Leveraging Functional Genomics and Engineering Approaches to Uncover the Molecular Mechanisms of Cnidarian–Dinoflagellate Symbiosis and Broaden Biotechnological Applications
Abstract
:1. Introduction
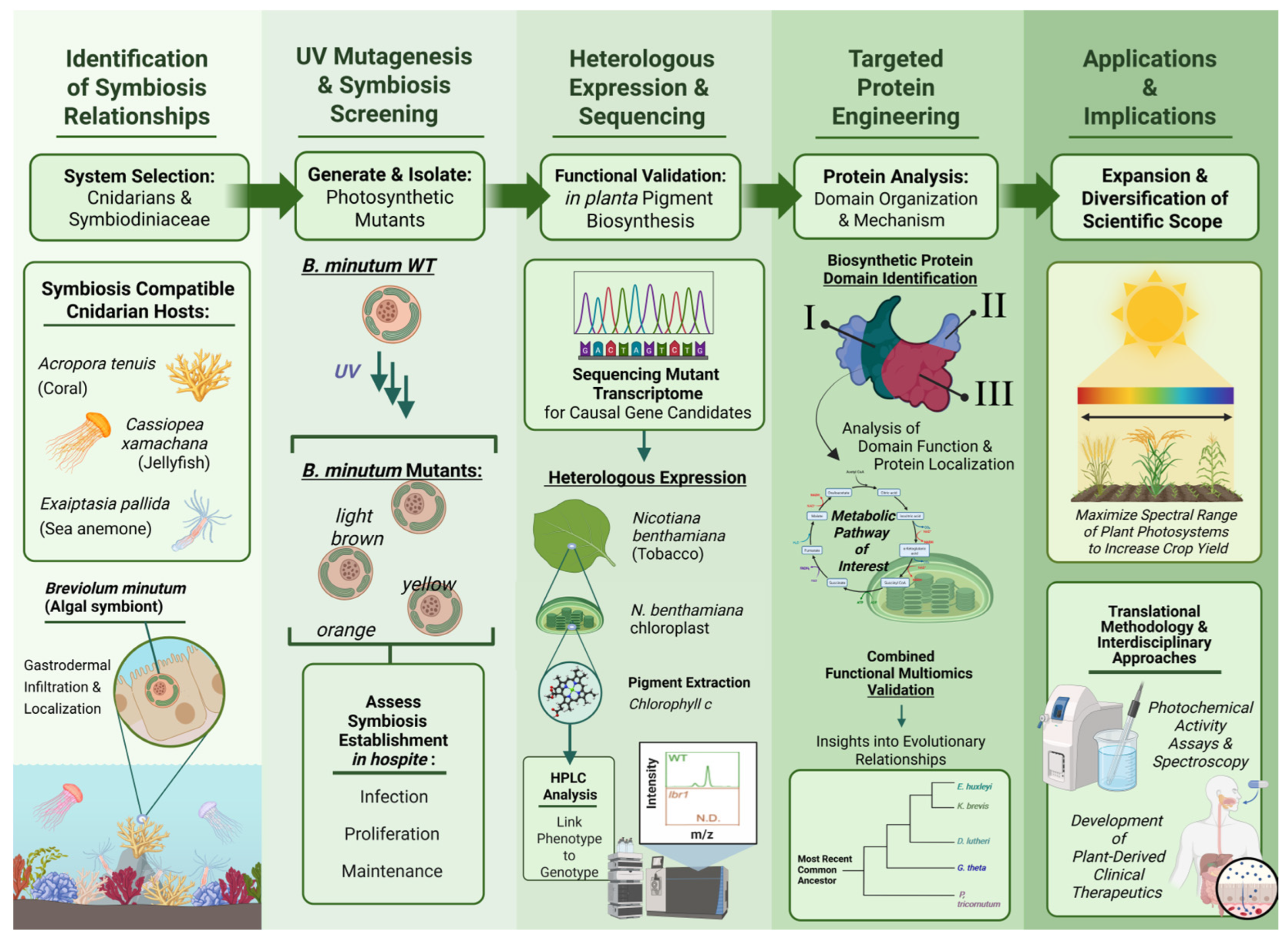
Functional Genomics to Decipher and Engineer Marine Symbioses
2. Photosynthesis in Marine Algal Symbiosis
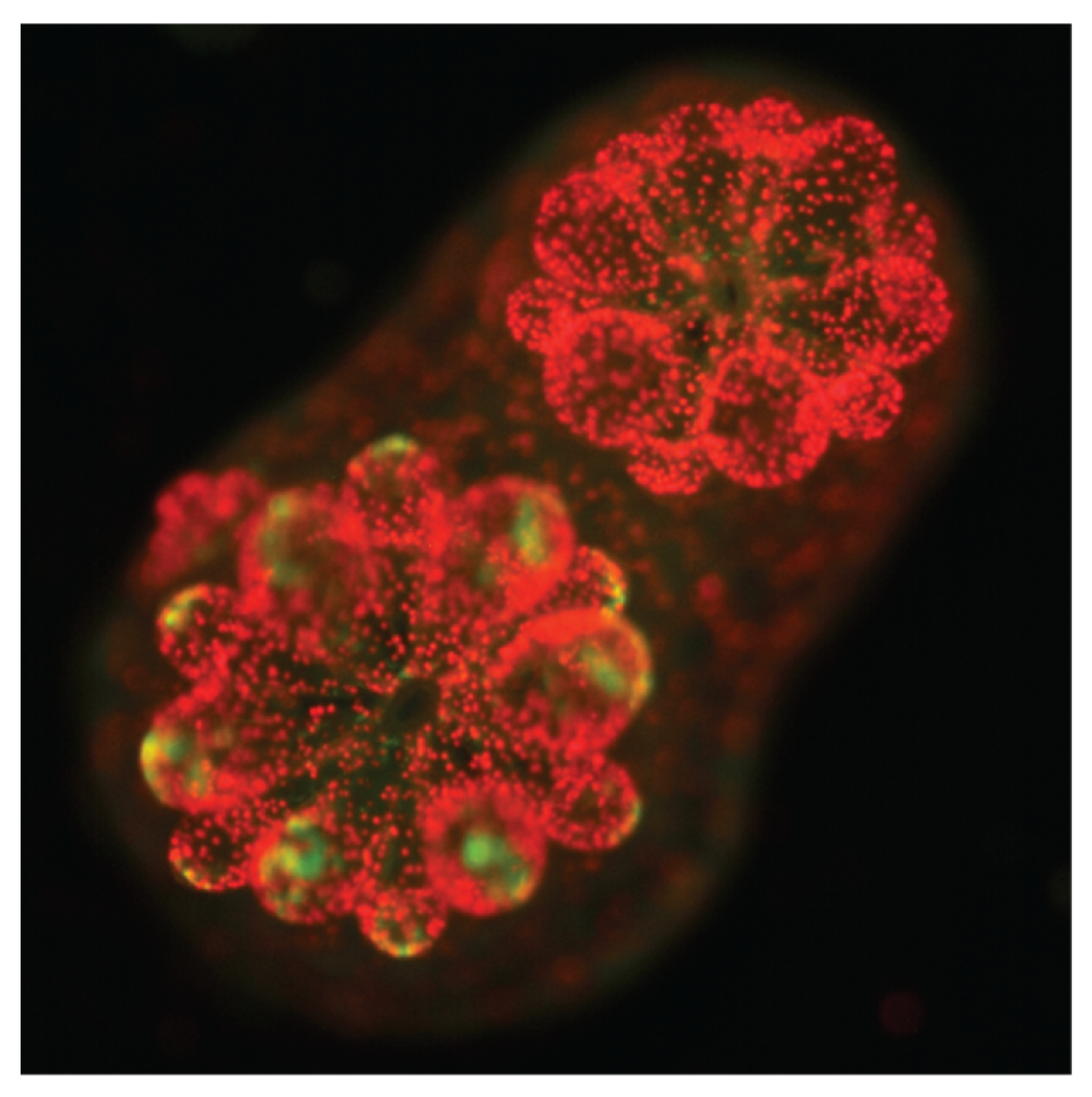
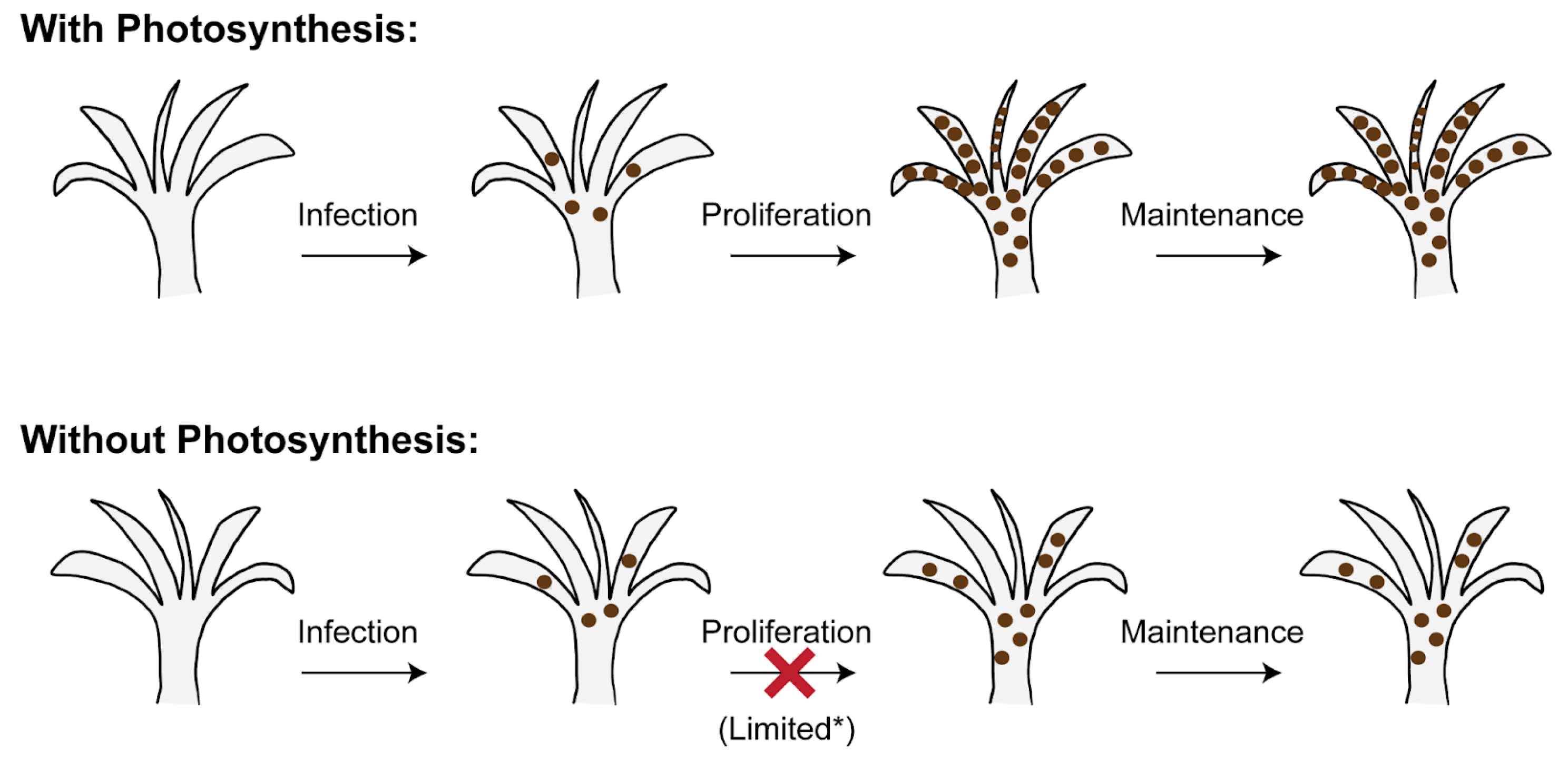
2.1. The Establishment of Cnidarian–Symbiodiniaceae Symbiosis Is Independent of Algal Photosynthesis
2.2. Other Molecular and Environmental Modulators of Symbiosis Establishment
3. Research Beyond the Role of Photosynthesis Within Cnidarian–Algal Symbiosis
3.1. Identification of Chlorophyll c Biosynthesis Pathway in Dinoflagellates
3.2. From Dinoflagellates to Plants for Enhanced Light Capture and Artificial Photosynthesis
4. Marine Pharmacology: A Sustainable Frontier for Therapeutic Discovery
Bridging Gaps in Biosynthetic Mechanisms Is Critical for Novel Therapeutic Development
5. Advancing Genomic Research in Marine Symbiosis and Beyond
5.1. Limitations in Genetic Engineering Tools for Studying Symbiodiniaceae Algae
5.2. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ALA | δ-Aminolevulinic acide |
| BmCHCLS | Breviolum minutum Chlorophyll c Synthase |
| Chls c | Chlorophyll c |
| CRISPR | Clustered Regularly Interspaced Short Palindromic Repeats |
| DCMU | 3-(3,4-dichlorophenyl)-1,1-dimethylurea |
| MAMP | Microbe Associated Molecular Patterns |
| RNAi | Ribonucleic Acid Interference |
| UHPLC-HRMS | Ultra-High-Performance Liquid Chromatography–High-Resolution Mass Spectrometry |
| USFDA | United States Food and Drug Administration |
| UV | Ultraviolet |
| 2OGD | 2-oxyglutarate-Fe(II) dioxygenase |
| B. minutum | Breviolum minutum |
| N. benthamiana | Nicotiana benthamiana |
| B. minutum Mutants | |
| lbr1 | Light Brown 1 |
| ora1 | Orange 1 |
| yel7-12 | Yellow 7-12 |
References
- Weis, V.M.; Allemand, D. Physiology. What Determines Coral Health? Science 2009, 324, 1153–1155. [Google Scholar] [CrossRef]
- Davy, S.K.; Allemand, D.; Weis, V.M. Cell Biology of Cnidarian-Dinoflagellate Symbiosis. Microbiol. Mol. Biol. Rev. 2012, 76, 229–261. [Google Scholar] [CrossRef]
- Gordon, B.R.; Leggat, W. Symbiodinium-Invertebrate Symbioses and the Role of Metabolomics. Mar. Drugs 2010, 8, 2546–2568. [Google Scholar] [CrossRef]
- Baker, A. Flexibility and Specificity in Coral-Algal Symbiosis: Diversity, Ecology, and Biogeography of Symbiodinium. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 661–689. [Google Scholar] [CrossRef]
- Yellowlees, D.; Rees, T.A.V.; Leggat, W. Metabolic Interactions between Algal Symbionts and Invertebrate Hosts. Plant Cell Environ. 2008, 31, 679–694. [Google Scholar] [CrossRef]
- Fisher, R.; O’Leary, R.A.; Low-Choy, S.; Mengersen, K.; Knowlton, N.; Brainard, R.E.; Caley, M.J. Species Richness on Coral Reefs and the Pursuit of Convergent Global Estimates. Curr. Biol. 2015, 25, 500–505. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O.; Poloczanska, E.S.; Skirving, W.; Dove, S. Coral Reef Ecosystems under Climate Change and Ocean Acidification. Front. Mar. Sci. 2017, 4, 158. [Google Scholar] [CrossRef]
