Unveiling the Potential of Spirulina Biomass—A Glimpse into the Future Circular Economy Using Green and Blue Ingredients
Abstract
:1. Introduction
2. Materials and Methods
3. Results
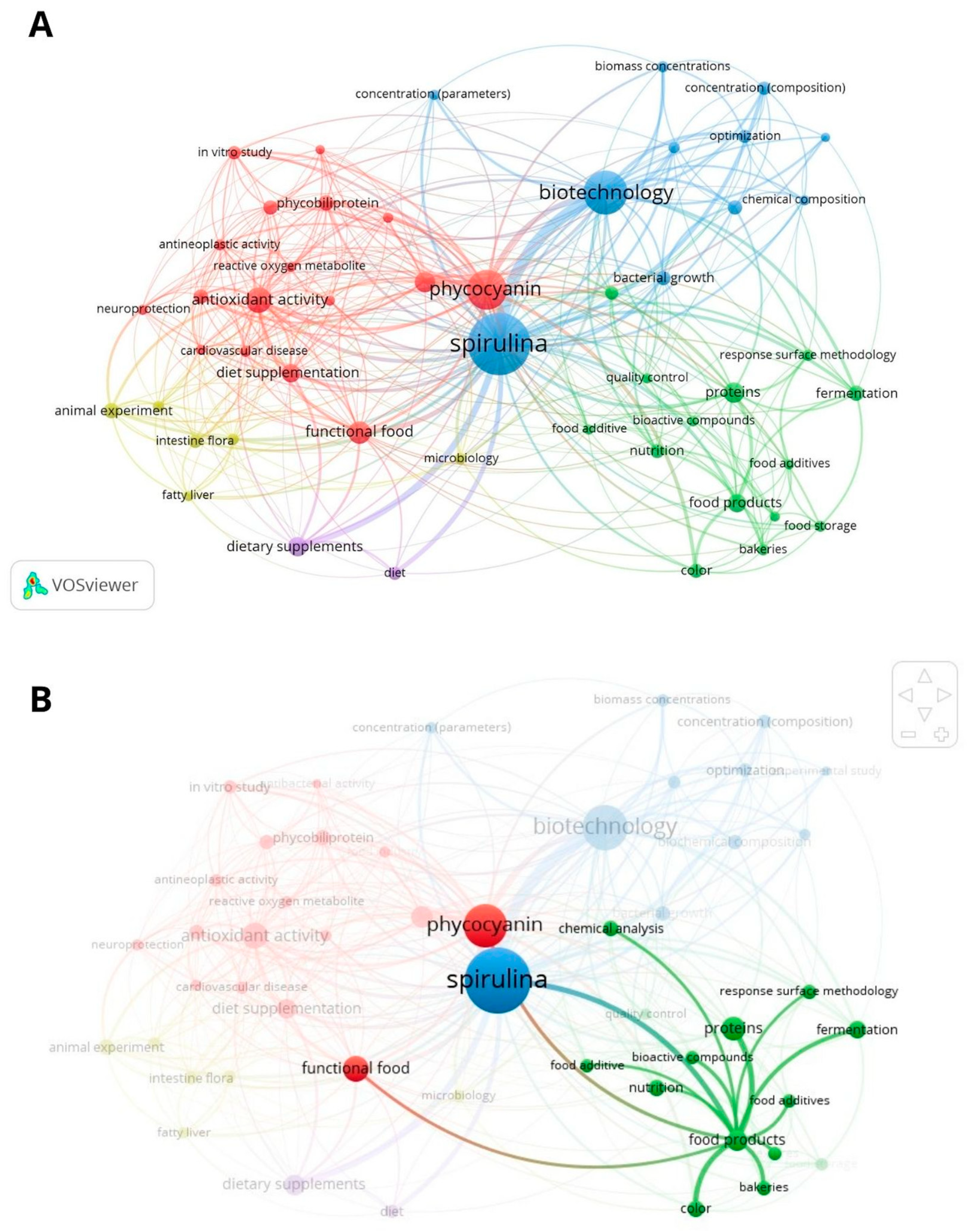
3.1. C-PC and Spirulina Biomass Applications in Food-Related Products
3.2. Health Effects
3.3. Knowledge Gaps and Further Research
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Swinburn, B.A.; Kraak, V.I.; Allender, S.; Atkins, V.J.; Baker, P.I.; Bogard, J.R.; Brinsden, H.; Calvillo, A.; Larijani, B.; Lobstein, T.; et al. The Global Syndemic of Obesity, Undernutrition, and Climate Change: The Lancet Commission Report. Lancet 2019, 393, 791–846. [Google Scholar] [CrossRef] [PubMed]
- Arashiro, L.T.; Boto-Ordóñez, M.; Van Hulle, S.W.H.; Ferrer, I.; Garfí, M.; Rousseau, D.P.L. Natural Pigments from Microalgae Grown in Industrial Wastewater. Bioresour. Technol. 2020, 303, 122894. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.R.; Khoo, K.S.; Chew, K.W.; Chang, C.K.; Munawaroh, H.S.H.; Kumar, P.S.; Huy, N.D.; Show, P.L. Perspective of Spirulina Culture with Wastewater into a Sustainable Circular Bioeconomy. Environ. Pollut. 2021, 284, 117492. [Google Scholar] [CrossRef] [PubMed]
- de Amarante, M.C.A.; Braga, A.R.C.; Sala, L.; Juliano Kalil, S. Colour Stability and Antioxidant Activity of C-Phycocyanin-Added Ice Creams after In Vitro Digestion. Food Res. Int. 2020, 137, 109602. [Google Scholar] [CrossRef] [PubMed]
- de Amarante, M.C.A.; Braga, A.R.C.; Sala, L.; Moraes, C.C.; Kalil, S.J. Design Strategies for C-Phycocyanin Purification: Process Influence on Purity Grade. Sep. Purif. Technol. 2020, 252, 117453. [Google Scholar] [CrossRef]
- Nakamoto, M.M.; Assis, M.; de Oliveira Filho, J.G.; Braga, A.R.C. Spirulina Application in Food Packaging: Gaps of Knowledge and Future Trends. Trends Food Sci. Technol. 2023, 133, 138–147. [Google Scholar] [CrossRef]
- Fratelli, C.; Burck, M.; Amarante, M.C.A.; Braga, A.R.C. Antioxidant Potential of Nature’s “Something Blue”: Something New in the Marriage of Biological Activity and Extraction Methods Applied to C-Phycocyanin. Trends Food Sci. Technol. 2021, 107, 309–323. [Google Scholar] [CrossRef]
- Silva-Neto, A.F.; Fratelli, C.; Pucci, V.G.; Boldarine, V.T.; Ferreira, Y.A.M.; Telles, M.M.; Braga, A.R.C.; Oyama, L.M. C-Phycocyanin Extracted from Spirulina Using a Green Solvent Approach Presents an Anti-Obesity Characteristic in Mice Fed a Hyperlipidic Diet. J. Funct. Foods 2023, 108, 105747. [Google Scholar] [CrossRef]
- Fratelli, C.; Bürck, M.; Silva-Neto, A.F.; Oyama, L.M.; De Rosso, V.V.; Braga, A.R.C. Green Extraction Process of Food Grade C-Phycocyanin: Biological Effects and Metabolic Study in Mice. Processes 2022, 10, 1793. [Google Scholar] [CrossRef]
- Aladaileh, S.H.; Khafaga, A.F.; Abd El-Hack, M.E.; Al-Gabri, N.A.; Abukhalil, M.H.; Alfwuaires, M.A.; Bin-Jumah, M.; Alkahtani, S.; Abdel-Daim, M.M.; Aleya, L.; et al. Spirulina platensis Ameliorates the Sub Chronic Toxicities of Lead in Rabbits via Anti-Oxidative, Anti- Inflammatory, and Immune Stimulatory Properties. Sci. Total Environ. 2020, 701, 134879. [Google Scholar] [CrossRef]
