399. [Google Scholar] [CrossRef]
- Dahham, R.Y.; Wei, H.; Pan, J. Improving Thermal Efficiency of Internal Combustion Engines: Recent Progress and Remaining Challenges. Energies 2022, 15, 6222. [Google Scholar] [CrossRef]
- Purayil, S.T.P.; Hamdan, M.O.; Al-Omari, S.A.B.; Selim, M.Y.E.; Elnajjar, E. Review of Hydrogen–Gasoline SI Dual Fuel Engines: Engine Performance and Emission. Energy Rep. 2023, 9, 4547–4573. [Google Scholar] [CrossRef]
- Bashir, S.S.; Siddiqi, T.O.; Kumar, D.; Ahmad, A. Physio-Biochemical, Agronomical, and Gene Expression Analysis Reveals Different Responsive Approach to Low Nitrogen in Contrasting Rice Cultivars for Nitrogen Use Efficiency. Mol. Biol. Rep. 2023, 50, 1575–1593. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Tian, H.; Song, S.; Tan, H.T.W.; Lee, J.T.E.; Zhang, J.; Sharma, P.; Tiong, Y.W.; Tong, Y.W. Effects of Digestate-Encapsulated Biochar on Plant Growth, Soil Microbiome and Nitrogen Leaching. J. Environ. Manag. 2023, 334, 117481. [Google Scholar] [CrossRef] [PubMed]
- Sztancs, G.; Kovacs, A.; Toth, A.J.; Mizsey, P.; Billen, P.; Fozer, D. Catalytic Hydrothermal Carbonization of Microalgae Biomass for Low-Carbon Emission Power Generation: The Environmental Impacts of Hydrochar Co-Firing. Fuel 2021, 300, 120927. [Google Scholar] [CrossRef]
- Liu, X.; Fan, Y.; Zhai, Y.; Liu, X.; Wang, Z.; Zhu, Y.; Shi, H.; Li, C.; Zhu, Y. Co-Hydrothermal Carbonization of Rape Straw and Microalgae: pH-Enhanced Carbonization Process to Obtain Clean Hydrochar. Energy 2022, 257, 124733. [Google Scholar] [CrossRef]
- Chen, J.; Ding, L.; Liu, R.; Xu, S.; Li, L.; Gao, L.; Wei, L.; Leng, S.; Li, J.; Li, J.; et al. Hydrothermal Carbonization of Microalgae-Fungal Pellets: Removal of Nutrients from the Aqueous Phase Fungi and Microalgae Cultivation. ACS Sustain. Chem. Eng. 2020, 8, 16823–16832. [Google Scholar] [CrossRef]
- Masoumi, S.; Dalai, A.K. Optimized Production and Characterization of Highly Porous Activated Carbon from Algal-Derived Hydrochar. J. Clean. Prod. 2020, 263, 121427. [Google Scholar] [CrossRef]
- Tsarpali, M.; Kuhn, J.N.; Philippidis, G.P. Hydrothermal Carbonization of Residual Algal Biomass for Production of Hydrochar as a Biobased Metal Adsorbent. Sustainability 2022, 14, 455. [Google Scholar] [CrossRef]
- Wang, T.; Liu, X.; Wang, D.; Gong, Z.; Si, B.; Zhai, Y. Persulfate Assisted Hydrothermal Processing of Spirulina for Enhanced Deoxidation Carbonization. Bioresour. Technol. 2021, 322, 124543. [Google Scholar] [CrossRef] [PubMed]
- Miliotti, E.; Casini, D.; Rosi, L.; Lotti, G.; Rizzo, A.M.; Chiaramonti, D. Lab-Scale Pyrolysis and Hydrothermal Carbonization of Biomass Digestate: Characterization of Solid Products and Compliance with Biochar Standards. Biomass Bioenergy 2020, 139, 105593. [Google Scholar] [CrossRef]