- Ainsworth, T.D.; Brown, B.E. Coral Bleaching. Curr. Biol. 2021, 31, R5–R6. [Google Scholar] [CrossRef]
- Douglas, A.E. Coral Bleaching—How and Why? Mar. Pollut. Bull. 2003, 46, 385–392. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O. Climate Change, Coral Bleaching and the Future of the World’s Coral Reefs. Mar. Freshw. Res. 1999, 50, 839. [Google Scholar] [CrossRef]
- Helgoe, J.; Davy, S.K.; Weis, V.M.; Rodriguez-Lanetty, M. Triggers, Cascades, and Endpoints: Connecting the Dots of Coral Bleaching Mechanisms. Biol. Rev. Camb. Philos. Soc. 2024, 99, 715–752. [Google Scholar] [CrossRef] [PubMed]
- Allen-Waller, L.; Barott, K.L. Symbiotic Dinoflagellates Divert Energy Away from Mutualism during Coral Bleaching Recovery. Symbiosis 2023, 89, 173–186. [Google Scholar] [CrossRef]
- Fitt, W.; Brown, B.; Warner, M.; Dunne, R. Coral Bleaching: Interpretation of Thermal Tolerance Limits and Thermal Thresholds in Tropical Corals. Coral Reefs 2001, 20, 51–65. [Google Scholar] [CrossRef]
- Dai, N.; Wang, Q.; Xu, B.; Chen, H. Remarkable Natural Biological Resource of Algae for Medical Applications. Front. Mar. Sci. 2022, 9, 912924. [Google Scholar] [CrossRef]
- Oliveira, C.Y.B.; Oliveira, C.D.L.; Müller, M.N.; Santos, E.P.; Dantas, D.M.M.; Gálvez, A.O. A Scientometric Overview of Global Dinoflagellate Research. Publications 2020, 8, 50. [Google Scholar] [CrossRef]
- Przybyla, L.; Gilbert, L.A. A New Era in Functional Genomics Screens. Nat. Rev. Genet. 2022, 23, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Fields, S.; Kohara, Y.; Lockhart, D.J. Functional Genomics. Proc. Natl. Acad. Sci. USA 1999, 96, 8825–8826. [Google Scholar] [CrossRef]
- Hieter, P.; Boguski, M. Functional Genomics: It’s All How You Read It. Science 1997, 278, 601–602. [Google Scholar] [CrossRef]
- Kim, H.S.; Kweon, J.; Kim, Y. Recent Advances in CRISPR-Based Functional Genomics for the Study of Disease-Associated Genetic Variants. Exp. Mol. Med. 2024, 56, 861–869. [Google Scholar] [CrossRef]
- Jinkerson, R.E.; Poveda-Huertes, D.; Cooney, E.C.; Cho, A.; Ochoa-Fernandez, R.; Keeling, P.J.; Xiang, T.; Andersen-Ranberg, J. Biosynthesis of Chlorophyll c in a Dinoflagellate and Heterologous Production in Planta. Curr. Biol. 2024, 34, 594–605.e4. [Google Scholar] [CrossRef]
- Razzaq, M.K.; Aleem, M.; Mansoor, S.; Khan, M.A.; Rauf, S.; Iqbal, S.; Siddique, K.H.M. Omics and CRISPR-Cas9 Approaches for Molecular Insight, Functional Gene Analysis, and Stress Tolerance Development in Crops. Int. J. Mol. Sci. 2021, 22, 1292. [Google Scholar] [CrossRef]
- Todor, H.; Silvis, M.R.; Osadnik, H.; Gross, C.A. Bacterial CRISPR Screens for Gene Function. Curr. Opin. Microbiol. 2021, 59, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Doench, J.G.; Chi, H. CRISPR Screens for Functional Interrogation of Immunity. Nat. Rev. Immunol. 2023, 23, 363–380. [Google Scholar] [CrossRef]
- Martín-Zamora, F.M.; Liang, Y.; Guynes, K.; Carrillo-Baltodano, A.M.; Davies, B.E.; Donnellan, R.D.; Tan, Y.; Moggioli, G.; Seudre, O.; Tran, M.; et al. Annelid Functional Genomics Reveal the Origins of Bilaterian Life Cycles. Nature 2023, 615, 105–110. [Google Scholar] [CrossRef]