- Salgado, M.T.S.F.; Fernandes e Silva, E.; Matsumoto, A.M.; Mattozo, F.H.; de Amarante, M.C.A.; Kalil, S.J.; de Souza Votto, A.P. C-Phycocyanin Decreases Proliferation and Migration of Melanoma Cells: In Silico and In Vitro Evidences. Bioorg. Chem. 2022, 122, 105757. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Liu, L.; Miron, A.; Klímová, B.; Wan, D.; Kuča, K. The Antioxidant, Immunomodulatory, and Anti-Inflammatory Activities of Spirulina: An Overview. Arch. Toxicol. 2016, 90, 1817–1840. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Title 21 Code of Federal Regulations (CFR) Part 320; National Archives and Records Administration: College Park, MD, USA, 2001.
- Chen, X.H. Effect of PH on the Emulsifying Activity of C-Phycocyanin. Mod. Food Sci. Technol. 2020, 36, 117–125. [Google Scholar] [CrossRef]
- Rodrigues, E.F.; Vendruscolo, L.P.; Bonfante, K.; Reinehr, C.O.; Colla, E.; Colla, L.M.; Fundo, P.; Reinehr, C.O. Phycocyanin as Substitute for Texture Ingredients in Ice Creams. Br. Food J. 2019, 122, 693–707. [Google Scholar] [CrossRef]
- Carocho, M.; Morales, P.; Ferreira, I.C.F.R. Natural Food Additives: Quo Vadis? Trends Food Sci. Technol. 2015, 45, 284–295. [Google Scholar] [CrossRef]
- Ramos-Souza, C.; Nass, P.; Jacob-Lopes, E.; Queiroz Zepka, L.; Rafaela Cavalcante Braga, A.; Vera De Rosso, V. Changing Despicable Me: Potential Replacement of Azo Dye Yellow Tartrazine for Pequi Carotenoids Employing Ionic Liquids as High-Performance Extractors. Food Res. Int. 2023, 174, 113593. [Google Scholar] [CrossRef] [PubMed]
- A.C. Nielsen Company. We Are What We Eat: Healthy Eating Trends around the World; Nielsen Company: New York, NY, USA, 2015. [Google Scholar]
- Oplatowska-Stachowiak, M.; Elliott, C.T. Food Colors: Existing and Emerging Food Safety Concerns. Crit. Rev. Food Sci. Nutr. 2017, 57, 524–548. [Google Scholar] [CrossRef]
- Landim Neves, M.I.; Silva, E.K.; Meireles, M.A.A.; Neves, M.I.L.; Silva, E.K.; Meireles, M.A.A. Natural Blue Food Colorants: Consumer Acceptance, Current Alternatives, Trends, Challenges, and Future Strategies. Trends Food Sci. Technol. 2021, 112, 163–173. [Google Scholar] [CrossRef]
- Fernandes, A.S.; Neves, B.V.; Mazzo, T.M.; Longo, E.; Jacob-Lopez, E.; Zepka, L.Q.; de Rosso, V.V. Bigels as Potential Inks for Extrusion-Based 3d Food Printing: Effect of Oleogel Fraction on Physical Characterization and Printability. Food Hydrocoll. 2023, 144, 108986. [Google Scholar] [CrossRef]
- Giaconia, M.A.; dos Ramos, S.P.; Pereira, C.F.; Lemes, A.C.; De Rosso, V.V.; Braga, A.R.C. Overcoming Restrictions of Bioactive Compounds Biological Effects in Food Using Nanometer-Sized Structures. Food Hydrocoll. 2020, 107, 105939. [Google Scholar] [CrossRef]
- Van Eck, N.J.; Waltman, L. Citation-Based Clustering of Publications Using CitNetExplorer and VOSviewer. Scientometrics 2017, 111, 1053–1070. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.A.V.; Freitas, B.C.B.; Rosa, G.M.; Moraes, L.; Morais, M.G.; Mitchell, B.G. Operational and Economic Aspects of Spirulina-Based Biorefinery. Bioresour. Technol. 2019, 292, 121946. [Google Scholar] [CrossRef]
- Kuddus, M.; Singh, P.; Thomas, G.; Al-Hazimi, A. Recent Developments in Production and Biotechnological Applications of C-Phycocyanin. Biomed Res. Int. 2013, 2013, 742859. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, N.T. Production of Phycocyanin—A Pigment with Applications in Biology, Biotechnology, Foods and Medicine. Appl Microbiol. Biotechnol. 2008, 80, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ashaolu, T.J.; Samborska, K.; Lee, C.C.; Tomas, M.; Capanoglu, E.; Tarhan, Ö.; Taze, B.; Jafari, S.M. Phycocyanin, a Super Functional Ingredient from Algae; Properties, Purification Characterization, and Applications. Int. J. Biol. Macromol. 2021, 193, 2320–2331. [Google Scholar] [CrossRef] [PubMed]