- Alcazar-Ruiz, A.; Villardon, A.; Dorado, F.; Sanchez-Silva, L. Hydrothermal Carbonization Coupled with Fast Pyrolysis of Almond Shells: Valorization and Production of Valuable Chemicals. Waste Manag. 2023, 169, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Mannarino, G.; Sarrion, A.; Diaz, E.; Gori, R.; De la Rubia, M.A.; Mohedano, A.F. Improved Energy Recovery from Food Waste through Hydrothermal Carbonization and Anaerobic Digestion. Waste Manag. 2022, 142, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wang, Q.; Cui, D.; Sun, H.; Yin, H.; Xu, F.; Wang, Z. Evaluation of Fuel Properties and Combustion Behaviour of Hydrochar Derived from Hydrothermal Carbonisation of Agricultural Wastes. J. Energy Inst. 2023, 108, 101209. [Google Scholar] [CrossRef]
- Ding, Y.; Li, D.; Lv, M.; Yuan, L.; Zhang, J.; Qin, S.; Wang, B.; Cui, X.; Guo, C.; Zhao, P. Influence of Process Water Recirculation on Hydrothermal Carbonization of Rice Husk at Different Temperatures. J. Environ. Chem. Eng. 2023, 11, 109364. [Google Scholar] [CrossRef]
- Ma, M.; Li, Y.; Chen, J.; Wang, F.; Yuan, L.; Li, Y.; Zhang, B.; Ye, D.; Han, D.; Jin, H.; et al. High-Cell-Density Cultivation of the Flagellate Alga Poterioochromonas malhamensis for Biomanufacturing the Water-Soluble β-1,3-Glucan with Multiple Biological Activities. Bioresour. Technol. 2021, 337, 125447. [Google Scholar] [CrossRef]
- Yimei, X.; Jinghan, W.; Song, X.; Zhanyou, C. β-Carotene Production from Dunaliella salina Cultivated with Bicarbonate as Carbon Source. J. Microbiol. Biotechnol. 2020, 30, 868–877. [Google Scholar] [CrossRef]
- Wang, S.; Lan, C.; Wang, Z.; Wan, W.; Zhang, H.; Cui, Q.; Song, X. Optimizing Eicosapentaenoic Acid Production by Grafting a Heterologous Polyketide Synthase Pathway in the Thraustochytrid aurantiochytrium. J. Agric. Food Chem. 2020, 68, 11253–11260. [Google Scholar] [CrossRef]
- Tang, Z.; Chen, W.; Chen, Y.; Hu, J.; Yang, H.; Chen, H. Preparation of Low-Nitrogen and High-Quality Bio-Oil from Microalgae Catalytic Pyrolysis with Zeolites and Activated Carbon. J. Anal. Appl. Pyrolysis 2021, 159, 105182. [Google Scholar] [CrossRef]
- Palomino, A.; Godoy-Silva, R.D.; Raikova, S.; Chuck, C.J. The Storage Stability of Biocrude Obtained by the Hydrothermal Liquefaction of Microalgae. Renew. Energy 2020, 145, 1720–1729. [Google Scholar] [CrossRef]
- Benavente, V.; Lage, S.; Gentili, F.G.; Jansson, S. Influence of Lipid Extraction and Processing Conditions on Hydrothermal Conversion of Microalgae Feedstocks—Effect on Hydrochar Composition, Secondary Char Formation and Phytotoxicity. Chem. Eng. J. 2022, 428, 129559. [Google Scholar] [CrossRef]