- Hirooka, S.; Itabashi, T.; Ichinose, T.M.; Onuma, R.; Fujiwara, T.; Yamashita, S.; Jong, L.W.; Tomita, R.; Iwane, A.H.; Miyagishima, S.-Y. Life Cycle and Functional Genomics of the Unicellular Red Alga Galdieria for Elucidating Algal and Plant Evolution and Industrial Use. Proc. Natl. Acad. Sci. USA 2022, 119, e2210665119. [Google Scholar] [CrossRef] [PubMed]
- Falk, D.A.; Watts, A.C.; Thode, A.E. Scaling Ecological Resilience. Front. Ecol. Evol. 2019, 7, 275. [Google Scholar] [CrossRef]
- Wang, Y.; Cai, Y.; Xie, Y.; Zhang, P.; Chen, L. A Quantitative Framework to Evaluate Urban Ecological Resilience: Broadening Understanding through Multi-Attribute Perspectives. Front. Ecol. Evol. 2023, 11, 1144244. [Google Scholar] [CrossRef]
- Venn, A.A.; Loram, J.E.; Douglas, A.E. Photosynthetic Symbioses in Animals. J. Exp. Bot. 2008, 59, 1069–1080. [Google Scholar] [CrossRef]
- Jinkerson, R.E.; Russo, J.A.; Newkirk, C.R.; Kirk, A.L.; Chi, R.J.; Martindale, M.Q.; Grossman, A.R.; Hatta, M.; Xiang, T. Cnidarian-Symbiodiniaceae Symbiosis Establishment Is Independent of Photosynthesis. Curr. Biol. 2022, 32, 2402–2415.e4. [Google Scholar] [CrossRef]
- Jones, R.J. Testing the ‘photoinhibition’ Model of Coral Bleaching Using Chemical Inhibitors. Mar. Ecol. Prog. Ser. 2004, 284, 133–145. [Google Scholar] [CrossRef]
- Zaquin, T.; Zaslansky, P.; Pinkas, I.; Mass, T. Simulating Bleaching: Long-Term Adaptation to the Dark Reveals Phenotypic Plasticity of the Mediterranean Sea Coral Oculina Patagonica. Front. Mar. Sci. 2019, 6, 662. [Google Scholar] [CrossRef]
- Jacobovitz, M.R.; Hambleton, E.A.; Guse, A. Unlocking the Complex Cell Biology of Coral-Dinoflagellate Symbiosis: A Model Systems Approach. Annu. Rev. Genet. 2023, 57, 411–434. [Google Scholar] [CrossRef] [PubMed]
- Nyholm, S.V.; McFall-Ngai, M.J. The Winnowing: Establishing the Squid-Vibrio Symbiosis. Nat. Rev. Microbiol. 2004, 2, 632–642. [Google Scholar] [CrossRef]
- Tivey, T.R.; Parkinson, J.E.; Weis, V.M. Host and Symbiont Cell Cycle Coordination Is Mediated by Symbiotic State, Nutrition, and Partner Identity in a Model Cnidarian-Dinoflagellate Symbiosis. MBio 2020, 11, e02626-19. [Google Scholar] [CrossRef]
- Xiang, T.; Jinkerson, R.E.; Clowez, S.; Tran, C.; Krediet, C.J.; Onishi, M.; Cleves, P.A.; Pringle, J.R.; Grossman, A.R. Glucose-Induced Trophic Shift in an Endosymbiont Dinoflagellate with Physiological and Molecular Consequences. Plant Physiol. 2018, 176, 1793–1807. [Google Scholar] [CrossRef] [PubMed]
- Russo, J.A.; Xiang, T.; Jinkerson, R.E. Protocol for the Generation of Symbiodiniaceae Mutants Using UV Mutagenesis. STAR Protoc. 2023, 4, 102627. [Google Scholar] [CrossRef]
- Rosset, S.L.; Oakley, C.A.; Ferrier-Pagès, C.; Suggett, D.J.; Weis, V.M.; Davy, S.K. The Molecular Language of the Cnidarian-Dinoflagellate Symbiosis. Trends Microbiol. 2021, 29, 320–333. [Google Scholar] [CrossRef]
- Douglas, A.; Smith, D.C. The Green Hydra Symbiosis. VIII. Mechanisms in Symbiont Regulation. Proc. R. Soc. Lond. 1984, 221, 291–319. [Google Scholar] [CrossRef]
