Production, Extraction and Partial Characterization of Natural Pigments from Chryseobacterium sp. kr6 Growing on Feather Meal Biomass
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganism and Cultivation Media
2.2. Production of Pigments
2.3. Extraction of Pigments
2.4. Color Analysis
2.5. Instrumental Analyses
2.6. Thiobarbituric Acid Reactive Substances (TBARS)
2.7. DPPH Radical Scavenging Assay
2.8. Antimicrobial Activity
3. Results and Discussion
3.1. Production of Pigments
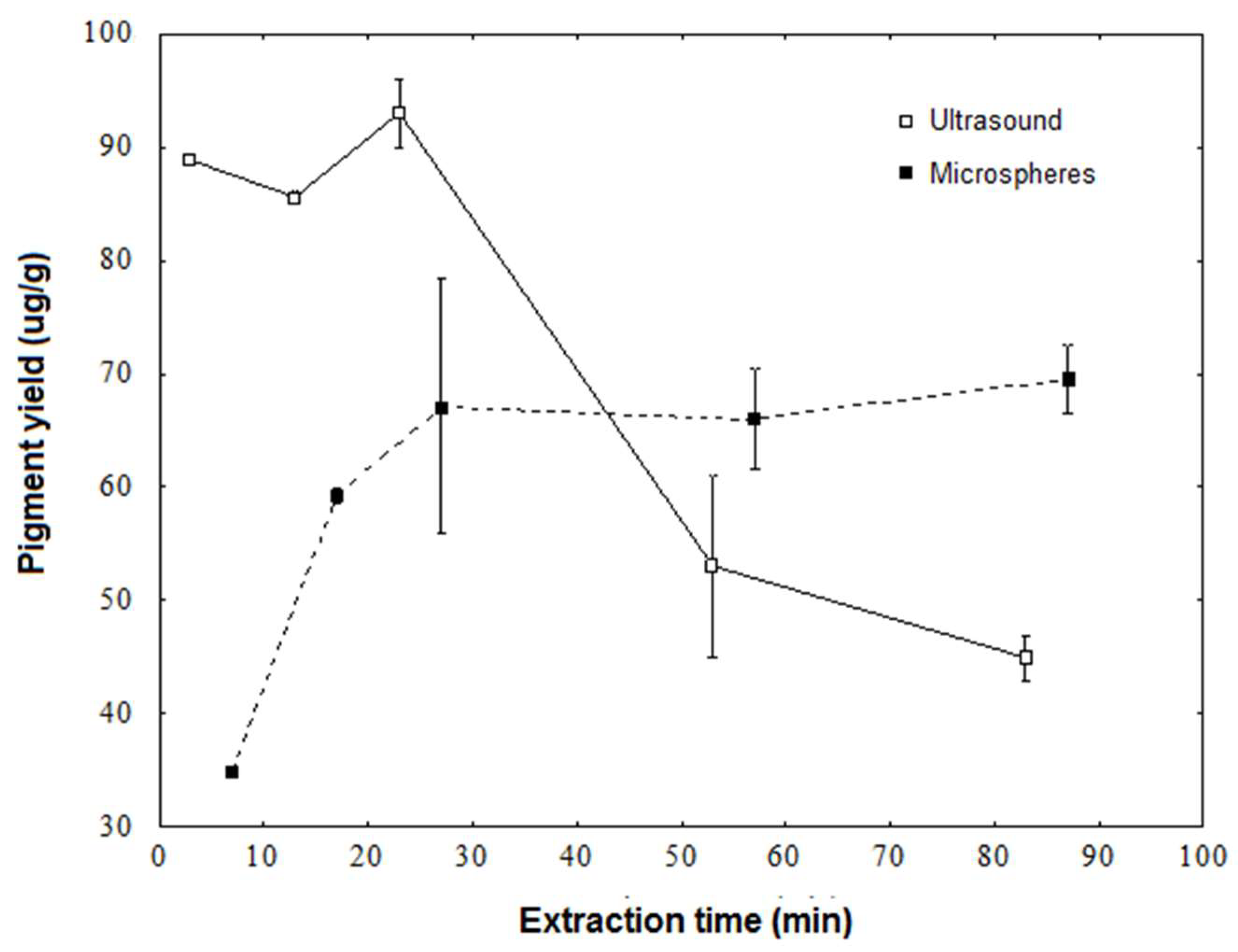
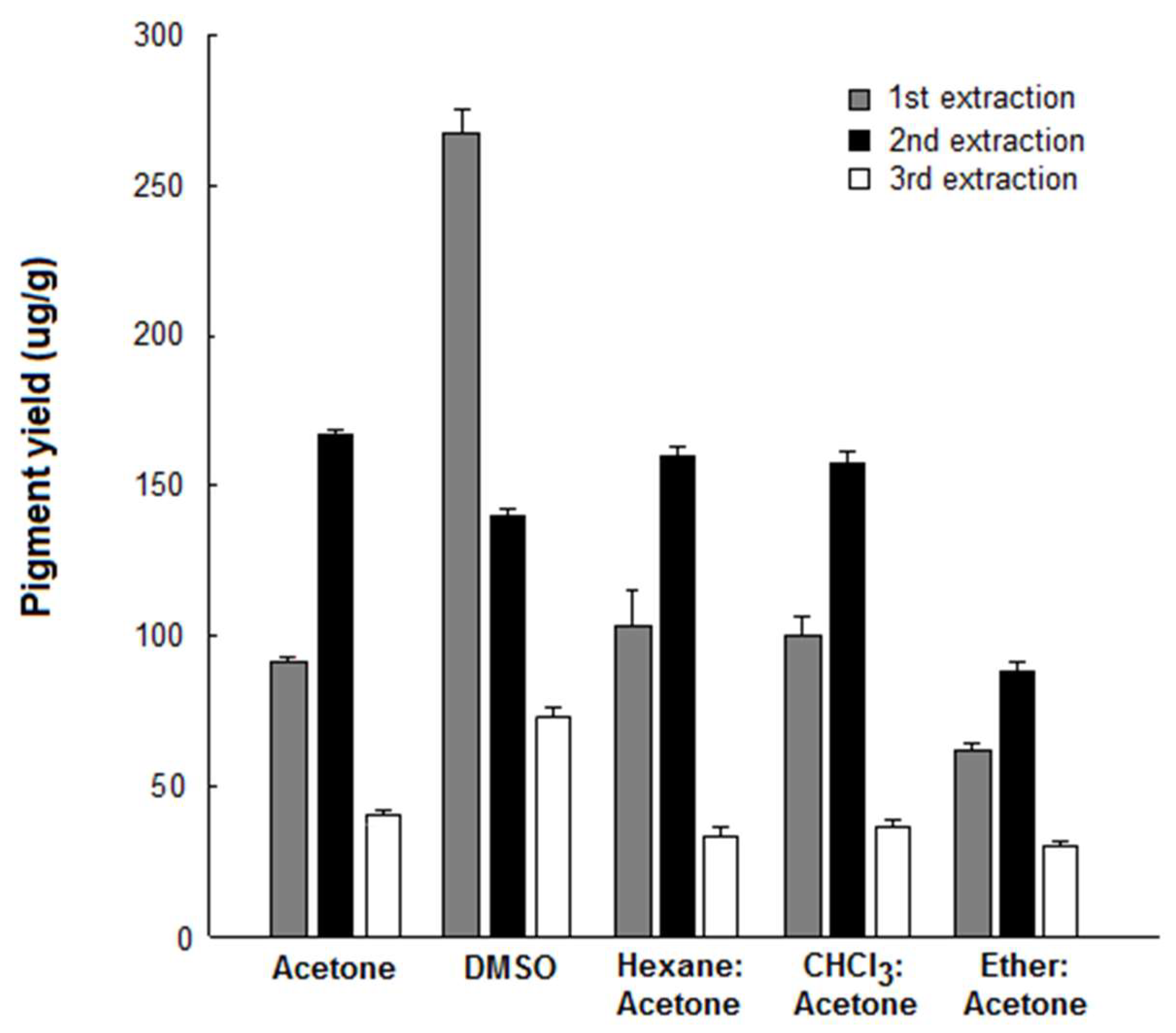
3.2. Pigment Extraction
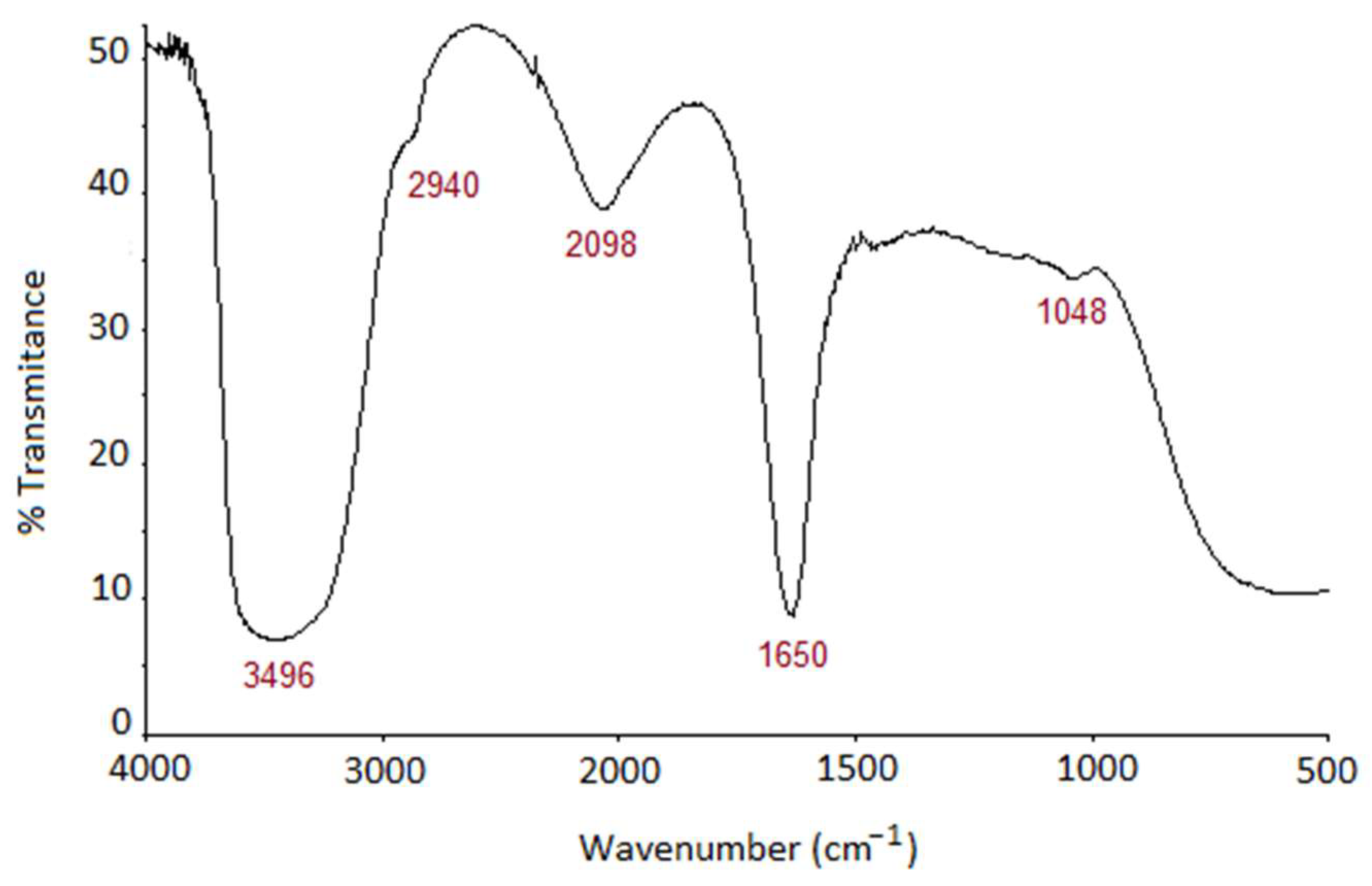
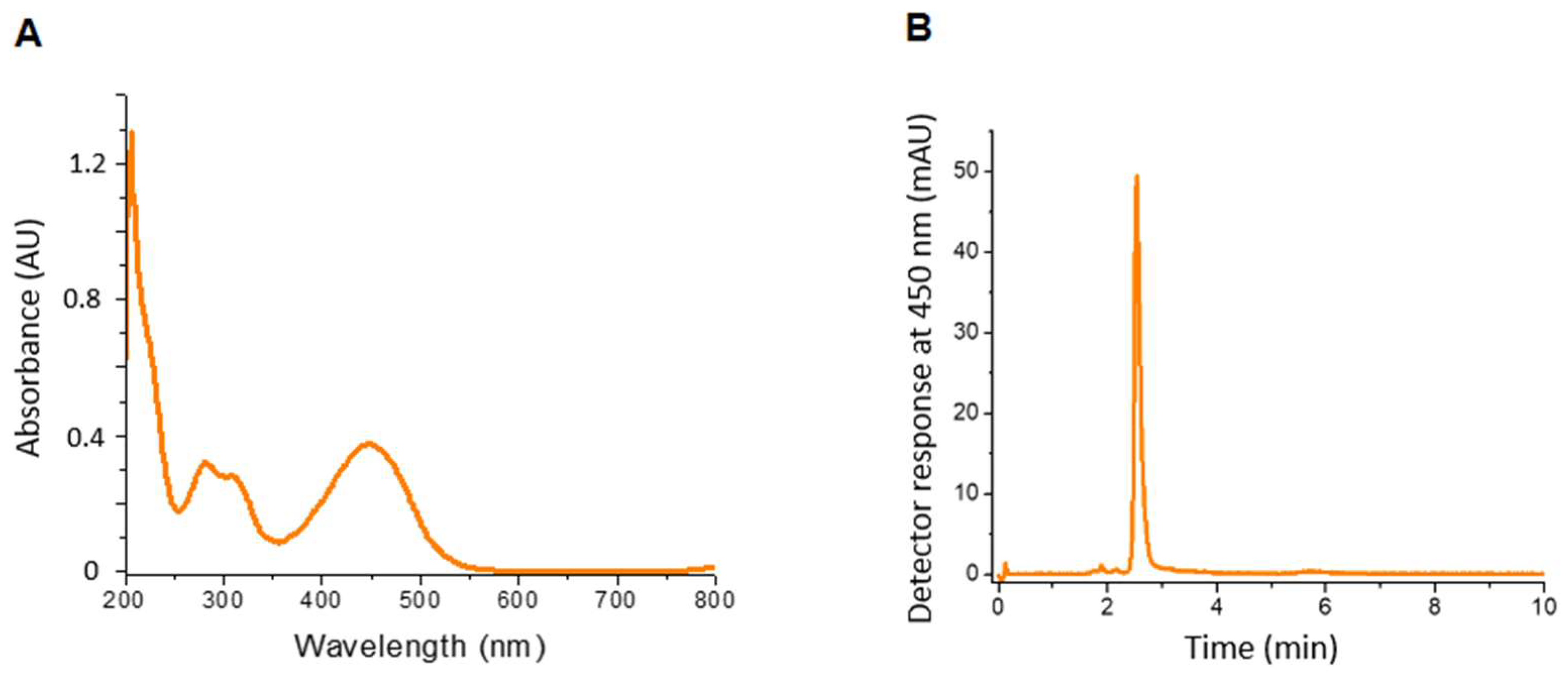
3.3. Characterization of the Pigment Extract
3.4. Antioxidant Activity
3.5. Antimicrobial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pailliè-Jiménez, M.E.; Stincone, P.; Brandelli, A. Natural pigments of microbial origin. Front. Sustain. Food Syst. 2020, 4, 590439. [Google Scholar] [CrossRef]
- Agarwal, H.; Bajpai, S.; Mishra, A.; Kohli, I.; Varma, A.; Fouillaud, M.; Dufossé, L.; Joshi, N.C. Bacterial pigments and their multifaceted roles in contemporary biotechnology and pharmacological applications. Microorganisms 2023, 11, 614. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, V.T.; Martegani, E.; Giaroni, C.; Baj, A.; Bolognese, F. Bacterial pigments: A colorful palette reservoir for biotechnological applications. Biotechnol. Appl. Biochem. 2022, 69, 981–1001. [Google Scholar] [CrossRef] [PubMed]
