Hydrochar Production by Hydrothermal Carbonization: Microwave versus Supercritical Water Treatment
Abstract
1. Introduction
2. Biomass and Biowaste: Sources and Composition
- CO2—Carbon Dioxide
- PM—Particulate Matter
- NH3—Ammonia
- CO—Carbon Monoxide
- PM2—Fine Particulate Matter
- TC—Total Carbon
- EC—Elemental Carbon
- Nox—Nitrogen Oxides
- VOCs—Volatile Organic Compounds
- CH4—Methane
- SO2—Sulfur Dioxide
- BC—Black Carbon
- OC—Organic Carbon [7].
2.1. Lignocellulose
2.2. Biomass Sources
2.3. Biomass Types and Conversion
- Wet Biomass: Contains more than 30 wt. % moisture. Examples include algae, sewage sludge, cattle manure, and industrial effluents.
- Dry Biomass: Contains less than 30 wt % moisture. Examples include woody, herbaceous, and agricultural biomass [63].
Hydrochar Yield and Physicochemical Characteristics
- Surface Area: Hydrochar’s surface area affects its reactivity and adsorption capacity.
- Porosity: Porous hydrochar provides sites for adsorption and catalysis.
- Fixed Carbon Content: Indicates the carbonaceous nature of hydrochar.
- Ash Load: The inorganic content in hydrochar.
- Nutrients and Minerals: Hydrochar may retain essential nutrients and minerals from the original biomass.
- Chemical Characteristics: Reaction temperature significantly influences hydrochar’s chemical properties [65].
3. Hydrochar
3.1. Properties and Applications of Hydrochar
3.2. Biochar
4. Biomass Conversion
4.1. Methods
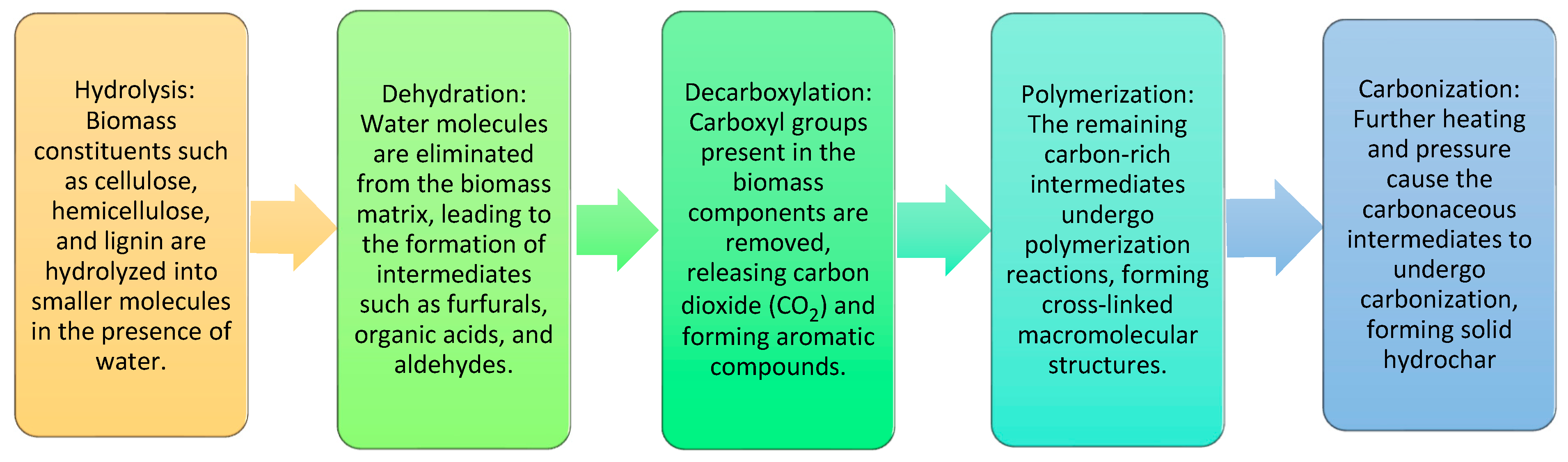
4.2. Hydrothermal Technology
4.3. Hydrothermal Carbonization (HTC)
4.4. Microwave-Assisted Hydrothermal Carbonization (MHTC)
4.5. Supercritical Water Treatment (SCWT)
- Temperature and Reaction Time: The HTC process is conducted at a range of temperatures from as low as 40 °C for food waste to as high as 900 °C for cattle manure compost. The reaction times vary from 0.5 h for materials like corn cob residue to 20 h for orange peel and mixed municipal solid waste.
- High Heat Value (HHV): The HHV, which indicates the energy content, varies widely. For instance, algae and corn cob residue have a higher HHV of 20–25 MJ/kg, suggesting a higher energy potential, while bamboo shoot shell has a lower HHV of 16–17 MJ/kg.
- Hydrochar Yield: The yield percentage indicates the efficiency of the conversion process. Switchgrass shows a wide yield range of 32–82%, possibly due to variations in process conditions or biomass properties. In contrast, rice husk and sewage sludge have more consistent yields of 65–67% and 60–65%, respectively [144,146].
5. Challenges and Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Z.; Shen, D.; Wu, C.; Gu, S. State-of-the-art on the production and application of carbon nanomaterials from biomass. Green Chem. 2018, 20, 5031–5057. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Vassileva, C.G. An overview of the behaviour of biomass during combustion: Part I. Phase-mineral transformations of organic and inorganic matter. Fuel 2013, 112, 391–449. [Google Scholar] [CrossRef]
- Ojewumi, M.E.; Obielue, B.I.; Emetere, M.E.; Awolu, O.O.; Ojewumi, E.O. Alkaline pre-treatment and enzymatic hydrolysis of waste papers to fermentable sugar. J. Ecol. Eng. 2018, 19, 211–217. [Google Scholar] [CrossRef]
- Van Wyk, J.P.H.; Mogale, M.A.; Moroka, K.S. Bioconversion of waste paper materials to sugars: An application illustrating the environmental benefit of enzymes. Biochem. Educ. 1999, 27, 227–228. [Google Scholar] [CrossRef]
- Ukaogo, P.O.; Ewuzie, U.; Onwuka, C.V. 21—Environmental pollution: Causes, effects, and the remedies. In Microorganisms for Sustainable Environment and Health; Elsevier: Amsterdam, The Netherlands, 2020; pp. 419–429. [Google Scholar]
- Jiao, H.; Ali, S.S.; Alsharbaty, M.H.; Elsamahy, T.; Abdelkarim, E.; Schagerl, M.; Al-Tohamy, R.; Sun, J. A critical review on plastic waste life cycle assessment and management: Challenges, research gaps, and future perspectives. Ecotoxicol. Environ. Saf. 2024, 271, 115942. [Google Scholar] [CrossRef] [PubMed]
- Giglio, L.; Randerson, J.T.; Van Der Werf, G.R. Analysis of daily, monthly, and annual burned area using the fourth-generation global fire emissions database (GFED4). J. Geophys. Res. Biogeosci. 2013, 118, 317–328. [Google Scholar] [CrossRef]
- Antar, M.; Lyu, D.; Nazari, M.; Shah, A.; Zhou, X.; Smith, D.L. Biomass for a sustainable bioeconomy: An overview of world biomass production and utilization. Renew. Sustain. Energy Rev. 2021, 139, 110691. [Google Scholar] [CrossRef]
- Zhang, B.; Jiang, Y.; Balasubramanian, R. Synthesis, formation mechanisms and applications of biomass-derived carbonaceous materials: A critical review. J. Mater. Chem. A 2021, 9, 24759–24802. [Google Scholar] [CrossRef]
- Wang, S.; Dai, G.; Yang, H.; Luo, Z. Lignocellulosic biomass pyrolysis mechanism: A state-of-the-art review. Prog. Energy Combust. Sci. 2017, 62, 33–86. [Google Scholar] [CrossRef]
- Wang, J.; Nie, P.; Ding, B.; Dong, S.; Hao, X.; Dou, H.; Zhang, X. Biomass derived carbon for energy storage devices. J. Mater. Chem. A 2017, 5, 2411–2428. [Google Scholar] [CrossRef]
- Chen, Q.; Tan, X.; Liu, Y.; Liu, S.; Li, M.; Gu, Y.; Zhang, P.; Ye, S.; Yang, Z.; Yang, Y. Biomass-derived porous graphitic carbon materials for energy and environmental applications. J. Mater. Chem. A 2020, 8, 5773–5811. [Google Scholar] [CrossRef]
- Song, B.; Lin, R.; Lam, C.H.; Wu, H.; Tsui, T.H.; Yu, Y. Recent advances and challenges of inter-disciplinary biomass valorization by integrating hydrothermal and biological techniques. Renew. Sustain. Energy Rev. 2021, 135, 110370. [Google Scholar] [CrossRef]
- Cha, H.G.; Choi, K.S. Combined biomass valorization and hydrogen production in a photoelectrochemical cell. Nat. Chem. 2015, 7, 328–333. [Google Scholar] [CrossRef]
- Zhang, C.; Su, H.; Baeyens, J.; Tan, T. Reviewing the anaerobic digestion of food waste for biogas production. Renew. Sustain. Energy Rev. 2014, 38, 383–392. [Google Scholar] [CrossRef]