- Aihara, Y.; Maruyama, S.; Baird, A.H.; Iguchi, A.; Takahashi, S.; Minagawa, J. Green Fluorescence from Cnidarian Hosts Attracts Symbiotic Algae. Proc. Natl. Acad. Sci. USA 2019, 116, 2118–2123. [Google Scholar] [CrossRef]
- Roth, M.S. The Engine of the Reef: Photobiology of the Coral–Algal Symbiosis. Front. Microbiol. 2014, 5, 422. [Google Scholar] [CrossRef]
- Rehman, A.U.; Szabó, M.; Deák, Z.; Sass, L.; Larkum, A.; Ralph, P.; Vass, I. Symbiodinium Sp. Cells Produce Light-Induced Intra- and Extracellular Singlet Oxygen, Which Mediates Photodamage of the Photosynthetic Apparatus and Has the Potential to Interact with the Animal Host in Coral Symbiosis. New Phytol. 2016, 212, 472–484. [Google Scholar] [CrossRef] [PubMed]
- Xiang, T.; Nelson, W.; Rodriguez, J.; Tolleter, D.; Grossman, A.R. Symbiodinium Transcriptome and Global Responses of Cells to Immediate Changes in Light Intensity When Grown under Autotrophic or Mixotrophic Conditions. Plant J. 2015, 82, 67–80. [Google Scholar] [CrossRef]
- Oliveira, C.Y.B.; Abreu, J.L.; Santos, E.P.; Matos, Â.P.; Tribuzi, G.; Oliveira, C.D.L.; Veras, B.O.; Bezerra, R.S.; Müller, M.N.; Gálvez, A.O. Light Induces Peridinin and Docosahexaenoic Acid Accumulation in the Dinoflagellate Durusdinium Glynnii. Appl. Microbiol. Biotechnol. 2022, 106, 6263–6276. [Google Scholar] [CrossRef] [PubMed]
- Fitt, W.K.; Cook, C.B. Photoacclimation and the Effect of the Symbiotic Environment on the Photosynthetic Response of Symbiotic Dinoflagellates in the Tropical Marine Hydroid Myrionema Amboinense. J. Exp. Mar. Bio. Ecol. 2001, 256, 15–31. [Google Scholar] [CrossRef]
- Sun, Y.; Jiang, L.; Gong, S.; Guo, M.; Yuan, X.; Zhou, G.; Lei, X.; Zhang, Y.; Yuan, T.; Lian, J.; et al. Impact of Ocean Warming and Acidification on Symbiosis Establishment and Gene Expression Profiles in Recruits of Reef Coral Acropora Intermedia. Front. Microbiol. 2020, 11, 532447. [Google Scholar] [CrossRef]
- Grottoli, A.G.; Dalcin Martins, P.; Wilkins, M.J.; Johnston, M.D.; Warner, M.E.; Cai, W.-J.; Melman, T.F.; Hoadley, K.D.; Pettay, D.T.; Levas, S.; et al. Coral Physiology and Microbiome Dynamics under Combined Warming and Ocean Acidification. PLoS ONE 2018, 13, e0191156. [Google Scholar] [CrossRef] [PubMed]
- Galston, A.W. Photosynthesis as a Basis for Life Support on Earth and in Space. Bioscience 1992, 42, 490–493. [Google Scholar] [CrossRef]
- Grayson, K.J.; Faries, K.M.; Huang, X.; Qian, P.; Dilbeck, P.; Martin, E.C.; Hitchcock, A.; Vasilev, C.; Yuen, J.M.; Niedzwiedzki, D.M.; et al. Augmenting Light Coverage for Photosynthesis through YFP-Enhanced Charge Separation at the Rhodobacter Sphaeroides Reaction Centre. Nat. Commun. 2017, 8, 13972. [Google Scholar] [CrossRef]
- Hitchcock, A.; Hunter, C.N.; Sobotka, R.; Komenda, J.; Dann, M.; Leister, D. Redesigning the Photosynthetic Light Reactions to Enhance Photosynthesis—The PhotoRedesign Consortium. Plant J. 2022, 109, 23–34. [Google Scholar] [CrossRef]
- Vullev, V.I. From Biomimesis to Bioinspiration: What’s the Benefit for Solar Energy Conversion Applications? J. Phys. Chem. Lett. 2011, 2, 503–508. [Google Scholar] [CrossRef]