- Braga, A.R.C.; da Figueira, F.S.; da Silveira, J.T.; de Morais, M.G.; Costa, J.A.V.; Kalil, S.J. Improvement of Thermal Stability of C-Phycocyanin by Nanofiber and Preservative Agents. J. Food Process. Preserv. 2016, 40, 1264–1269. [Google Scholar] [CrossRef]
- Fratelli, C.; Nunes, M.C.; De Rosso, V.V.; Raymundo, A.; Braga, A.R.C. Spirulina and Its Residual Biomass as Alternative Sustainable Ingredients: Impact on the Rheological and Nutritional Features of Wheat Bread Manufacture. Front. Food Sci. Technol. 2023, 3, 1258219. [Google Scholar] [CrossRef]
- Martelli, F.; Cirlini, M.; Lazzi, C.; Neviani, E.; Bernini, V. Edible Seaweeds and Spirulina Extracts for Food Application: In Vitro and In Situ Evaluation of Antimicrobial Activity towards Foodborne Pathogenic Bacteria. Foods 2020, 9, 1442. [Google Scholar] [CrossRef] [PubMed]
- Stanic-Vucinic, D.; Minic, S.; Nikolic, M.R.; Velickovic, T.C. Spirulina Phycobiliproteins as Food Components and Complements. In Microalgal Biotechnology; InTechOpen: Rijeka, Croatia, 2018. [Google Scholar]
- Braga, A.R.C.; Nunes, M.C.; Raymundo, A. The Experimental Development of Emulsions Enriched and Stabilized by Recovering Matter from Spirulina Biomass: Valorization of Residue into a Sustainable Protein Source. Molecules 2023, 28, 6179. [Google Scholar] [CrossRef] [PubMed]
- Utama-ang, N.; Kuatrakul, I.; Walter, P.; Rattanapitigorn, P.; Kawee-ai, A. Effect of Instant Jasmine Rice Coating Combining Spirulina with Edible Polymers on Physicochemical Properties, Textural Properties and Sensory Acceptance. Sci. Rep. 2022, 12, 7699. [Google Scholar] [CrossRef] [PubMed]
- Ismail, H.A.; El-Sawah, T.H.; Ayyash, M.; Adhikari, B.; Elkot, W.F. Functionalization of Ricotta Cheese with Powder of Spirulina platensis: Physicochemical, Sensory, and Microbiological Properties. Int. J. Food Prop. 2023, 26, 1968–1983. [Google Scholar] [CrossRef]
- Elkot, W.F.; Elmahdy, A.; El-Sawah, T.H.; Alghamdia, O.A.; Alhag, S.K.; Al-Shahari, E.A.; AL-Farga, A.; Ismail, H.A. Development and Characterization of a Novel Flavored Functional Fermented Whey-Based Sports Beverage Fortified with Spirulina platensis. Int. J. Biol. Macromol. 2024, 258, 128999. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.K.; Singh, P.; Srivastava, S.; Zanwar, S.; Dar, A.H.; Singh, R.; Lal, A. Box–Behnken Design Based Statistical Modelling to Study the Effects of Spirulina (Arthrospira platensis) Incorporation on Nutritional Standards of Vegan Snack Product. J. Agric. Food Res. 2023, 14, 100700. [Google Scholar] [CrossRef]
- Rose, H.; Bakshi, S.; Kanetkar, P.; Lukose, S.J.; Felix, J.; Yadav, S.P.; Gupta, P.K.; Paswan, V.K. Development and Characterization of Cultured Buttermilk Fortified with Spirulina plantensis and Its Physico-Chemical and Functional Characteristics. Dairy 2023, 4, 271–284. [Google Scholar] [CrossRef]
- Wang, M.; Yin, Z.; Sun, W.; Zhong, Q.; Zhang, Y.; Zeng, M. Microalgae Play a Structuring Role in Food: Effect of Spirulina platensis on the Rheological, Gelling Characteristics, and Mechanical Properties of Soy Protein Isolate Hydrogel. Food Hydrocoll. 2023, 136, 108244. [Google Scholar] [CrossRef]
- Gün, D.; Çelekli, A.; Bozkurt, H.; Kaya, S. Optimization of Biscuit Enrichment with the Incorporation of Arthrospira platensis: Nutritional and Sensory Approach. J. Appl. Phycol. 2022, 34, 1555–1563. [Google Scholar] [CrossRef]