- Raganati, F.; Procentese, A.; Montagnaro, F.; Olivieri, G.; Marzocchella, A. Butanol Production from Leftover Beverages and Sport Drinks. Bioenergy Res. 2015, 8, 369–379. [Google Scholar] [CrossRef]
- Cao, Y.; He, M.; Dutta, S.; Luo, G.; Zhang, S.; Tsang, D.C.W. Hydrothermal carbonization and liquefaction for sustainable production of hydrochar and aromatics. Renew. Sustain. Energy Rev. 2021, 152, 111722. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, Z.; Shen, B.; Liu, L. Insights into biochar and hydrochar production and applications: A review. Energy 2019, 171, 581–598. [Google Scholar] [CrossRef]
- Kumar, M.; Olajire Oyedun, A.; Kumar, A. A review on the current status of various hydrothermal technologies on biomass feedstock. Renew. Sustain. Energy Rev. 2018, 81, 1742–1770. [Google Scholar] [CrossRef]
- Fernández-Sanromán, Á.; Lama, G.; Pazos, M.; Rosales, E.; Sanromán, M.Á. Bridging the gap to hydrochar production and its application into frameworks of bioenergy, environmental and biocatalysis areas. Bioresour. Technol. 2021, 320, 124399. [Google Scholar] [CrossRef]
- Cavali, M.; Junior, N.L.; de Sena, J.D.; Woiciechowski, A.L.; Soccol, C.R.; Belli Filho, P.; Bayard, R.; Benbelkacem, H.; de Castilhos Junior, A.B. A review on hydrothermal carbonization of potential biomass wastes, characterization and environmental applications of hydrochar, and biorefinery perspectives of the process. Sci. Total Environ. 2023, 857, 159627. [Google Scholar] [CrossRef] [PubMed]
- Reijnders, L. Conditions for the sustainability of biomass based fuel use. Energy Policy 2006, 34, 863–876. [Google Scholar] [CrossRef]
- Siwal, S.S.; Zhang, Q.; Devi, N.; Saini, A.K.; Saini, V.; Pareek, B.; Gaidukovs, S.; Thakur, V.K. Recovery processes of sustainable energy using different biomass and wastes. Renew. Sustain. Energy Rev. 2021, 150, 111483. [Google Scholar] [CrossRef]
- Kataya, G.; Cornu, D.; Bechelany, M.; Hijazi, A.; Issa, M. Biomass Waste Conversion Technologies and Its Application for Sustainable Environmental Development—A Review. Agronomy 2023, 13, 2833. [Google Scholar] [CrossRef]
- Nazir, G.; Rehman, A.; Hussain, S.; Mahmood, Q.; Fteiti, M.; Heo, K.; Ikram, M.; Din, M.A. Towards a sustainable conversion of biomass/biowaste to porous carbons for CO2 adsorption: Recent advances, current challenges, and future directions. Green Chem. 2023, 25, 4951–4980. [Google Scholar] [CrossRef]
- Mäki-Arvela, P.; Holmbom, B.; Salmi, T.; Murzin, D.Y. Recent progress in synthesis of fine and specialty chemicals from wood and other biomass by heterogeneous catalytic processes. Catal. Rev. 2007, 49, 197–340. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Yang, D.-P.; Li, Z.; Liu, M.; Zhang, X.; Chen, Y.; Xue, H.; Ye, E.; Luque, R. Biomass-Derived Carbonaceous Materials: Recent Progress in Synthetic Approaches, Advantages, and Applications. ACS Sustain. Chem. Eng. 2019, 7, 4564–4585. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, Y.-J.; Hao, X.; Peng, P.; Shi, J.-Y.; Peng, F.; Sun, R.-C. Hydrothermal synthesis and applications of advanced carbonaceous materials from biomass: A review. Adv. Compos. Hybrid Mater. 2020, 3, 267–284. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, Y.; Qian, F.; Zhou, C.; Zhang, S.; Chen, J. Role of Hydrochar Properties on the Porosity of Hydrochar-based Porous Carbon for Their Sustainable Application. ACS Sustain. Chem Eng. 2015, 3, 833–840. [Google Scholar] [CrossRef]
- Leng, L.; Yang, L.; Leng, S.; Zhang, W.; Zhou, Y.; Peng, H.; Li, H.; Hu, Y.; Jiang, S.; Li, H. A review on nitrogen transformation in hydrochar during hydrothermal carbonization of biomass containing nitrogen. Sci. Total Environ. 2021, 756, 143679. [Google Scholar] [CrossRef]
- Goyal, A.; Ghosh, B.; Eveleigh, D. Characteristics of fungal cellulases. Bioresour. Technol. 1991, 36, 37–50. [Google Scholar] [CrossRef]
- Oshins, C.; Michel, F.; Louis, P.; Richard, T.L.; Rynk, R. Chapter 3–The composting process. In The Composting Handbook; Academic Press: Cambridge, MA, USA, 2022; pp. 51–101. [Google Scholar]
- Alvarez, J.V.L.; Larrucea, M.A.; Bermúdez, P.A.; Chicote, B.L. Biodegradation of paper waste under controlled composting conditions. Waste Manag. 2009, 29, 1514–1519. [Google Scholar] [CrossRef]
- Nigam, P.S.; Singh, A. Production of liquid biofuels from renewable resources. Prog. Energy Combust. Sci. 2011, 37, 52–68. [Google Scholar] [CrossRef]
- Nitsos, C.K.; Matis, K.A.; Triantafyllidis, K.S. Optimization of hydrothermal pretreatment of lignocellulosic biomass in the bioethanol production process. ChemSusChem 2013, 6, 110–122. [Google Scholar] [CrossRef]
- Umenweke, G.; Ighalo, J.; Anusi, M.; Itabana, B.; Ekeh, L. Selected Thermo-Chemical Biorefining: Evaluation of the Current Trends and Progressions. Eur. J. Sustain. Dev. Res. 2021, 5, em0154. [Google Scholar] [CrossRef]
- Akinbile, C.O.; Yusoff, M.S. Environmental Impact of Leachate Pollution on Groundwater Supplies in Akure, Nigeria. Int. J. Environ. Sci. Dev. 2011, 2, 81–86. [Google Scholar] [CrossRef]
- Zaccariello, L.; Battaglia, D.; Morrone, B.; Mastellone, M.L. Hydrothermal carbonization of digestate and leachate in a lab-scale batch reactor. Chem. Eng. Trans. 2021, 86, 91–96. [Google Scholar]
- Zhou, C.; Wang, Y. Recent progress in the conversion of biomass wastes into functional materials for value-added applications. Sci. Technol. Adv. Mater. 2020, 21, 787–804. [Google Scholar] [CrossRef]
- Lou, X.F.; Nair, J. The impact of landfilling and composting on greenhouse gas emissions—A review. Bioresour. Technol. 2009, 100, 3792–3798. [Google Scholar] [CrossRef]
- Weitz, K.A.; Thorneloe, S.A.; Nishtala, S.R.; Yarkosky, S.; Zannes, M. The impact of municipal solid waste management on greenhouse gas emissions in the United States. J. Air Waste Manag. Assoc. 2002, 52, 1000–1011. [Google Scholar] [CrossRef]