- Fiedor, J.; Burda, K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef] [PubMed]
- Saini, D.K.; Chakdar, H.; Pabbi, S.; Shukla, P. Enhancing production of microalgal biopigments through metabolic and genetic engineering. Crit. Rev. Food Sci. Nutr. 2020, 60, 391–405. [Google Scholar] [CrossRef] [PubMed]
- Venil, C.K.; Dufossé, L.; Devi, P.R. Bacterial pigments: Sustainable compounds with market potential for pharma and food industry. Front. Sustain. Food Syst. 2020, 4, 100. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, M.S.; Lee, B.Y.; Lee, P.C. Generation of structurally novel short carotenoids and study of their biological activity. Sci. Rep. 2016, 6, 21987. [Google Scholar] [CrossRef]
- Liu, J.; Luo, Y.; Guo, T.; Tang, C.; Chai, X.; Zhao, W.; Bai, J.; Lin, Q. Cost-effective pigment production by Monascus purpureus using rice straw hydrolysate as substrate in submerged fermentation. J. Biosci. Bioeng. 2020, 129, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Brandelli, A. Bacterial keratinases: Useful enzymes for bioprocessing agroindustrial wastes and beyond. Food Bioprocess Technol. 2008, 1, 105–116. [Google Scholar] [CrossRef]
- Silveira, S.T.; Casarin, F.; Gemelli, S.; Brandelli, A. Thermodynamics and kinetics of heat inactivation of a novel keratinase from Chryseobacterium sp. strain kr6. Appl. Biochem. Biotechnol. 2010, 162, 548–560. [Google Scholar] [CrossRef]
- Jiménez, M.E.P.; Pinilla, C.M.B.; Rodrigues, E.; Brandelli, A. Extraction and partial characterisation of antioxidant pigment produced by Chryseobacterium sp. kr6. Nat. Prod. Res. 2019, 33, 1541–1549. [Google Scholar] [CrossRef] [PubMed]