- Özbal, B.; Çelekli, A.; Gün, D.; Bozkurt, H. Effect of Arthrospira platensis Incorporation on Nutritional and Sensory Attributes of White Chocolate. Int. J. Gastron. Food Sci. 2022, 28, 100544. [Google Scholar] [CrossRef]
- Üstün-Aytekin, Ö.; Çoban, I.; Aktaş, B. Nutritional Value, Sensory Properties, and Antioxidant Activity of a Traditional Kefir Produced with Arthrospira platensis. J. Food Process Preserv. 2022, 46, e16380. [Google Scholar] [CrossRef]
- Niccolai, A.; Venturi, M.; Galli, V.; Pini, N.; Rodolfi, L.; Biondi, N.; Granchi, L.; Tredici, M.R. Vegetable Oils Protect Phycocyanin from Thermal Degradation during Cooking of Spirulina-Based “Crostini”. LWT 2021, 138, 110776. [Google Scholar] [CrossRef]
- Sözeri Atik, D.; Gürbüz, B.; Bölük, E.; Palabıyık, İ. Development of Vegan Kefir Fortified with Spirulina platensis. Food Biosci. 2021, 42, 101050. [Google Scholar] [CrossRef]
- Uribe-Wandurraga, Z.N.; Igual, M.; García-Segovia, P.; Martínez-Monzó, J. Influence of Microalgae Addition in Formulation on Colour, Texture, and Extrusion Parameters of Corn Snacks. Food Sci. Technol. Int. 2020, 26, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Fradinho, P.; Niccolai, A.; Soares, R.; Rodolfi, L.; Biondi, N.; Tredici, M.R.; Sousa, I.; Raymundo, A. Effect of Arthrospira platensis (Spirulina) Incorporation on the Rheological and Bioactive Properties of Gluten-Free Fresh Pasta. Algal. Res. 2020, 45, 101743. [Google Scholar] [CrossRef]
- Grahl, S.; Strack, M.; Mensching, A.; Mörlein, D. Alternative Protein Sources in Western Diets: Food Product Development and Consumer Acceptance of Spirulina-Filled Pasta. Food Qual. Prefer. 2020, 84, 103933. [Google Scholar] [CrossRef]
- Martelli, F.; Alinovi, M.; Bernini, V.; Gatti, M.; Bancalari, E. Arthrospira platensis as Natural Fermentation Booster for Milk and Soy Fermented Beverages. Foods 2020, 9, 350. [Google Scholar] [CrossRef] [PubMed]
- Mostolizadeh, S.; Moradi, Y.; Mortazavi, M.S.; Motallebi, A.A.; Ghaeni, M. Effects of Incorporation Spirulina platensis (Gomont, 1892) Powder in Wheat Flour on Chemical, Microbial and Sensory Properties of Pasta. Iran J. Fish Sci. 2020, 19, 410–420. [Google Scholar] [CrossRef]
- Pan-utai, W.; Iamtham, S. Extraction, Purification and Antioxidant Activity of Phycobiliprotein from Arthrospira platensis. Process Biochem. 2019, 82, 189–198. [Google Scholar] [CrossRef]
- Saharan, V.; Jood, S. Effect of Storage on Spirulina platensis Powder Supplemented Breads. J. Food Sci. Technol. 2021, 58, 978–984. [Google Scholar] [CrossRef] [PubMed]
- Niccolai, A.; Venturi, M.; Galli, V.; Pini, N.; Rodolfi, L.; Biondi, N.; D’Ottavio, M.; Batista, A.P.; Raymundo, A.; Granchi, L.; et al. Development of New Microalgae-Based Sourdough “Crostini”: Functional Effects of Arthrospira platensis (Spirulina) Addition. Sci. Rep. 2019, 9, 19433. [Google Scholar] [CrossRef] [PubMed]
- Çelekli, A.; Alslibi, Z.A.; Bozkurt, H. üseyin Influence of Incorporated Spirulina platensis on the Growth of Microflora and Physicochemical Properties of Ayran as a Functional Food. Algal Res. 2019, 44, 101710. [Google Scholar] [CrossRef]
- Barkallah, M.; Dammak, M.; Louati, I.; Hentati, F.; Hadrich, B.; Mechichi, T.; Ayadi, M.A.; Fendri, I.; Attia, H.; Abdelkafi, S. Effect of Spirulina platensis Fortification on Physicochemical, Textural, Antioxidant and Sensory Properties of Yogurt during Fermentation and Storage. LWT 2017, 84, 323–330. [Google Scholar] [CrossRef]