- Sankaran, R.; Markandan, K.; Khoo, K.S.; Cheng, C.K.; Ashokkumar, V.; Deepanraj, B.; Show, P.L. The Expansion of Lignocellulose Biomass Conversion Into Bioenergy via Nanobiotechnology. Front. Nanotechnol. 2021, 3, 793528. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Friedl, J.; Vahidi, M.; Rowlings, D.W.; Bai, Z.; Dunn, K.; O’Hara, I.M.; Zhang, Z. Effects of hydrochar derived from hydrothermal treatment of sludge and lignocellulose mixtures on soil properties, nitrogen transformation, and greenhouse gases emissions. Chemosphere 2022, 307, 135792. [Google Scholar] [CrossRef]
- Xue, Y.; Bai, L.; Chi, M.; Xu, X.; Chen, Z.; Yu, K.; Liu, Z. Co-hydrothermal carbonization of pretreatment lignocellulose biomass and polyvinyl chloride for clean solid fuel production: Hydrochar properties and its formation mechanism. J. Environ. Chem. Eng. 2022, 10, 106975. [Google Scholar] [CrossRef]
- Ajdary, R.; Tardy, B.L.; Mattos, B.D.; Bai, L.; Rojas, O.J. Plant Nanomaterials and Inspiration from Nature: Water Interactions and Hierarchically Structured Hydrogels. Adv. Mater. 2021, 33, 2001085. [Google Scholar] [CrossRef]
- Scheller, H.V.; Ulvskov, P. Hemicelluloses. Annu. Rev. Plant Biol. 2010, 61, 263–289. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Growth of the plant cell wall. Nat. Rev. Mol. Cell Biol. 2005, 66, 850–861. [Google Scholar] [CrossRef]
- Simmons, T.J.; Mortimer, J.C.; Bernardinelli, O.D.; Pöppler, A.C.; Brown, S.P.; de Azevedo, E.R.; Dupree, R.; Dupree, P. Folding of xylan onto cellulose fibrils in plant cell walls revealed by solid-state NMR. Nat. Commun. 2016, 7, 13902. [Google Scholar] [CrossRef]
- Pauly, M.K.K. Plant cell wall polymers as precursors for biofuels. Curr. Opin. Plant Biol. 2010, 13, 305–312. [Google Scholar] [CrossRef]
- Qaseem, M.F.; Shaheen, H.; Wu, A.M. Cell wall hemicellulose for sustainable industrial utilization. Renew. Sustain. Energy Rev. 2021, 144, 110996. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Williams, C.K.; Davison, B.H.; Britovsek, G.; Cairney, J.; Eckert, C.A.; Frederick, W.J., Jr.; Hallett, J.P.; Leak, D.J.; Liotta, C.L.; et al. The path forward for biofuels and biomaterials. Science 2006, 311, 484–489. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, J. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef]
- Sun, H.; Gao, Z.; Zhang, L.; Wang, X.; Gao, M.; Wang, Q. A comprehensive review on microbial lipid production from wastes: Research updates and tendencies. Environ. Sci. Pollut. Res. 2023, 30, 79654–79675. [Google Scholar] [CrossRef]
- Meez, E.; Rahdar, A.; Kyzas, G.Z. Sawdust for the removal of heavy metals from water: A review. Molecules 2021, 36, 4318. [Google Scholar] [CrossRef]
- Julkipli, J.; Babel, S.; Bilyaminu, A.M.; Rene, E.R. Hydrogen and biodiesel production from food waste: A review. Environ. Chem. Lett. 2024, 22, 585–607. [Google Scholar] [CrossRef]
- Güleç, F.; Williams, O.; Kostas, E.T.; Samson, A.; Lester, E. A comprehensive comparative study on the energy application of chars produced from different biomass feedstocks via hydrothermal conversion, pyrolysis, and torrefaction. Energy Convers. Manag. 2022, 270, 116260. [Google Scholar] [CrossRef]
- Jain, A.; Balasubramanian, R.; Srinivasan, M.P. Hydrothermal conversion of biomass waste to activated carbon with high porosity: A review. Chem. Eng. J. 2016, 283, 789–805. [Google Scholar] [CrossRef]
- Benavente, V.; Lage, S.; Gentili, F.G.; Jansson, S. Influence of lipid extraction and processing conditions on hydrothermal conversion of microalgae feedstocks—Effect on hydrochar composition, secondary char formation and phytotoxicity. Chem. Eng. J. 2022, 428, 129559. [Google Scholar] [CrossRef]
- Zheng, C.; Ma, X.; Yao, Z.; Chen, X. The properties and combustion behaviors of hydrochars derived from co-hydrothermal carbonization of sewage sludge and food waste. Bioresour. Technol. 2019, 285, 121347. [Google Scholar] [CrossRef]
- Hoang, A.T.; Varbanov, P.S.; Nižetić, S.; Sirohi, R.; Pandey, A.; Luque, R.; Ng, K.H.; Pham, V.V. Perspective review on Municipal Solid Waste-to-energy route: Characteristics, management strategy, and role in circular economy. J. Clean. Prod. 2022, 359, 131897. [Google Scholar] [CrossRef]
- González Fernández, L.A.; Castillo Ramos, V.; Sánchez Polo, M.; Medellín Castillo, N.A. Fundamentals in applications of algae biomass: A review. J. Environ. Manag. 2023, 338, 117830. [Google Scholar] [CrossRef] [PubMed]
- Tasca, A.L.; Puccini, M.; Gori, R.; Corsi, I.; Galletti, A.M.R.; Vitolo, S. Hydrothermal carbonization of sewage sludge: A critical analysis of process severity, hydrochar properties and environmental implications. Waste Manag. 2019, 93, 1–13. [Google Scholar] [CrossRef]
- Gao, Y.; Remón, J.; Matharu, A.S. Microwave-assisted hydrothermal treatments for biomass valorisation: A critical review. Green Chem. 2021, 23, 3502–3525. [Google Scholar] [CrossRef]
- Sharma, H.B.; Sarmah, A.K.; Dubey, B. Hydrothermal carbonization of renewable waste biomass for solid biofuel production: A discussion on process mechanism, the influence of process parameters, environmental performance and fuel properties of hydrochar. Renew. Sustain. Energy Rev. 2020, 123, 109761. [Google Scholar] [CrossRef]
- Hrnčič, M.K.; Kravanja, G.; Knez, Ž. Hydrothermal treatment of biomass for energy and chemicals. Energy 2016, 116, 1312–1322. [Google Scholar] [CrossRef]
- Nizamuddin, S.; Baloch, H.A.; Griffin, G.; Mubarak, N.; Bhutto, A.W.; Abro, R.; Mazari, S.A.; Ali, B.S. An overview of effect of process parameters on hydrothermal carbonization of biomass. Renew. Sustain. Energy Rev. 2017, 73, 1289–1299. [Google Scholar] [CrossRef]
- Langone, M.; Basso, D. Process waters from hydrothermal carbonization of sludge: Characteristics and possible valorization pathways. Int. J. Environ. Res. Public Health 2020, 17, 6618. [Google Scholar] [CrossRef]
- Bhaskar, T.; Bhavya, B.; Singh, R.; Naik, D.V.; Kumar, A.; Goyal, H.B. Thermochemical Conversion of Biomass to Biofuels. In Biofuels; Academic Press: Cambridge, MA, USA, 2011; pp. 51–77. [Google Scholar]