- Su, J.; Vayssieres, L. A Place in the Sun for Artificial Photosynthesis? ACS Energy Lett. 2016, 1, 121–135. [Google Scholar] [CrossRef]
- Myśliwa-Kurdziel, B.; Latowski, D.; Strzałka, K. Chlorophylls c—Occurrence, Synthesis, Properties, Photosynthetic and Evolutionary Significance. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2019; pp. 91–119. ISBN 9780081027523. [Google Scholar] [CrossRef]
- Reinbothe, S.; Reinbothe, C. The Regulation of Enzymes Involved in Chlorophyll Biosynthesis. Eur. J. Biochem. 1996, 237, 323–343. [Google Scholar] [CrossRef]
- Averina, S.G.; Velichko, N.V.; Pinevich, A.A.; Senatskaya, E.V.; Pinevich, A.V. Non-a Chlorophylls in Cyanobacteria. Photosynthetica 2019, 57, 1109–1118. [Google Scholar] [CrossRef]
- Wang, P.; Grimm, B. Connecting Chlorophyll Metabolism with Accumulation of the Photosynthetic Apparatus. Trends Plant Sci. 2021, 26, 484–495. [Google Scholar] [CrossRef]
- Peterhansel, C.; Niessen, M.; Kebeish, R.M. Metabolic Engineering towards the Enhancement of Photosynthesis. Photochem. Photobiol. 2008, 84, 1317–1323. [Google Scholar] [CrossRef]
- Liu, Y.; Gui, Z.; Liu, J. Research Progress of Light Wavelength Conversion Materials and Their Applications in Functional Agricultural Films. Polymers 2022, 14, 851. [Google Scholar] [CrossRef] [PubMed]
- Eckhardt, U.; Grimm, B.; Hörtensteiner, S. Recent Advances in Chlorophyll Biosynthesis and Breakdown in Higher Plants. Plant Mol. Biol. 2004, 56, 1–14. [Google Scholar] [CrossRef]
- Rebeiz, C.A.; Benning, C.; Bohnert, H.J.; Daniell, H.; Hoober, J.K.; Lichtenthaler, H.K.; Portis, A.R.; Tripathy, B.C. The Chloroplast: Basics and Applications; Springer: Dordrecht, The Netherlands, 2010; p. 426. [Google Scholar]
- Wei, S.; Zhang, W.; Fu, R.; Zhang, Y. Genome-Wide Characterization of 2-Oxoglutarate and Fe(II)-Dependent Dioxygenase Family Genes in Tomato during Growth Cycle and Their Roles in Metabolism. BMC Genomics 2021, 22, 126. [Google Scholar] [CrossRef]
- Bolte, K.; Bullmann, L.; Hempel, F.; Bozarth, A.; Zauner, S. Maier Protein Targeting into Secondary Plastids 1. J. Eukaryot. Microbiol. 2009, 56, 9–15. [Google Scholar] [CrossRef]
- Cavalier-Smith, T. Principles of Protein and Lipid Targeting in Secondary Symbiogenesis: Euglenoid, Dinoflagellate, and Sporozoan Plastid Origins and the Eukaryote Family Tree. J. Eukaryot. Microbiol. 1999, 46, 347–366. [Google Scholar] [CrossRef]
- Raven, J.A. Cells inside Cells: Symbiosis and Continuing Phagotrophy. Curr. Biol. 2013, 23, R530–R531. [Google Scholar] [CrossRef]
- Liu, G.; Gao, F.; Gao, C.; Xiong, Y. Bioinspiration toward Efficient Photosynthetic Systems: From Biohybrids to Biomimetics. Chem Catal. 2021, 1, 1367–1377. [Google Scholar] [CrossRef]
- Huang, G.; Xu, J.; Markides, C.N. High-Efficiency Bio-Inspired Hybrid Multi-Generation Photovoltaic Leaf. Nat. Commun. 2023, 14, 3344. [Google Scholar] [CrossRef]