- da Costa, S.F.F.; dos Passos Ramos, S.; Bürck, M.; Braga, A.R.C. Development of Eco-Friendly Solid Shampoo Containing Natural Pigments: Physical-Chemical, Microbiological Characterization and Analysis of Antioxidant Activity. Ind. Biotechnol. 2023, 19, 337–346. [Google Scholar] [CrossRef]
- El Baky, H.H.A.; El Baroty, G.S.; Ibrahem, E.A. Functional Characters Evaluation of Biscuits Sublimated with Pure Phycocyanin Isolated from Spirulina and Spirulina Biomass. Nutr. Hosp. 2015, 32, 231–241. [Google Scholar] [CrossRef]
- Mohammadi-Gouraji, E.; Soleimanian-Zad, S.; Ghiaci, M. Phycocyanin-Enriched Yogurt and Its Antibacterial and Physicochemical Properties during 21 Days of Storage. LWT 2019, 102, 230–236. [Google Scholar] [CrossRef]
- Belén García, A.; Longo, E.; Bermejo, R. The Application of a Phycocyanin Extract Obtained from Arthrospira platensis as a Blue Natural Colorant in Beverages. J. Appl. Phycol. 2021, 33, 3059–3070. [Google Scholar] [CrossRef]
- Hadiyanto, S.H.; Hadiyanto, H.; Suttrisnorhadi, S. Response Surface Optimization of Ultrasound Assisted Extraction (UAE) of Phycocyanin from Microalgae Spirulina platensis. Emir. J. Food Agric. 2016, 28, 227–234. [Google Scholar] [CrossRef]
- Moraes, C.C.; de Burkert, J.F.M.; Kalil, S.J.; De Medeiros Burkert, J.F.; Kalil, S.J. C-Phycocyanin Extraction Process for Large-Scale Use. J. Food Biochem. 2010, 34, 133–148. [Google Scholar] [CrossRef]
- Chaiklahan, R.; Chirasuwan, N.; Bunnag, B. Stability of Phycocyanin Extracted from Spirulina sp.: Influence of Temperature, PH and Preservatives. Process Biochem. 2012, 47, 659–664. [Google Scholar] [CrossRef]
- Martelli, G.; Folli, C.; Visai, L.; Daglia, M.; Ferrari, D. Thermal Stability Improvement of Blue Colorant C-Phycocyanin from Spirulina platensis for Food Industry Applications. Process Biochem. 2014, 49, 154–159. [Google Scholar] [CrossRef]
- Ramos, S.d.P.; Trindade, L.G.d.; Mazzo, T.M.; Longo, E.; Bonsanto, F.P.; de Rosso, V.V.; Braga, A.R.C. Electrospinning Composites as Carriers of Natural Pigment: Screening of Polymeric Blends. Processes 2022, 10, 2737. [Google Scholar] [CrossRef]
- Paramanya, A.; Oyesiji Abiodun, A.; Shamsul Ola, M.; Ali, A. Enhancing the Quality and Antioxidant Capacity of Phycocyanin Extracted from Spirulina platensis PCC 7345: A Quality-by-Design Approach. Arab. J. Chem. 2024, 17, 105653. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, Z.; Li, W.; Tian, H.; Yuan, L.; Yang, X. Stability of High Internal-Phase Emulsions Prepared from Phycocyanin and Small-Molecule Sugars. J. Sci. Food Agric. 2024, 104, 2917–2927. [Google Scholar] [CrossRef] [PubMed]
- Rekha, V.S.; Sankar, K.; Rajaram, S.; Karuppiah, P.; Dawoud, T.M.S.; Syed, A.; Elgorban, A.M. Unveiling the Impact of Additives on Structural Integrity, Thermal and Color Stability of C-Phycocyanin—Agar Hydrocolloid. Food Chem. 2024, 448, 139000. [Google Scholar] [CrossRef] [PubMed]
- Faieta, M.; Chen, C.; Colaruotolo, L.A.; Huynh, L.; Corradini, M.G.; Pittia, P. High-Pressure Processing (HPP) of Phycocyanin Extract Solutions: Enhancing Stability through Molecular Interactions. LWT 2024, 198, 115965. [Google Scholar] [CrossRef]
- Muys, M.; Sui, Y.; Schwaiger, B.; Lesueur, C.; Vandenheuvel, D.; Vermeir, P.; Vlaeminck, S.E. High Variability in Nutritional Value and Safety of Commercially Available Chlorella and Spirulina Biomass Indicates the Need for Smart Production Strategies. Bioresour. Technol. 2019, 275, 247–257. [Google Scholar] [CrossRef] [PubMed]
- ANVISA Novos Alimentos, Novos Ingredientes, Probióticos e Enzimas Aprovados; Agência Nacional de Vigilância Sanitária: Brasília, Brazil, 2019.