- Toptas Tag, A.; Duman, G.; Yanik, J. Influences of feedstock type and process variables on hydrochar properties. Bioresour. Technol. 2018, 250, 337–344. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Rangabhashiyam, S.; Dulta, K.; Umeh, C.T.; Iwuozor, K.O.; Aniagor, C.O.; Eshiemogie, S.O.; Iwuchukwu, F.U.; Igwegbe, C.A. Recent advances in hydrochar application for the adsorptive removal of wastewater pollutants. Chem. Eng. Res. Des. 2022, 184, 419–456. [Google Scholar] [CrossRef]
- Khosravi, A.; Zheng, H.; Liu, Q.; Hashemi, M.; Tang, Y.; Xing, B. Production and characterization of hydrochars and their application in soil improvement and environmental remediation. Chem. Eng. J. 2022, 430, 133–142. [Google Scholar] [CrossRef]
- Berge, N.D.; Li, L.; Flora, J.R.; Ro, K.S. Assessing the environmental impact of energy production from hydrochar generated via hydrothermal carbonization of food wastes. Waste Manag. 2015, 43, 203–217. [Google Scholar] [CrossRef]
- Islam, M.T.; Sultana, A.I.; Chambers, C.; Saha, S.; Saha, N.; Kirtania, K.; Reza, M.T. Recent Progress on Emerging Applications of Hydrochar. Energies 2022, 15, 9340. [Google Scholar] [CrossRef]
- Huang, J.; Feng, Y.; Xie, H.; Wu, P.; Wang, M.; Wang, B.; Zhang, Q.; Zhang, S.; Liu, Z. A bibliographic study reviewing the last decade of hydrochar in environmental application: History, status quo, and trending research paths. Biochar 2023, 5, 12. [Google Scholar] [CrossRef]
- Bolisetty, S.; Peydayesh, M.; Mezzenga, R. Sustainable technologies for water purification from heavy metals: Review and analysis. Chem. Soc. Rev. 2019, 48, 463–487. [Google Scholar] [CrossRef] [PubMed]
- Dieguez-Alonso, A.; Funke, A.; Anca-Couce, A.; Rombolà, A.G.; Ojeda, G.; Bachmann, J.; Behrendt, F. Towards biochar and hydrochar engineering-influence of process conditions on surface physical and chemical properties, thermal stability, nutrient availability, toxicity and wettability. Energies 2018, 11, 496. [Google Scholar] [CrossRef]
- Mulabagal, V.; Baah, D.A.; Egiebor, N.O.; Chen, W.Y. Biochar from Biomass: A Strategy for Carbon Dioxide Sequestration, Soil Amendment, Power Generation, and CO2 Utilization. In Handbook of Climate Change Mitigation and Adaptation; Springer: New York, NY, USA, 2015; pp. 1–31. [Google Scholar]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Su, J.-Z.; Wang, C.-C.; Zhang, M.-Y.; Zong, X.-B.; Huang, X.-F.; Deng, Z.-H.; Xiang, P. Advances and prospectives of iron/biochar composites: Application, influencing factors and characterization methods. Ind. Crop. Prod. 2023, 205, 117496. [Google Scholar] [CrossRef]
- Guo, M.; Song, W.; Tian, J. Biochar-Facilitated Soil Remediation: Mechanisms and Efficacy Variations. Front. Environ. Sci. 2020, 8, 521512. [Google Scholar] [CrossRef]
- Tan, X.; Liu, Y.; Zeng, G.; Wang, X.; Hu, X.; Gu, Y.; Yang, Z. Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere 2015, 125, 70–85. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhong, H.; Liu, G.; Dai, Z.; Brookes, P.C.; Xu, J. Remediation of heavy metal contaminated soils by biochar: Mechanisms, potential risks and applications in China. Environ. Pollut. 2019, 252, 846–855. [Google Scholar] [CrossRef]
- Rajput, V.D.; Minkina, T.; Ahmed, B.; Singh, V.K.; Mandzhieva, S.; Sushkova, S.; Bauer, T.; Verma, K.K.; Shan, S.; van Hullebusch, E.D.; et al. Nano-biochar: A novel solution for sustainable agriculture and environmental remediation. Environ. Res. 2022, 210, 112891. [Google Scholar] [CrossRef]
- He, M.; Xu, Z.; Hou, D.; Gao, B.; Cao, X.; Ok, Y.S.; Rinklebe, J.; Bolan, N.S.; Tsang, D.C.W. Waste-derived biochar for water pollution control and sustainable development. Nat. Rev. Earth Environ. 2022, 3, 444–460. [Google Scholar] [CrossRef]
- Kambo, H.S.; Dutta, A. A comparative review of biochar and hydrochar in terms of production, physico-chemical properties and applications. Renew. Sustain. Energy Rev. 2015, 45, 359–378. [Google Scholar] [CrossRef]
- Cavali, M.; Junior, N.L.; de Almeida Mohedano, R.; Belli Filho, P.; da Costa, R.H.; de Castilhos Junior, A.B. Biochar and hydrochar in the context of anaerobic digestion for a circular approach: An overview. Sci. Total Environ. 2022, 822, 153614. [Google Scholar] [CrossRef] [PubMed]
- Knodel, N. A Framework for Agricultural Decarbonisation: Environmental Assessment from Seed to Soil of a Cradle-to-Cradle Farm System with Industrial Hemp and Pyrogenic Carbon Capture & Storage. SSRN Electron. J. 2022. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Cui, L.; Kammann, C.; Wrage-Monnig, N.; Estavillo, J.M.; Fuertes-Mendizabal, T.; Cayuela, M.L.; Sigua, G.; Novak, J.; Spokas, K.; et al. Feedstock choice, pyrolysis temperature and type influence biochar characteristics: A comprehensive meta-data analysis review. Biochar 2020, 2, 421–438. [Google Scholar] [CrossRef]
- Amalina, F.; Razak, A.S.A.; Krishnan, S.; Zularisam, A.W.; Nasrullah, M. A comprehensive assessment of the method for producing biochar, its characterization, stability, and potential applications in regenerative economic sustainability—A review. Clean. Mater. 2022, 3, 100045. [Google Scholar] [CrossRef]
- Igalavithana, A.D.; Ok, Y.S.; Usman, A.R.A.; Al-Wabel, M.I.; Oleszczuk, P.; Lee, S.S. The effects of biochar amendment on soil fertility. In Agricultural and Environmental Applications of Biochar: Advances and Barriers; Wiley: Hoboken, NJ, USA, 2015; pp. 123–144. [Google Scholar]
- Ding, Y.; Liu, Y.; Liu, S.; Li, Z.; Tan, X.; Huang, X.; Zeng, G.; Zhou, L.; Zheng, B. Biochar to improve soil fertility. A review. Agron. Sustain. Dev. 2016, 36, 36. [Google Scholar] [CrossRef]
- Song, C.; Shan, S.; Yang, C.; Zhang, C.; Zhou, X.; Ma, Q.; Yrjala, K.; Zheng, H.; Cao, Y. The comparison of dissolved organic matter in hydrochars and biochars from pig manure. Sci. Total Environ. 2020, 720, 137423. [Google Scholar] [CrossRef] [PubMed]
- Brtnicky, M.; Datta, R.; Holatko, J.; Bielska, L.; Gusiatin, Z.M.; Kucerik, J.; Hammerschmiedt, T.; Danish, S.; Radziemska, M.; Mravcova, L.; et al. A critical review of the possible adverse effects of biochar in the soil environment. Sci. Total Environ. 2021, 796, 148756. [Google Scholar] [CrossRef] [PubMed]