- Riffel, A.; Lucas, F.; Heeb, P.; Brandelli, A. Characterization of a new keratinolytic bacterium that completely degrades native feather keratin. Arch. Microbiol. 2003, 179, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Chen, D.; Han, Y.; Chen, Z.; Gu, F. Optimization of carotenoids extraction from Rhodobacter sphaeroides. LWT Food Sci. Technol. 2008, 41, 1082–1088. [Google Scholar] [CrossRef]
- Ngamwonglumlert, L.; Devahastin, S.; Chiewchan, N. Natural colorants: Pigment stability and extraction yield enhancement via utilization of appropriate pretreatment and extraction methods. Crit. Rev. Food Sci. Nutr. 2017, 57, 3243–3259. [Google Scholar] [CrossRef] [PubMed]
- Myers, R.H.; Montgomery, R.C. Response Surface Methodology: Process and Product Optimization Using Designed Experiments; Wiley: New York, NY, USA, 2002. [Google Scholar]
- Silveira, S.T.; Daroit, D.J.; Brandelli, A. Pigment production by Monascus purpureus in grape waste using factorial design. LWT Food Sci. Technol. 2008, 41, 170–174. [Google Scholar] [CrossRef]
- Caivano, J.L.; Buera, M.P. Color in Food: Technological and Psycophysical Aspects; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Ohkawa, H.; Ohishi, H.; Yagi, K. Assay for lipid peroxide in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuveliver, M.E.; Berset, C. Use of free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Kimura, H.; Sashihara, T.; Matsusaki, H.; Sonomoto, K.; Ishizaki, A. Novel bacteriocin of Pediococcus sp. ISK-1 isolated from well-aged bed of fermented rice bran. Ann. N. Y. Acad. Sci. 1998, 864, 345–348. [Google Scholar] [CrossRef]