- FDA. Spirulina Extract. In 21 Code of Federal Legislations; FDA: White Oak, MD, USA, 2001; p. 73.1530. [Google Scholar]
- Ferrazzano, G.F.; Papa, C.; Pollio, A.; Ingenito, A.; Sangianantoni, G.; Cantile, T. Cyanobacteria and Microalgae as Sources of Functional Foods to Improve Human General and Oral Health. Molecules 2020, 25, 5164. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Feng, Y.; Zheng, J.; Addy, M.; Zhang, L.; Ren, D. The Immune-enhancing Potential of Peptide Fractions from Fermented Spirulina platensis by Mixed Probiotics. J. Food Biochem. 2020, 44, e13245. [Google Scholar] [CrossRef] [PubMed]
- Aissaoui, O.; Amiali, M.; Bouzid, N.; Belkacemi, K.; Bitam, A. Effect of Spirulina platensis Ingestion on the Abnormal Biochemical and Oxidative Stress Parameters in the Pancreas and Liver of Alloxan-Induced Diabetic Rats. Pharm. Biol. 2017, 55, 1304–1312. [Google Scholar] [CrossRef] [PubMed]
- Bitam, A.; Aissaoui, O. Spirulina platensis, Oxidative Stress, and Diabetes. In Diabetes; Elsevier: Amsterdam, The Netherlands, 2020; pp. 325–331. [Google Scholar]
- Moradi, S.; Ziaei, R.; Foshati, S.; Mohammadi, H.; Nachvak, S.M.; Rouhani, M.H. Effects of Spirulina Supplementation on Obesity: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Complement. Ther. Med. 2019, 47, 102211. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, R.; Saidpour, A.; Mottaghi, A. The Effects of Spirulina Supplementation on Metabolic Syndrome Components, Its Liver Manifestation and Related Inflammatory Markers: A Systematic Review. Complement. Ther. Med. 2019, 42, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Cui, Y.; Fan, X.; Qi, P.; Liu, C.; Zhou, X.; Zhang, X. Anti-Obesity Effects of Spirulina platensis Protein Hydrolysate by Modulating Brain-Liver Axis in High-Fat Diet Fed Mice. PLoS ONE 2019, 14, e0218543. [Google Scholar] [CrossRef] [PubMed]
- Ragaza, J.A.; Hossain, M.S.; Meiler, K.A.; Velasquez, S.F.; Kumar, V. A Review on Spirulina: Alternative Media for Cultivation and Nutritive Value as an Aquafeed. Rev. Aquac. 2020, 12, 2371–2395. [Google Scholar] [CrossRef]
- Silvestris, E.; de Pergola, G.; Rosania, R.; Loverro, G. Obesity as Disruptor of the Female Fertility. Reprod. Biol. Endocrinol. 2018, 16, 22. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Han, Z.; Liu, S.-J.J.; Hao, X.; Zhang, X.-J.J.; Wang, X.-Y.Y.; Zhou, C.-J.J.; Ma, Y.-Z.Z.; Liang, C.-G.G. Phycocyanin Improves Reproductive Ability in Obese Female Mice by Restoring Ovary and Oocyte Quality. Front. Cell Dev. Biol. 2020, 8, 595373. [Google Scholar] [CrossRef] [PubMed]
- Mrozik, K.M.; Blaschuk, O.W.; Cheong, C.M.; Zannettino, A.C.W.; Vandyke, K. N-Cadherin in Cancer Metastasis, Itsemerging Role in Haematologicalmalignancies and Potential as a Therapeutictarget in Cancer. BMC Cancer 2018, 18, 939. [Google Scholar] [CrossRef] [PubMed]
- Cheirsilp, B.; Maneechote, W.; Srinuanpan, S.; Angelidaki, I. Microalgae as Tools for Bio-Circular-Green Economy: Zero-Waste Approaches for Sustainable Production and Biorefineries of Microalgal Biomass. Bioresour. Technol. 2023, 387, 129620. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ríos, A.; Butnar, I.; Margallo, M.; Laso, J.; Borrion, A.; Aldaco, R. Carbon Accounting of Negative Emissions Technologies Integrated in the Life Cycle of Spirulina Supplements. Sci. Total Environ. 2023, 890, 164362. [Google Scholar] [CrossRef] [PubMed]
- Becker, E.W. Micro-Algae as a Source of Protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Sala, L.; Moraes, C.C.; Kalil, S.J. Cell Pretreatment with Ethylenediaminetetraacetic Acid for Selective Extraction of C-Phycocyanin with Food Grade Purity. Biotechnol. Prog. 2018, 34, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Sarada, R.; Pillai, M.G.; Ravishankar, G.A. Phycocyanin from Spirulina sp.: Influence of Processing of Biomass on Phycocyanin Yield, Analysis of Efficacy of Extraction Methods and Stability Studies on Phycocyanin. Process Biochem. 1999, 34, 795–801. [Google Scholar] [CrossRef]
- Ghosh, T.; Katiyar, V. Edible Food Packaging: An Introduction. In Nanotechnology in Edible Food Packaging; Springer: Singapore, 2021; pp. 1–23. [Google Scholar]
- Pradeep, H.N.; Nayak, C.A. Enhanced Stability of C-Phycocyanin Colorant by Extrusion Encapsulation. J. Food Sci. Technol. 2019, 56, 4526–4534. [Google Scholar] [CrossRef] [PubMed]

| Food Product | Technological Function | Spirulina Ratio | Physicochemical Characteristics | Antioxidant Activity | Main Effects | Reference |
|---|---|---|---|---|---|---|
| Fermented whey-based sports beverage | Nutritional composition, probiotic profile, rheology, and sensory acceptance | 0.25%, 0.5%, and 0.75% (w/w) | Total solids, protein, fiber, vitamins, and minerals were higher in the samples containing Spirulina and insignificant differences in fat or ash compared to the control samples | 1 TPC: 65.15, 68.19, and 70.05 mg 2 GAE/100 g in the samples loaded with Spirulina versus 60.75, 63.80 GAE/100 g in the control samples | 0.5% of Spirulina resulted in good sensory acceptance. Spirulina are directly related to textural quality of the final products and pH decrease. All fermented beverage samples presented more than 7 log CFU/mL probiotic bacteria throughout storage | [35] |