- Wancura, J.H.; Brondani, M.; Vezaro, F.D.; Martins-Vieira, J.C.; Moreira, B.P.; dos Santos, M.S.; Abaide, E.R.; de Castilhos, F.; Mayer, F.D. Motivations to produce biofuels from rice bran: An overview involving a recent panorama. Ind. Crop. Prod. 2023, 203, 117–170. [Google Scholar] [CrossRef]
- Martín, M.; Taifouris, M.; Galán, G. Lignocellulosic biorefineries: A multiscale approach for resource exploitation. Bioresour. Technol. 2023, 385, 129–397. [Google Scholar] [CrossRef] [PubMed]
- Vickram, A.S.; Saravanan, A.; Kumar, P.S.; Thamarai, P.; Yasodha, S.; Jamuna, G.; Rangasamy, G. An integrated approach to the sustainable development and production of biofuel from biopolymers and algal biomass derived from wastewater. Fuel 2023, 349, 128691. [Google Scholar] [CrossRef]
- Yamakawa, C.K.; Qin, F.; Mussatto, S.I. Biomass Conversion Technologies. Greenh. Gas Balances Bioenergy Syst. 2018, 119, 107–139. [Google Scholar]
- Velvizhi, G.; Jacqueline, P.J.; Shetti, N.P.; Latha, K.; Mohanakrishna, G.; Aminabhavi, T.M. Emerging trends and advances in valorization of lignocellulosic biomass to biofuels. J. Environ. Manag. 2023, 345, 118527. [Google Scholar] [CrossRef] [PubMed]
- Garba, A. Biomass Conversion Technologies for Bioenergy Generation: An Introduction. Available online: www.intechopen.com (accessed on 1 December 2020). [CrossRef]
- Rosendahl, L. 12—Biomass resources, fuel preparation and utilization for improving the fuel flexibility of advanced power plants. In Advanced Power Plant Materials, Design and Technology; Woodhead Publishing: Southton, UK, 2010; pp. 312–331. [Google Scholar]
- Hussin, F.; Hazani, N.N.; Khalil, M.; Aroua, M.K. Environmental life cycle assessment of biomass conversion using hydrothermal technology: A review. Fuel Process. Technol. 2023, 246, 107747. [Google Scholar] [CrossRef]
- Bernardo, M.; Correa, C.R.; Ringelspacher, Y.; Becker, G.C.; Lapa, N.; Fonseca, I.; Esteves, I.A.; Kruse, A. Porous carbons derived from hydrothermally treated biogas digestate. Waste Manag. 2020, 105, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Toufiqur, M.; Charles, R.; Coronella, J.; Advisor, D. Upgrading Biomass by Hydrothermal and Chemical Conditioning. Ph.D. Thesis, University of Nevada, Reno, NV, USA, 2013. [Google Scholar]
- Peterson, A.A.; Vogel, F.; Lachance, R.P.; Fröling, M.; Antal, M.J.; Tester, J.W. Thermochemical biofuel production in hydrothermal media: A review of sub- and supercritical water technologies. Energy Environ. Sci. 2008, 1, 32–65. [Google Scholar] [CrossRef]
- Rudra, S.; Jayathilake, M. 5.08—Hydrothermal Liquefaction of Biomass for Biofuel Production. In Comprehensive Renewable Energy, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2022; Volume 1–9, pp. 165–186. [Google Scholar]
- Ipiales, R.P.; Sarrion, A.; Diaz, E.; de la Rubia, M.A.; Diaz-Portuondo, E.; Coronella, C.J.; Mohedano, A.F. Swine manure management by hydrothermal carbonization: Comparative study of batch and continuous operation. Environ. Res. 2023, 245, 118062. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Zhang, H.; Lü, F.; Shao, L.; He, P. From food waste and its digestate to nitrogen self-doped char and methane-rich syngas: Evolution of pyrolysis products during autogenic pressure carbonization. J. Hazard. Mater. 2022, 424, 127249. [Google Scholar] [CrossRef] [PubMed]
- Amalina, F.; Krishnan, S.; Zularisam, A.W.; Nasrullah, M. Biochar and sustainable environmental development towards adsorptive removal of pollutants: Modern advancements and future insight. Process Saf. Environ. Prot. 2023, 173, 715–728. [Google Scholar] [CrossRef]
- Harry, I.; Ibrahim, H.; Thring, R.; Idem, R. Catalytic subcritical water liquefaction of flax straw for high yield of furfural. Biomass Bioenergy 2014, 71, 381–393. [Google Scholar] [CrossRef]
- Funke, A.; Ziegler, F. Hydrothermal carbonization of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuels Bioprod. Biorefin. 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Román, S.; Libra, J.; Berge, N.; Sabio, E.; Ro, K.; Li, L.; Ledesma, B.; Álvarez, A.; Bae, S. Hydrothermal carbonization: Modeling, final properties design and applications: A review. Energies 2018, 11, 216. [Google Scholar] [CrossRef]
- Ramke, H.G.; Blöhse, D.; Lehmann, H.J.; Fettig, J. Hydrothermal Carbonization of Organic Waste. In Proceedings of the Twelfth International Waste Management and Landfill Symphosium, Cagliari, Italy, 5–9 October 2009. [Google Scholar]
- Wang, J.X.; Chen, S.W.; Lai, F.Y.; Liu, S.Y.; Xiong, J.B.; Zhou, C.F.; Huang, H.J. Microwave-assisted hydrothermal carbonization of pig feces for the production of hydrochar. J. Supercrit. Fluids 2020, 162, 104858. [Google Scholar] [CrossRef]
- Wang, Y.J.; Yu, Y.; Huang, H.J.; Yu, C.L.; Fang, H.S.; Zhou, C.H.; Yin, X.; Chen, W.H.; Guo, X.C. Efficient conversion of sewage sludge into hydrochar by microwave-assisted hydrothermal carbonization. Sci. Total Environ. 2022, 803, 149874. [Google Scholar] [CrossRef] [PubMed]
- Yameen, M.Z.; Naqvi, S.R.; Juchelková, D.; Khan, M.N.A. Harnessing the power of functionalized biochar: Progress, challenges, and future perspectives in energy, water treatment, and environmental sustainability. Biochar 2024, 6, 25. [Google Scholar] [CrossRef]
- Li, Y.; Tsend, N.; Li, T.; Liu, H.; Yang, R.; Gai, X.; Wang, H.; Shan, S. Microwave assisted hydrothermal preparation of rice straw hydrochars for adsorption of organics and heavy metals. Bioresour. Technol. 2019, 273, 136–143. [Google Scholar] [CrossRef]
- Elaigwu, S.E.; Rocher, V.; Kyriakou, G.; Greenway, G.M. Removal of Pb2+ and Cd2+ from aqueous solution using chars from pyrolysis and microwave-assisted hydrothermal carbonization of Prosopis Africana shell. J. Ind. Eng. Chem. 2014, 20, 3467–3473. [Google Scholar] [CrossRef]
- Calcio Gaudino, E.; Cravotto, G.; Manzoli, M.; Tabasso, S. From waste biomass to chemicals and energy: Via microwave-assisted processes. Green Chem. 2019, 21, 1202–1235. [Google Scholar] [CrossRef]