- Hagaggi, N.S.; Abdul-Raouf, U.M. Production of bioactive β-carotene by the endophytic bacterium Citricoccus parietis AUCs with multiple in vitro biological potentials. Microb. Cell Fact. 2023, 22, 90. [Google Scholar] [CrossRef]
- Tinoi, J.; Rakariyatham, N.; Deming, R.L. Simplex optimization of carotenoid production by Rhodotorula glutinis using hydrolyzed mung bean waste flour as substrate. Process Biochem. 2005, 40, 2551–2557. [Google Scholar] [CrossRef]
- Bertsch, A.; Coello, N. A biotechnological process for test and recycling poultry feathers as a feed ingredient. Bioresour. Technol. 2005, 96, 1703–1708. [Google Scholar] [CrossRef] [PubMed]
- Aksu, Z.; Eren, A.T. Carotenoid production by the yeast Rhodotorula mucilaginosa: Use of agricultural wastes as carbon source. Process Biochem. 2005, 40, 2985–2991. [Google Scholar] [CrossRef]
- Park, P.K.; Kim, E.Y.; Chu, K.H. Chemical disruption of yeast cells for the isolation of carotenoid pigments. Sep. Purif. Technol. 2007, 53, 148–152. [Google Scholar] [CrossRef]
- Balachandran, S.; Kentish, E.; Mawson, R.; Ashokkumar, M. Ultrasonic enhancement of the supercritical extraction from ginger. Ultrason. Sonochem. 2006, 13, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Zhang, G.; Wang, H.; Long, Z.; Wei, T.; Li, Q. Progress in ultrasound-assisted extraction of the value-added products from microorganisms. World J. Microbiol. Biotechnol. 