| Vegan emulsions | Nutritional composition, natural pigment, and stabilizing agent | 3% (w/w) proteins in the emulsions were composed by residual biomass (after extracting C-phycocyanin) at 0%, 25%, 50%, 75%, and 100%) and chickpea protein | Predominantly elastic behavior; shear tinning behavior typical of protein-stabilized emulsions. Emulsions with 100% residual biomass showed highest adhesiveness and firmness; at 75% and 50%, these parameters decreased; at 25%, no significant difference were assed compared to control. | 3 DPPH ranged from 15.4 ± 0.16 to 43.0 ± 1.5 and 4 FRAP from 169.38 ± 5.75 to 343.91 ± 21.12, respectively | Residual biomass led to a decrease in droplet sizes of the emulsions and improved the antioxidant activity of the formulations. The formulations’ color was stable alongside 30 days of tests | [32] |
| Vegan snacks | Nutritional composition and sensorial attributes | 0%, 4%, and 8% combined with finger millet and gram flour | Moisture content ranged from 1% to 3%; ash from 1% to 3.5%; from 2% to 4.5% in the extruded product. The percentage of protein increased with the addition of the Spirulina powder | - | Protein denaturation diminished with Spirulina incorporation in the snacks, observed after the frying process. Spirulina increased the protein and ash content while decreased the fat content due to high fiber and protein of the formulations. The shelf life that maintained the sensory attributes in acceptable scores was 90 days. The overall acceptability decreased as the storage time increased | [36] |
| Ricotta cheese | Nutritional composition, microstructure improvement, and sensorial attributes | 0%, 0.25%, 0.5%, 0.75%, and 1.0% (w/w) | Total solids, protein, fat, ash, carbohydrate, fiber, minerals, and pH showed a significant increase; however, the acidity was comparatively lower compared to control samples | TPC: 7.03 ± 0.08 mg versus 5.11 ± 0.07 mg (GAE/g); DPPH: 20.27 ± 0.80% versus 15.25 ± 0.41% in the samples with Spirulina at 1% and control ricotta cheese | Spirulina led to increment in hardness at the start and the end of 21 days of storage, while decreased cohesiveness, gumminess, and chewiness values. Incorporation of 0.75% was the optimum concentration for structure and sensory acceptance | [34] |
| Buttermilk | Nutritional composition and fermentation process improvement | 0%, 0.25%, 0.5%, and 1% | Protein significantly increased from 1.71 to 1.83 g/100 g and fat was not affected comparing control and 0.25% Spirulina concentration | DPPH ranged from 41.99% to 48.19%; TPC ranged from 2.44 to 4.21 mg/g GAE both essays for control and 0.25% Spirulina loaded | The best sensory acceptance was achieved for buttermilk containing 0.25% Spirulina. The microbial findings from this study indicate that Spirulina might promote the proliferation of lactic acid bacteria for prebiotic algae-based | [37] |
| Soy protein isolate hydrogel | Rheological properties and microstructure improvement | 0% to 8% | Hydrogels presented predominantly elastic behavior and as the concentration of Spirulina increased, the entanglement of molecules within the hydrogel also rose | - | Pre-thermal treatment followed by high-speed shearing led to Spirulina cell wall damage, releasing protein and carbohydrates that formed dense hydrogels. Spirulina enhanced the rigidity and compactness of the hydrogels | [38] |
| Biscuit | Texture parameter improvement | 0%, 1%, 2.5%, and 4% | Spirulina at 4% improved protein and amino acid content (e.g., alanine, lysine, glutamic acid, aspartic acid, arginine, serine, threonine, tryptophan, and valine) of biscuits | - | Hardness and crispness of the biscuits were improved at 4% Spirulina incorporation. The same were observed in the color, aroma, taste, and texture parameters | [39] |
| White chocolate | Nutritional composition and sensory attributes | 0.5%, 1%, 2%, and 4% | Improved viscosity at all concentrations, except compared to control | - | No significant (p > 0.05) difference in sensory acceptance compared to control | [40] |
| Kefir | Nutritional composition and functional properties | 0.05%, 0.1%, 0.5%, 1%, and 2% (w/v) | - | DPPH: 22.2% for 1% concentration sample | The overall acceptability decreased as the Spirulina ratio increased | [41] |