- Khan, N.; Mohan, S.; Dinesha, P. Regimes of hydrochar yield from hydrothermal degradation of various lignocellulosic biomass: A review. J. Clean. Prod. 2021, 288, 125629. [Google Scholar] [CrossRef]
- Castello, D.; Kruse, A.; Fiori, L. Supercritical water gasification of hydrochar. Chem. Eng. Res. Des. 2014, 92, 1864–1875. [Google Scholar] [CrossRef]
- Lu, Y.; Savage, P.E. Supercritical water gasification of lipid-extracted hydrochar to recover energy and nutrients. J. Supercrit. Fluids 2015, 99, 88–94. [Google Scholar] [CrossRef]
- Ojewumi, M.E.; Oyekunle, D.T.; Amaefule, C.V.; Omoleye, J.A.; Ogunbiyi, A.T. Investigation into Alternative Energy Sources from Waste Citrus Peel (Orange): Approach to Environmental Protection. J. Phys. Conf. Ser. 2019, 1378, 022066. [Google Scholar] [CrossRef]
- Usman, M.; Chen, H.; Chen, K.; Ren, S.; Clark, J.H.; Fan, J.; Luo, G.; Zhang, S. Characterization and utilization of aqueous products from hydrothermal conversion of biomass for bio-oil and hydro-char production: A review. Green Chem. 2019, 21, 1553–1572. [Google Scholar] [CrossRef]
- Lee, C.S.; Conradie, A.V.; Lester, E. Review of supercritical water gasification with lignocellulosic real biomass as the feedstocks: Process parameters, biomass composition, catalyst development, reactor design and its challenges. Chem. Eng. J. 2021, 415, 128837. [Google Scholar] [CrossRef]
- Ambaye, T.G.; Djellabi, R.; Vaccari, M.; Prasad, S.M.; Aminabhavi, T.; Rtimi, S. Emerging technologies and sustainable strategies for municipal solid waste valorization: Challenges of circular economy implementation. J. Clean. Prod. 2023, 423, 138708. [Google Scholar] [CrossRef]
- Tripathi, P.M.; Basu, S. Novel two-phase method for supercritical water flow. arXiv 2020, arXiv:2011.12017. [Google Scholar]
- Auxéméry, A.; Philippot, G.; Suchomel, M.R.; Testemale, D.; Aymonier, C. Stabilization of Tetragonal Zirconia Nanocrystallites Using an Original Supercritical-Based Synthesis Route. Chem. Mater. 2020, 32, 8169–8181. [Google Scholar] [CrossRef]
- Jin, H.; Zhao, X.; Wu, Z.; Cao, C.; Guo, L. Supercritical water synthesis of nano-particle catalyst on TiO2 and its application in supercritical water gasification of biomass. J. Exp. Nanosci. 2017, 12, 72–82. [Google Scholar] [CrossRef]
- Si, H.; Zhao, C.; Wang, B.; Liang, X.; Gao, M.; Jiang, Z.; Yu, H.; Yang, Y.; Gu, Z.; Ogino, K.; et al. Liquid-solid ratio during hydrothermal carbonization affects hydrochar application potential in soil: Based on characteristics comparison and economic benefit analysis. J. Environ. Manag. 2023, 335, 117567. [Google Scholar] [CrossRef]
- Aljvanieh, M.G.; Geçgel, C.; Yabalak, E. Hydrochar synthesis from waste corncob using subcritical water and microwave-assisted carbonization methods and ammonium enrichment of synthesized hydrochars. Environ. Res. 2023, 226, 115715. [Google Scholar] [CrossRef] [PubMed]
- Nzediegwu, C.; Naeth, M.A.; Chang, S.X. Lead(II) adsorption on microwave-pyrolyzed biochars and hydrochars depends on feedstock type and production temperature. J. Hazard. Mater. 2021, 412, 125255. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Ye, J.; Chang, J.; Lou, H.; Zheng, X. Solid fuel production by hydrothermal carbonization of water-like phase of bio-oil. Fuel 2016, 180, 591–596. [Google Scholar] [CrossRef]
- Chen, Z.; Zheng, Z.; He, C.; Liu, J.; Zhang, R.; Chen, Q. Oily sludge treatment in subcritical and supercritical water: A review. J. Hazard. Mater. 2022, 433, 128761. [Google Scholar] [CrossRef] [PubMed]
- Waqas, M.; Hashim, S.; Humphries, U.W.; Ahmad, S.; Noor, R.; Shoaib, M.; Naseem, A.; Hlaing, P.T.; Lin, H.A. Composting Processes for Agricultural Waste Management: A Comprehensive Review. Processes 2023, 11, 731. [Google Scholar] [CrossRef]
- Bach, Q.V.; Tran, K.Q.; Skreiberg, Ø. Hydrothermal pretreatment of fresh forest residues: Effects of feedstock pre-drying. Biomass Bioenergy 2016, 85, 76–83. [Google Scholar] [CrossRef]
- Sivaranjanee, R.; Kumar, P.S.; Rangasamy, G. A recent advancement on hydrothermal carbonization of biomass to produce hydrochar for pollution control. Carbon Lett. 2023, 33, 1909–1933. [Google Scholar] [CrossRef]
- Elaigwu, S.E.; Greenway, G.M. Chemical, structural and energy properties of hydrochars from microwave-assisted hydrothermal carbonization of glucose. Int. J. Ind. Chem. 2016, 7, 449–456. [Google Scholar] [CrossRef]
- Kalderis, D.; Kotti, M.S.; Méndez, A.; Gascó, G. Characterization of hydrochars produced by hydrothermal carbonization of rice husk. Solid Earth 2014, 5, 477–483. [Google Scholar] [CrossRef]
- Demirbaş, A. Biomass resource facilities and biomass conversion processing for fuels and chemicals. Energy Convers. Manag. 2001, 42, 1357–1378. [Google Scholar] [CrossRef]
- Küçük, M.M.; Demirbaş, A. Biomass conversion processes. Energy Convers. Manag. 1997, 38, 151–165. [Google Scholar] [CrossRef]
- A Libra, J.; Ro, K.S.; Kammann, C.; Funke, A.; Berge, N.D.; Neubauer, Y.; Titirici, M.-M.; Fühner, C.; Bens, O.; Kern, J.; et al. Hydrothermal carbonization of biomass residuals: A comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels 2011, 2, 71–106. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, S.; Wang, B.; Wang, Q.; Yang, G.; Chen, J. Effect of Residence Time on Hydrothermal Carbonization of Corn Cob Residual. BioResources 2015, 10, 3979–3986. [Google Scholar] [CrossRef]
- Fernandez, M.E.; Ledesma, B.; Román, S.; Bonelli, P.R.; Cukierman, A.L. Development and characterization of activated hydrochars from orange peels as potential adsorbents for emerging organic contaminants. Bioresour. Technol. 2015, 183, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Zhou, Y.; Meng, F.; Zhang, Y.; Liu, Z.; Zhang, W.; Xue, G. Preparation and characterization of hydrochar from waste eucalyptus bark by hydrothermal carbonization. Energy 2016, 97, 238–245. [Google Scholar] [CrossRef]