2021, 37, 71. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S. Bacterial cell disruption: A key unit operation in the recovery of intracellular products. Biotechnol. Adv. 1991, 9, 217–240. [Google Scholar] [CrossRef] [PubMed]
- Poddar, K.; Padhan, B.; Sarkar, D.; Sarkar, A. Purification and optimization of pink pigment produced by newly isolated bacterial strain Enterobacter sp. PWN1. SN Appl. Sci. 2021, 3, 105. [Google Scholar] [CrossRef]
- Gang, C.; Zora, D. Carotenoids are degraded by free radicals but do not affect lipid peroxidation in unilamellar liposomes under different oxygen tensions. FEBS Lett. 2001, 505, 151–154. [Google Scholar]
- Amorim, L.F.A.; Fangueiro, R.; Gouveia, I.C. Characterization of bioactive colored materials produced from bacterial cellulose and bacterial pigments. Materials 2022, 15, 2069. [Google Scholar] [CrossRef]
- Wahidin, M.A.; Aruldass, C.A.; Hamzah, M.A.A.M.; Setu, S.A.; Ahmad, W.A. Flexirubin-type pigment production from Chryseobacterium artocarpi CECT 8497 and its application as natural ink. J. Energy Environ. Sustain. 2017, 3, 62–65. [Google Scholar]
- Shutova, V.V.; Tyutyaev, E.V.; Churin, A.A.; Ponomarev, V.Y.; Belyakova, G.A.; Maksimov, G.V. IR and Raman spectroscopy in the study of carotenoids of Caldophora rivularis algae. Biophysics 2016, 61, 601–605. [Google Scholar] [CrossRef]