- Hann, E.C.; Overa, S.; Harland-Dunaway, M.; Narvaez, A.F.; Le, D.N.; Orozco-Cárdenas, M.L.; Jiao, F.; Jinkerson, R.E. A Hybrid Inorganic-Biological Artificial Photosynthesis System for Energy-Efficient Food Production. Nat. Food 2022, 3, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Luo, D.; Luesch, H. Advances in Exploring the Therapeutic Potential of Marine Natural Products. Pharmacol. Res. 2019, 147, 104373. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, A.; Joseph, A.; Nair, B.G. Promising Bioactive Compounds from the Marine Environment and Their Potential Effects on Various Diseases. J. Genet. Eng. Biotechnol. 2022, 20, 14. [Google Scholar] [CrossRef]
- Blunt, J.W.; Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2018, 35, 8–53. [Google Scholar] [CrossRef]
- Yang, J.; Tan, S.; Ge, S.; Yang, M.; Liu, H.; Liu, W.; Zhang, K.; Zhang, Z.; Wang, Z.-H.; Shi, J.; et al. Cyanobacteria-Probiotics Symbionts for Modulation of Intestinal Inflammation and Microbiome Dysregulation in Colitis. Proc. Natl. Acad. Sci. USA 2024, 121, e2403417121. [Google Scholar] [CrossRef]
- Datta, D.; Nath Talapatra, S.; Swarnakar, S. Bioactive Compounds from Marine Invertebrates for Potential Medicines—An Overview. Int. Lett. Nat. Sci. 2015, 34, 42–61. [Google Scholar] [CrossRef]
- Stonik, V.A. Marine Natural Products: A Way to New Drugs. Acta Naturae 2009, 1, 15–25. [Google Scholar] [CrossRef]
- Mayer, A.M.S.; Guerrero, A.J.; Rodríguez, A.D.; Taglialatela-Scafati, O.; Nakamura, F.; Fusetani, N. Marine Pharmacology in 2014–2015: Marine Compounds with Antibacterial, Antidiabetic, Antifungal, Anti-Inflammatory, Antiprotozoal, Antituberculosis, Antiviral, and Anthelmintic Activities; Affecting the Immune and Nervous Systems, and Other Miscellaneous Mechanisms of Action. Mar. Drugs 2019, 18, 5. [Google Scholar] [CrossRef] [PubMed]
- Malve, H. Exploring the Ocean for New Drug Developments: Marine Pharmacology. J. Pharm. Bioallied Sci. 2016, 8, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Hanif, N.; Murni, A.; Tanaka, C.; Tanaka, J. Marine Natural Products from Indonesian Waters. Mar. Drugs 2019, 17, 364. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.-H.; Wang, Y.-J.; Sheng, J.; Wang, F.; Zheng, Y.; Lin, X.-K.; Sun, M. Antitumor Peptides from Marine Organisms. Mar. Drugs 2011, 9, 1840–1859. [Google Scholar] [CrossRef]
- Romano, G.; Almeida, M.; Varela Coelho, A.; Cutignano, A.; Gonçalves, L.G.; Hansen, E.; Khnykin, D.; Mass, T.; Ramšak, A.; Rocha, M.S.; et al. Biomaterials and Bioactive Natural Products from Marine Invertebrates: From Basic Research to Innovative Applications. Mar. Drugs 2022, 20, 219. [Google Scholar] [CrossRef]
- Rudi, K.; Zhao, L. Grand Challenges in Understanding Gut Microbes. Front. Microbiol. 2021, 12, 752829. [Google Scholar] [CrossRef]
- Diaz, J.; Reese, A.T. Possibilities and Limits for Using the Gut Microbiome to Improve Captive Animal Health. Anim. Microbiome 2021, 3, 89. [Google Scholar] [CrossRef]
- Kim, D.; Hofstaedter, C.E.; Zhao, C.; Mattei, L.; Tanes, C.; Clarke, E.; Lauder, A.; Sherrill-Mix, S.; Chehoud, C.; Kelsen, J.; et al. Optimizing Methods and Dodging Pitfalls in Microbiome Research. Microbiome 2017, 5, 52. [Google Scholar] [CrossRef]
- Walter, J.; Armet, A.M.; Finlay, B.B.; Shanahan, F. Establishing or Exaggerating Causality for the Gut Microbiome: Lessons from Human Microbiota-Associated Rodents. Cell 2020, 180, 221–232. [Google Scholar] [CrossRef]