- Nizamuddin, S.; Mubarak, N.M.; Tiripathi, M.; Jayakumar, N.S.; Sahu, J.N.; Ganesan, P. Chemical, dielectric and structural characterization of optimized hydrochar produced from hydrothermal carbonization of palm shell. Fuel 2016, 163, 88–97. [Google Scholar] [CrossRef]
- Guo, N.; Li, M.; Sun, X.; Wang, F.; Yang, R. Enzymatic hydrolysis lignin derived hierarchical porous carbon for supercapacitors in ionic liquids with high power and energy densities. Green Chem. 2017, 19, 2595–2602. [Google Scholar] [CrossRef]
- Sharma, R.; Jasrotia, K.; Singh, N.; Ghosh, P.; Srivastava, S.; Sharma, N.R.; Singh, J.; Kanwar, R.; Kumar, A. A Comprehensive Review on Hydrothermal Carbonization of Biomass and its Applications. Chem. Afr. 2020, 3, 1–19. [Google Scholar]
- Mochidzuki, K.; Sato, N.; Sakoda, A. Production and Characterization of Carbonaceous Adsorbents from Biomass Wastes by Aqueous Phase Carbonization. Adsorption 2005, 11, 669–673. [Google Scholar]
- Masoumi, S.; Borugadda, V.B.; Nanda, S.; Dalai, A.K. Hydrochar: A review on its production technologies and applications. Catalysts 2021, 11, 939. [Google Scholar] [CrossRef]
- Padhye, L.P.; Bandala, E.R.; Wijesiri, B.; Goonetilleke, A.; Bolan, N. Hydrochar: A Promising Step Towards Achieving a Circular Economy and Sustainable Development Goals. Front. Chem. Eng. 2022, 4, 867228. [Google Scholar] [CrossRef]
- Islam, M.A.; Sharif, M.; Limon, H.; Romić, M.; Islam, A. Hydrochar-based soil amendments for agriculture: A review of recent progress. Arab. J. Geosci. 2021, 14, 102. [Google Scholar] [CrossRef]
- Ojewumi, M.E.; Ehinmowo, A.B.; Obanla, O.R.; Durodola, B.M.; Ezeocha, R.C. Comparative analysis on the bleaching of crude palm oil using activated groundnut hull, snail shell, and rice husk. Heliyon 2021, 7, e07747. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Fan, Y.; Zhao, X.; E, J.; Xu, W.; Zhang, F.; Liao, G.; Leng, E.; Liu, S. Experimental investigation on gasification characteristic of food waste using supercritical water for combustible gas production: Exploring the way to complete gasification. Fuel 2020, 263, 116753. [Google Scholar] [CrossRef]
| Aspect | Biochar | Hydrochar | Reference |
|---|---|---|---|
| Definition | Biochar is a high-carbon, fine-grained residue produced via pyrolysis—the thermal decomposition of biomass without oxygen. It results in a mixture of solids (biochar), liquid (bio-oil), and gas (syngas) products. | Hydrochar is produced through hydrothermal carbonization (HTC) or liquefaction (HTL). HTC and HTL yield hydrochar, which is a distinct category of biochar. These processes involve subjecting wet biomass to high temperatures and pressure in the presence of water. | [65,66,71] |
| Stability and Carbon Sequestration | Biochar is a stable solid rich in pyrogenic carbon. It can endure in soil for thousands of years, making it suitable for carbon sequestration (PyCCS) and climate change mitigation. | Hydrochar also exhibits stability and can persist in soil. Its refractory nature contributes to carbon storage, although its longevity may not match biochar. | [67,72] |
| Composition and Properties | Biochar typically contains a mix of carbon, ash, and other elements. Its properties depend on the feedstock and pyrolysis conditions. | Hydrochar has a similar carbonaceous composition but may differ in alkali, alkaline earth, and heavy metal content. It often possesses a larger surface area and higher heating value than biochar produced at the same operating temperature. | [69] |
| Applications |
|
| [70] |
| Environmental Considerations: | Biochar can alter soil pH and introduce chemical characteristics that impact microorganisms. | Hydrochar research continues to explore its benefits and drawbacks. | [71,72] |
| S/N | Challenges | Issue | Impact | Reference |
|---|---|---|---|---|
| 1 | Low Yield | The yield of hydrochar obtained from supercritical water conditions is relatively low. | This affects the overall efficiency of the process and limits the effective utilization of valuable biomass resources. | [105,106,107] |
| 2 | Gaseous Product Yield | The gaseous products generated during supercritical water hydrochar production often have low selectivity for hydrogen gas. | Inefficient utilization of the biomass feedstock and suboptimal conversion efficiencies. | [108] |
| 3 | Energy Consumption | The process requires high-energy input due to the extreme conditions (temperature and pressure) needed for supercritical water. | Increased operational costs and environmental footprint. | [109] |
| 4 | Ash Content and Composition | Hydrochar may contain ash from the biomass feedstock. | Ash content affects the quality of hydrochar and suitability for various applications, such as agricultural applications, energy production, and carbon sequestration. | [110] |
| 5 | Reaction Mechanisms and Kinetics | Understanding the complex reactions during supercritical water hydrochar production is challenging. | Lack of detailed knowledge hinders process optimization and control. | [111] |
| 6 | Resource Recycling and Valorization | Efficiently reusing and valorizing post-processing water and byproducts. | Resource management can enhance economic viability and environmental sustainability. | [112] |
| 7. | Scaling Up | Transitioning from lab-scale experiments to large-scale production. | Impact: Ensuring consistent product quality, safety, and cost-effectiveness. | [112] |
| 8. | Functionalization and Applications | Tailoring hydrochar properties for specific applications. | Unlocking its potential in agriculture, pollutant adsorption, catalyst support, bioenergy, and carbon sequestration. | [4,113] |
| S/N | Aspect | Supercritical Water Treatment | Microwave-Assisted Hydrothermal Carbonization | References |
|---|---|---|---|---|
| 1 | Process | Hydrothermal conversion using supercritical water. | Biomass conversion using microwave heating. | [118,119,120] |
| 2 | Temperature | Requires supercritical water conditions (high temperature and pressure). | Higher temperatures due to microwave energy absorption. | [121,122,123,124] |