- Butnariu, M. Methods of analysis (extraction, separation, identification and quantification) of carotenoids from natural products. J. Ecosys. Ecograph. 2016, 6, 193. [Google Scholar] [CrossRef]
- Ashenafi, E.L.; Nyman, M.C.; Shelly, J.T.; Mattson, N.S. Spectral properties and stability of selected carotenoid and chlorophyll compounds in different solvent systems. Food Chem. Adv. 2023, 2, 100178. [Google Scholar] [CrossRef]
- Schöner, T.A.; Fuchs, S.W.; Schönau, C.; Bode, H.B. Initiation of the flexirubin biosynthesis in Chitinophaga pinensis. Microb. Biotechnol. 2014, 7, 232–241. [Google Scholar] [CrossRef]
- Park, S.A.; Ahn, S.Y.; Choi, K.Y. Functional microbial pigments isolated from Chryseobacterium and Deinococcus species for bio-paint application. Biotechnol. Bioprocess. Eng. 2020, 25, 394–402. [Google Scholar] [CrossRef]
- Aruldass, C.A.; Aziz, A.; Venil, C.K.; Khasim, A.R.; Ahmad, W.A. Utilization of agro-industrial waste for the production of yellowish-orange pigment from Chryseobacterium artocarpi CECT 8497. Int. Biodeter. Biodegrad. 2016, 113, 342–349. [Google Scholar] [CrossRef]
- Shindo, K.; Misawa, N. New and rare carotenoids isolated from marine bacteria and their antioxidant activities. Mar. Drugs 2014, 12, 1690–1698. [Google Scholar] [CrossRef]
- Venil, C.K.; Khasim, A.R.; Aruldass, C.A.; Ahmad, W.A. Microencapsulation of flexirubin-type pigment by spray drying. Characterization and antioxidant activity. Int. Biodeterior. Biodegrad. 2016, 113, 350–356. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Mogadem, A.; Almamary, M.A.; Mahat, N.A.; Jemon, K.; Ahmad, W.A.; Ali, I. Antioxidant activity evaluation of flexirubin-type pigment from Chryseobacterium artocarpi CECT 8497 and related docking study. Molecules 2021, 26, 979. [Google Scholar] [CrossRef]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their action mechanisms. RCS Adv. 2015, 5, 27986–28006. [Google Scholar]
- Amorim, L.F.A.; Fangueiro, R.; Gouveia, I.C. Novel functional material incorporating flexirubin in polyvinyl alcohol-kefiran/polycaprolactone nanofibers. J. Appl. Polym. Sci. 2022, 139, e53208. [Google Scholar] [CrossRef]
- Patki, J.M.; Singh, S.; Singh, S.; Padmadas, N.; Dasgupta, D. Analysis of the applicative potential of pigments extracted from bacterial isolates of mangrove soil as topical UV protectants. Braz. J. Pharm. Sci. 2021, 57, e19127. [Google Scholar] [CrossRef]
- Dahal, R.H.; Chaudhary, D.K.; Kim, D.U.; Pandey, R.P.; Kim, J. Chryseobacterium antibioticum sp. nov. with antimicrobial activity against Gram-negative bacteria, isolated from Arctic soil. J. Antibiot. 2021, 74, 115–123. [Google Scholar] [CrossRef]
- Venil, C.K.; Khasim, A.R.; Aruldass, C.A.; Ahmad, W.A. Safety evaluation of flexirubin from Chryseobacterium artocarpi CECT 8497: Acute, subacute toxicity and mutagenicity studies. Process Saf. Environ. Prot. 2017, 112, 362–370. [Google Scholar] [CrossRef]

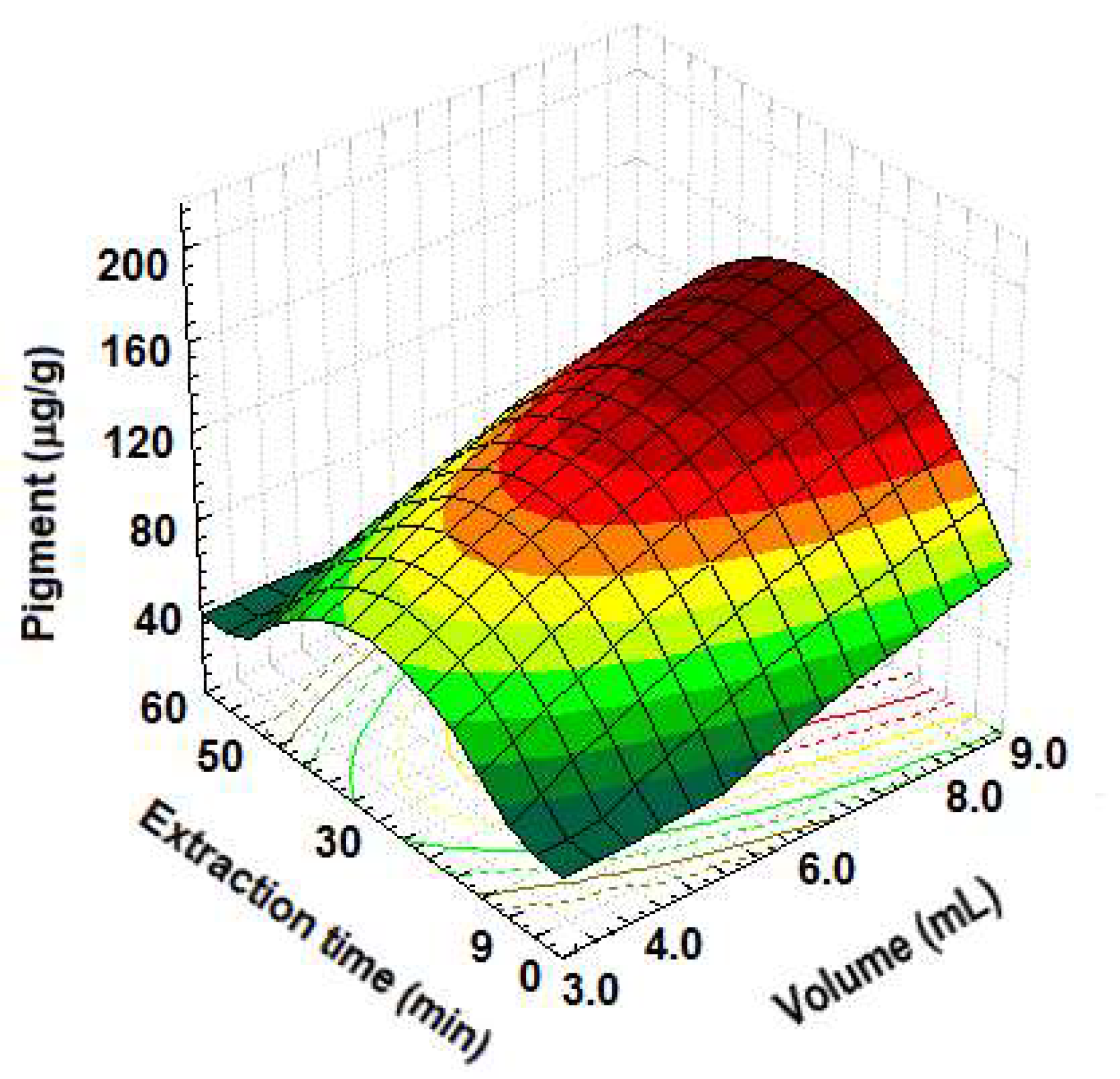

| Run | Solvent | Volume (mL) | Extractions (n) | Time (min) | Yield (μg/g) 1 |
|---|---|---|---|---|---|
| 1 | Acetone | 3 | 3 | 10 | 15.83 d |