| Sl No. | Microalgal Strain | Reactor Type | Light Intensity | Wastewater Type | NH4+ Removal Efficiency (%) | NO3− Removal Efficiency (%) | P Removal Efficiency (%) | Biomass Productivity (mg. L−1. day−1) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 1. | Chlorella vulgaris | Mini— Photobioreactor | 11,000 Lux | Dairy Processing Wastewater | 91.4 | 79.4 | 96.4 | 530 | [16] |
| Chlorella pyrenoidosa | 92.5 | 83.1 | 92.2 | 470 | |||||
| Chlorella minutissima | 95.7 | 94.4 | 88.5 | 600 | |||||
| 2. | Synechocystis sp. | Glass Bubbling Bottles | 60 µmol m−2 s−1 | Diluted Swine Wastewater | 73.7 | - | 71.6 | 260 | [17] |
| 3. | Spirulina platensis FACHB-341 | Conical Glass Flask | 2000–4000 Lux | Domestic Wastewater | >99 | - | 88.6 | 33.4 | [18] |
| Scenedesmus obliquus FACHB-417 | 4000–8000 Lux | 93.8 | - | >99 | 38 | ||||
| 4. | Scenedesmus sp. | Conical Glass Flask | 95 µmol m−2 s−1 | Fertilizer Plant Wastewater | 92.8 | 83.6 | 97 | 3.1 | [19] |
| 5. | Chlorella pyrenoidosa | Erlemeyer Flask | 120 µmol m−2 s−1 | Domestic Wastewater Treatment Plant | >98 | 78.3 | >78 | 47.3 | [20] |
| Scenedesmus Obliquus | >98 | 81.3 | >78 | 60 | |||||
| Chlorella sorokiniana | >98 | 85.5 | >88 | 50.7 | |||||
| 6. | Chlorella vulgaris | Column Photobioreactor | 6300 Lux | Wastewater Treatment Plant | >90 | >90 | >90 | 9.28 | [21] |
| 7. | Tetraselmis sp. | L—Shaped Glass Tubes | 50 µmol m−2 s−1 | Municipal Wastewater (Activated Sludge) | 98 | - | 82 | 157 | [22] |
| Parachlorella kessleri | 98 | - | 20 | 101 | |||||
| 8. | Navicula venata | Glass based Batch Reactor | 100 µmol m−2 s−1 | Municipal Wastewater | 96.9 | - | 99.8 | 7.1 | [23] |
| 9. | Neochloris sp. | Conical Glass Flask | Natural Sunlight (11:13 h/h Day: Night Cycle) | Poultry Slaughterhouse Wastewater | - | 95 | 79.3 | 119.2 | [24] |
| Sl. No. | Feedstock (F) | Pyrolysis Temperature (°C) | Aromatic Hydrocarbon Yield | Main Aromatic Hydrocarbon | Catalyst (C) Used | C:F Ratio (wt:wt) | Reference |
|---|---|---|---|---|---|---|---|
| 1. | Nanochloropsis sp. (NS) | 800 | 9.9 | Toluene | - | - | [77] |
| Waste Tires (WT) | 59.2 | Xylene | |||||
| NS:WT (1:4 wt/wt) | 63 | Toluene | |||||
| NS:WT (2:3 wt/wt) | 60.6 | ||||||
| NS:WT (1:1 wt/wt) | 56.3 | ||||||
| NS:WT (3:2 wt/wt) | 52.8 | ||||||
| NS:WT (4:1 wt/wt) | 49.2 | ||||||
| 2. | Spirulina | 600 | 7.2 | Toluene | CaO:HZSM–5 (1:3 wt/wt) | 1:1 | [78] |
| Oil Shale | 3 | ||||||
| Spirulina: Oil Shale (1:1 wt/wt) | 15.9 | ||||||
| 3. | Chlorella sorokiniana (CS) | 500 | 19 | Toluene | - | - | [79] |