| Rice coating | Nutritional composition texture and sensorial attributes | 2% Spirulina, combined with 20% maltodextrin and 1% carboxymethyl cellulose (w/v) | Spirulina incremented the protein content in 64.5% compared to control samples. Moisture content increased up to 67.4% because of the hydrophilic behavior of maltodextrin, carboxymethyl cellulose and proteins. | TPC: 137.3 µg GAE/g and FRAP: 3.8 mg Fe2+/g | Hardness, adhesiveness, springiness, gumminess, and chewiness decreased in the samples with Spirulina due to the presence of these other polymers. The softness was appreciated. Overall acceptability of 7.1, corresponding to like moderately. Unfavorable odor in the rice fortified with 3 to 5% (w/v) Spirulina | [33] |
| Crostini | Nutritional composition | 6% | - | - | C-PC was protected from thermal degradation by tocopherol | [42] |
| Vegan kefir (soy- or almond-milk-based) | Nutritional composition and functional properties | 0.25% and 0.50% | No significant color differences (p > 0.05) | DPPH: 12.03 ± 4.41 was the greatest value (soy milk at 0.5%) 5 ABTS: 46.90 ± 2.41 was the greatest value (soy milk at 0.25%) | Spirulina improved the prebiotic potential and bioactive quality of food | [43] |
| Emulsion | Low-fat oil-in-water food emulsions and coloring | 1% (Dunaliella; Chlorella or Spirulina) | Spirulina: pH slightly decreased during storage; the emulsion were stable | - | Spirulina: greenish color differences from 25 to 60 days of storage were observed | [44] |
| Gluten-free fresh pasta | Nutritional composition, rheological and functional properties, and appearance | 2 and 3% | Higher antioxidant activity; high digestibility in vitro; good mechanical properties | DPPH: 70.33% ± 4.36 and 6 VCEAC: 0.77 μg/g ± 0.02 | Spirulina biomass enhanced the nutritional quality of pasta without affecting its cooking and texture quality properties | [45] |
| Pasta | Nutritional composition | 10, 30, and 50% of Spirulina-soy-extrudate | - | - | Generally, pasta was accepted Familiarity with Spirulina was related to acceptance | [46] |
| Milk and fermented soy beverages | Boost on fermentation | 0.25% and 0.50% | The lightness was increased at both concentration | - | Spirulina improved lactic acid bacteria strains and viscosity at 0.25% | [47] |
| Pasta | Nutritional composition and rheological and functional properties | 0.25, 0.5, 0.75, and 1% | Improved mineral content | - | Greater color, aroma, and overall acceptability of pasta (0.25% Spirulina) compared to the control | [48] |
| Yogurt | Nutritional composition, functional properties, and coloring | 0.1, 0.3, and 0.5% oven-dried biomass and 1, 5, and 10% fresh biomass | pH decreased with biomass incorporation; water-holding capacity ranged from 53 to 62% | - | Yogurt products showed the ability to retain water after the fermentation process | [49] |
| Bread | Nutritional composition | 2, 4, and 6% | - | DPPH: 16.51 ± 0.85 at 6% Spirulina biomass ratio was the greatest value | The overall acceptability decreased as the Spirulina ratio increased | [50] |
| Crostini | Nutritional composition, functional properties, and appearance | 2%, 6% and 10% | Improved protein, phycocyanin, and total phenolic content | DPPH: ranged from 57% to 61% and VCEAC: from 0.60 to 0.64 μg/g | Color and global acceptance were greater at 2% Spirulina concentration and lower than the control | [51] |
| Ayran (Yogurt) | Boost on fermentation capability and probiotic bacteria | 0.25%, 0.5%, and 1% | Improved protein at 1%. Viscosity decreased in the first seven days of storage, then it increased | - | Enhancement in the growth of probiotic bacteria and nutritional value of ayran | [52] |
| Yogurt | Nutritional composition, rheological properties, and coloring | 0.25, 0.5, 0.75, and 1% | Higher antioxidant activity, protein, fat, and dietary fiber contents were reported | DPPH: 52.41 ± 2.61 at 0.25% Spirulina | 0.25% Spirulina concentration was the best formulation. The color showed no tendency to lighten during storage | [53] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bürck, M.; Fratelli, C.; Campos Assumpção de Amarante, M.; Braga, A.R.C. Unveiling the Potential of Spirulina Biomass—A Glimpse into the Future Circular Economy Using Green and Blue Ingredients. Biomass 2024, 4, 704-719. https://doi.org/10.3390/biomass4030039
Bürck M, Fratelli C, Campos Assumpção de Amarante M, Braga ARC. Unveiling the Potential of Spirulina Biomass—A Glimpse into the Future Circular Economy Using Green and Blue Ingredients. Biomass. 2024; 4(3):704-719. https://doi.org/10.3390/biomass4030039
Chicago/Turabian StyleBürck, Monize, Camilly Fratelli, Marina Campos Assumpção de Amarante, and Anna Rafaela Cavalcante Braga. 2024. "Unveiling the Potential of Spirulina Biomass—A Glimpse into the Future Circular Economy Using Green and Blue Ingredients" Biomass 4, no. 3: 704-719. https://doi.org/10.3390/biomass4030039
APA StyleBürck, M., Fratelli, C., Campos Assumpção de Amarante, M., & Braga, A. R. C. (2024). Unveiling the Potential of Spirulina Biomass—A Glimpse into the Future Circular Economy Using Green and Blue Ingredients. Biomass, 4(3), 704-719. https://doi.org/10.3390/biomass4030039