| 2 | DMSO | 3 | 3 | 20 | 57.45 c |
| 3 | Acetone | 6 | 3 | 20 | 125.36 a |
| 4 | DMSO | 6 | 3 | 10 | 90.45 b |
| 5 | Acetone | 3 | 6 | 20 | 70.50 c |
| 6 | DMSO | 3 | 6 | 10 | 61.01 c |
| 7 | Acetone | 6 | 6 | 20 | 137.29 a |
| 8 | DMSO | 6 | 6 | 20 | 104.55 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gemelli, S.; Silveira, S.T.; Pailliè-Jiménez, M.E.; Rios, A.d.O.; Brandelli, A. Production, Extraction and Partial Characterization of Natural Pigments from Chryseobacterium sp. kr6 Growing on Feather Meal Biomass. Biomass 2024, 4, 530-542. https://doi.org/10.3390/biomass4020028
Gemelli S, Silveira ST, Pailliè-Jiménez ME, Rios AdO, Brandelli A. Production, Extraction and Partial Characterization of Natural Pigments from Chryseobacterium sp. kr6 Growing on Feather Meal Biomass. Biomass. 2024; 4(2):530-542. https://doi.org/10.3390/biomass4020028
Chicago/Turabian StyleGemelli, Sabrine, Silvana Terra Silveira, Maria Elisa Pailliè-Jiménez, Alessandro de Oliveira Rios, and Adriano Brandelli. 2024. "Production, Extraction and Partial Characterization of Natural Pigments from Chryseobacterium sp. kr6 Growing on Feather Meal Biomass" Biomass 4, no. 2: 530-542. https://doi.org/10.3390/biomass4020028
APA StyleGemelli, S., Silveira, S. T., Pailliè-Jiménez, M. E., Rios, A. d. O., & Brandelli, A. (2024). Production, Extraction and Partial Characterization of Natural Pigments from Chryseobacterium sp. kr6 Growing on Feather Meal Biomass. Biomass, 4(2), 530-542. https://doi.org/10.3390/biomass4020028