| 3 | Reaction Rate | Slower due to hydrothermal process (due to conventional heating). | Faster due to selective, fast, and homogeneous heating. | [123,125] |
| 4 | Product Characteristics |
|
| [124,125,126,127] |
| 5 | Energy Efficiency |
|
| [126,127,128,129] |
| 6 | Uniform Heating |
|
| [130] |
| 7 | Scale-Up and Industrial Applications: | Also, scalable but may require more space and time. |
| [130] |
| 8. | Applications | Agriculture, pollutant adsorption, bioenergy. | Energy, pharmaceuticals, and chemistry sectors. | [131,132] |
| Aspect | Supercritical Water Treatment Method | Microwave-Assisted Hydrothermal Carbonization | Reference |
|---|---|---|---|
| Advantages |
|
| [133,134] |
| Disadvantages |
|
| [134,135,136,137,138] |
| Biomass Source | Temperature (°C)/Reaction Time (Hour) | High Heat Value (HHV) (MJ/kg) | Hydrochar Yield % | Reference |
|---|---|---|---|---|
| Rice husk | 300/6 | 16–18 | 65–67 | [135,137] |
| Algae | 190–210/2 | 20–25 | 25–46 | [136,139] |
| Sewage sludge | 200–250/5 | 20–23 | 60–65 | [137] |
| Corn cob residue | 245–250/0.5 | 20–25 | 45–50 | [138] |
| Orange peel | 180–200/20 | ------ | 37 | [139] |
| Water hyacinth | 220–240/0.5 | 17–20 | 61 | [140] |
| Palm shell | 180–200/0.5 | 26–27 | 4565 | [141] |
| Bamboo shoot shell | 200–210/0.5 | 16–17 | 56 | [142] |
| Switchgrass | 300–400/1–2 | 18–22 | 32–82 | [142] |
| Mix wood | 215–295/1 | 20–232 | 70–50 | [143] |
| Sawdust | 240–250/2 | 20–23 | 40 | [143] |
| Cattle manure compost | 400–900/3 | 17–20 | 50 | [144] |
| Food waste | 40–250/3 | 20–23 | 47 | [145] |
| Paper | 240–250/20 | 30–32 | 29–30 | [146] |
| Mixed municipal solid waste | 240–250/20 | 23–25 | 74 | [146] |
| Article | Perspectives | Gaps | Summary | Reference | |
|---|---|---|---|---|---|
| 1 | Hydrochar: A Review on Its Production Technologies and Applications. Catalysts, 11(8), 939. | Comprehensive Overview: The review offers a comprehensive overview of hydrochar, covering its characteristics, production mechanisms, and activation methods.
|
| This paper provides a valuable foundation for understanding hydrochar, but further research is needed to address gaps and optimize its practical implementation. | [147] |
| 2. | A bibliographic study reviewing the last decade of hydrochar in environmental application: history, status quo, and trending research paths. Biochar 5, 12 (2023). |
|
| This study provides valuable insights into hydrochar research trends, but addressing gaps related to long-term effects, standardization, and economic viability remains crucial for practical implementation. | [148] |
| 3. | Hydrochar: A Promising Step Towards Achieving a Circular Economy and Sustainable Development Goals. Frontiers in Chemical Engineering, 4, 867228. |
|
| While the article recognizes hydrochar’s promise, addressing gaps related to water, soil, emissions, and economics will enhance its practical implementation. | [149] |
| 4 | Hydrochar-based soil amendments for agriculture: a review of recent progress. Arabian Journal of Geoscience 14, 102 (2021). |
|
| While hydrochar shows promise as a soil amendment, addressing gaps related to water dynamics, economics, and long-term effects will enhance its practical adoption in agriculture. | [150] |
| 5. | A Comprehensive Review on Hydrothermal Carbonization of Biomass and its Applications. Chemistry Africa 3, 1–19 (2020). |
|
| This review sheds light on HTC’s environmental remediation potential and industrial applications, but addressing gaps related to economics and optimal conditions is essential. | [151] |
| 6. | Microwave-assisted hydrothermal treatments for biomass valorization: a critical review. Green Chemistry, 23(10), 3502–3525. |
|
| While the review highlights the potential of microwave-assisted hydrothermal treatments, addressing gaps related to process optimization and understanding combined effects will enhance its practical application. | [152] |
| 7. | A comprehensive review on hydrothermal carbonization of biomass and its applications. Chemistry Africa, 3, 1–19. | This study investigates the hydrothermal carbonization of biomass in supercritical water conditions. It explores the effects of temperature, pressure, and residence time on hydrochar yield and properties. The research provides insights into the potential of supercritical water treatment for hydrochar production. | While the study offers valuable insights, gaps exist in understanding the mechanisms underlying hydrochar formation under supercritical conditions. Further research could elucidate reaction pathways and optimize process parameters to enhance hydrochar yield and quality. | This review provides an extensive overview of hydrothermal carbonization (HTC) of biomass. It covers HTC process mechanisms, hydrochar properties, and applications. The study emphasizes the potential of hydrochar in wastewater treatment, carbon sequestration, and soil improvement. However, gaps related to economic feasibility and environmental impact require further investigation. In essence, the review highlights the promise of HTC-derived hydrochar while identifying areas for future research. | [153] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ojewumi, M.E.; Chen, G. Hydrochar Production by Hydrothermal Carbonization: Microwave versus Supercritical Water Treatment. Biomass 2024, 4, 574-598. https://doi.org/10.3390/biomass4020031
Ojewumi ME, Chen G. Hydrochar Production by Hydrothermal Carbonization: Microwave versus Supercritical Water Treatment. Biomass. 2024; 4(2):574-598. https://doi.org/10.3390/biomass4020031
Chicago/Turabian StyleOjewumi, Modupe Elizabeth, and Gang Chen. 2024. "Hydrochar Production by Hydrothermal Carbonization: Microwave versus Supercritical Water Treatment" Biomass 4, no. 2: 574-598. https://doi.org/10.3390/biomass4020031
APA StyleOjewumi, M. E., & Chen, G. (2024). Hydrochar Production by Hydrothermal Carbonization: Microwave versus Supercritical Water Treatment. Biomass, 4(2), 574-598. https://doi.org/10.3390/biomass4020031