| Polystyrene (PS) | 77.5 | ||||||
| Waste Tire (WT) | 45.1 | ||||||
| CS:PS:WT (1:1:1 wt/wt) | 77.2 | ||||||
| 4. | CV | 800 | 9.5 | Not Available | ZSM—5 | 1:5 | [80] |
| Rural Solid Waste (RSW) | 18 | ||||||
| CV:RSW (1:1 wt/wt) | 17.6 | ||||||
| 5. | Algal Biomass (AB) | 500 | 62.2 | 2,6—Dimethyl Naphthalene | ZSM—5 | 2:1 | [4] |
| Cedar Wood (CW) | 85.1 | 1—Methyl, Napthalene | |||||
| Digested Sludge (DS) | 72.3 | ||||||
| AB:CW:DS (1:1:1 wt/wt) | 89.4 | 2—Methyl, Napthalene |
| Sl. No. | Feedstock | Temperature (°C) | Biocrude Yield (wt%) | Bio—Oil Characteristics | Reference | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | H | N | S | O | HHV a | |||||
| 1. | Chlorella pyrenoidosa (CP) | 43.1 | 73.6 | 8.5 | 7.6 | 0.6 | 9.8 | 35.4 | [73] | |
| CP:SPR (3:1 wt:wt) | 300 | 40 | 75.2 | 8.1 | 7 | 0.5 | 9.2 | 35.4 | ||
| Sweet Potato Residue (SPR) | 37 | 70.9 | 6.7 | 0.4 | BDL b | 22.1 | 29.6 | |||
| 2. | Nannochloropsis sp. (NS) | 350 | 45.1 | 75.8 | 7.5 | 5 | BDL | 11.6 | 35.9 | [110] |
| NS:RS (1:1 wt:wt) | 42.2 | 77.2 | 7.1 | 4.6 | BDL | 11.1 | 32.6 | |||
| Rice Straw (RS) | 32.5 | 77.8 | 6.6 | 2.4 | BDL | 13.3 | 33.6 | |||
| 3. | Chlorella vulgaris (CV) | 350 | 18.3 | 66.2 | 8.1 | 5 | 0.7 | 20.1 | 31.9 | [111] |
| Green Waste (GW) | 4.4 | 76.6 | 3.1 | 7.4 | 0.1 | 12.8 | 32.5 | |||
| CV:GW (1:1 wt:wt) | 10.9 | 75 | 4.5 | 8.5 | 0.2 | 11.7 | 32.5 | |||
| Sewage Sludge (SS) | 12 | 71.3 | 3.8 | 8.4 | 1.7 | 14.7 | 32.1 | |||
| CV:SS (1:1 wt:wt) | 16.4 | 72.1 | 4.4 | 8.4 | 1 | 14.1 | 32.9 | |||
| 4. | Chlorella vulgaris (CV) | 21 | 65.4 | 8.8 | 6.5 | 0.7 | 18.6 | 29.6 | [112] | |
| CV:SB (1:1 wt:wt) | 250 | 20.9 | 62.3 | 8.6 | 6.2 | 0.4 | 22.5 | 28 | ||
| Sugarcane Bagasse (SB) | 12 | 58 | 6 | 0.2 | 0.1 | 35.7 | 23.2 | |||
| Sl. No. | Feedstock | Temperature (°C) | Hydrochar Yield a | Hydrochar Characteristics | Reference | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C b | H | N | S | O | Ash | HHV b | |||||
| 1. | Chlorella sp. | 220 | 28 | 49.3 | 7.4 | 9.6 | 0.9 | 31.7 | 1.2 | 21.2 | [131] |
| 2. | Nannochloropsis gladina | 222 | 40 | 49.4 | 7 | 5.9 | 0.5 | 32.1 | 5.1 | 22.1 | [132] |
| 3. | Picochlorum oculatum | 200 | 30.8 | 65.1 | 7.5 | 6 | 0.4 | 21.1 | 8.1 | 28.9 | [133] |
| 4. | Spirulina platensis | 180 | 47.3 | 46.1 | 6.7 | 8.7 | 0.6 | 31.8 | 6.1 | 19.4 | [134] |
| 5. | Farm Digestate | 250 | 51 | 62.9 | 5.6 | 1.7 | 0.4 | 17.2 | 12.3 | 26.5 | [135] |
| 6. | Almond Shells | 220 | 62 | 50.7 | 6 | 0.4 | BDL c | 43 | 1.4 | 20.3 | [136] |
| 7. | Food Waste | 230 | 10 | 54.8 | 6.1 | 2.3 | 0.2 | 22.3 | 14.3 | 23.7 | [137] |