- Wang, G.-J.; Gao, C.-F.; Wei, D.; Wang, C.; Ding, S.-Q. Acute Pancreatitis: Etiology and Common Pathogenesis. World J. Gastroenterol. 2009, 15, 1427–1430. [Google Scholar] [CrossRef]
- Chang, L.; Chey, W.D.; Imdad, A.; Almario, C.V.; Bharucha, A.E.; Diem, S.; Greer, K.B.; Hanson, B.; Harris, L.A.; Ko, C.; et al. American Gastroenterological Association-American College of Gastroenterology Clinical Practice Guideline: Pharmacological Management of Chronic Idiopathic Constipation. Gastroenterology 2023, 164, 1086–1106. [Google Scholar] [CrossRef]
- Saha, L. Irritable Bowel Syndrome: Pathogenesis, Diagnosis, Treatment, and Evidence-Based Medicine. World J. Gastroenterol. 2014, 20, 6759–6773. [Google Scholar] [CrossRef]
- Guo, M.; Wang, X. Pathological Mechanism and Targeted Drugs of Ulcerative Colitis: A Review. Medicine 2023, 102, e35020. [Google Scholar] [CrossRef] [PubMed]
- Chávez, M.N.; Moellhoff, N.; Schenck, T.L.; Egaña, J.T.; Nickelsen, J. Photosymbiosis for Biomedical Applications. Front. Bioeng. Biotechnol. 2020, 8, 577204. [Google Scholar] [CrossRef] [PubMed]
- Radjasa, O.K.; Vaske, Y.M.; Navarro, G.; Vervoort, H.C.; Tenney, K.; Linington, R.G.; Crews, P. Highlights of Marine Invertebrate-Derived Biosynthetic Products: Their Biomedical Potential and Possible Production by Microbial Associants. Bioorg. Med. Chem. 2011, 19, 6658–6674. [Google Scholar] [CrossRef] [PubMed]
- Gerwick, W.H.; Moore, B.S. Lessons from the Past and Charting the Future of Marine Natural Products Drug Discovery and Chemical Biology. Chem. Biol. 2012, 19, 85–98. [Google Scholar] [CrossRef]
- Proksch, P.; Edrada-Ebel, R.; Ebel, R. Drugs from the Sea—Opportunities and Obstacles. Mar. Drugs 2003, 1, 5–17. [Google Scholar] [CrossRef]
- Molinski, T.F.; Dalisay, D.S.; Lievens, S.L.; Saludes, J.P. Drug Development from Marine Natural Products. Nat. Rev. Drug Discov. 2009, 8, 69–85. [Google Scholar] [CrossRef]
- Costas, E.; Goyanes, V. Architecture and Evolution of Dinoflagellate Chromosomes: An Enigmatic Origin. Cytogenet. Genome Res. 2005, 109, 268–275. [Google Scholar] [CrossRef]
- Gornik, S.G.; Hu, I.; Lassadi, I.; Waller, R.F. The Biochemistry and Evolution of the Dinoflagellate Nucleus. Microorganisms 2019, 7, 245. [Google Scholar] [CrossRef]
- Lin, S. A Decade of Dinoflagellate Genomics Illuminating an Enigmatic Eukaryote Cell. BMC Genomics 2024, 25, 932. [Google Scholar] [CrossRef]
- De Saeger, J.; Coulembier Vandelannoote, E.; Lee, H.; Park, J.; Blomme, J. Genome Editing in Macroalgae: Advances and Challenges. Front. Genome Ed. 2024, 6, 1380682. [Google Scholar] [CrossRef]
- Marinov, G.K.; Chen, X.; Swaffer, M.P.; Xiang, T.; Grossman, A.R.; Greenleaf, W.J. Genome-Wide Distribution of 5-Hydroxymethyluracil and Chromatin Accessibility in the Breviolum Minutum Genome. Genome Biol. 2024, 25, 115. [Google Scholar] [CrossRef]
- Marinov, G.K.; Trevino, A.E.; Xiang, T.; Kundaje, A.; Grossman, A.R.; Greenleaf, W.J. Transcription-Dependent Domain-Scale Three-Dimensional Genome Organization in the Dinoflagellate Breviolum Minutum. Nat. Genet. 2021, 53, 613–617. [Google Scholar] [CrossRef] [PubMed]
- LaJeunesse, T.C.; Parkinson, J.E.; Gabrielson, P.W.; Jeong, H.J.; Reimer, J.D.; Voolstra, C.R.; Santos, S.R. Systematic Revision of Symbiodiniaceae Highlights the Antiquity and Diversity of Coral Endosymbionts. Curr. Biol. 2018, 28, 2570–2580.e6. [Google Scholar] [CrossRef] [PubMed]
- Gornik, S.G.; Maegele, I.; Hambleton, E.A.; Voss, P.A.; Waller, R.F.; Guse, A. Nuclear Transformation of a Dinoflagellate Symbiont of Corals. Front. Mar. Sci. 2022, 9, 1035413. [Google Scholar] [CrossRef]
- Ciche, T.A.; Goffredi, S.K. General Methods to Investigate Microbial Symbioses. In Methods for General and Molecular Microbiology; ASM Press: Washington, DC, USA, 2014; pp. 394–419. ISBN 9781683671619. [Google Scholar] [CrossRef]
- Gutiérrez, S.; Lauersen, K.J. Gene Delivery Technologies with Applications in Microalgal Genetic Engineering. Biology 2021, 10, 265. [Google Scholar] [CrossRef]
- Neupert, J.; Shao, N.; Lu, Y.; Bock, R. Genetic Transformation of the Model Green Alga Chlamydomonas Reinhardtii. Methods Mol. Biol. 2012, 847, 35–47. [Google Scholar] [CrossRef]
- Zaslavskaia, L.A.; Lippmeier, J.C.; Kroth, P.G.; Grossman, A.R.; Apt, K.E. Transformation of the Diatom Phaeodactylum tricornutum (Bacillariophyceae) with a Variety of Selectable Marker and Reporter Genes. J. Phycol. 2000, 36, 379–386. [Google Scholar] [CrossRef]
- Pairs, P.I.; Dundon, M.L.; Narváez-Vásquez, J.; Orozco-Cárdenas, M.L.; Xiang, T.; Jinkerson, R.E.; Rao, M.P. Cell Wall Digestion of the Dinoflagellate Breviolum Minutum. J. Appl. Phycol. 2024, 36, 181–189. [Google Scholar] [CrossRef]
- Gaubert, J.; Greff, S.; Thomas, O.P.; Payri, C.E. Metabolomic Variability of Four Macroalgal Species of the Genus Lobophora Using Diverse Approaches. Phytochemistry 2019, 162, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.R.; Purton, S. Genetic Engineering of Eukarygtic Algae: Progress and Prospects. J. Phycol. 1997, 33, 713–722. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mannur, G.; Taepakdee, A.; Ho, J.P.; Xiang, T. Leveraging Functional Genomics and Engineering Approaches to Uncover the Molecular Mechanisms of Cnidarian–Dinoflagellate Symbiosis and Broaden Biotechnological Applications. Phycology 2025, 5, 14. https://doi.org/10.3390/phycology5020014
Mannur G, Taepakdee A, Ho JP, Xiang T. Leveraging Functional Genomics and Engineering Approaches to Uncover the Molecular Mechanisms of Cnidarian–Dinoflagellate Symbiosis and Broaden Biotechnological Applications. Phycology. 2025; 5(2):14. https://doi.org/10.3390/phycology5020014
Chicago/Turabian StyleMannur, Gagan, Ashley Taepakdee, Jimmy Pham Ho, and Tingting Xiang. 2025. "Leveraging Functional Genomics and Engineering Approaches to Uncover the Molecular Mechanisms of Cnidarian–Dinoflagellate Symbiosis and Broaden Biotechnological Applications" Phycology 5, no. 2: 14. https://doi.org/10.3390/phycology5020014
APA StyleMannur, G., Taepakdee, A., Ho, J. P., & Xiang, T. (2025). Leveraging Functional Genomics and Engineering Approaches to Uncover the Molecular Mechanisms of Cnidarian–Dinoflagellate Symbiosis and Broaden Biotechnological Applications. Phycology, 5(2), 14. https://doi.org/10.3390/phycology5020014