| 8. | Agricultural Waste | 240 | 42 | 63.7 | 5.8 | 0.8 | 0.2 | 27.9 | 1.6 | 25.3 | [138] |
| 9. | Rice Husk | 220 | 75.9 | 58.8 | 5.9 | 0.4 | BDL | 34.8 | 21.2 | 22 | [139] |
| Sl. No. | Microalgal Strain | Process Utilized | Target Bioproduct | Major Outcome | Reference |
|---|---|---|---|---|---|
| 1. | Chlorella pyrenoidosa | Dark fermentation of acid treated microalgal biomass (cultivated in wastewater) | Biohydrogen (Biofuel Application) | Biohydrogen Produced: 45.5 mL H2/g Volatile Solids | [20] |
| Scenedesmus obliquus | Biohydrogen Produced: 38.4 mL H2/g Volatile Solids | ||||
| Chlorella sorokiniana | Biohydrogen Produced: 34.8 mL H2/g Volatile Solids | ||||
| 2. | Poterioochromonas malhamensis | Microalgae cultivated in Bold Basal media (with optimized NH4Cl content) | Carbohydrate (Food Preservative and Pharmaceutical Applications) | Chrysolaminarin Productivity: 8.2 g. L−1. day−1 | [140] |
| 3. | Dunaliella salina | Microalgae grown in Artificial Sea Water medium (Carbon Source = NaHCO3) | Pigment Production (Food and Nutraceutical Applications) | β—Carotene Produced: 4.6 mg. L−1. day−1 | [141] |
| 4. | Aurantiochytrium sp. | Genetically engineered microalgae grown in PYG medium | Ω-3 Polyunsaturated Fatty Acids (Health Supplement Application) | Eicosapentaenoic Acid Produced: 0.5 g. L−1. day−1 | [142] |
| 5. | Nannochloropsis sp. | Catalytic Pyrolysis (Catalyst Used = Zeolite HY) | Bio-Oil (Valuable precursor for high value compounds) | Bio-oil Yield = 38.3 wt%; Aromatic Hydrocarbon Content of Bio-oil = 96.3 area% | [143] |
| 6. | Chlorella vulgaris | HTL | Biocrude Oil (improved properties for utilization as fuel and/or fuel additive) | Biocrude Oil Yield = 36.2 wt% (C = 68.2 wt%; H = 8.7 wt%; N = 7 wt%; O = 16.1 wt%; S = BDL a) | [144] |
| Arthrospira platensis | Biocrude Oil Yield = 39.6 wt% (C = 70.8 wt%; H = 9 wt%; N = 6.6 wt%; O = 13.6 wt%; S = BDL) | ||||
| 7. | Chlorella vulgaris | HTC | Hydrochar (Utility as Soil Amendment; electrode material and more) | Hydrochar Yield = 38 wt%; (C = 49.1 wt%; H = 6.2 wt%; N = 4.1 wt%; O = 11.5 wt%; S = BDL; Ash = 29.1 wt%) | [145] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chakraborty, S.; Dunford, N.T. Algae: Nature’s Renewable Resource for Fuels and Chemicals. Biomass 2024, 4, 329-348. https://doi.org/10.3390/biomass4020016
Chakraborty S, Dunford NT. Algae: Nature’s Renewable Resource for Fuels and Chemicals. Biomass. 2024; 4(2):329-348. https://doi.org/10.3390/biomass4020016
Chicago/Turabian StyleChakraborty, Sourabh, and Nurhan Turgut Dunford. 2024. "Algae: Nature’s Renewable Resource for Fuels and Chemicals" Biomass 4, no. 2: 329-348. https://doi.org/10.3390/biomass4020016





